![]() 2 Laser treatment of vascular lesions
2 Laser treatment of vascular lesions
Summary and Key Features
• Vascular lesions are one of the most common indications for laser treatment
• Treatment of vascular lesions implements the theory of selective photothermolysis, confining thermal injury to the target of interest
• Pulsed dye laser remains the gold standard treatment for port-wine stains, and while most improve, the minority clear completely
• Early laser treatment improves port-wine stain response
• Alexandrite laser can be implemented for treatment of hypertrophic, or pulsed dye laser resistant, port-wine stains
• Indications for laser treatment of hemangiomas includes ulcerated lesions and involuted lesions with residual telangiectasias and / or textural change
• The role of laser treatment for proliferating hemangiomas remains less clear, and may be most beneficial for superficial hemangiomas
• Deeper-penetrating near-infrared lasers may be implemented to treat select venous malformations
• Vascular lasers and intense pulsed light are the treatment of choice for the background erythema and telangiectasias associated with rosacea
• Poikiloderma of Civatte can be successfully treated with intense pulsed light, or a combination of vascular and pigment selective lasers
Introduction and history
Three components are necessary for selective photothermolysis: (1) a laser wavelength with preferential absorption of the target chromophore, (2) appropriate pulse duration matched to the target size, and (3) a fluence that both treats the target and minimizes non-specific thermal related injury. The ideal pulse duration is equal to or somewhat shorter than the thermal relaxation time of the target vessel. The thermal relaxation time is defined as the time for 50% of the heat to dissipate from the target of interest. A pulse duration that is too short may not be effective, whereas one that is too long may cause heat to dissipate to surrounding structures and cause unwanted thermal injury. The classic target chromophore for vascular lesions has been oxyhemoglobin, which has the greatest absorption peaks at 418, 542, and 577 nm (Fig. 2.1). The laser light is absorbed by oxyhemoglobin, and converted to heat, which is transferred to the vessel wall causing coagulation and vessel closure. Other hemoglobin species have more recently been recognized as appropriate targets, depending on the vascular lesion. For example, venous lesions may benefit from wavelengths of light that target deoxyhemoglobin. The alexandrite laser at 755 nm is close to a deoxyhemoglobin absorption peak and has been used for refractory or hypertrophic PWS, a venocapillary malformation. Methemoglobin absorption has also been recognized as a potential target chromophore.
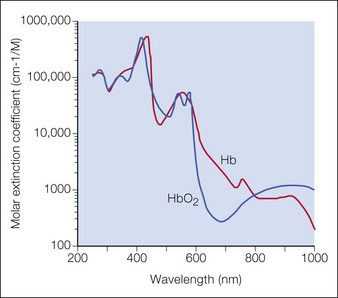
Figure 2.1 Optical absorption of hemoglobin.
Source: Dr Scott Prahl, http://omlc.ogi.edu/spectra/hemoglobin.
The most commonly used vascular lasers and light sources include:
Vascular anomalies classification
PWS, a type of capillary malformation, and hemangiomas are the two most common vascular anomalies that present for laser treatment (Table 2.1).
