Chapter 18 Laser use in percutaneous coronary intervention
 Excimer laser catheters may be useful for treating coronary lesions which can be crossed with a guidewire but not with a balloon catheter.
Excimer laser catheters may be useful for treating coronary lesions which can be crossed with a guidewire but not with a balloon catheter. Laser balloon angioplasty may be useful for treating immediate vessel recoil or severe dissection. However, the introduction of coronary stents has essentially obviated the need for laser balloon angioplasty.
Laser balloon angioplasty may be useful for treating immediate vessel recoil or severe dissection. However, the introduction of coronary stents has essentially obviated the need for laser balloon angioplasty.INTRODUCTION
The presence of a chronic total coronary artery occlusion continues to be a major problem for interventional cardiology. Around 10% of all attempted coronary angioplasties are performed for chronic total coronary occlusion.1 The procedural success rate is lower for patients with chronic total occlusions who undergo coronary angioplasty when compared with patients who have stenosed, but patent, vessels. Depending to some extent on the nature of the occlusion and its duration, the success rate is usually in the order of 50–70%2,3 – this is clearly much lower than for stenosed vessels. Failure to treat a chronic total occlusion may be due to the inability to cross the lesion with a guidewire or to inability to advance the balloon catheter across the lesion once the wire has crossed successfully. When treating a chronic total occlusion, the conventional technique uses a stiff guidewire and advancement of the balloon catheter close to the tip of the guidewire for additional rigidity. A variety of technologies have been introduced without a major improvement in the success rates. These include guidewires with olive-shaped tips, drills of various velocities, radio frequency heat applicators and laser devices. Two laser technologies will be considered further: first the laser guidewires and second the over-the-wire Excimer laser catheter.
LASER GUIDEWIRES
An Argon laser heated balltip (hot tip) guidewire has been used for the initial passage through chronic total occlusions.4 In some cases, it has been successful when conventional systems had failed. Vessel perforation is a possible complication of the technique. Further developments in hot tip recanalisation have not occurred and this technology is now rarely used.
The results of the bare Argon laser instrument Lastac are again comparable to those achieved with less costly mechanical means, although the cases undertaken may have been slightly more complex.5 A beneficial mechanical component is undoubtedly present with these catheters, which have many of the features which may be useful when treating chronic total occlusions (e.g. stiffness, bluntness). Randomised prospective trials against conventional technologies have not been carried out and this equipment now has very limited application.
EXCIMER LASER CATHETER
In the Excimer Laser Coronary Angioplasty Registry, 172 chronic total occlusions were treated in 162 patients (10.3% of the 1569 patients entered). Once a guidewire crossed an occlusion, the overall laser success rate for treatment of chronic total occlusions was 83%. The extent of stenosis decreased from 100% to 55 *b+ 26%.6 In 74% of patients, adjunctive percutaneous balloon coronary angioplasty was used after laser treatment. A final procedural success, defined as less than 50% residual stenosis and no major complication (death, myocardial infarction or CABG) was achieved in 90%. Major complications were infrequent: one death, 1.9% myocardial infarction and 1.2% requirement for emergency bypass surgery. The results suggest that Excimer laser angioplasty may be useful for treating chronic total occlusions that can be crossed with a guidewire but not with the balloon catheter. A role has also been suggested when the occlusion has been confirmed to be extremely long.6
Excimer laser angioplasty continues to develop, particularly as an adjunct to conventional balloon angioplasty or coronary stenting in the treatment of chronic total occlusions. The data suggest that failure of laser angioplasty occurs because of low catheter flexibility and the need for good guidewire support when treating total occlusions – once the catheter has reached the target area, the morphology of the lesion seems to be of only minor importance for the success of the procedure.7 An alternative strategy when treating totally occluded arteries, which can be crossed with a guidewire, but not the balloon catheter is to use the Rotablator system. This technology, which uses a high-speed rotational burr, produces ablation of tissue. By using burrs of increasing diameter, the lesion may be successfully treated by the rotablator technique alone. Frequently, coronary balloon angioplasty or coronary stenting are required after the initial rotablator therapy. Currently there are no randomised studies comparing Excimer laser angioplasty with Rotablator in the treatment of chronic total occlusions which can be crossed with a guidewire but not a balloon catheter.
