Left Atrial Occluders/Isolation
Occlusion of the left atrial appendage (LAA) has the potential for significant change in the strategies for stroke prevention in patients with nonvalvular atrial fibrillation (AF).1–4 These strategies are predicated on the putative mechanism of stroke in these patients.5 Multiple anecdotal cases6 (Figure 132-1) and a series of pathologic and echocardiographic studies have documented that the LAA is the nidus for thrombus, resulting in stroke in up to 90% of cases in this setting.5 The hypothesis of the link between thrombus in the LAA and subsequent stroke has been substantiated in the randomized PROTECT-AF trial,1,2 in which LAA occlusion alone was found to be noninferior to anticoagulation for stroke prevention. It must be remembered in this regard that other sources of thromboembolic stroke exist; for example, the left atrium (LA) itself in the setting of significant mitral valve and other structural heart disease, complicated patent foramen ovale, left ventricular thrombus, mobile aortic atheroma, and carotid arterial disease. In these later situations, chronic anticoagulant therapy can be effective in preventing stroke or systemic thromboembolism, but LAA occlusion by itself would have no beneficial effect.
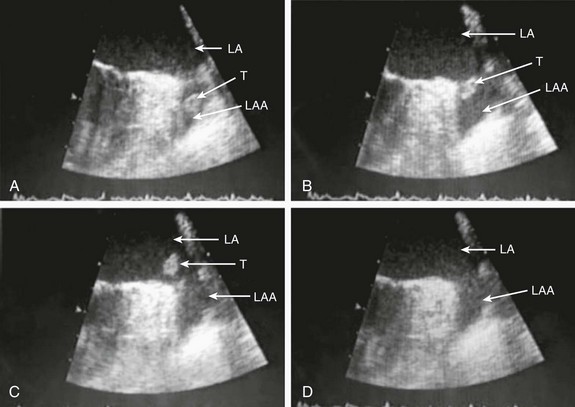
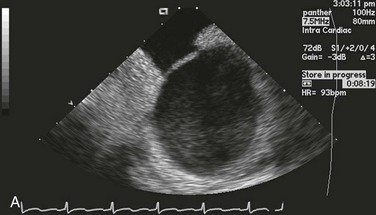
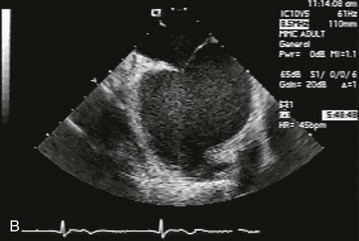
Figure 132-1 A, Echocardiographic assessment documenting thrombus (T) in the left atrial appendage (LAA). During the study, the thrombus is seen to migrate from the LAA out into the left atrium (LA) and then embolize from the LA (B-D).
Given the well-known relationship between an increasing incidence of atrial fibrillation with advancing age, as well as the increase in incidence of stroke with advancing age, the numbers of patients at risk is estimated to increase dramatically.7–12 Between 10% and 30% of strokes occur in the setting of diagnosed or undiagnosed AF.
Stroke prevention in the setting of AF has been the focus of intense investigation resulting in multiple professional societal guidelines10–12 and the evaluation and testing of risk scores.13–18 The most common risk score until recently was the CHADS2 score, which combined clinical factors that are useful in predicting the occurrence of stroke. This system has been superseded by CHADS2 VASc (Table 132-1).