Table 2.1 Comparison of infantile hemangioma and port-wine stain
| Infantile hemangioma | Port-wine stain | |
|---|---|---|
| Onset |
Port-wine stain birthmarks
Overview
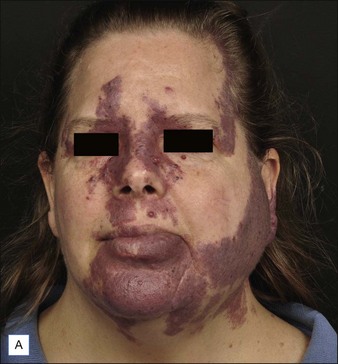
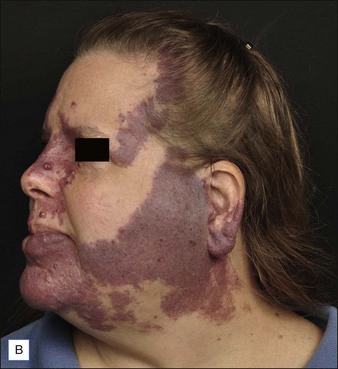
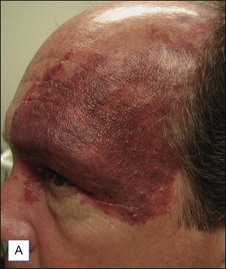
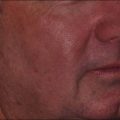
PWS are vascular malformations that are composed of ectatic capillaries and post-capillary venules in the superficial vascular plexus. PWS vessels are characterized by diminished vascular tone and decreased density of nerves, especially those with autonomic function. In most cases, PWS are congenital, though in rare cases they may be acquired. PWS are found in approximately 0.3% of newborns. They tend to occur on the head and neck, although they may appear anywhere on the body. PWS persist throughout life and many thicken with time (Fig. 2.2). Geronemus et al reported that the mean age of hypertrophy is 37 years and, by the fifth decade, approximately 65% of lesions had become hypertrophied or nodular. There may be associated soft tissue overgrowth, leading to functional impairment in areas such as the lip or eyelid. Vascular blebs often form and may bleed with minimal trauma. These lesions are often considered disfiguring and many patients or their families seek treatment. PWS vessels vary in size from 7–300 µm with older patients tending to have larger vessels.
Treatment
The PDL is the most commonly used laser to treat PWS. Treatments are typically done at 4–6 week intervals, and it is not uncommon for 10 or more treatments to be performed initially until a plateau is reached or the lesion clears (Fig. 2.3). Larger spot sizes allow for greater depth of penetration and so the clinician should select the largest spot size that will provide sufficient fluence to achieve the desired end point, while confining the treatment to the area of interest. It is advisable to determine the fluence threshold on the darkest portion of the PWS with 1 or 2 test pulses before treating the entire lesion. The fluence is adjusted to achieve the desired end point. For the PDL, the desired end point is immediate purpura. A confluent gray color signifies that the fluence is too high. A cookbook approach to treatment may result in complications.
Pearl 5
Know, follow, and trust the desired clinical treatment end point, not a setting number.
Use longer pulse durations and / or lower fluences for darker skin.
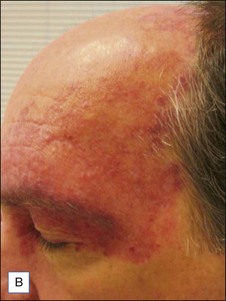
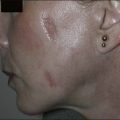
The alexandrite laser is typically used for PDL-resistant lesions, though it may be implemented as a first-line treatment for hypertrophic violaceous lesions in adults (Fig. 2.4). The end point is a subtle gray-blue discoloration followed by deeper purpura that takes several minutes to develop. A sustained gray color indicates that the fluence is too high, and there is a risk of scarring. Care must be taken not to overlap pulses as scarring can occur. Note that the range of appropriate fluences for alexandrite laser use is quite broad.
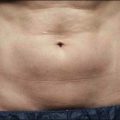
Venous malformations
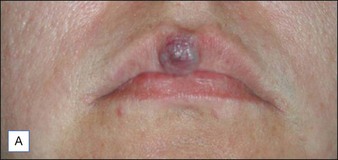
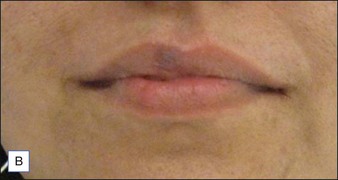
Laser treatment can be indicated for small and discrete venous malformations, for example on the lip (Fig. 2.5). The goal is to decrease the size of lesion and at times complete clearance may be possible, though venous malformations have a tendency for recanalization. Multiple treatment modalities are used for larger lesions including surgery and sclerotherapy. Laser treatment may also be performed for larger venous malformations with the goal of debulking prior to surgery. Laser treatment of venous malformations requires a deeper penetrating laser, and the near-infrared lasers, specifically diode or Nd : YAG, are most commonly implemented. Treatment of these lesions is complex and best handled by experienced surgeons. Scherer and Waner described the benefits of Nd : YAG laser therapy for complex venous malformations including tissue shrinkage, improved color, and induction of dermal fibrosis, thus reducing the risk of skin loss in surgery and sclerotherapy. In their hands, swelling lasted approximately 2 weeks, and blistering, dyspigmentation, and scarring occurred in <5% of patients.
