CHAPTER 5 Latissimus Dorsi Flap Breast Reconstruction
Key Points
Latissimus dorsi (LD) myocutaneous flaps, first described by Tassini,1 have been used in various reconstructive procedures for decades. In the setting of immediate or delayed breast reconstruction2 LD flaps have several characteristics that can make them excellent options.
Patient Selection
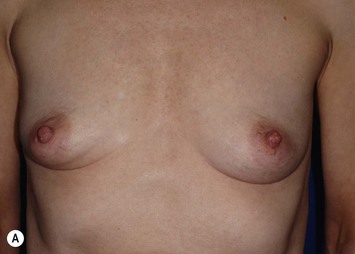
Patients who have undergone radiotherapy can also benefit from the use of an LD flap in breast reconstruction. In these patients, the skin island of an LD flap can replace the constricted, irradiated skin of the breast; and the muscle of an LD flap can cover an implant, thereby decreasing the risk of capsular contracture and implant infection.3–5 Patients who are at an increased risk of mastectomy skin flap necrosis, such as tobacco smokers, may also benefit from an LD flap, which can provide additional muscle coverage of a tissue expander or implant and a robust skin paddle. Furthermore, focal skin and soft tissue defects caused by previous partial mastectomy and radiotherapy that cannot be corrected with implants alone often can be repaired with an LD flap.6,7
Indications
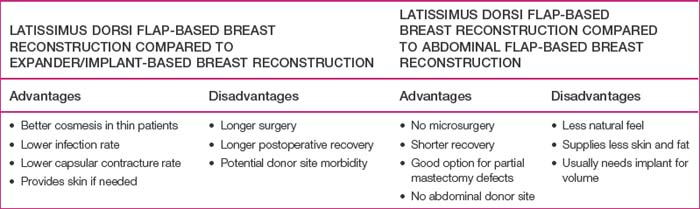
Every patient who desires breast reconstruction must undergo a thorough history and examination to help determine which reconstruction technique should be used. Specific details should be obtained regarding previous surgery to the abdomen, chest and axilla, previous history of radiation, and the patient’s preference for reconstruction method and willingness to undergo major surgery. Many reconstructive surgeons prefer abdominal flaps such as transverse rectus abdominis myocutaneous, deep inferior epigastric, or superficial inferior epigastric artery flaps for autologous tissue-based breast reconstruction. However, patients may not desire or have adequate tissue for an abdominal flap harvest. Furthermore, previous abdominal surgeries such as abdominoplasty, laparotomy, or liposuction may reduce the reliability of an abdominal flap or preclude abdominal flap-based breast reconstruction. In such cases, an LD flap offers a reliable alternative to an abdominal flap. In addition, if an implant is going to be included in the reconstruction, using an LD flap over the implant can improve the contour of the reconstructed breast,8 particularly in thin patients, in whom implant-based reconstructions tend to have less esthetic results (Table 5.1).
Operative Technique
Preoperative evaluation and markings
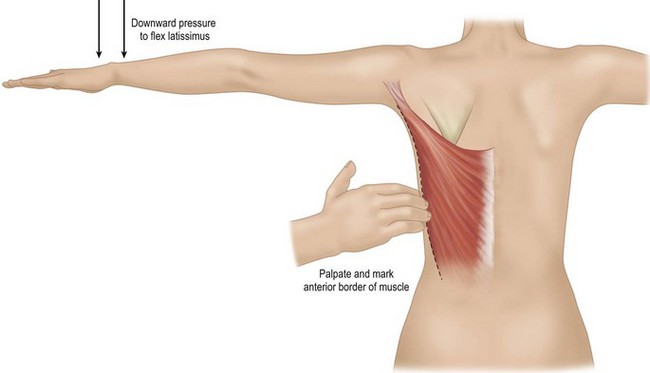
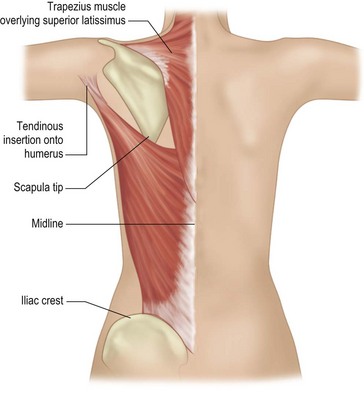
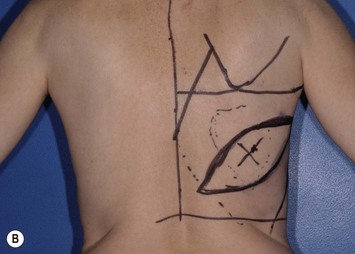
While the patient is awake and standing or sitting upright, the reconstructive surgeon uses a provocative maneuver to evaluate the contractility of the LD muscle (Fig. 5.1). The patient adducts her arm; as the LD muscle contracts, the reconstructive surgeon palpates and marks the muscle’s anterior border. The tip of the scapula, posterior iliac crest, and midline are also marked to further delineate the muscle’s topography (Fig. 5.2). If the patient has a history of lymph node sampling and the LD muscle does not contract when the patient adducts her arm, the thoracodorsal nerve may be injured or transected, and the adjacent thoracodorsal vascular pedicle may also be injured.