Table 132-1
Prediction of Stroke and Thromboembolism
| Risk Factor | Points |
| CHADS2 score | |
| Congestive Heart Failure | 1 |
| Hypertension | 1 |
| Age > 75 years | 1 |
| Diabetes | 1 |
| Prior stroke, TIA | 2 |
| CHA2DS2-VASc score | |
| Congestive heart failure | 1 |
| Hypertension | 1 |
| Age ≥ 75 years | 2 |
| Diabetes | 1 |
| Stroke, TIA, TE | 2 |
| Vascular disease | 1 |
| Age 65 to 74 years | 1 |
| Female sex | 1 |
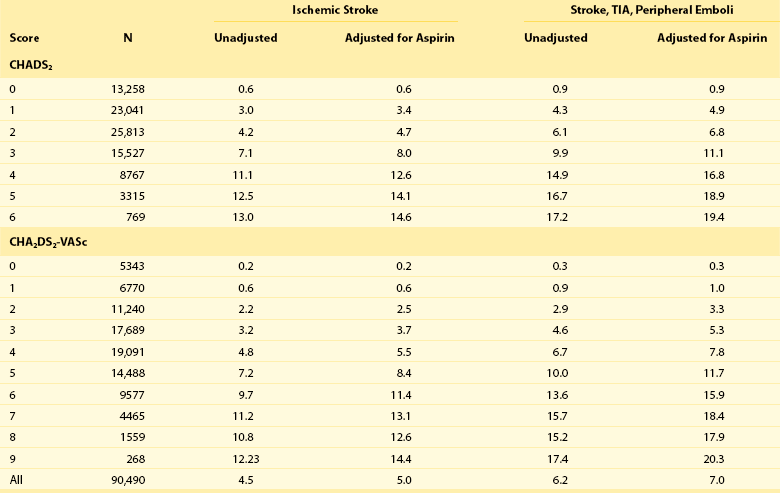
One of the largest studies evaluating risk stratification for both ischemic stroke and bleeding included 182,678 patients with atrial fibrillation in the Swedish National Registry.16 In a subset of 90,490 patients without warfarin throughout follow-up, the rate of stroke, transient ischemic attack, or peripheral embolization per 100 years at risk when adjusted for aspirin administration ranged from 0.9 to 19.4 using the CHADS2 score and 0.3 to 20.3 using the CHADS2DS2–VASc score (Table 132-2). There was improved predictive performance for the CHADS2DS2–VASc score for the composite thromboembolism endpoint. This expanded score appears to be of special importance in patients with lower CHADS2 scores of 0 to 1, and it is based on an incremental improvement in the predictive value of the added risk factors of age, sex, and the presence of vascular disease.17 In addition, it has important implications for identifying the need for anticoagulant therapy in these groups with lowest risk. Although other scoring systems have been developed,13 none of them have been widely used because of only having modest predictive value.
Table 132-2
Stroke or Thromboembolism per 100 Patient-Years at Risk in Relation to CHADS2 and CHA2DS2-VASc Scores in 90,490 Patients Without Warfarin Throughout Follow-Up16
There has been controversy regarding the specific risk score that identifies a positive benefit or risk for anticoagulation; however, in general a CHADS2 score of 2 or greater is an indication,10,11 and in some documents 1 or greater is also strongly considered.12 It is important to remember that undertreatment with the occurrence of stroke is generally considered more harmful than overtreatment with potential bleeds.
Conventional therapy has centered on warfarin,10,11 with which there were many problems, including absolute or relative contraindications, potential for bleeding, medication interactions, variability in dosing and effect, and the need for chronic intermittent monitoring. These issues resulted in the finding that warfarin was used in approximately 50% of patients in whom it was indicated; newer agents have been tested in large randomized trials involving in aggregate greater than 50,000 patients.19–29 In general, these new agents have been found to be more effective than warfarin with either somewhat less or similar bleeding risk, without the need for long-term monitoring of international normalized ratio (INR); adoption of these new agents remains highly variable. AF ablation, when successful, can allow the discontinuation of anticoagulation in low-risk patients. Nevertheless, convincing scientific data supporting its discontinuation in patients at moderate and high risk are not available. Current guidelines continue to mandate ongoing anticoagulation based on baseline stroke risk, regardless of the success of the ablation.
1. Some patients might have absolute or relative contraindications to both warfarin and the new anticoagulant agents.
2. The new agents might be associated with somewhat less bleeding, but the slope of the curve is only decreased and bleeding potential still increases over time.
3. The occurrence of gastrointestinal, pulmonary, and other side effects of new agents is not trivial.
4. In patients with higher CHADS2 or CHADS2 VASc scores, even after ablation, anticoagulation is recommended.
5. The life-long need for anticoagulants with the potential for side effects or drug-drug interactions and costs is substantial.
6. A substantial number of patients might develop coronary artery disease over time, requiring additional antiplatelet therapy and thus increasing the risk of bleeding known to accompany triple drug therapy.
1. It must be equally or more effective than alternative anticoagulation in clinical practice for stroke prevention, as demonstrated by large randomized clinical trials. The availability of data from well-executed, adequately powered randomized controlled trials is extremely important in this regard, although only one such trial has been published.
