CHAPTER 79 Late Decompression of Patients with Spinal Cord Injury
Nonoperative care was the earliest standard treatment modality for patients with spinal cord injury. This approach was superseded for a time by delayed surgery on the basis of data that suggested nonoperative treatment, or early surgery, did not reliably improve neurologic outcome and may be associated with increased risks of complications.1–17 With improvements in imaging techniques18,19 and our understanding of the primary and secondary mechanisms of spinal cord injury,12,17,20–27 there is increasingly greater emphasis on early pharmacologic and surgical intervention.28–44
According to the National Acute Spinal Cord Injury Study (NASCIS) III, methylprednisolone may improve outcome if given as a loading dose of 30 mg/kg over 15 minutes and continued at 5.4 mg/kg/hr for 24 hours in acute nonpenetrating spinal cord injuries presenting at less than 3 hours and for 48 hours in injuries presenting at 3 to 8 hours.28,45,46 Preliminary data from further prospective studies are largely negative but are under way. Rabinowitz and colleagues47 reported surgical decompression is more effective for improving neurologic recover in beagle dogs than methylprednisolone alone.
Despite the lack of a clear definition of “early” surgery, the current consensus is that early decompression and stabilization of traumatic spinal cord injuries with cord compression provides an improved physiologic environment for neurologic recovery. Earlier concerns of increased complications with early surgery are unsupported. Surgical decompression should therefore be done as soon as the patient is medically stable. This more aggressive approach may improve neurologic recovery in some patients and decrease patient mortality and morbidity from pulmonary, skin, and gastrointestinal complications, as well as length of hospitalization and time to mobilization and rehabilitation in most patients.18,31–40,43,48–50 The Spine Trauma Study Group has defined early intervention as within 24 hours of the injury.51 Chen and colleagues52 found no statistical difference in outcomes between traumatic central cord syndrome (TCCS) patients treated operatively less than 4 days and greater than 4 days from injury. Clearly, timing of surgical intervention remains controversial.
As a result of improved acute care and rehabilitation, more patients with spinal cord injury are living longer and more productive lives; however, with increased longevity after traumatic spinal cord injuries, some patients with incomplete spinal cord lesions will present late with chronic cord compression and a neurologic status that has plateaued or is deteriorating. Experimental53,54 and clinical14,39,55–67 evidence suggests that these patients may receive improved cord function from decompression even as late as 9 years after spinal cord injury.15 Patients with complete cord lesions may also benefit from root recovery after late decompression.* Other late complications of traumatic spinal cord injury such as spasticity,70 syringomyelia,71–74 and Charcot arthropathy75–79 of the spine may cause neurologic deterioration or pain that may also benefit from late decompression.
Experimental Studies
An acute traumatic injury to the spinal cord may leave varying amounts of physiologically viable tissue in an incomplete lesion that may progressively deteriorate due to secondary injury.44,53,54,80–83 The amount of viable tissue that remains and the ability of the tissue to function partly depend on the primary mechanism of the injury such as the characteristics of the initial force, as well as on the secondary mechanisms of injury caused by the combination of elevated levels of intracellular calcium, endogenous opiates, and inflammatory mediators, lipid peroxidation, edema, glutamatergic excitotoxicity, and mechanical factors producing continued compression and cord ischemia.†
Several experimental models have been developed to determine how much injury the cord can tolerate and how long it can be compressed anteriorly and still recover neurologic function.‡ Allen,22 in 1911, developed a standardized weight-drop model to produce graded spinal cord lesions in vivo. Pathologic evaluation of the injured cords revealed central hemorrhage and progressive necrosis that was thought to be the underlying mechanism behind neurologic deterioration in these dogs. Some dogs regained the ability to ambulate after myelotomy and evacuation of the hemorrhagic tissue. Other animal experiments have subsequently been done using the weight-drop method, and others have used dynamic loading with a piston device or a graded constriction method using clips, constriction bands, or balloons.* Collectively, these experiments suggest that surgical decompression, preferably early but also late, may result in varying degrees of neurologic recovery.
†References 20, 21, 25, 27, 28, 46, 58, 82–85.
‡References 22, 39, 44, 53, 54, 58, 80, 81, 86–97.
The duration of compression and timing of decompression are not the only factors affecting the potential for improved neurologic function in these animal studies. Other factors such as force of the initial compression, amount of cord displacement, and the kinetic energy of the applied load affect the severity of the injury. Dolan and colleagues90 and Guha and colleagues92 used modified aneurysmal clips to constrict the T1 cord of rats with graded forces over different time periods. Clinical neurologic recovery varied exponentially according to the force of injury and linearly according to the duration of compression. They concluded that the major determinant of recovery was the initial force of injury. Although earlier decompression produced significantly more neurologic recovery, later decompression has the potential to salvage physiologically viable tissue that survived the initial injury and subsequent compression. Sommerson and Stokes98 showed that compression injuries with less than 1-mm deformation of the spinal cord in rodents result in linear displacement of the cord relative to the applied force. This linear relationship becomes nonlinear if the cord displacement exceeds 1 mm. The result of this study suggests the possibility of a similar relationship between a traumatic compressive lesion and neurologic deterioration. Late decompression may, therefore, prevent further neurologic deficit owing to increasing cord deformation from progressive deformity or instability. Kobrine and colleagues88,89 compressed the T6 spinal cord level in monkeys using an inflatable extradural balloon at different compression rates. Monkeys who had slow balloon inflation had return of function based on spinal cord evoked potentials even after several minutes of compression.88 In contrast, monkeys that had rapid balloon inflation and acute cord compression showed return of response in 1 hour if compression lasted for 1 minute but no recovery up to 1 hour if compression lasted for 3, 5, 7, or 15 minutes.89 Although this study can be criticized for not checking responses beyond 1 hour, the authors concluded that recovery of spinal cord function depended on the rapidity and length of time of compression. Therefore lower energy injuries or progressive deficits due to cord compression after an acute injury may have potential for neurologic recovery even with late decompression.