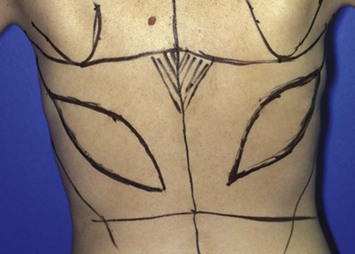
If the breast reconstruction requires a skin island, the reconstructive surgeon can design and transpose a template of the size, shape, orientation, and location of the anticipated skin island onto the skin that overlies the LD muscle. The location of the anticipated skin paddle is critical in determining the relative location of the muscle and skin island in the reconstructed breast during inset. The template should also reflect the 90–110° of rotation the flap will undergo from the patient’s back to her chest during the reconstruction (Fig. 5.3). Attaching the template to a towel and using the axilla as a pivot point can help the surgeon confirm the flap will reach its intended final location. To ensure that the flap donor site can be closed primarily, the reconstructive surgeon should pinch together the anticipated incision lines. In most patients, primary closure without significant tension can be performed if the skin island is less than 10 cm wide. If a skin island is not required for breast reconstruction, the reconstructive surgeon can harvest the LD flap through a small incision in the posterior axilla and use endoscopy to limit the number of incisions made on the patient’s back.
Flap elevation
Full-muscle myocutaneous flap elevation
If the patient has undergone axillary lymph node sampling, the reconstructive surgeon should identify the vascular pedicle anteriorly though the mastectomy defect while the patient is supine. Once the pedicle has been identified and preserved, the reconstructive surgeon should mobilize the flap as much as possible from the surrounding soft tissues (Box 5.1).
Box 5.1 Surgical steps in latissimus dorsi (LD) flap harvest
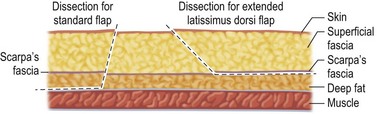
Figure 5.4 shows the dissection planes for the standard myocutaneous and extended myocutaneous LD flaps. To facilitate a smooth transition between the mastectomy skin and the skin island inset, the reconstructive surgeon incises the skin island at a slight outward angle through the subcutaneous fat. Sufficient subcutaneous fat is preserved at the edge of the donor site to prevent a depressed scar and/or fasciodesis. When harvesting an extended LD flap, the reconstructive surgeon creates a plane just deep to Scarpa’s fascia; the flap’s volume is increased by including a rim of deep fat below Scarpa’s fascia.9,10 In contrast, when harvesting a standard LD myocutaneous flap, the reconstructive surgeon dissects directly down to the investing muscle fascia. Dissection over the LD muscle is continued to its peripheral margins. The anterior margin of the LD muscle is identified and separated from the serratus anterior muscle. Because the serratus and external oblique muscles coalesce with the LD muscle inferiorly and posteriorly, it is easier to identify the anterior border of the LD muscle cephalad and dissect the muscle from the chest wall caudally. Once the LD muscle’s anterior border is freed, the plane deep to the LD muscle is developed caudally to avoid injuring the vascular pedicle. The large lumbar and intercostal perforating vessels entering the posterior surface of the LD muscle are isolated and ligated.