2. The risk-to-benefit ratio must be favorable. This issue is complex because, as with any invasive procedure, there will be at least some early procedural risks not present when only medication is prescribed; with the latter, there may be longer-term safety risks from hemorrhage.
3. The procedure must be performable in a substantial percentage of the patient candidates.
4. The procedure must be able available in a variety of institutions with well-trained specialists.
5. The approach should also be cost effective, without an adverse effect on quality of life. If these criteria can be met, LAA occlusion will play a substantial role for stroke prevention in patients with nonvalvular AF who are at increased risk of stroke.
A variety of approaches for LAA occlusion has been developed and is being tested. An important initial consideration regarding feasibility relates to the detailed anatomy of the LAA itself,30–35 which is highly variable—often with multiple lobes and marked trabeculation. The orifice is usually asymmetrical with an oval or elliptical shape. This structure is universally located between the left upper pulmonary vein and the mitral annulus, but the three-dimensional spatial orientation of the body of the appendage is also variable. For example, its three-dimensional shape could be straight or have varying degrees of angulation or spiraling. Classification schemes of the orientation and location of the tip of the LAA have been developed and could have some application, although they are not widely used.34 Using computed tomographic imaging, Wang et al.35 identified specific features (Figure 132-2). Perhaps most importantly for device selection and use is either the presence of a marked bend in the proximal or middle portion of the dominant lobe or the redundant folding back of the LAA on itself. Such anatomical orientation can affect the ability to deploy a transseptal device. In patients without such an obvious bend, several different specific types were categorized depending on the number of lobes, the length of the dominant lobe, and the take-off of the lobes relative to the origin of the LAA. The angulation, length, and number of lobes therefore remain an extremely important consideration for the selection of potential closure approaches.
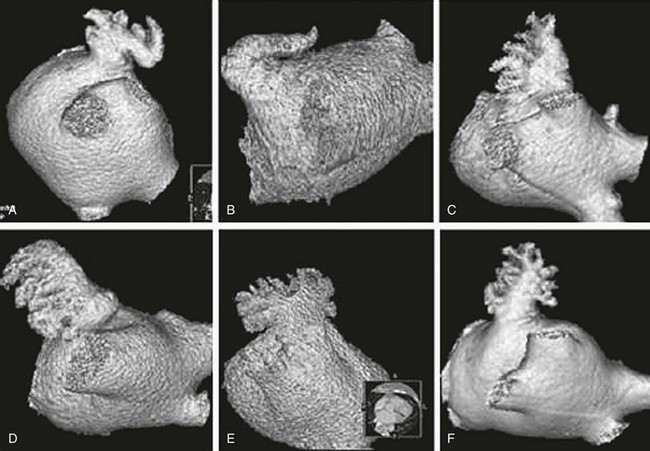
Figure 132-2 Assessment of the shape of the left atrial appendage can affect selection of a specific device. Wang et al. have developed a classification scheme based on computed tomographic imaging. The “chicken wing” configuration (A, B) may be difficult to fully intubate and deliver a device. The other subtypes—windsock (C, D), califlower (E) and cactus (F)—can also affect the selection of specific devices.35
Another important consideration is fragility (Figure 132-3). The LAA has been described as “our most lethal human attachment.”36 The thickness of the LA wall itself varies substantially. Using 64-slice multidetector computed tomography, the average thickness of the LAA excluding fat was 1.89 ± 0.48 mm but ranged from 0.5 to 3.5 mm.37 Histologic assessment of the LAA itself documented small crevasses or pits or areas of wall thinning within the trabeculated appendage that could be transilluminated. Such paper-thin walls are highly vulnerable to manipulation or to the placement of anchors to maintain device position and prevent device displacement.
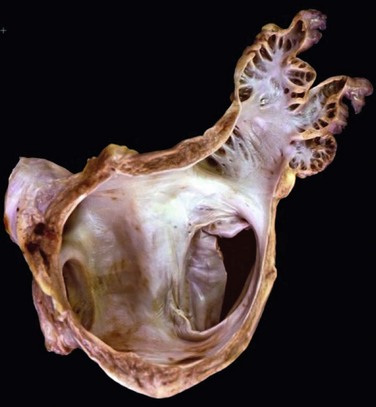
Figure 132-3 Autopsy specimen of the left atrial appendage (LAA). The crypts within the pectinate muscles and the tip of the LAA are often extremely thin and possibly translucent.