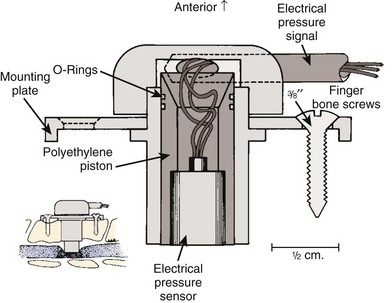
Bohlman and colleagues53,54 developed an animal model to demonstrate that late decompression of a traumatic incomplete spinal cord injury that occurred after either cord contusion or compression may result in neurologic recovery. Incomplete paralysis was produced in eight beagles by remote control of a pressure transducer (Figs. 79-1 to 79-4) and maintained for 3 to 8 weeks until recovery plateaued. After cord compression was relieved, all animals recovered completely by 10 weeks after injury. Recovery was faster in the five with complete decompression versus the three with lesser degree of ongoing compression from the transducer. Microscopic examination was normal in two dogs, central gray necrosis in two, peripheral demyelinization in two, and lacerations in three. The weight-drop method was used to contuse the cord in seven dogs using 257 to 722 gm/cm of energy. Variable levels of quadriparesis occurred, and all dogs recovered by 6 weeks after injury. Thirteen dogs developed central cord syndromes (forepaw paralysis greater than hindpaw) with either method. Central gray matter damage was the most common finding. The gray matter seemed more sensitive to deforming forces of external degeneration of the ascending sensory posterior columns, whereas others showed damage of the anterior and lateral corticospinal tracts, indicating descending wallerian degeneration. However, histopathologic damage of the spinal cord did not always correspond to the degree of clinical paralysis in these dogs. For example, there was normal histology in three dogs, two with quadriplegia for 1 to 6 weeks, and one with grade 3 paralysis. Because these dogs recovered neurologic function after contusion and compression, it appeared that deformation of the cord produced a physiologic block but the cord remained viable. In the contusion model, greater contusion forces caused proportionately increased neurologic damage and recovery time, whereas in the compression model anterior decompression allowed for faster and more complete recovery.
Clinical Studies
Although there are likely significant differences between the mechanical and biologic properties of the spinal cord in human versus animals, the animal studies suggest that the characteristics of the initial force and the amount and duration of cord compression alter neurologic recovery. Persistent compression may cause progressive mechanical injury and diminished blood flow to the cord in the region of the anterior spinal artery, thus preventing improvement with nonoperative treatment. Early decompression of incomplete cord injury restores blood flow and provides faster and more complete recovery, but late decompression can achieve varying degrees of the same result in cases of residual compression. Decompression in the region of the lesion in a patient with an incomplete spinal cord injury may provide functional recovery of motor roots and the spinal cord at that level and below. Patients with complete cord injuries are not expected to improve distal neurologic function from late decompression, but they may recover nerve roots near or above the level of injury and prevent ascending nerve root loss. The majority of clinical studies that have tried to determine the optimum timing of surgery for traumatic spinal cord injury are retrospective16,31–40,43,48,49 with only a few prospective studies.16,17,40,43,99 These studies have inconsistent definition of early versus late surgery that may have contributed to conflicting findings in which some studies found a benefit from early decompression and stabilization, whereas others found no advantage. However, if 4 weeks is considered late, there are studies, albeit retrospective, that have shown reversal of neurologic deficits in incomplete lesions.*
Cervical Spine Lesions
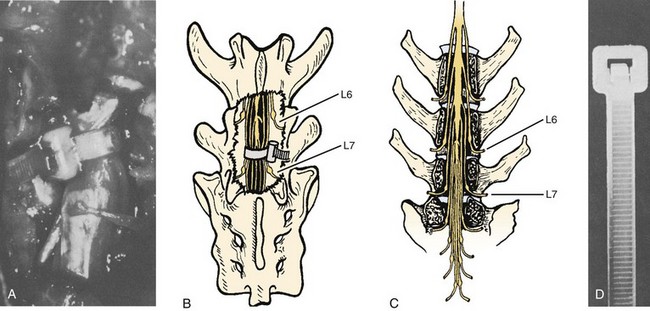
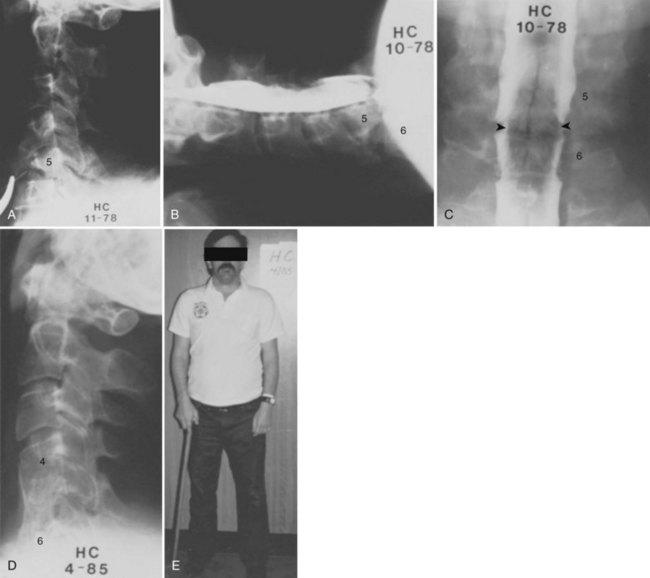
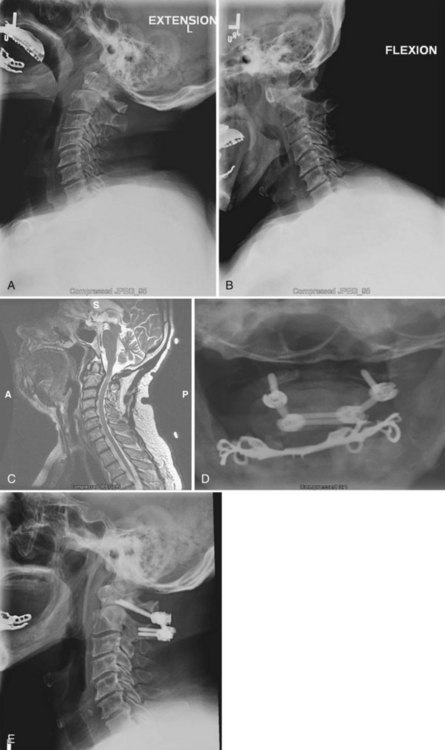
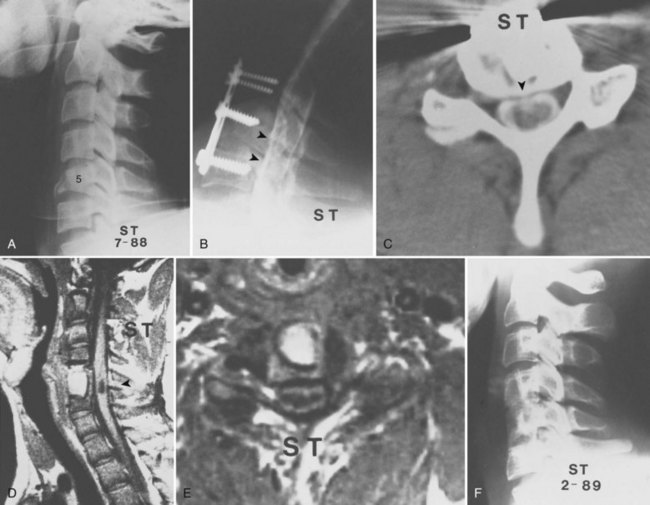
Bohlman and colleagues15,55 demonstrated that later anterior decompression can result in reversible neurologic function in patients with incomplete cord injuries (Fig. 79–5). In one study, 58 patients with incomplete cord injuries underwent late anterior cervical decompression and fusion with iliac crest autograft an average of 13 months (range, 1 month to 9 years) from injury. Twenty-nine patients who could not walk preoperatively became functional ambulators, and six patients who could walk before surgery had improvement in the ability to walk. Thirty-nine patients had improvement in motor root function in the upper extremities. Improvement was less in the patients who had decompression more than 12 months after injury.