LASER BALLOON ANGIOPLASTY
Conventional balloon coronary angioplasty improves luminal dimensions by producing fracture of the atheromatous plaque and by stretching the plaque-free wall. It is associated with dissection of the media and the formation of intimal flaps. These changes create local flow abnormalities which are associated with varying degrees of mural thrombus formation. In the vast majority of cases, dissection and thrombus formation do not result in acute vessel occlusion. During the next few months, however, the vascular reponse to injury may lead to restenosis. The aim of the laser balloon angioplasty is to create a large, smooth vascular lumen, which may lead to better short- and long-term results than conventional balloon angioplasty. In theory, this could potentially be achieved by thermal welding of dissection flaps, the elimination of vascular recoil, the elimination of coronary vasospasm, the reduction in platelet activation and the inhibition of smooth muscle cell proliferation.8
Laser balloon angioplasty has been shown to be effective in the management of acute failure of balloon coronary angioplasty, due to either immediate vessel recoil or severe dissection with impaired flow (‘impending closure’). Despite this early success rate, however, the late angiographic restenosis rate of laser balloon angioplasty is very similar to conventional balloon angioplasty.8 This is despite laser balloon angioplasty producing larger vessel lumens in the acute stage. With the introduction of coronary stents, the potential role for laser balloon angioplasty seems to have been taken over. The use of coronary stents will usually solve the acute problem of vessel recoil or severe dissection resulting in threatened occlusion. In addition, coronary stents are associated with a lower angiographic restenosis rate. Therefore, there would now appear to be no role for the technique of laser balloon angioplasty.
TRANSMYOCARDIAL LASER REVASCULARISATION (TMLR)
This is a technique that creates transmural channels in ischaemic myocardium using laser ablation. Before the advent of coronary angioplasty and coronary artery bypass surgery, attempts were made at direct transmyocardial revascularisation.9,10 A variety of tubes and needles were used to create transmural channels, with limited success in terms of clinical improvement. The concept was based on the knowledge of myocardial sinusoids and the thebesian system. These communications were thought to allow the direct perfusion of the myocardium by blood from the left ventricle. Other techniques included the creation of myocardial neovascularisation by internal thoracic artery implantation directly into the myocardium.11 In principle, TMLR incorporates the direct perfusion and neovascularisation of these other techniques. Although the exact mechanism of action of TMLR is unknown, suggestions include direct perfusion through patent channels, denervation produced by the surgical procedure and new vessel formation (angiogenesis).12 At the moment, the most likely mechanism for symptomatic improvement seems to be angiogenesis.
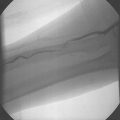
The technique of TMLR is usually carried out through a left anterolateral thoracotomy. The pericardium is opened and dissected free of the heart and the area of reversible ischaemia is exposed. This is determined pre-operatively by myocardial perfusion scanning (nuclear techniques or positron emission tomography). There is no requirement for cardiopulmonary bypass. The original laser used was the high energy carbon dioxide system (The Heart Laser, PLC Medical Systems). The laser probe is placed on the surface of the heart and fired when the ventricle is maximally distended with blood and electrically quiescent. The laser energy is absorbed by the blood within the left ventricle and this produces an acoustic image analogous to steam which is readily visible on transoesophageal echocardiography. Clearly, this appearance on the echocardiogram denotes transmyocardial penetration. The channels created by the CO2 laser are approximately 1 mm in diameter and are created in a distribution of approximately one per square centimeter (Fig. 18.1). Haemorrhage from the channel is controlled with direct finger pressure or an epicardial suture if pressure is not adequate. TMLR can also be performed using a Holmium: YAG laser or an Excimer laser.
Uncontrolled studies using the CO2 laser have suggested an overall improvement both subjectively and objectively following TMLR. In a USA multicentre, uncontrolled phase II clinical trial, based on 200 patients, the operative mortality was 9%.12 All patients had angina refractory to medical therapy, documented evidence of reversible ischaemia by radionuclide scanning and were untreatable by conventional forms of revascularisation. There was a significant improvement in the Canadian Cardiovascular Score (CCS) for angina at 3, 6 and 12 months following the procedure. Taking a decrease of two CCS classes as a clinically significant improvement, 75% of those assessed post-operatively achieved this. In addition, there was a significant decrease in the number of perfusion defects in the treated left ventricular wall. Patients who had at least one year of follow up experienced a significant decrease in the number of admissions for angina from 2.5 per patient-year before treatment to 0.5 per patient-year in the year following surgery.