Imaging the Left Atrial Appendage
Echocardiographic Assessment
During follow-up, TEE is used to document adequacy of closure of the LAA. In the experience with the Watchman device, TEE is performed at 45 days after implantation.1,2 If closure at that time is complete, the short-term anticoagulation therapy can be discontinued.
Real-time three-dimensional TEE is being used with increasing frequency and helps to overcome some of the limitations of inadequate spatial resolution and sensitivity seen with two-dimensional TEE. LAA dimensions and geometry can be more accurately assessed with three-dimensional TEE than with two-dimensional TEE.32
Intracardiac echocardiographic imaging (ICE) is also used for LAA imaging and has the advantage of avoiding the need for either deep conscious sedation or general anesthesia used for TEE; it also helps to avoid the potential for rare but serious complications seen with TEE (e.g., esophageal trauma). In addition, ICE can be performed by the interventional operator during the procedure, thus avoiding the need for additional physician support needed for the TEE. ICE imaging can identify details of the intraatrial septum accurately (Figure 132-4); it can also be used to guide the placement of guidewires within the LAA. ICE is also important for measurement of LAA dimensions for device sizing and placement, as well as for monitoring the degree and severity of residual flow and device stability before final deployment. Two ICE views are required for characterizing the LAA. Although the superior to inferior dimensions can be seen from an RA imaging venue, the anterior to posterior dimensions are best imaged from a location below the tricuspid valve. It must be remembered that the LAA dimensions obtained by ICE might be different from those obtained by two-dimensional TEE because of the different spatial angulation of the imaging planes.
Specific Devices and Approaches
In the field of LAA occlusion, there is only one complete, published, randomized clinical trial involving transvenous approaches to left atrial appendage occlusion, with a second clinical trial having completed enrollment.1 Other information available comes from single-center or multicenter registries or experiences.
There are several different approaches for LAA occlusion. The oldest approach is surgical incision or obliteration, with the latter later using a variety of techniques including clips, staples, or sutures. The Left Atrial Appendage Occlusion Study evaluated feasibility, safety, and efficacy of LAA occlusion at the time of elective coronary artery bypass graft surgery38; 77 patients at risk for stroke were randomized to LAA exclusion with either sutures or staples versus control. Occlusion was successful in 29 patients (66%) versus an important limitation was the high rate of incomplete exclusion of the LAA. The specific utility of surgical LAA obliteration is controversial.39 A metaanalysis involving 1400 patients and five clinical trials appeared to show no clear benefit for surgical exclusion of LAA; only one showed a benefit, three were neutral, and one demonstrated increased risk from the exclusion itself. A new study, the Canadian multicenter trial,40 is planned to enroll 4700 patients undergoing clinically indicated cardiac surgery with the aim of evaluating LAA occlusion versus no LAA occlusion. All patients will continue to receive usual antithrombotic therapy. The primary outcome will be the composite of stroke or non–central nervous system embolization. The specific surgical LAA occlusion technique will be at the individual operator’s discretion.
Thorascopic obliteration of the left atrial appendage has been demonstrated in 15 high-risk patients.41 The procedure could be completed in 14 patients, and one patient required conversion to an open procedure. Feasibility was documented, but its efficacy requires further study depending on the continued clinical need for this approach given the fact that new strategies are being developed.
A recent approach uses a clip placed at the time of open heart surgery. This procedure (Atri Clip, Intra Cure, Inc, Cinncinati, OH) (42,43) was initially evaluated in 34 patients,42 which documented complete occlusion of the LAA and stable device position in all patients. A subsequent trial (EXCLUDE) of 70 patients undergoing elective surgery using a median sternotomy was documented with 96% intraprocedural success.43 Sixty-one patients underwent imaging during follow up, and 98% were found to have a successful LAA occlusion.
There are several important points to be considered in evaluating this trial.
1. The trial, which included 707 patients who were randomized in a 2 : 1 device-to-control drug ratio met the 1-year specified primary noninferiority efficacy endpoint (3.0 vs 4.9 events per 100 patient-years, respectively, with an RR of 0.62 (95% Bayesian credible interval, 0.3 to 1.25).