In a similar study by Benzel and Larson,61 41 of 51 (81%) patients with incomplete myelopathies and whose neurologic function had plateaued had improved spinal cord function after decompression and fusion as late as 115 and 173 days in 2 patients. Among eight patients with motor complete lesions preoperatively with no useful function, six regained useful function, with four able to walk with assistance. The return of neurologic function in these patients began promptly after decompression, suggesting a cause-and-effect relationship. These studies suggest that late anterior decompression and stabilization can result in reversible neurologic deficits of both upper and lower extremity roots and distal cord function in the lower extremities.
Reduction of pain and restoration of even a single functional nerve root level may greatly improve the quality of life in complete quadriplegic patients. Return of sensory perception improves the chances of preventing pressure sores, and arm functional recovery of one or more levels may allow independent ambulation. Several studies38,39,55–57,61,62,69 have shown that these are realistic goals even when decompression is done as late as after 21 years.62 Patients with complete cord injuries have far less predictable and dramatic recovery. The mechanisms of recovery are poorly understood, but it appears that the injury falls along a continuum of an incomplete lesion where spinal cord fibers are anatomically but not functionally intact. Dimitrijevic101 proposed the term discomplete quadriplegia to describe this concept. Multiple factors including persistent compression, instability, and progressive deformity may diminish the healing environment of the cord and cause secondary injury. Nevertheless, decompression appears to have a limited but positive effect on recovering function of remaining viable motor roots and cord tissue.
Anderson and Bohlman69 performed anterior cervical decompression and fusion with iliac crest autograft in 51 patients with complete motor quadriplegia in an attempt to gain improvements of the motor roots in the upper extremities and thereby to improve function. Five patients died in less than 1 year. The average time from injury to decompression was 15 months (range, 1 month to 8 years), and 46 patients were followed for an average of 5 years (range, 2 to 13 years). Neurologic improvement of at least two new functional motor root levels occurred in 7 patients, and 18 patients had one-level recovery. Six patients had increased motor strength. Therefore anterior decompression resulted in neurologic improvement in 67% of the patients. Results were better in patients younger than age 53 years, those who had decompression less than 18 months after injury, and those who had neurologic levels cephalad to the site of skeletal injury rather than those with functioning motor roots at or caudad to the level of injury.
Benzel and Larson61 reported on 35 patients with complete quadriplegia who also underwent late surgery to improve neurologic function. Twenty-five patients underwent anterior decompression with or without posterior fusion and foraminotomy, whereas 10 had posterior fusion alone. Recovery of nerve root function only occurred in the patients who had anterior decompression (15 of 35 patients). No patient with both motor and sensory complete lesions recovered long tract function. However, of the 11 patients with motor injuries and sensory perception, 5 improved significantly and 2 were able to walk. It is highly unusual to have return of long tract motor function in patients with motor complete injuries, but it highlights the potential for late decompression to allow the cord maximal opportunity to recover neurologic function.
Thoracolumbar and Cauda Equina Lesions
Benzel and Larson61 described the results of anterior decompression and fusion via the lateral extracavity approach in 105 patients with thoracic and lumbar spinal cord injuries. There was significant improvement in the neurologic status of patients with incomplete lesions. Four of 10 patients with motor complete injuries with some sensory perception improved neurologically, with one able to ambulate. The anterior approach was deemed superior to a nonoperative or posterior stabilization approach for anterior compressive lesions.
Similarly, Maiman and colleagues56 reported the cases of 20 patients with incomplete thoracolumbar spinal cord injuries who underwent late anterior decompression via a lateral extracavity approach. Operations were done on average at 23 months and as late as 5 years after the initial injury or prior surgery. Thirteen patients had a mass in the canal, and seven patients had kyphotic deformities. Seventeen patients had substantial neurologic improvement. All but three of the nonambulatory patients with canal masses and the seven patients with kyphosis became ambulatory. The authors concluded that anterior decompression with appropriate stabilization affords the best opportunity for neurologic improvement in thoracolumbar spinal cord injuries.
Injuries to the cauda equina and conus medullaris with ongoing compression may also benefit from late decompression. The peripheral nerve roots of the cauda equina are fairly resilient, and conus medullaris injuries have significant potential for neurologic recovery, especially bladder control after decompression.57,59 Bradford and McBride64 studied 59 patients with incomplete deficits after thoracolumbar and cauda equina injuries. Eighteen patients who had anterior decompression were treated an average of 15.8 months (range, 1 to 79 months) after their injury. Anterior decompression was shown to be superior to posterior decompression, with 88% versus 64% neurologic improvement, respectively.