In a registry report from European and Asian centres performing TMLR using the carbon dioxide laser, the operative mortality was 9.7%.13 Benefit in terms of improvement in exercise performance was clinically significant, but less impressive than in the US uncontrolled study and less than benefits observed following other revascularisation procedures. Less than 50% of patients achieved an improvement of at least two angina classes compared with the 75% in the USA study. Complications of the technique include peri-operative bleeding and infection, left ventricular failure in the early post-operative period and cardiac arrhythmias – both supraventricular and ventricular.
TMLR is still a new technology which is being used more widely in Europe, the USA and Asia. It is clear that the benefits of the procedure in terms of improvements in angina and exercise tolerance will need to be sufficient to justify the peri-operative morbidity and mortality if the procedure is to become universally accepted. The results of a USA multicentre randomised controlled trial of TMLR against medical management have been submitted to the Food and Drug Administration Advisory Committee. They initially recommended non-approval for use in the USA due to the lack of a definitive explanation for the underlying mechanism, although many other well accepted techniques in clinical practice still lack full insight into the pathophysiological mechanism. In addition, there were concerns about the conduct of the trial in that the design allowed cross-over from medical therapy to TMLR and follow up data was incomplete. A follow up submission was eventually approved. There have been two large randomised controlled trials of TMLR – one from the US and one from the UK. The US trial randomised 198 patients to continued medication or TMLR plus medication.14 There was a high cross-over rate from medical therapy to TMLR and the 12-month data only includes 64 patients from the TMLR group and 23 from the control group. An improvement of at least two angina classes was found at 12 months in 72% of the TMLR group and 13% of control patients. There was an operative mortality of 3% for TMLR with no significant difference in survival at 12 months between the two groups. The results of the UK trial were less favourable.15 In this study 188 patients with severe angina associated with coronary artery disease unsuitable for conventional revascularisation were randomised to continued medication or TMLR plus medication. There were no cross-overs and almost complete follow-up data. At 12 months, the CCS angina score decreased by at least two classes in 25% of the TMLR patients and 4% of the control group. There was a peri-operative mortality of 5% for TMLR with no significant difference in survival at 12 months between the two groups. The morbidity associated with the TMLR procedure included wound/respiratory infection (33%) transient arrhythmia (usually atrial fibrillation) (15%) and left ventricular failure (12%). Exercise capacity measured using treadmill exercise times and 12 minutes’ walk distance, was greater in the TMLR patients, although the difference between the two groups did not reach statistical significance. TMLR, therefore, seems to produce symptomatic improvement in this patient population, although an increase in exercise capacity has not been consistently demonstrated. Since the subjective improvement seems to be greater than the objective measurements, there are reservations regarding the widespread introduction of TMLR into clinical practice. It carries a peri-operative mortality of between 3% and 10% as well as a significant procedural morbidity.
PERCUTANEOUS MYOCARDIAL REVASCULARISATION (PMR)
With further developments in technology, it became possible to perform direct myocardial laser revascularisation using a percutaneous approach. A Holmium: YAG laser was developed in order to deliver energy directly to the endocardial surface of the left ventricular cavity (percutaneous myocardial revascularisation, CardioGenesis). Clearly, from the patient’s point of view, this technique is much less invasive. It obviates the need for a general anaesthetic and thoracotomy with a consequent reduction in the length of hospital stay. Following TMLR, the mean length of stay is between 10 and 12 days, whereas patients can normally be discharged from hospital 24 to 48 hours after PMR.