2. There was an early safety hazard in the device limb. The primary safety end point occurred significantly more frequently in the device group at 7.4 versus 4.4 events per 100 patient-years. The RR was 1.69, with a 95% Bayesian credible interval of 1.01 to 3.19. This primary safety end point was the result of periprocedural events, which included most commonly 22 pericardial effusions at 4.8% and stroke in 0.9% (typically owing to air embolization). In contrast to this early safety device concern, the control group had a high prevalence of major bleeding and hemorrhagic stroke compared with the device group: 4.1% control versus 3.5% with the device and hemorrhagic stroke, and 2.5% in the control group versus 0.2% with the device.
3. With expanded operator experience in an expanded continued access protocol, the pericardial effusion rate has diminished significantly to 2.2%, and strokes appear to have been eliminated.
4. In the final analysis of the PROTECT-AF trial, at 1588 patient-years follow-up, primary event rates were 3.0% and 4.3% per year in the LAA closure group versus the warfarin group (RR, 0.71; 95% credible interval, 0.44% to 1.30% per year). When a landmark type analysis was performed to isolate the risk contribution of LAA closure from the complications of implantation, LAA closure was found to be superior to warfarin.
5. Patients randomized to device received warfarin for the first 45 days to facilitate endothelialization of the device surface. This dosing potentially exposed patients to bleeding risk in the device group related to the short-term use of warfarin. Device success rate was seen in approximately 95%, and 95% of the patients randomized to device were able to discontinue warfarin.
6. A final issue relates to the completeness of closure.44,45 In the PROTECT-AF trial, 445 patients with a successful device implantation underwent TEE at 45 days. Of these patients, 389 had 12-months studies.44 Device flow was categorized as no residual flow, minor flow (<1 mm), moderate flow (1 to 3 mm), and major flow (>3 mm). The prevalence of any flow decreased from 40.9% at 45 days to 33.8% at 6 months and then to 32.1% at 12 months. A moderate leak (1 to 3 mm) was the most common at all three time points. The primary efficacy event rates and ischemic stroke and systemic embolism were not different between patients with any residual flow and those with no residual flow (Table 132-3). Not all studies have documented a decrease in leak size over time.45 In a smaller analysis of 58 patients with the Watchman device, a “peri-device gap” was noted in 29.3% at 45 days, but the incidence had increased to 34.5% at 1 year. This issue of incomplete sealing of the LAA has important implications, particularly the relationship with subsequent events.
An important consideration with the Watchman device has been the recommendation that patients treated with the device should receive warfarin for 45 days to mitigate stroke risk during ongoing occluder endothelialization. Recently, a multicenter registry of patients in whom warfarin was contraindicated has been reported.46 This registry included 150 patients with an average CHADS2 score of 2.8 who were treated only with aspirin indefinitely and clopidogrel for 6 months but no anticoagulant therapy. Based on the expected event rate, the patients in this registry had a 77% reduction in ischemic stroke. The ability to use devices safely and effectively in those patients who cannot take anticoagulants would have major implications for the field.
Other single or multicenter registries have been developed using other devices, most commonly the Amplatzer cardiac plug.9 Although there are no data from randomized controlled trials, multiple reports are available. The current device is self-expanding, with a lobe and a disc connected by a central waist. In the European Registry, the device could be implanted in 132 of 137 patients (96%).4 As was true with the PROTECT-AF trial, initial complications included pericardial effusion and ischemic stroke. It was also studied in patients in whom warfarin was contraindicated. In this small experience, procedural success was achieved in 95% of patients. Other transseptal devices are currently undergoing testing. As is true with the Watchman device, periprocedural complications are uncommon but have been documented and include pericardial effusion and rare device embolizations. These complications prolong hospital stay, but they do not typically result in death or myocardial infarction. Currently, selection of device strategy relates more to operator experience and exposure to the technology than it does to any comparative data.
Another category of devices combines both transseptal and epicardial approaches. The LARIAT device has three components47: (1) magnet-tipped guide wires (0.025 and 0.035 inches), (2) a 12-French suture delivery device, and (3) a 20-mm compliant occlusion balloon.