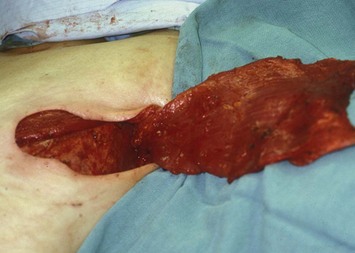
Dissection of the LD muscle proceeds cephalad toward the neurovascular bundle, which enters the deep surface of the LD muscle 8–10 cm inferior to the axillary line and 2–3 cm lateral to the muscle’s anterior border. The anterior branch of the bundle that supplies the serratus anterior muscle group is identified and preserved unless it prevents adequate flap rotation. The pedicle dissected is typically 8–10 cm long, but complete dissection of the pedicle often is not necessary to allow for an adequate arc of rotation; therefore, pedicle dissection should proceed only to a point that enables adequate flap rotation without tension on the vascular pedicle (Fig. 5.5).
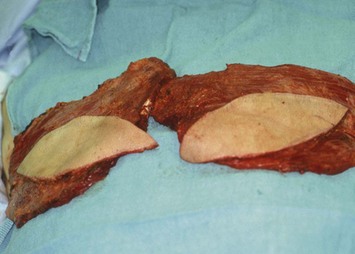
Fig. 5.5 Elevated bilateral myocutaneous latissimus dorsi flaps, prior to inset into the mastectomy defects.
The tendinous insertion into the humerus is exposed by dissecting cephalad and circumferentially near the LD insertion. The reconstructive surgeon can divide the insertion to increase the arc of rotation and reduce any axillary bulging that the rotated proximal muscle may cause.11 If the insertion is completely divided, care must be taken to prevent traction injury to the vascular pedicle.
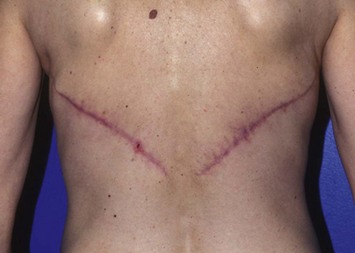
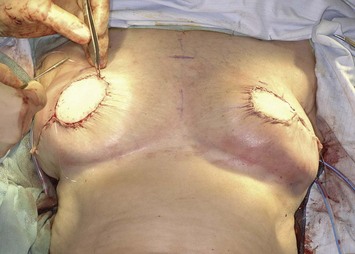
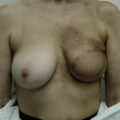
Two large, closed-suction drains are placed in a dependent location in the donor site. Drains are typically left in place for 1–3 weeks and removed once the output decreases to fewer than 30 ml of drainage per 24 hours. Placing quilting or progressive tension sutures from the donor site skin flaps to the chest wall may decrease the amount of drainage and incidence of seroma formation by closing off the dead space left by flap dissection and preventing shear forces.13 Primary skin closure can be performed without significant tension in almost all cases in which the skin paddle is less than 10 cm wide (Fig. 5.6).
Split LD flap elevation
A split (segmental) LD flap can be useful in reconstructions for partial mastectomy defects. The anatomical basis for splitting the LD muscle is well established.14,15 The skin paddle is marked as in Figure 5.3 and incised, and the anterior border of the LD muscle is identified. The lateral descending branch of the thoracodorsal pedicle is identified on the undersurface of the muscle, and intramuscular dissection is performed medial to the lateral vascular pedicle. The remaining muscle’s vascular pedicle is sacrificed to allow for flap rotation; however, the muscle’s nerve branches are preserved to provide motor function.