Although laminectomy is rarely indicated in spinal cord injuries, Ramamurthi and colleagues66 observed improvement in bladder function in 14 of 27 patients and motor function in 8 of 27 patients who had decompressive laminectomy between 6 and 12 weeks after conus and cauda equina injuries. Compression was due to displaced lamina, subarachnoid adhesions after traumatic fractures involving T12 to L4.
Late Surgical Procedures
Neural Compression, Deformity, and Pain
Patients may present late with neural compression, deformity, and pain because of failure to adequately stabilize a fracture, decompress the nerve roots or cord, or reduce dislocations. Despite controversy between the benefits of early versus late surgery, anterior decompression and stabilization leads to improved neurologic recovery over that expected with either a conservative, nonoperative approach or a posterior stabilization-only approach in patients with anterior compressive lesions.* Laminectomy is contraindicated in most instances because it does not adequately decompress the spinal cord when the compression is anterior and predisposes the patient to spinal instability and further neurologic deterioration.55,59,105
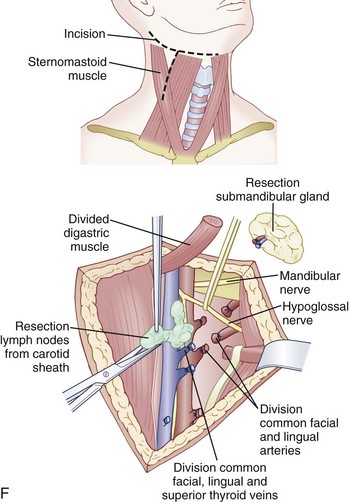
In the cervical spine, anterior cervical discectomy and interbody fusion using the Robinson technique safely and reliably treats central and lateral disc herniations.8,110 Vertebral body burst fractures with cord compression due to malunion or kyphosis respond well to corpectomies via an anterior approach. Supplemental posterior fixation may be required depending on the associated posterior osseous and ligamentous injuries.
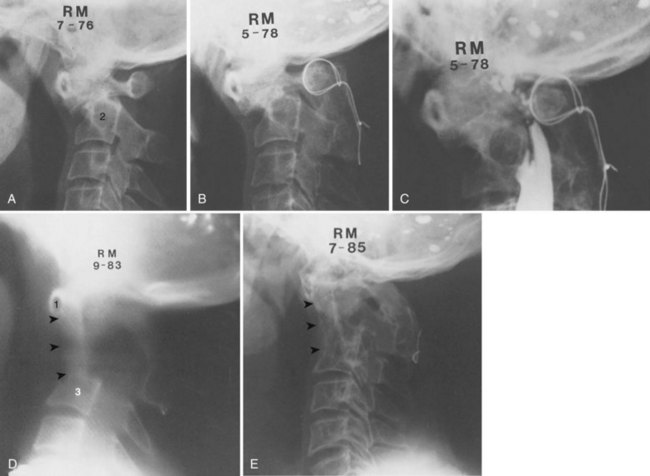
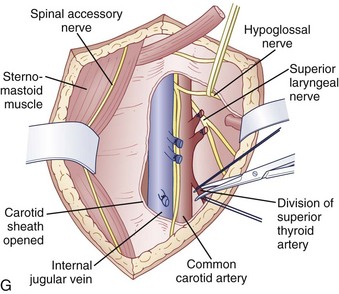
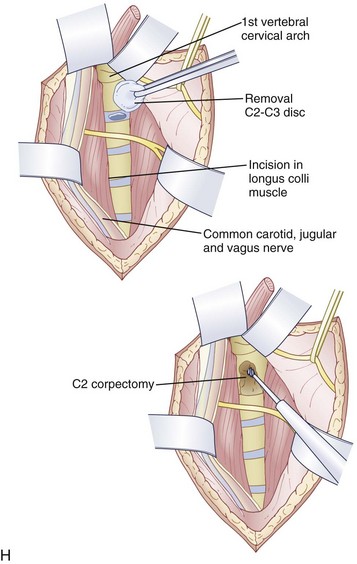
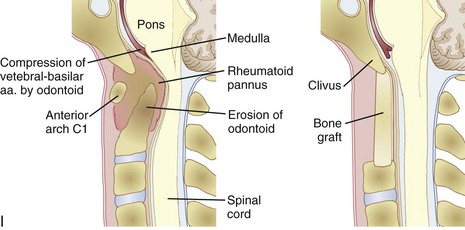
An anterior approach is also indicated in patients with a healed malunion of the odontoid or axis body fracture and anterior cord compression with myelopathy. We prefer the extrapharyngeal111 approach to the transoral112 approach with supplemental posterior C1 to C3 arthrodesis (Fig. 79–6). Alternatively, if the posterior atlantodental interval is less than 13 mm, it may be treacherous to fuse the axis to the atlas by passing instruments beneath the atlas posteriorly. This maneuver risks further neurologic injury that could be fatal. Instead, these patients can be treated with laminectomy of the arch of the atlas followed by arthrodesis between the occiput and C3. Use of C1 lateral mass screws and C2 translamina screws offer alternative fixation techniques as well (Fig. 79–7).
Thoracic and midlumbar neural compression are treated with the same principled approach as in the cervical spine. The site of neural compression dictates the approach, with anterior decompression preferred when there is proven anterior pathology.59,104,105,110 The deformity pattern and the osseous and ligamentous pathology dictate stabilization procedures. Treatment can include a single anterior113 or posterior114–116 approach, a combined anterior and posterior approach, and a simultaneous anterior and posterior approach with the patient in the lateral decubitus position.117
Late pain and paralysis may occur after thoracolumbar fractures with canal compromise in patients with or without neurologic deficits at the time of their injuries.57,62,107 Bohlman and colleagues62 performed anterior decompression of 45 patients with late pain and paralysis at an average of 4.5 years (range, 3 months to 21 years) after the initial injury. Twenty-one of the 25 patients with neurologic deficits noted neurologic improvement. Patients with less than severe paralysis had greater recovery. Forty-one of the 45 (91%) patients noted pain relief, with complete relief in 30 and partial in 11. In a similar study, Transfeldt and colleagues57 noted pain relief in 83% of their population after delayed anterior decompression of thoracolumbar and cauda equina injuries. In rare instances, isolated intercostal pain without thoracic cord compression may respond to posterior hemilaminectomy and foraminotomy to decompress the nerve root. At the L4 and L5 levels, the anterior retroperitoneal approach may be technically challenging, so a laminectomy and foraminotomies may be performed and the dural sac gently mobilized to remove bone or disc material.