Transseptal access is obtained as described previously. In contrast to the fact that transseptal access techniques are used widely in both interventional and electrophysiology settings, transpericardial access typically has been performed only in the latter setting. General anesthesia is often used for transpericardial access. A subxyphoid approach is most common.48 Skin entry is initiated approximately 2 cm below the subxyphoid process. A blunt-tipped 18-gauge Tuohy epidural needle is advanced, directing it toward the left shoulder. As the needle approaches the cardiac silhouette, small volumes of contrast are injected until the pericardium is entered, at which time the contrast is visualized as a thin film layered over the cardiac chambers. Aspiration of the needle confirms the absence of cardiac chamber penetration. A guide wire is advanced and can be seen to wrap around the cardiac structures. Using fluoroscopic imaging in the left anterior oblique position is important because it allows differentiation of an intrapericardial versus a right ventricular outflow tract position. Over this a 14-French sheath is advanced. After obtaining dual access, a LAA angiogram is performed. Next, the 0.024-inch guide wire is advanced into the LAA and positioned at the tip while the 0.035-inch wire is advanced through the 14-French cannula into the epicardial space. The suture delivery device is advanced over the epicardial wire and positioned over the LAA. When adequate positioning has been documented, the suture is tightened and then ligated. Limitations of this technique include the presence of a thrombus in the LAA, projected inability to be able to reach the tip of the LAA by virtue of very superior orientation, and pericardial disease that limits direct pericardial access. Small uncontrolled series of patients have been described with successful closure in more than 95%.
References
1. Holmes, DR, Reddy, VY, Turi, ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009; 374:534–542.
2. Reddy, VY, Holmes, D, Doshi, SK, et al. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011; 123:417–424.
3. Bayard, YL, Omran, H, Neuzil, P, et al. PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) for prevention of cardioembolic stroke in non-anticoagulation eligible atrial fibrillation patients: results from the European PLAATO study. EuroIntervention. 2010; 6:220–226.
4. Park, JW, Bethencourt, A, Sievert, H, et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv. 2011; 77:700–706.
5. Blackshear, JL, Odell, JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996; 61:755–759.
6. Parekh, A, Jaladi, R, Sharma, S, et al. The case of the disappearing left atrial appendage thrombus. Circulation. 2006; 114:e513–e514.
7. Go, AS, Hylek, EM, Phillips, KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001; 285:2370–2375.
8. Miyasaka, Y, Barnes, ME, Gersh, BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota 1980-2000, and implications on the projections for future prevalence. Circulation. 2006; 114:119–125.
9. Heeringa, J, van der Kuip, DA, Hofman, A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006; 27:949–953.
10. Camm, AJ, Kirchhof, P, Lip, GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010; 31(19):2369–2429.
11. Fuster, V, Ryden, LE, Cannom, DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol. 2006; 48:854–906.
12. Skanes, AC, Healey, JS, Cairns, JA, et al. Focused 2012 Update of the Canadian Cardiovasc Society Atrial Fibrillation Guidelines; Recommendations for stroke prevention and rate/rhythm control. Canadian J Cardiol. 2012; 28:125–136.
13. Comparison of 12 risk stratification schemes to predict stroke in patients with nonvalvular atrial fibrillation. Stroke. 2008; 39:1901–1910.
14. Gage, BF, Waterman, AD, Shannon, W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001; 285:2864–2870.
15. Lip, GY, Nieuwlaat, R, Pisters, R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010; 137:263–272.
16. Friberg, L, Rosenquist, M, Lip, GY. Evaluation of risk stratification schemes for ischemic stroke and bleeding in 182,678 patients with atrial fibrillation. The Swedish Atrial Fibirllation Cohort Study. European Heart J. 2012; 33:1500–1510.
17. Olesen, JB, Torp-Pedersen, C, Hansen, ML, et al. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost. 2012; 107:1172–1179.
18. Lip, GY, Frison, L, Halperin, JL, et al. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010; 41:2731–2738.
19. Hylek, EM, Go, AS, Chang, Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. New Engl J Med. 2003; 349:1019–1026.
20. Connolly, S, Pogue, J, Hart, R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006; 367:1903–1912.
21. Connolly, SJ, Pogue, J, Hart, RG, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. New Engl J Med. 2009; 360:2066–2078.
22. Singer, DE, Albers, GW, Dalen, JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008; 133:546S–592S.
23. Fang, MC, Go, AS, Chang, Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med. 2007; 120:700–705.
24. Sudlow, M, Thomson, R, Thwaites, B, et al. Prevalence of atrial fibrillation and eligibility for anticoagulants in the community. Lancet. 1998; 352:1167–1171.