Thoracodorsal artery perforator flap elevation
Like a split LD flap, a thoracodorsal artery perforator flap15–18 can be used to repair partial breast defects without compromising function of the remaining LD muscle. Preoperative ultrasonography or computed tomography angiography is usually used to help identify perforators on which to base the flap. The LD flap can be based on a row of perforators approximately 2 cm from the anterior margin of the flap just caudal to its scapular tip. Once the perforators are identified, a skin paddle is designed around the selected perforator. The skin island should be incised on only one side of the flap, preferably its anterior portion. Once the perforator is identified, the remainder of the skin island is elevated, and intramuscular dissection proceeds to the point at which the perforator meets with the thoracodorsal vascular pedicle. The LD muscle’s nerve branches are dissected from the vascular pedicle and preserved. All other vascular branches are divided until adequate pedicle length is attained. Great care must be taken to prevent traction injury to the perforators and vascular pedicle.
Flap insetting
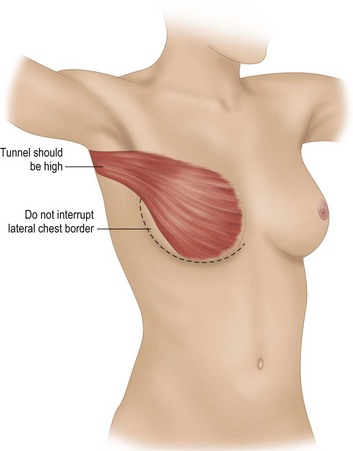
A subcutaneous tunnel between the LD muscle donor site and the mastectomy defect should be created if one is not already present (Fig. 5.7). This tunnel is made as high in the axilla as possible while still allowing adequate flap insetting to help recreate the breast’s anterior axillary fold and prevent excessive tissue bulk in the axilla. When an implant is included, the reconstructive surgeon uses interrupted sutures to attach the edge of the LD muscle to the lateral chest wall and prevent migration of the implant into the axilla or donor site (Fig. 5.8). Securing the muscle to the chest wall laterally also helps prevent traction on the vascular pedicle, particularly if the muscle insertion has been transected.
Immediate reconstruction
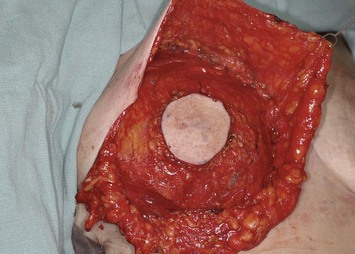
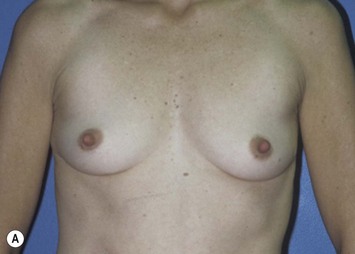
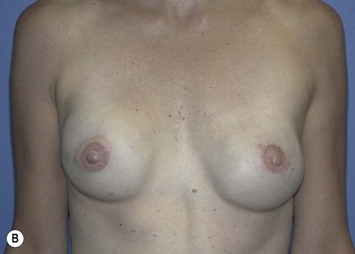
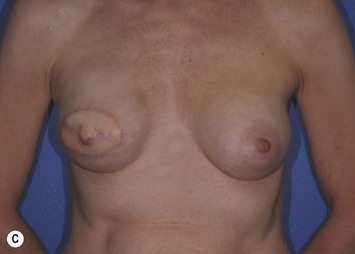
The pectoralis major muscle is often maintained on the chest wall following mastectomy, and an implant can be placed above or beneath it. We prefer to elevate the pectoralis major muscle and place the implant beneath it to provide superior implant coverage and improve the contour transition from the chest wall to the implant along the superior pole of the reconstructed breast. In this case, the LD muscle is sutured ventral to the elevated pectoralis major muscle in a ‘pants-over-vest’ fashion (Fig. 5.9). The borders of the LD muscle are secured to the chest wall within the mastectomy pocket. Inserting the transected LD muscle into the pectoralis major tendon can improve the axillary contour of the reconstructed breast. As much of the flap inset as possible should be performed before placement of the expander or implant. Carefully insetting the LD muscle is important to correctly position the reconstructed breast and maximize symmetry between the reconstructed and contralateral breast. The LD flap inset must also allow the skin island to be appropriately located on the reconstructed breast to provide skin coverage within the mastectomy flap skin edges. If the LD flap is not large enough to fully cover the implant, the LD muscle can be sutured to the inferior border of the elevated pectoralis major muscle to create adequate submuscular space for the implant. Closed-suction drains are placed between the subcutaneous mastectomy skin flaps and LD muscle and within the submuscular pocket. The skin edges are trimmed to their final dimensions, and the skin island is fully inset (Fig. 5.10). Nipple and areola reconstruction are performed at a later stage (Figs 5.11 and 5.12).
