8
Ligament Healing
1. Define and discuss the inflammatory response to injury.
2. Describe the phases of healing and sequence of events characteristic of each phase.
3. Identify the five cardinal signs of inflammation.
4. Describe the effects of immobilization on ligaments.
5. Discuss the effects of stress and exercise on ligaments.
6. Identify and discuss practical clinical applications of stress deprivation and protected motion during phases of ligament healing.
LIGAMENT ANATOMY
Interestingly, certain ligaments (e.g., ligamentum nuchae) contain greater amounts of elastin, which in turn contributes to different mechanical properties than those of ligaments with less elastin. Although considered hypovascular, ligaments demonstrate relative uniform microvascularity that originates from their origin and insertion sites.24
Ligaments have a rich sensory innervation of specialized mechanoreceptors and free nerve endings that contribute to proprioception and pain, respectively. Ligament attachment to bone is by direct or indirect transition. Direct ligament insertion into bone represents a gradual change from specific ligament fiber to fibrocartilage to calcified fibrocartilage to bone. With indirect insertion, the superficial layers of ligament fibers attach directly in the periosteum, whereas the deep fibers transition to bone by way of Sharpey’s perforating fibers.24
INJURY AND REPAIR
As with other vascular tissues, extraarticular ligaments heal in a highly structured, organized, and predictable fashion. Generally, the sequential cascade of events overlaps four stages of repair: Phase I, hemostasis and degeneration; Phase II, inflammation; Phase III, proliferation and migration; and Phase IV, remodeling and degeneration.11
In contrast, intraarticular ligaments, although demonstrating an intense vascular response to injury, do not heal spontaneously. The environment of intraarticular synovial fluid tends to dilute hematoma formation between the ends of the injured ligaments while preventing fibrin clot organization and ultimately limiting the intrinsic healing mechanism.24
Phase II is marked by a release of extremely potent chemical mediators of vasodilation, cell wall permeability, and pain in response to fibrin clot formation. Prostaglandins, histamine, bradykinins, and serotonin are mobilized to the trauma site to increase capillary permeability and profuse dilation of blood vessels. This action allows migration of specific inflammatory polymorphonuclear cells and lymphocytes to the injured tissue to initiate the action of ingestion (phagocytosis) to remove bacteria and dead tissue. The predominant cell types present during the acute inflammatory phase are neutrophils and lymphocytes. Monocytes are referred to as macrophages as they become phagocytes.24
The production of type III collagen, extracellular matrix, and, within 2 days, proteoglycans by fibroblasts initiate the beginning of Phase III matrix and cellular proliferation. Fibroblasts rapidly synthesize new extracellular matrix containing high concentrations of water; GAGs; and relatively weak, fragile, and immature type III collagen. Neovascularization (angiogenesis) begins as granulation tissue tenuously attaches to the damaged gap. Gradually the concentration of water, GAGs, and type III collagen decreases over several weeks. Inflammatory cytokines are slowly removed from the injured site. Fibroblastic activity synthesizes type I collagen during this highly cellular phase of repair. There is a marked decrease in vascularity within the repair tissue as the collagen concentration increases. Matrix organization continues as the fibrils of type I collagen slowly arrange and align in response to appropriately applied stress. As the density of collagen, elastin, and proteoglycans increase, the tensile properties of repaired tissue also increase.23
Important consideration must be given to the fact that intrinsically repaired extraarticular ligament does not return to normal, biochemically or biomechanically. Even after considerable time and remodeling of dense connective tissue, ultimate tensile strength may approach only 50% to 70% of normal ligaments up to 1 year after injury.16,24
The most common injury of joints are ligament sprains. The knee and ankle joints are common areas of sprains, with the incidence of knee ligament sprains, particularly those of the medial collateral ligament (MCL), occurring in as many as 25% to 40% of all knee injuries.7,19
Not all ligaments heal at the same rate or to the same degree.2 For example, the anterior cruciate ligament (ACL) does not appear to heal as well as the MCL of the knee.25 Factors affecting ligament healing include blood supply and function.12,25