25. Go, AS, Hylek, EM, Borowsky, LH, et al. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999; 131:927–934.
26. Connolly, SJ, Ezekowitz, MD, Yusuf, S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. New Engl J Med. 2009; 361:1139–1151.
27. Wallentin, L, Yusuf, S, Ezekowitz, MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010; 376(9745):975–983.
28. Patel, MR, Mahaffey, KW, Garg, J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365(10):883.
29. Granger, CB, Alexander, JH, McMurray, JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365(11):981–992.
30. Veinot, JP, Harrity, PJ, Gentile, F, et al. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. 1997; 96:3112–3115.
31. Sharma, S, Devine, W, Anderson, RH, et al. The determination of atrial arrangement by examination of appendage morphology in 1842 heart specimens. Br Heart J. 1988; 60:227–231.
32. Shah, SJ, Bardo, DM, Sugeng, L, et al. Real-time three-dimensional transesophageal echocardiography of the left atrial appendage: initial experience in the clinical setting. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2008; 21:1362–1368.
33. Budge, LP, Shaffer, KM, Moorman, JR, et al. Analysis of in vivo left atrial appendage morphology in patients with atrial fibrillation: a direct comparison of transesophageal echocardiography, planar cardiac CT, and segmented three-dimensional cardiac CT. Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing. 2008; 23:87–93.
34. Lacomis, JM, Goitein, O, Deible, C, et al. Dynamic multidimensional imaging of the human left atrial appendage. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2007; 9:1134–1140.
35. Wang, Y, Di Biase, L, Horton, RP, et al. Left atrial appendage studied by computed tomography to help planning for appendage closure device placement. Journal of cardiovascular electrophysiology. 2010; 21:973–982.
36. Johnson, WD, Ganjoo, AK, Stone, CD, et al. The left atrial appendage: our most lethal human attachment! Surgical implications. Eur J Cardiothorac Surg. 2000; 17:718–722.
37. Beinart, R, Abbara, S, Blum, A, et al. Left atrial wall thickness variability measured by CT scans in patients undergoing pulmonary vein isolation. J Cardiovasc Electrophysiol. 2011; 22:1232–1236.
38. Healey, JS, Crystal, E, Lamy, A, et al. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. American heart journal. 2005; 150:288–293.
39. Dawson, AG, Asopa, S, Dunning, J. Should patients undergoing cardiac surgery with atrial fibrillation have left atrial appendage exclusion? Interactive cardiovascular and thoracic surgery. 2010; 10:306–311.
40. CANNECTIN Pilot Project. www.cannectin.ca.
41. Blackshear, JL, Johnson, WD, Odell, JA, et al. Thoracoscopic extracardiac obliteration of the left atrial appendage for stroke risk reduction in atrial fibrillation. J Am Coll Cardiol. 2003; 42:1249–1252.
42. Salzberg, SP, Plass, A, Emmert, MY, et al. Left atrial appendage clip occlusion: early clinical results. The Journal of thoracic and cardiovascular surgery. 2010; 139:1269–1274.
43. Ailawadi, G, Gerdisch, MW, Harvey, RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. The Journal of thoracic and cardiovascular surgery. 2011; 142:1002–1009.
44. Viles-Gonzalez, JF, Kar, S, Douglas, P, et al. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol. 2012; 59(10):923–929.
45. Bai, R, Horton, RP, L, DIB, et al. Intraprocedural and long-term incomplete occlusion of the left atrial appendage following placement of the WATCHMAN device: a single center experience. J Cardiovasc Electrophysiol. 2012; 23:455–461.
46. Reddy, V, Neuzil, P, Miller, MA, et al. First Formal Analysis of the “ASA Plavix Registry” (ASAP): Watchman Left Atrial Appendage Closure in Atrial Fibrillation Patients with Contradiction to Oral Anticoagulation. LBCT HRS May. 2012.
47. Bartus, K, Bednarek, J, Myc, J, et al. Feasibility of closed-chest ligation of the left atrial appendage in humans. Heart rhythm : the official journal of the Heart Rhythm Society. 2011; 8:188–193.
48. Syed, F, Lachman, N, Christensen, K, et al. The pericardial space: obtaining access and an approach to fluoroscopic anatomy. Card Electrophysiol Clin. 2010; 2:9–23.