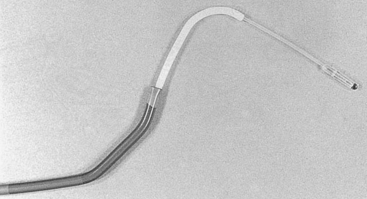
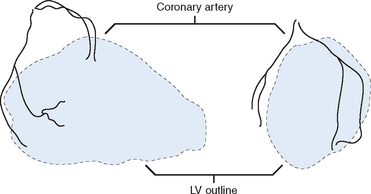
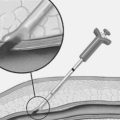
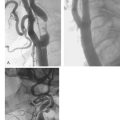
With the CardioGenesis PMR system, access to the left ventricle is gained via a 9 F sheath introduced into the right femoral artery. The equipment essentially consists of a ‘guiding catheter’, a ‘laser catheter’ (which has a right angle bend towards its tip) and a ‘laser fibre’, which is passed through the laser catheter (Fig. 18.2). It is essential that the patient and the X-ray table do not move once the views in which to work have been selected. The two views typically selected are 40 degrees right anterior oblique and 50 degrees left anterior oblique, often with up to 10 degrees of cranial angulation. Whilst a biplane facility is preferred, the procedure can be easily carried out on single-plane equipment, although it may take slightly longer. The ‘guiding catheter’ is advanced to the left ventricular cavity and pre-procedure left ventricular angiograms are performed in the two selected views. In addition, pre-procedure coronary angiograms are usually carried out in the same projections. Both the coronary angiograms (at end-diastole) and the left ventricular angiograms (at end-diastole) are traced onto acetate sheets fixed over the viewing screens (Fig. 18.3). These then act as ‘maps’ during the procedure. The area or areas to be treated are determined prior to the procedure using myocardial perfusion scanning. Reversible ischaemia may be present in the inferior, anterior, or lateral walls, or may involve the interventricular septum or left ventricular apex. All of these sites are accessible for treatment.
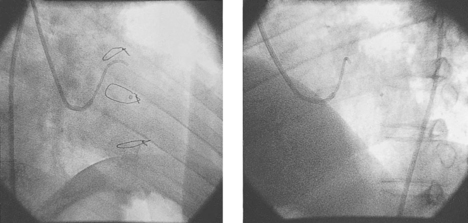
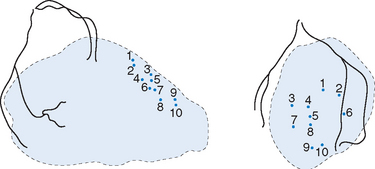
The ‘laser catheter’ is advanced through the ‘guiding catheter’ which has been positioned within the left ventricle. The ‘guiding catheter’ is available in a variety of curves and the one selected depends on the size and shape of the left ventricular cavity as well as the area to be treated. By manipulating the ‘guiding catheter’ and/or the ‘laser catheter’ all sites within the left ventricle can be accessed. Once in the appropriate position, the ‘laser fibre’ is advanced through the ‘laser catheter’ until there is contact with the endocardial surface of the left ventricle. This can often be felt, but can be seen as the laser catheter ‘backs away’ from the left ventricular wall and frequently ventricular ectopics are produced. Usually the right anterior oblique view is preferred for demonstrating contact with the anterior and inferior walls, and the left anterior oblique view for contact with the lateral and septal walls. The ‘laser fibre’ should be perpendicular to the left ventricular wall (Fig. 18.4). The laser is then activated, which typically produces 3 mm of penetration into the left ventricular myocardium: the ‘laser fibre’ is then advanced slightly and re-activated, which produces a channel of around 6 mm in total into the wall. The laser fibre is then withdrawn into the laser catheter and a new site is selected by manipulating the ‘guiding catheter’ and/or the ‘laser catheter’. Clearly, it is important to determine prior to the procedure that the part of the left ventricle to be treated is at least 8 mm thick, using transthoracic echocardiography, to reduce the risk of left ventricular perforation. The apex of the left ventricle is usually thinner than the rest of the ventricle, and, therefore, most operators will only be using one ‘burst’ of laser energy rather than two (that is, only 3 mm deep channel) when treating the apical area. When a channel is created, the site is recorded by marking it on the acetate sheet in the two views. Typically, channels are created at about 1 cm intervals. When treating the anterior and inferior walls, the ‘map’ of channels is usually best demonstrated in the left anterior oblique view, whereas for the lateral and septal walls, the right anterior oblique view is preferred (Fig. 18.5). A total of 10–15 channels are typically created in each of the areas demonstrated to have evidence of reversible ischaemia (inferior, anterior, lateral or septal).