Three key conditions must be present for ligaments to properly remodel or heal:2
1. Torn ligament ends must be in contact with each other.
2. Progressive, controlled stress must be applied to the healing tissues to orient scar tissue formation.
3. The ligament must be protected against excessive forces during the remodeling process.
The continuum of the healing process outlined here is ligament specific, and healing is related to blood supply, degree of injury, and mechanical stresses applied to the ligament.2
Nonsurgical Repair versus Surgical Repair
Ligaments can be repaired surgically or allowed to heal conservatively without surgery, depending on the degree of injury and involvement of supporting tissues. Investigators have shown that untreated ligaments heal by way of scar tissue proliferation rather than true ligament regeneration.8 Untreated ligament tears are biochemically inferior, possessing a large portion of type III immature collagen, and generally are not healed even at 40 weeks after injury.8
Grade I: Microscopic tearing of the ligament without producing joint laxity
Grade II: Tearing of some ligament fibers with moderate laxity
Grade III: Complete rupture of the ligament with profound instability and laxity
Grade I and II ligament sprains are most common, with only 15% of all knee sprains classified as grade III.3 Generally, grade I and II ligament sprains can be treated with protective bracing and comprehensive and progressive rehabilitation with appropriate strengthening to provide dynamic muscular support. With grade I and II ligament sprains of the knee (ACL, MCL, posterior cruciate ligament [PCL], and lateral collateral ligament [LCL]), good to excellent results can be anticipated in 90% of those cases treated nonsurgically.2
Repair versus Nonrepair
By virtue of its extracapsular anatomy, the MCL provides for a greater periarticular vascular response and the ability to protect the ligament from unwanted forces (e.g., varus, valgus, and internal and external rotation), and allows for an appropriate environment to stimulate healing and propagation of motion, collagen synthesis organization and orientation, proteoglycan concentration, and joint function. Therefore all three distinct grades (grades I, II, and III) of isolated MCL tears appear to heal uneventfully without surgical repair. Even though a fibrous repair gap may exist between torn ends of the ligament, resulting in inferior mechanical resistance to tensile loads, the greater cross-sectional area of the healed ligament provides for biochemical properties (e.g., ultimate tensile load to failure) that more closely resemble an uninjured ligament.24
Effects of Immobilization
Immobilization, surgery, injury, and rehabilitation of ligaments must take into consideration not only the healing response of the ligament itself, but also that of the ligament–bone interface. Stress deprivation of the ligament and ligament–bone complexes resulting from prolonged immobilization after injury or surgery can have significant and profound negative effects. Joint stiffness after immobilization is related to adhesion formation, active shortening of dense connective tissue (ligament),and decreases in water content.2 Studies show a gradual deterioration in ligament strength, loss of bone, weakening of cartilage and tendons, significant muscle atrophy, and negative effects on joint mechanics after periods of immobilization (Box 8-1).1 Immobilization also affects ligament–bone complexes. Studies report that loss of bone directly beneath the junction of ligament and bone reduces the strength of both the insertion site and entire ligament–bone complex.15
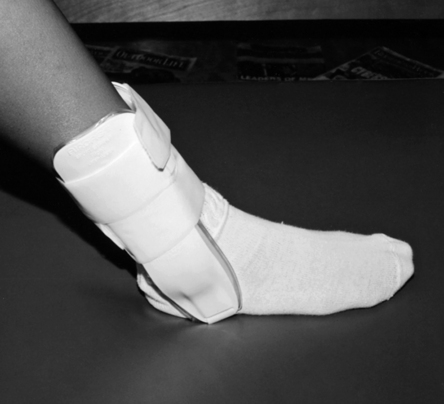
Rigidly immobilized joints produce chemical and morphologic changes in ligaments 2 and 4 weeks following injury, respectively.9 After 8 weeks of immobilization, ligaments lose 20% of their weight; significant atrophy results, and marked infiltration of periarticular connective tissue is observed surrounding the ligament.9 Although immobilization may be needed to promote healing of damaged tissues, the extended use of rigid immobilization should be limited. As an alternative, limited range of motion braces (Fig. 8-1) can be used to protect healing structures and decrease unwanted external forces, as well as allow for progressive motion of involved joints to minimize the negative effects of immobilization.
The effects of immobilization on dense connective tissue have been described by Cummings and Reynolds5 as follows: Following only 2 weeks of immobilization, animal studies revealed increased GAG synthesis and concentration, decreased water content, thickening of the joint capsule and ligaments, adaptive muscle shortening and adhesion formation of unopposed articular surfaces. After 4 weeks of immobilization, the biochemical and morphologic changes become more pronounced, with a reduction of GAGs, fissures in articular cartilage, increased ligament stiffness, and decreased capsular remodeling. After 6 weeks of immobilization, joint mobility becomes significantly limited, with thickening noted in the joint capsule, ligaments, and cartilage and decreased ligamentous compliance. In summary, joint immobilization causes a progression of dense connective tissue remodeling, resulting in joint stiffness.