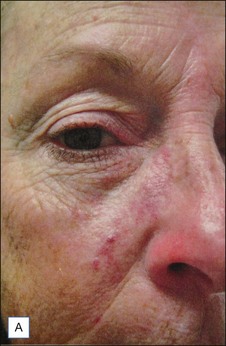
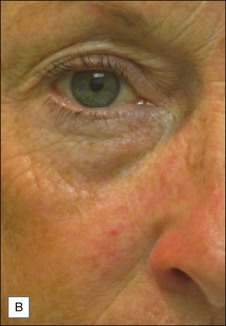
Rosacea and telangiectasias
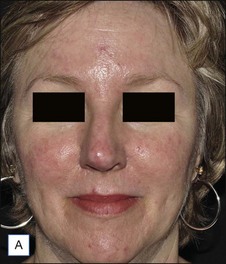
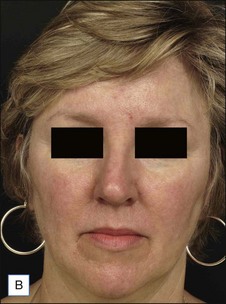
![]() Typically 3–4 monthly non-purpuric laser treatment sessions with PDL produce a significant reduction in erythema and telangiectasias. (Fig. 2.6) Typical settings include 7–10 mm spot, 6 ms pulse duration, 6–9 J/cm2 with epidermal cooling. Lower energies are used with larger spot sizes. Lower fluences should be used for patients with intense facial erythema. Lower fluences and longer pulse durations are advisable in darker skin types. Parameters vary by device. Residual telangiectasias can then be treated with purpuric or non-purpuric settings. Pulse durations of 6 ms and above are typically non-purpuric. Pulse durations of 1.5 ms and 3 ms are likely to induce purpura. Telangiectasia may respond in fewer treatment sessions, and purpuric treatments may be more effective. The end point for treating vessels is vessel clearance, a transient blue coagulum (Video 1), or purpura.
Typically 3–4 monthly non-purpuric laser treatment sessions with PDL produce a significant reduction in erythema and telangiectasias. (Fig. 2.6) Typical settings include 7–10 mm spot, 6 ms pulse duration, 6–9 J/cm2 with epidermal cooling. Lower energies are used with larger spot sizes. Lower fluences should be used for patients with intense facial erythema. Lower fluences and longer pulse durations are advisable in darker skin types. Parameters vary by device. Residual telangiectasias can then be treated with purpuric or non-purpuric settings. Pulse durations of 6 ms and above are typically non-purpuric. Pulse durations of 1.5 ms and 3 ms are likely to induce purpura. Telangiectasia may respond in fewer treatment sessions, and purpuric treatments may be more effective. The end point for treating vessels is vessel clearance, a transient blue coagulum (Video 1), or purpura.