The results of a multicentre randomised prospective trial of PMR (PACIFIC trial) were reported in 1999.16 The patients included in the study had angina refractory to medical therapy, associated with coronary artery disease and were not suitable for conventional forms of revascularisation. They had evidence of reversible myocardial ischaemia on myocardial perfusion scans and left ventricular ejection fractions of at least 30%. The study included 221 patients from several US sites and one UK site. Subjects were randomised to medication alone (n=111) or to PMR in addition to usual medication (n=110). At six-month follow up there was a mean reduction of 1.4 CCS angina classes in the PMR group compared with 0.125 in the control group (p=0.001). There was also a 30% increase in treadmill exercise time at six months in the PMR group compared with 5% in the control group, from baseline valves of a little over 400 seconds (p <0.001). Notably, there were no peri-procedural deaths in the PMR group. The morbidity associated with the procedure was also low. Of the 110 patients who underwent PMR, one developed cardiac tamponade requiring percutaneous drainage and one developed atrioventricular block requiring permanent pacing. In an uncontrolled longitudinal study there was a significant improvement in angina class and increase in exercise time three months and six months following PMR.17 A randomized sham-controlled study using this PMR system was reported last year.18 This study, known as the BELIEF (Blinded Evaluation of Laser Intervention electively For Angina Pectoris) study, recruited 82 patients with refractory angina in CCS class 3 or 4. It was designed to assess whether the improvement in symptoms of angina following PMR were attributable to placebo effect and clinician bias. The cohort recruited to this study was similar to the patients in the PACIFIC study. Patients were randomized to PMR with optimal medical therapy (n=40) or optimal medical therapy plus a sham procedure (n=42). Both the treating clinician and the patient were blinded to group allocation. There was one death in the sham group attributed to myocardial infarction. Follow up was completed in 79 patients at 6 and 12 months, with the primary outcome being improvement in CCS class. At both these timepoints, significantly more PMR patients improved by two or more CCS classes (40% vs 12% at 6 month, p<0.01 and 35% vs 14% at 12 months, p=0.04). There was no significant difference in exercise parameters, however, the trial was not powered to detect this. There was an improvement in angina specific health-related quality of life (p<0.05) as measured by the Seattle Angina Questionnaire. This data supports the conclusions reached in the unblinded trials of PMR as to improvement in angina scores using the Cardiogenesis laser system. They are, however, contrary to the negative findings of the single-blinded DIRECT (Direct myocardial laser revascularisation In Regeneration of Endomyocardial channels) trial. This study was reported in abstract form at the Transcatheter Cardiovascular Therapeutics conference in 2000. The subjects were randomized to placebo (n=102), low dose laser treatment (n=98) or high dose laser treatment (n=98). There was no difference in the groups at 6 or 12 months as regards to exercise parameters or angina scores. The laser used in this study was the Biosense DMR system which differs from the Cardiogenesis system in that electromechanical mapping of the left ventricular cavity is used to identify areas to be treated by laser as opposed to angiographic and perfusion data, and in that the DMR laser is a ‘contact’ system such that the depth of the channels created is significantly less. These factors may account for the differences between these two studies. There is also data to suggest that patient selection for PMR has a significant effect on treatment success. Stone et al.19 reported a prospective multicentre study of 141 patients who were randomized after attempted PCI of a chronic total occlusion in a native coronary artery (with no other lesions needing PCI or CABG) to optimal medical management with or without PMR. Angina improved significantly in both groups but there was no difference between the groups (p=0.86) nor was a difference found in exercise parameters in the 71 patients of the 141 recruited who completed baseline and six-month tests (p=0.73). Although this patient group also suffered CCS class 3/4 angina they were potentially revascularisable by CABG and are less likely to have the diffuse coronary artery disease of those patients in the BELIEF and PACIFIC studies above. The evidence discussed above suggests that PMR using the Cardiogenesis laser system is an effective treatment for patients with chronic refractory angina which is not controlled by conventional means, and that PMR is likely to be preferred to TMLR in patients who have no other revascularisation option, since it is much less invasive and is likely to have a lower morbidity and mortality. TMLR may, however, still have a useful role – perhaps as an adjunct to coronary artery bypass surgery. There are many patients who have obstructed coronary vessels, some of which are suitable for bypass grafting and some of which are not. In these patients a combination of bypass grafting to some territories and TMLR to other regions may be the optimal therapy. This is currently a Class II recommendation for TMLR treatment in the Society of Cardiothoracic Surgeons Practice Guidelines.20 Similarly PMR may be undertaken in conjunction with coronary angioplasty/stenting in the future – for example, with angioplasty/stenting of the LAD artery and PMR of the inferior wall since the right coronary artery is diffusely diseased. It is likely that the use of laser myocardial revascularisation, whether TMLR or PMR, will increase in the coming years.
1 Ketre K, Holubkov R, Kelsey S, et al. Percutaneous transluminal coronary angioplasty in 1985–88 and 1977-81. The National Heart, Lung, and Blood Institute Registry. N Engl J Med. 1988;318:265-270.