During the first 2 weeks, adaptive muscle shortening appears to be the primary limiting factor in joint mobility. As immobilization extends to 4 weeks, changes in GAG synthesis and water concentration become more pronounced, resulting in a loss of normal fiber lubrication, spacing, and connective tissue disorganization. As the immobilization period approaches 6 weeks, morphologic and biochemical changes become more evident as the dense connective tissue remodels in a shortened position.24
Specifically, immobilization causes more pronounced mechanical and biochemical changes in ligament–bone complex insertion sites compared with ligament substance alone. Generally the area of bone directly beneath the ligament–bone insertion site becomes osteoporotic with osteoclastic activity, resulting in pronounced bone resorption, loss of cortex, and reduced strength of the entire ligament–bone insertion complex.24
Exercise
As stated, stress deprivation of ligaments, because of immobilization, results in atrophy.8,9,16,18,25 Conversely, motion, stress, and general physical activity prescribed for healing ligaments produce hypertrophy and increased tensile strength.2,9 Research shows that ligament and ligament–bone complex strength is related to the mode and duration of exercise used during rehabilitation.2 Tipton23 has shown that endurance types of exercise are more effective in producing larger diameter collagen than nonendurance types of exercise. In addition, the long-term detrimental effects of prolonged immobilization on ligament–bone insertion sites are reversible.2 In fact, the effects of mobilization and exercise are seen 4 months to 1 year after immobilization.2
EFFECTS OF REMOBILIZATION AND EXERCISE
As stated, ligament substance once injured may regain only 50% to 70% of normal tensile strength after 1 year or more of remodeling.24 Ligament–bone insertion complexes tend to remodel and regain tensile strength more slowly than ligament tissue after immobilization and therapeutically directed reconditioning. The clinical significance of delayed ligament–bone insertion healing compared with midsubstance tissue repairs is manifested by the relative increase in avulsion injury during this protracted healing interval.24
Generally, ligament substance and ligament–bone complexes are sensitive to exercise. These tissues become stronger depending on the type and duration of exercise prescribed. The orientation, composition, synthesis, and concentration of type I collagen and ultimate load to failure, increase in tensile strength, and stiffness of ligament and ligament–bone complexes are observed with appropriately directed exercise.24
Generally, after injury to ligaments, appropriate controlled motion and exercise stimulates ligament repair by improving matrix organization and composition, increasing the weight of injured ligaments, and promoting normalized collagen synthesis and strength.24
Continuous Passive Motion
Motion, exercise, and protected progressive stress can influence and determine the degree and type of healing that occur after trauma and subsequent immobilization.17 Studies have demonstrated that healing is dramatically different in immobilized joints compared with those moved passively through limited motion.10 Gelberman and colleagues10 have shown that joints moved passively have well-organized, longitudinally oriented collagen fibers in which no adhesions are present. Conversely, joints that were immobilized demonstrated scar tissue and adhesions.
The concept of early protected motion applied to healing soft tissues has resulted in the development of a technique termed continuous passive motion (CPM) (Fig. 8-2). CPM is used in the treatment of the following: knee joint contractures; postoperative ACL reconstructions; joint effusions; knee, elbow, and ankle fractures (after immobilization); joint arthrosis; and total knee arthroplasty.14 Early motion after surgery or immobilization acts to enhance and facilitate connective tissue strength, size, and shape; evacuate joint hemarthrosis (bloody effusion within the joint space); improve joint nutrition; inhibit adhesions; initiate normal joint kinematics; reduce articular surface changes; and minimize other deleterious effects of prolonged immobilization.14 With postoperative ACL reconstruction, no stretching out of the graft occurs when CPM is used.4,6,17,20 CPM can be used postoperatively; applied in the operating room; or be done a few days after surgery, immediately upon cast removal, or during the early phases of rehabilitation.
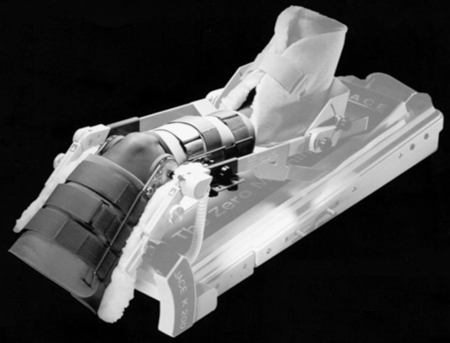
CPM devices have been designed for use on many body parts.14 The knee is the most common, with the ankle, shoulder, elbow, wrist, hand, and hip joints also benefiting from CPM. The CPM machine is calibrated in cycles per minute and degrees of motion. Progressive increases in the cycle mode and degrees of motion are made gradually so as not to initiate pain or increase the time necessary for healing.
Although CPM may be helpful to decrease postoperative pain following ACL reconstruction, systematic reviews suggest that there is no substantial advantage to using CPM, and no difference in long-term outcomes. It is recommend as an adjunct rather than a replacement for a postoperative exercise program.21,27
Practical Considerations
The time constraints and healing mechanics of ligaments are well documented.2,3,8,13,15,16,22,25,28 Careful consideration must be given to the progression of therapeutic exercises and functional activities for patients with ligament injuries. Usually the absence of pain and swelling is an exceedingly poor indicator of healing tissue. With an ACL reconstruction, pain and swelling normally subside within a few weeks, but return of functional joint motion requires a couple of months. Strength values gradually increase, with muscle hypertrophy following slowly.