Other vascular lesions
Cherry angiomas
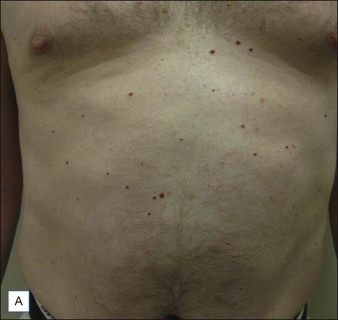
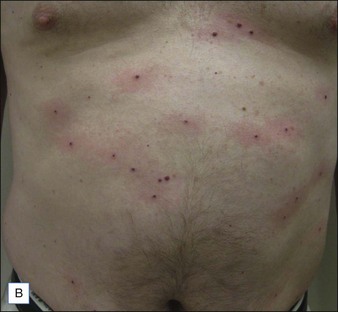
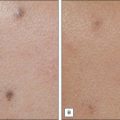
Cherry angiomas are the most common vascular growths of the skin. Cherry angiomas are benign proliferations that tend to be highly responsive to laser treatment. The PDL is commonly used to treat angiomas. The end point is purpura (Fig. 2.7). Clearance often occurs with one treatment although there are resistant lesions and larger lesions may require multiple treatments. The KTP laser or IPL may also be utilized.
Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524–527.
Bagazgoitia L, Torrelo A, Gutiérrez JC, et al. Propranolol for infantile hemangiomas. Pediatric Dermatology. 2011;28:108–114.
Batta K, Goodyear HM, Moss C, et al. Randomized controlled study of early PDL treatment of uncomplicated childhood haemangiomas: results of a 1-year analysis. Lancet. 2002;360:521–527.
Chapas AM, Eickhorst K, Geronemus RG. Efficacy of early treatment of facial port wine stains in newborns: a review of 49 cases. Lasers in Surgery and Medicine. 2007;39:563–568.
David LR, Malek M, Argenta LC. Efficacy of pulse dye laser therapy for the treatment of ulcerated hemangiomas: a review of 78 patients. British Journal of Plastic Surgery. 2003;56:317–327.
Finn MC, Glowacki J, Mulliken JB. Congenital vascular lesions: clinical application of a new classification. Journal of Pediatric Surgery. 1983;18:894–899.
Izikson L, Nelson JS, Anderson RR. Treatment of hypertrophic and resistant port wine stains with a 755 nm laser: a case series of 20 patients. Lasers in Surgery and Medicine. 2009;41:427–432.
Jia W, Sun V, Tran N, et al. Long-term blood vessel removal with combined laser and topical rapamycin antiangiogenic therapy: implications for effective port wine stain treatment. Lasers in Surgery and Medicine. 2010;42:105–112.
Madan V, Ferguson F. Using the ultra-long pulse width pulsed dye laser and elliptical spot to treat resistant nasal telangiectasia. Lasers in Medical Science. 2010;25:151–154.
Maguiness SM, Frieden IJ. Current management of infantile hemangiomas. Seminars in Cutaneous Medicine Surgery. 2010;29:106–114.
Nguyen CM, Yohn JJ, Weston WL, et al. Facial port wine stains in childhood: prediction of the rate of improvement as a function of age of the patient, size, and location of the port wine stain and the number of treatments with the pulsed dye (585nm) laser. British Journal of Dermatology. 1998;138:821–825.
Ni N, Langer P, Wagner R, Guo S. Topical timolol for periocular hemangioma: report of further study. Archives of Ophthalmology. 2011;129:377–379.
Rusciani A, Motta A, Fino P, et al. Treatment of poikiloderma of civatte using intense pulsed light source: 7 years of experience. Dermatologic Surgery. 2008;34:314–319.
Scherer K, Waner M. Nd: YAG lasers (1,064nm) in the treatment of venous malformations of the face and neck: challenges and benefits. Lasers in Medical Science. 2007;22:119–126.
Tournas JA, Lai J, Truitt Anne, et al. Combined benzoporphyrin derivative monoacid ring A photodynamic therapy and pulsed dye laser for port wine stain birthmarks. Photodiagnosis and Photodynamic Therapy. 2009;6:195–199.
Wall TL, Grassi AM, Avram MM. Clearance of multiple venous lakes with an 800-nm diode laser: a novel approach. Dermatologic Surgery. 2007;33:100–103.
Yang MU, Yaroslavsky AN, Farinelli WA, et al. Long pulsed neodynmium:yttrium-aluminum-garnet laser treatment for port wine stains. Journal of the American Academy of Dermatology. 2005;52:480–490.