2 Hamm CW, Kupper W, Kuck K, Hofmann D, Bleifield W. Recanalisation of chronic, totally occluded coronary arteries by new angioplasty systems. Am J Cardiol. 1990;66:1459-1463.
3 Bell MR, Berger PB, Bresnahan JF, Reeder GS, Bailey KR, Holmes DR. Initial and long-term outcome of 354 patients after coronary balloon angioplasty of total coronary artery occlusions. Circulation. 1992;85:1003-1011.
4 Bowes RJ, Oakley GD, Fleming JS, et al. Early clinical experience with a hot tip laser wire in patients with chronic coronary artery occlusion. J Invas Cardiol. 1990;2:241-245.
5 Mast EG, Plokker HW, Ernst JM, et al. Percutaneous recanalisation of chronic total occlusions: experience with the direct Argon laser assisted angioplasty system (LASTAC). Herz. 1990;15:241-244.
6 Holmes DR, Forrester JS, Litvack F, et al. Chronic total obstruction and short-term outcome: the Excimer laser coronary angioplasty registry experience. Mayo Clin Pro. 1992;68:5-10.
7 Baumbach A, Haase K, Karsch K. Usefulness of morphologic parameters in predicting the outcome of coronary Excimer laser angioplasty. Am J Cardiol. 1991;68:1310-1315.
8 Safian RD, Reis GJ, Pomerantz RM. Laser balloon angioplasty: potential clinical applications. Herz. 1990;15:299-306.
9 Sen PK, Udwadia TE, Kinare SG, et al. Transmyocardial acupuncture: a new approach to myocardial revascularisation. J Thorac Cardiovasc Surg. 1965;50:181-189.
10 Khazei All, Kime WP, Papadopoulous C, et al. Myocardial canalization: a new method of myocardial revascularisation. Ann Thorac Surg. 1968;6:163-171.
11 Vineberg A. Clinical and experimental studies in the treatment of coronary artery insufficiency by internal mammary artery implant. J Int Coll Surg. 1954;22:503-518.
12 Horvath KA, Cohn LH, Cooley DA. Transmyocardial laser revascularisation: results of a multicentre trial with transmyocardial laser revascularisation used as sole therapy for end-stage coronary artery disease. J Thorac Cardiovasc Surg. 1997;113:645-654.
13 Burns SM, Sharples ID, Tait S, et al. The transmyocardial laser revascularisation international registry report. Eur Heart J. 1999;20:31-37.
14 March RJ. Transmyocardial laser revascularisation with the CO2 laser: one year results of a randomised controlled trial. Semin Thorac Cardiovasc Surg. 1999;11:12-18.
15 Schofield PM, Sharples LD, Caine N, et al. Transmyocardial laser revascularisation in patients with refractory angina: a randomised controlled trial. The Lancet. 1999;353:519-524.
16 Oesterle SN, Yeung A, Ali N, et al. The CardioGenesis percutaneous myocardial revascularisation (PMR) randomised trial: initial clinical results. J Am Cardiol. 1999;33((2)Suppl. A):380A. (Abstract).
17 Lauer B, Junghans U, Stahl F, Kluge R, Oesterle S, Schuler G. Catheter-based percutaneous myocardial laser revascularisation in patients with end-stage coronary artery disease. Eur Heart J. 1998;19 Abstract suppl.:589.. (Abstract).
18 Salem M, Rotevatn S, Stavnes S, Brekke M, Vollset SE, Nordrehaug JE. Usefulness and Safety of Percutaneous Myocardial Laser Revscularisation for Refractory Angina Pectoris. Am J Cardiol. 2004;93:1086-1091.
19 Stone GW, Terstein PS, Rubenstein R, Schmidt D, Whitlow P, Kosinski E, Mishkel G, Power GA. A Prospective, Multicenter, Randomised Trial of Percutaneous Transmyocardial Laser Revascularisation in Patients with Nonrecanalizable Chronic Total Occlusions. JACC. 2002;39:1581-1587.
20 Bridges CR, Horvath KA, Nugent WC, Shahian DM, Haan CK, Shemin RJ, Allen KB, Edwards FH. The Society of Thoracic Surgeons Practice Guidelines Series: Transmyocardial Laser Revascularization. Ann Thorac Surg. 2004;77:1494-1502.