Pitfalls and How to Correct
Seroma
The large potential space created during LD flap harvest can contribute to donor site seroma, the most common complication of LD flap-based breast reconstruction.5,8,10,13,19 Multiple long-term drains and quilting sutures can be used to prevent seromas.13 If a seroma develops after all surgical drains have been removed, serial aspirations are generally the initial treatment step, and frequently, the seroma will resolve quickly. If not, a new drain can be placed; often, this treatment can be supplemented with diluted fibrin sealant injections,20 sclerotherapy,21 or steroid injections.22 Using compression garments or wrapping the donor site with elastic bandages is an important adjunct to all treatment options. If the seroma persists, surgical decortication of the seroma pseudocapsule23 can be performed with the use of quilting sutures and closed-suction drains.
Axillary node dissection
Weak or absent LD muscle function in patients who have undergone axillary node sampling is suggestive of thoracodorsal nerve and/or vascular pedicle injury; however, a functional LD muscle does not always indicate an intact vascular pedicle. Therefore, in patients who have undergone axillary node sampling, the reconstructive surgeon should confirm the patency of the pedicle before elevating the LD flap. If the thoracodorsal vessels have been injured or transected proximal to the branch to the serratus anterior muscle, an LD flap can be elevated based on this serratus branch with retrograde arterial inflow.24 On the other hand, if the thoracodorsal and serratus branch vessels have been injured or transected, the reconstructive surgeon may choose to microsurgically repair the vessels or use a reconstructive technique that does not involve the ipsilateral LD muscle.
Vascular pedicle injury
Despite preventative measures, vascular pedicle injury can occur in LD flap breast reconstruction. Common causes include injury caused by traction and inadvertently dividing the pedicle from the flap while attempting to divide the thoracodorsal nerve. Depending on the injury, microsurgically repairing the flap vessel to the remaining thoracodorsal vessels can often be performed. If too much pedicle is sacrificed, converting to a free flap with the internal mammary arteries used as donor vessels or with the use of vein grafts to the subscapular axis vessels can be performed. If the injury is proximal to the serratus branch, the LD flap may be transferred based on retrograde flow through the serratus branch24 to provide flap vascularity.
Postoperative Care
To reduce the risk of bleeding and prevent undue tension on the flap inset or vascular pedicle, patients should limit movement of the arm on the side of the reconstruction for 1–2 weeks. Thereafter, progressive range of motion exercises are initiated to help prevent shoulder stiffness. Prolonged shoulder stiffness, if it occurs, can be effectively treated with physical therapy. Patients generally regain full shoulder function and are able to resume their normal daily activities after 3 or 4 weeks.25,26
1 Maxwell GP. Iginio Tansini and the origin of the latissimus dorsi musculocutaneous flap. Plast Reconstr Surg. 1980;65:686.
2 Bostwick J, Nahai F, Wallace JG, Vasconez LO. Sixty latissimus dorsi flaps. Plast Reconstr Surg. 1979;63:31.
3 Spear SL, Boehmler JH, Taylor NS, Prada C. The role of the latissimus dorsi flap in reconstruction of the radiated breast. Plast Reconstr Surg. 2007;119:1.
4 Chang DW, Barnea Y, Robb GL. Effects of an autologous flap combined with an implant for breast reconstruction: an evaluation of 1000 consecutive reconstructions of previously irradiated breasts. Plast Reconstr Surg. 2008;122:356.
5 Pinsolle V, Grinfeder C, Mathoulin-Pelissier S, Faucher A. Complications analysis of 266 immediate breast reconstruction. J Plast Reconstr. Aesth Surg. 2006;59:1017.
6 Clough KB, Kroll SS, Audretsch W. An approach to the repair of partial mastectomy defects. Plast Reconstr Surg. 1999;104:409.