Spasticity
Uninhibited muscle contractions below the level of injury in spinal cord–injured patients lead to spasticity of the lower abdominal wall, extremities, or bladder. Surgery aims to reduce the amount of local sensory input to the lower motor cells that have lost their regulatory input from the central nervous system (Fig. 79–8). The most common operation is a dorsal “T”-shaped myelotomy through the two dorsal columns to disrupt the reflex arc between the entering sensory axon as it traverses to the motor neuron. This can be selective to counteract the abdomen, extremity, or bladder. Some surgeons prefer a percutaneous dorsal rhizotomy. Patients choosing surgery for a spastic bladder should undergo preoperative urologic evaluation and be counseled on the possibility of losing erectile function or reflex bladder emptying. The spastic bladder can be controlled by sectioning the second, third, and fourth sacral segments or only the second and third sacral nerve roots in selected patients. Sphincterotomy before the neurosurgical procedures may relieve the bladder symptoms.70
Charcot Arthropathy
The German physician Kronig was the first to describe neuropathic arthropathy affecting the spine in a diabetic patient, 16 years after Charcot’s original description of the joint disease in 1868.77 Although uncommon, spinal cord patients are at risk for developing Charcot arthropathy of the spine. The etiology is unclear but seems to be predicted by loss of protective sensation.79 Ensuing trauma may subsequently incite an inflammatory cascade, followed by fracture, erosion, and sclerosis. Abundant hypertrophic callus forms at the site of destruction of the spine that may progress to form a ball-and-socket joint in advanced cases. Patients find it difficult to balance themselves because of the collapsing spine and may develop pain, neurologic dysfunction, instability, and deformity.78,79 It is controversial whether fixation should routinely extend to the sacrum to prevent the development of another pseudarthrosis below the fixation. Standaert and colleagues79 reported one of four patients developing destruction below the instrumented L4 vertebra. Brown and colleagues76 reported eight cases treated with circumferential fusion: Two patients developed Charcot arthropathy below the lowest instrumented level. In general, posterior fixation to the sacrum is often sufficient,75,76,79 but in some cases a combined anterior approach to debulk the hypertrophic tissue at the site of the disease supplemented with strut grafting and posterior instrumented fusion is necessary to achieve adequate correction and arthrodesis.76
Pearls
Pitfalls
Key Points
1 Anderson PA, Bohlman HH. Anterior decompression and arthrodesis of the cervical spine: Long-term motor improvement: II. Improvement in complete traumatic quadriplegia. J Bone Joint Surg Am. 1992;74:683-692.
2 Bohlman HH, Anderson PA. Anterior decompression and arthrodesis of the cervical spine: Long-term motor improvement: I. Improvement in incomplete traumatic quadriparesis. J Bone Joint Surg Am. 1992;74:671-682.
3 Bohlman HH, Bahniuk E, Raskulinecz G, Field G. Mechanical factors affecting recovery from incomplete cervical spinal cord injury: A preliminary report. Johns Hopkins Med J. 1979;145:115-125.
4 Bohlman HH, Freehafer A, Dejak J. The results of treatment of acute injuries of the upper thoracic spine with paralysis. J Bone Joint Surg Am. 1985;67:360-369.
5 McAfee PC, Bohlman HH, Yaun HS. Anterior decompression of traumatic thoracolumbar fractures with incomplete neurological deficit using a retroperitoneal approach. J Bone Joint Surg Am. 1985;67:89-104.
1 Katoh S, Masry WS, Jaffray D, et al. Neurologic outcome in conservatively treated patients with incomplete closed traumatic cervical spinal cord injuries. Spine. 1996;21:2345-2351.
2 Hughes JT. The Edwin Smith Surgical Papyrus: An analysis of the first case reports of spinal cord injuries. Paraplegia. 1988;26:71-82.
3 Guttman L. Spinal cord injuries: Comprehensive management and research. Oxford/London/Edinburgh/Melbourne: Blackwell Scientific Publications; 1973. 122-157
4 Davies WE, Morris JH, Hill V. An analysis of conservative (non-surgical) management of thoracolumbar fractures and fracture-dislocations with neural damage. J Bone Joint Surg Am. 1980;62:1324-1328.
5 Donovan WH, Kopaniky D, Stolzmann E, et al. The neurological and skeletal outcome in patients with closed cervical spinal cord injury. J Neurosurg. 1987;66:690-694.
6 Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial treatment of closed injuries of the spine with paraplegia and tetraplegia: I. Paraplegia. 1969;7:179-192.
7 Marshall LF, Knowton S, Garfin SR, et al. Deterioration following spinal cord injury: A multicenter study. J Neurosurg. 1987;66:400-404.
8 Bradford DS, Asbarnia BA, Winter RB, Seljeskog EL. Surgical stabilization of fracture and fracture dislocations of the thoracic spine. Spine. 1977;2:185-196.
9 Harris P, Karmi MZ, McClemont E, et al. The prognosis of patients sustaining severe cervical spine injury (C2-C7 inclusive). Paraplegia. 1980;18:324-330.
10 Heiden JS, Weiss MH, Rosenberg AW, et al. Management of cervical spinal cord trauma in Southern California. J Neurosurg. 1975;43:732-736.
11 Maynard FM, Reynolds GG, Fountain S, et al. Neurological prognosis after traumatic quadriplegia: Three-year experience of California Regional Spinal Cord Injury Care System. J Neurosurg. 1979;50:611-616.
12 Wagner FC, Chehrazi B. Early decompression and neurological outcome in acute cervical spinal cord injuries. J Neurosurg. 1982;56:699-705.
13 Hamell E, Karimi-Nejad A, Frowein FA, et al. Results of conservative and surgical early treatment of cervical spine injuries. In: Wullenweber R, Brock M, Hamer J, et al, editors. Advances in Neurosurgery, vol 4. Berlin: Springer-Verlag; 1977:185-190.