As the outward clinical signs point to healing, the ligament, being a dynamic tissue, continues to remodel and mature for up to 1 year. Protection of the joint is critical during healing. Functional knee braces with range-limiting devices can have a protective effect for MCL injuries, however no evidence exists that supports the routine use of functional bracing following ACL reconstruction.26,27 Initiating progressive resistance exercises after knee ligament injury or surgery, while maintaining joint protection, can be challenging. Placing the resistance (weight) above the joint line during straight leg raises (Fig. 8-3) after ACL surgery can be the first phase of progressive resistance while protecting the healing ligament and retraining quadriceps activation.
To strengthen hip adduction with an MCL sprain, resistance initially is applied above the knee joint line so as not to overstress the healing MCL (Fig. 8-4). As the time constraints of healing allow, progressive strengthening of the adductors can involve loading the joint more distally, if joint protection is applied. Awareness of the time necessary for healing and duration of immobilization after trauma, weight bearing status, and degree of injury guide the physical therapist assistant (PTA) in making clinical recommendations about the progression of exercise and the placement of force during rehabilitation.
Developing a progressive therapeutic exercise and functional activities program with ankle sprains is similarly challenging. Generally, a grade II anterior talofibular (ATF) ligament sprain does not produce significant pain or functional limitations after a few weeks of conservative treatment involving splinting, crutch walking (non–weight bearing progressing to full weight bearing), ice, compression, and elevation. The PTA should protect the ligament for many weeks after injury because the process of ligament healing occurs slowly (Fig. 8-5).
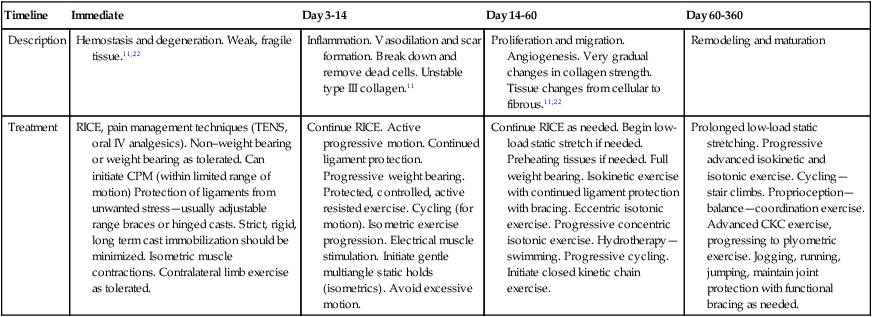
Table 8-1 outlines the stages of ligament healing and subsequent therapeutic interventions that enhance the healing of ligament tissues.
GLOSSARY
Table 8-1
Therapeutic Considerations During Stages of Ligament Healing
| Timeline | Immediate | Day 3-14 | Day 14-60 | Day 60-360 |
| Description | Hemostasis and degeneration. Weak, fragile tissue.11,22 | Inflammation. Vasodilation and scar formation. Break down and remove dead cells. Unstable type III collagen.11 | Proliferation and migration. Angiogenesis. Very gradual changes in collagen strength. Tissue changes from cellular to fibrous.11,22 | Remodeling and maturation |
| Treatment | RICE, pain management techniques (TENS, oral IV analgesics). Non–weight bearing or weight bearing as tolerated. Can initiate CPM (within limited range of motion) Protection of ligaments from unwanted stress—usually adjustable range braces or hinged casts. Strict, rigid, long term cast immobilization should be minimized. Isometric muscle contractions. Contralateral limb exercise as tolerated. | Continue RICE. Active progressive motion. Continued ligament protection. Progressive weight bearing. Protected, controlled, active resisted exercise. Cycling (for motion). Isometric exercise progression. Electrical muscle stimulation. Initiate gentle multiangle static holds (isometrics). Avoid excessive motion. | Continue RICE as needed. Begin low-load static stretch if needed. Preheating tissues if needed. Full weight bearing. Isokinetic exercise with continued ligament protection with bracing. Eccentric isotonic exercise. Progressive concentric isotonic exercise. Hydrotherapy—swimming. Progressive cycling. Initiate closed kinetic chain exercise. | Prolonged low-load static stretching. Progressive advanced isokinetic and isotonic exercise. Cycling—stair climbs. Proprioception—balance—coordination exercise. Advanced CKC exercise, progressing to plyometric exercise. Jogging, running, jumping, maintain joint protection with functional bracing as needed. |