7 Kronowitz SJ, Feledy JA, Hunt KK, et al. Determining the optimal approach to breast reconstruction after partial mastectomy. Plast Reconstr Surg. 2006;117:1.
8 Moore TS, Farrell LD. Latissimus dorsi myocutaneous flap for breast reconstruction: long-term results. Plast Reconstr Surg. 1992;89:666.
9 Hofkin JAB, Silfverskiold KL. Breast reconstruction without an implant: results and complications using an extended latissimus dorsi flap. Plast Reconstr Surg. 1987;79:58.
10 Chang DW, Youssef A, Cha S, Reece GP. Autologous breast reconstruction with the extended latissimus dorsi flap. Plast Reconstr Surg. 2002;110:751.
11 Gerber B, Krause A, Reimer T, Muller H, Friese K. Breast reconstruction with latissimus dorsi flap: improved aesthetic results after transection of its humeral insertion. Plast Reconstr Surg. 1999;103:1876.
12 Akhtar S. Our early experience in the use of tissue glue to reduce the incidence of seroma formation from the latissimus dorsi flap donor site. Plast Reconstr Surg. 2005;116:347.
13 Rios JL, Pollock T, Adams WP. Progressive tension sutures to prevent seroma formation after latissimus dorsi harvest. Plast Reconstr Surg. 2003;112:1779.
14 Tobin GT, Schusterman M, Peterson GH, Nichols G, Bland KI. The intramuscular neurovascular anatomy of the latissimus dorsi muscle: the basis for splitting the flap. Plast Reconstr Surg. 1981;67:637.
15 Schaverien M, Saint-Cyr M, Arbique G, Brown SA, Rohrich RJ. Three- and four-dimensional arterial and venous anatomies of the thoracodorsal artery perforator flap. Plast Reconstr Surg. 2008;121:1578.
16 Heitmann C, Guerra A, Metzinger SW, Levin LS, Allen RJ. The thoracodorsal artery perforator flap: anatomic basis and clinical applications. Ann Plast Surg. 2003;51:23.
17 Levine JL, Soueid NE, Allen RJ. Algorithm for autologous breast reconstruction for partial mastectomy defects. Plast Reconstr Surg. 2005;116:762.
18 Hamdi M, Van Landuyt K, Monstrey S, Blondeel P. Pedicled perforator flaps in breast reconstruction: a new concept. Br J Plast Surg. 2004;57:531.
19 Tomita K, Yano K, Masuoka T, Matsuda K, Takada A, Hosokawa K. Postoperative seroma formation in breast reconstruction with latissimus dorsi flaps. Ann Plast Surg. 2007;59:149.
20 Butler CE. Treatment of refractory donor-site seromas with percutaneous instillation of fibrin sealant. Plast Reconstr Surg. 2006;117:976.
21 Shermak MA, Rotellini-Coltvet LA, Chang D. Seroma development following body contouring surgery for massive weight loss: patient risk factors and treatment strategies. Plast Reconstr Surg. 2008;122:280.
22 Taghizadeh R, Shoaib T, Hart AM, Weiler-Mithoff EM. Triamcinolone reduces seroma re-accumulation in the extended latissimus dorsi donor site. J Plast Reconstr Aesth Surg. 2008;61:636.
23 Roje Z, Roje Z, Karanovic N, Utrobicic I. Abdominoplasty complications: a comprehensive approach of chronic seroma with pseudobursa. Aesth Plast Surg. 2006;30:611.
24 Fisher J, Bostwick J, Powell RW. Latissimus dorsi blood supply after thoracodorsal vessel division: the serratus collateral. Plast Reconstr Surg. 1983;72:502.
25 Russel RC, Pribaz J, Zook EG, Leighton WD, Erikkson E, Smith CJ. Functional evaluation of latissimus dorsi donor site. Plast Reconstr Surg. 1986;78:336.
26 Spear SL, Hess CL. A review of the biomechanical and functional changes in the shoulder following transfer of the latissimus dorsi muscles. Plast Reconstr Surg. 2005;115:2070.