14 Larson SJ, Holst RA, Hemmy DC, et al. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45:628-637.
15 Bohlman HH, Anderson PA. Anterior decompression and arthrodesis of the cervical spine: Long-term motor improvement: I. Improvement in incomplete traumatic quadriparesis. J Bone Joint Surg Am. 1992;74:671-682.
16 Waters RL, Adkins RH, Yakura JS, et al. Effect of surgery on motor recovery following traumatic spinal cord injury. Spinal Cord. 1988;34:244-249.
17 Tator CH, Duncan EG, Edmonds VE, et al. Comparison of surgical and conservative management in 208 patients with acute spinal cord injury. Can J Neurosci. 1987;14:60-69.
18 Petitjean ME, Mousselard H, Pointillart V, et al. Thoracic spinal trauma and associated injuries: Should early spinal decompression be considered? J Trauma. 1995;39:368-372.
19 Quencer RM, Sheldon JJ, Post MJD, et al. MRI of the chronically injured cervical spinal cord. AJR Am J Roentgenol. 1986;147:125-132.
20 Agrawal SK, Fehlings MG. Mechanisms of secondary injury to spinal cord axons in vitro: Role of Na+, Na(+)-K(+)-ATPase, the NA(+)-H+ exchanger, and the Na(+)-C++ exchanger. J Neurosci. 1996;16:545-552.
21 Agrawal SK, Fehlings MG. Role of NMDA and non-NMDA iconotopic glutamate receptors in traumatic spinal cord axonal injury. J Neurosci. 1997;17:1055-1063.
22 Allen AR. Surgery of experimental lesion of spinal cord equivalent to brush injury of fracture dislocation of spinal column: A preliminary report. JAMA. 1911;57:878-880.
23 Hung TK, Chang GL, Chang JL, et al. Stress-strain relationship and neurological sequelae of uniaxial elongation of the spinal cord of cats. Surg Neurol. 1981;15:471-476.
24 Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15-26.
25 Young W. The post-injury responses in trauma and ischemia: Secondary injury or protective mechanisms? Cent Nerv Syst Trauma. 1987;4:27-51.
26 Osterholm JL, Mathews GL. Altered norepinephrine metabolism following experimental spinal cord injury: I. Relationship to hemorrhagic necrosis and post-wounding neurological deficits. J Neurosurg. 1972;36:386-394.
27 Anderson DK, Means ED, Waters TR. Signal cord energy metabolism in normal and postlaminectomy cats. J Neurosurg. 1980;52:387-391.
28 Bracken MB, Shephard MJ, Colins EF, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spine Cord Injury Study. JAMA. 1997;277:1597-1604.
29 Geisler FH, Dorsey FC, Coleman WP. Past and current clinical studies with GM-1 ganglioside in acute spinal cord injury. Ann Emerg Med. 1993;22:1041-1047.
30 Pitts LH, Ross A, Chase GA, et al. Treatment with thyrotropin-releasing hormone (TRH) in patients with traumatic spinal cord injuries. J Neurotrauma. 1995;12:235-243.
31 Jacobs RR, Asher MA, Snider RK. Thoracolumbar spinal injuries: A comparative study of recumbent and operative treatment in 100 patients. Spine. 1980;5:463-477.
32 Mizra SK, Krengel WFIII, Chapman JR, et al. Early versus delayed surgery for acute cervical spinal cord injury. Clin Orthop. 1999;359:104-114.
33 Aebi M, Mohler J, Zach GA, et al. Indication, surgical technique, and results of 100 surgically treated fractures and fracture-dislocations of the cervical spine. Clin Orthop. 1986;203:244-257.
34 Wiberg J, Hauge HN. Neurological outcome after surgery for thoracic and lumbar spine injuries. Acta Neurochir. 1988;91:106-112.
35 Wilberger JE. Diagnosis and management of spinal cord trauma. J Neurotrauma. 1991;8(Suppl):S21-S30.
36 Papadopoulos SM, Selden NR, Quint DJ, et al. Immediate spinal cord decompression for cervical spinal cord injury: Feasibility and outcome. J Trauma. 2002;52:323-332.
37 Murphy KP, Opitz JL, Cabanela ME, et al. Cervical fractures and spinal cord injury: Outcome of surgical and nonsurgical management. Mayo Clin Proc. 1990;65:949-959.
38 Weinshel SS, Maiman DJ, Back P, et al. Neurologic recovery in quadriplegia following operative treatment. J Spinal Disord. 1990;3:244-249.
39 Levi L, Wolf A, Rigamonti D, et al. Anterior decompression in cervical spine trauma: Does timing of surgery affect the outcome? Neurosurgery. 1991;29:216-222.
40 Vaccaro AR, Daugherty RJ, Sheehan TP, et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine. 1997;22:2609-2613.
41 Wolf A, Levi L, Marvis S, et al. Operative management of bilateral facet dislocation. J Neurosurg. 1991;75:883-890.
42 Krengel WFIII, Anderson PA, Henley MB. Early stabilization and decompression for incomplete paraplegia due to a thoracic-level spinal cord injury. Spine. 1993;18:2080-2087.
43 Duh MS, Shephard MJ, Silberger JE, et al. The effectiveness of surgery on the treatment of acute spinal cord and its relation to pharmacological treatment. Neurosurgery. 1994;35:240-249.
44 Carlson GD, Gorden CD, Oliff HS, et al. Sustained spinal cord compression: I. Time-dependent effect on long-term pathophysiology. J Bone Joint Surg Am. 2003;85:86-94.
45 Heary RF, Vaccaro R, Mesa JJ, et al. Thoracolumbar infections in penetrating injuries to the spine. Orthop Clin North Am. 1996;27:69-81.
46 Carlson GD, Gorden CD, Nakazawa S, et al. Sustained spinal cord compression: II. Effect of methylprednisone on regional blood flow and recovery of somatosensory evoked potentials. J Bone Joint Surg Am. 2003;85:95-101.
47 Rabinowitz RS, Eck JC, Harper JrM, et al. Urgent surgical decompression compared to methylprednisolone for the treatment of acute spinal cord injury: A randomized prospective study in beagle dogs. Spine. 2008;33:2260-2268.
48 Schlegel J, Bayley J, Yaun HA, et al. Timing of surgical decompression and fixation of acute spinal fractures. J Orthop Trauma. 1996;10:323-330.
49 Campagnolo DI, Esquieres RE, Kopacz KJ. Effect of timing of stabilization on length of stay and medical complications following spinal cord injury. J Spinal Cord Med. 1997;20:331-334.
50 Zigler JE, Cdapen DA. Epidemiology of spinal cord injury: A perspective on the problem. In: Levine AM, Eismont FJ, Garfin S, et al, editors. Spine Trauma. Philadelphia: WB Saunders; 1998:2-8.
51 Fehlings MG, Arvin B. The timing of surgery in patients with central spinal cord injury. J Neurosurg Spine. 2009;10:1-2.
52 Chen L, Yang H, Yang T, et al. Effectiveness of surgical treatment for traumatic central cord syndrome. J Neurosurg Spine. 2009;10:3-8.
53 Bohlman HH, Bahniuk E, ield G, Raskulinecz G. Spinal cord monitoring of experimental incomplete cervical spinal cord injury. Spine. 1981;6:428-436.
54 Bohlman HH, Bahniuk E, Raskulinecz G, Field G. Mechanical factors affecting recovery from incomplete cervical spinal cord injury: A preliminary report. Johns Hopkins Med J. 1979;145:115-125.
55 Bohlman HH, Freehafer A. Later anterior decompression of spinal cord injuries. J Bone Joint Surg Am. 1979;57:1025.
56 Maiman DJ, Larson SJ, Benzel EC. Neurological improvement associated with late decompression of the thoracolumbar spinal cord. Neurosurgery. 1984;14:302-307.
57 Transfeldt EE, White D, Bradford DS, et al. Delayed anterior decompression in patients with spinal cord and cauda equina injuries of the thoracolumbar spine. Spine. 1990;15:953-957.
58 Zhang Y, Hillered L, Olsson Y, et al. Time course of energy pertubation after compression trauma to the spinal cord: An experimental study in the rat using microdialysis. Surg Neurol. 1993;39:297-304.
59 McAfee PC, Bohlman HH, Yaun HS. Anterior decompression of traumatic thoracolumbar fractures with incomplete neurological deficit using a retroperitoneal approach. J Bone Joint Surg Am. 1985;67:89-104.
60 Chen TY, Lee ST, Lui TN, et al. Efficacy of surgical treatment in traumatic central cord syndrome. Surg Neurol. 1997;48:435-440.
61 Benzel EC, Larson SJ. Functional recovery after decompressive spine operation for cervical spine fractures. Neurosurgery. 1987;20:742-746.
62 Bohlman HH, Kirkpatrick JS, Delamarter RB, et al. Anterior decompression for late pain and paralysis after fractures of the thoracolumbar spine. Clin Orthop. 1994;300:24-29.
63 Bradford DS, Akbarnia BA, Winter RB, Seljeskog EL. Surgical stabilization of fracture and fracture dislocations of the thoracic spine. Spine. 1977;2:185-196.
64 Bradford DS, McBride GG. Surgical management of thoracolumbar spine fractures with incomplete neurologic deficits. Clin Orthop Rel Res. 1987;218:201-216.
65 Clohisy JC, Akbarnia BA, Bucholz RD, et al. Neurologic recovery associated with anterior decompression of spine fractures at the thoracolumbar junction (T12-L1). Spine. 1992;17:S321-S330.
66 Ramamurthi B, Ramamurthi R, Narayanan R. Late laminectomy in traumatic paraplegia. Surg Neurol. 1983;20:414-416.
67 Schneider RC, Kahn EA. Chronic neurologic sequelae of acute trauma to the spine and spinal cord: I. The significance of the acute-flexion or “tear-drop” fracture-dislocation of the cervical spine. J Bone Joint Surg Am. 1956;38:985-997.
68 Yablon EG, Palumbo M, Spatz E, et al. Nerve root recovery in complete injuries of the cervical spine. Spine. 1991;16:S519-S521.
69 Anderson PA, Bohlman HH. Anterior decompression and arthrodesis of the cervical spine. Long-term motor improvement: II. Improvement in complete traumatic quadriplegia. J Bone Joint Surg Am. 1992;74:683-692.
70 Bohlman HH, Ducker T. Late surgical procedures. In: Rothman RH, Simeone FA, editors. The Spine. Philadelphia: WB Saunders; 1992:983-991.
71 Lee TT, Alameda GJ, Gromelski EB, et al. Surgical treatment of post-traumatic myelopathy associated with syringomyelia. J Neurosurg. 2000;92:149-154.
72 Rossier AB, Foo D, Shillito J, et al. Post-traumatic cervical syringomyelia: Incidence, clinical presentation, electrophysiological studies, syrinx protein and results of conservative and operative treatment. Brain. 1985;108:439-461.
73 Schurch B, Wichmann W, Rossier AB. Post-traumatic syringomyelia (cystic myelopathy): A prospective study of 449 patients with spinal cord injury. J Neurol Neurosurg Psychiatry. 1996;60:61-67.
74 Sgouros S, Williams B. Management and outcome of post-traumatic syringomyelia. J Neurosurg. 1996;85:197-205.
75 Arnold PM, Back PN, Stillerman CB, et al. Surgical management of lumbar neuropathic spinal arthropathy (Charcot joint) after traumatic thoracic paraplegia: Report of two cases. J Spinal Disord. 1995;8:357-362.
76 Brown CW, Jones B, Donaldson DH, et al. Neuropathic (Charcot) arthropathy of the spine after traumatic spinal paraplegia. Spine. 1992;17(Suppl)):S103-S108.
77 Gupta R. A short history of neuropathic arthropathy. Clin Orthop. 1993;296:43-49.
78 Sobel JW, Bohlman HH, Freehafer AA. Charcot’s arthropathy of the spine following spinal cord injury: A report of 5 cases. J Bone Joint Surg Am. 1985;67:771-776.
79 Standaert C, Cardenas DD, Anderson P. Charcot spine as late complication of traumatic cord injury. Arch Phys Med Rehabil. 1997;78:221-225.
80 Tarlov IM, Klinger H. Spinal cord compression studies: I. Experimental techniques to produce acute and gradual compression. Arch Neurol Psychiatry. 1953;70:813-819.
81 Tarlov IM. Spinal cord compression studies: III. Time limits for recovery after gradual compression in dogs. Arch Neurol Psychiatry. 1954;71:588-597.
82 Tarlov IM. Acute spinal cord compression paralysis. J Neurosurg. 1972;36:10-20.
83 Liu XZ, Xu XM, Hu R, et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395-5406.
84 Demopoulos HB, Flamm ES, Pietronigro DD, et al. The free radical pathology and the microcirculation in the major central nervous system disorders. Acta Physiol Scand. 1980;492:S91-S119.
85 Lu J, Ashwell KW, Waite P. Advances in secondary spinal cord injury: Role of apoptosis. Spine. 2000;25:1859-1866.
86 Croft TJ, Brodkey JS, Nulsen FE. Reversible spinal cord trauma: A model for electrical monitoring of spinal cord function. J Neurosurg. 1972;36:402-406.
87 Thienprasit P, Bantli H, Bloedel JR, et al. Effect of delayed local cooling on experimental spinal cord injury. J Neurosurg. 1975;42:150-154.
88 Kobrine AI, Evans DE, Rizzoli HV. Correlation of spinal cord blood flow and function in experimental compression. Surg Neurol. 1978;10:54-59.
89 Kobrine AI, Evans DE, Rizzoli HV. Experimental acute balloon compression of the spinal cord: Factors affecting disappearance and return of the spinal evoked response. J Neurosurg. 1979;51:841-845.
90 Dolan EJ, Tator CH, Endrenyi L. The value of decompression for acute experimental spinal cord compression injury. J Neurosurg. 1980;53:749-755.
91 Aki T, Toya S. Experimental study on changes of the spinal-evoked potential and circulatory dynamics following spinal cord compression and decompression. Spine. 1911;9:800-809.
92 Guha A, Tator CH, Endrenyi L, et al. Decompression of the spinal cord improves recovery after acute experimental spinal cord compression injury. Paraplegia. 1987;25:324-339.
93 Nystrom B, Berglund JE. Spinal cord restitution following compression injuries in rats. Acta Neurol Scand. 1988;78:467-472.
94 Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury: Recovery after immediate and delayed compression. J Bone Joint Surg Am. 1995;77:1042-1049.
95 Carlson GD, Minato Y, Okada A, et al. Early time-dependent decompression for spinal cord injury: Vascular mechanisms of recovery. J Neurotrauma. 1997;14:951-962.
96 Dimar JRI, Glassman SD, Raque GH, et al. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine. 1999;24:1623-1633.
97 Tarlov IM, Klinger H. Spinal cord compression studies: II. Time limits for recovery after acute compression in dogs. Arch Neurol Psychiatry. 1954;71:271-290.
98 Sommerson SK, Stokes BT. Functional analysis of an electromechanical spinal cord injury device. Exp Neurol. 1987;96:82-96.
99 Vale FL, Burns J, Jackson AB, et al. Combined medical and surgical treatment after acute spinal cord injury: Results of a progressive pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87:239-246.
100 Brodkey JS, Miller CJJr, Marmody RM. The syndrome of acute central cervical spinal cord injury revisited. Surg Neurol. 1980;14:251-257.
101 Dimitrijevic M. Neurophysiology in spinal cord injury. Paraplegia. 1987;25:205-208.
102 Bohlman HH, Zdeblick TA. Anterior excision of herniated thoracic disks. J Bone Joint Surg Am. 1988;70:1038-1047.
103 McAfee PC, Bohlman HH. One-stage anterior cervical decompression and posterior stabilization with circumferential arthrodesis: An analysis of twenty-four cases. J Bone Joint Surg Am. 1989;71:78-88.
104 Bohlman HH, Eismont FJ. Surgical techniques of anterior decompression and fusion for spinal cord injuries. Clin Orthop. 1981;154:57-67.
105 Bohlman HH, Freehafer A, Dejak J. The results of treatment of acute injuries of the upper thoracic spine with paralysis. J Bone Joint Surg Am. 1985;67:360-369.
106 Bohlman HH. Acute fractures and dislocations of the cervical spine: An analysis of 300 hospitalized patients and review of the literature. J Bone Joint Surg Am. 1979;61:1119-1142.
107 Bohlman HH. Late progressive paralysis and pain following fractures of the thoracolumbar spine. A report of 10 patients. J Bone Joint Surg Am. 1976;58:723.
108 Bohlman HH. Treatment of thoracic and lumbar fractures and dislocations: Current concepts review. J Bone Joint Surg Am. 1985;67:192-200.
109 Bohlman HH. Cervical spondylosis with moderate to severe myelopathy: Report of 17 cases treated by Robinson anterior cervical discectomy and fusion. Spine. 1977;2:151-162.
110 Chin KR, Ozuna R. Options in the surgical treatment of cervical spondylotic myelopathy. Curr Opin Orthop. 2000;11:151-157.
111 McAfee PC, Bohlman HH, Riley L, et al. Anterior extraoral approach to the atlas and axis. J Bone Joint Surg Am. 1987;69:1371-1383.
112 Ashraf J, Crockard HA. Transoral fusion for high cervical fractures. J Bone Joint Surg Br. 1990;72:76-79.
113 Kostuik JP, Matsusaki H. Anterior stabilization, instrumentation, and decompression for post-traumatic kyphosis. Spine. 1978;14:379-386.
114 Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop. 1985;194:142-152.
115 Heinig CF. Eggshell procedure. In: Luque ER, editor. Segmental Spinal Instrumental. Thorofare, NJ: Slack; 1984:221-234.
116 Lehmer SM, Keppler L, Biscup RS, et al. Posterior transvertebral osteotomy for adult thoracolumbar kyphosis. Spine. 1994;19:2060-2067.
117 Pascal-Mousselard H, Klein JR, Schwab FJ. Simultaneous anterior and posterior approaches to the spine for revision surgery: Current indications and techniques. J Spinal Disord. 1999;12:206-213.