Chapter 22 Laser Stapedotomy
 Videos corresponding to this chapter are available online at www.expertconsult.com.
Videos corresponding to this chapter are available online at www.expertconsult.com.
The surgical treatment of otosclerosis has been evolutionary in nature. Multiple techniques are now available to accomplish the same goal, which is illustrated by the number of chapters devoted to this subject in this book. The small fenestra technique evolved as a less invasive method to accomplish stapedectomy.1 It allows a smaller opening into the inner ear with the potential for less surgical trauma. Many techniques have been used in the past to accomplish a small fenestra, including a manual pick method, a hand drill, a microdrill, and a laser technique.2 The laser technique was developed by Perkins3 in the early 1980s. Several different lasers have been used to perform the small fenestra, including argon, KTP, and CO2.4–6 A hand-held fiberoptic probe or a micromanipulator can be used to deliver the laser beam, and each method has its champions.7
PREOPERATIVE EVALUATION
The surgical candidate should have a conductive hearing loss on audiogram and confirmed by tuning fork of at least 15 dB. Acoustic reflexes can be used as a simple screening tool for conductive hearing loss owing to superior canal dehiscence, and should be absent. The tympanic membrane should be intact, and there should be no evidence of ongoing infection in the ear. The patient should not have evidence of cochlear hydrops (see the section on pitfalls). The patient should have no medical contraindications to a short surgical procedure performed under local anesthesia. See Chapter 21 for indications and contraindications of stapes surgery.
SURGERY
Operating Room Setup and Preparation
A support bar (Fig. 22-1) is attached to the operating table to keep the drapes off the patient’s face. By attaching the bar directly to the table, the bar moves in conjunction with the table. Using a floor-mounted device, such as a Mayo stand, does not allow the coordination of movement between the two items and can result in limitations of table movement. The operative site is prepared by surrounding the ear with a plastic drape (1030; 3M) that keeps the hair out of the operative field. The ear and a margin of the plastic drape are prepared with povidone-iodine (Betadine) solution, and the povidone-iodine solution is allowed to fill the ear canal. Intravenous midazolam (Versed) is used for sedation. This medication is given in 0.5 mg increments until the patient is sleepy and relaxed. Typically, the patient requires 4 to 5 mg. Intravenous meperidine (Demerol), given in 12.5 mg increments, can be used to supplement the midazolam. An antiemetic such as ondansetron (Zofran) is also administered during surgery. The amount of sedation is titrated to the point that the patient is somnolent, yet appropriately responsive. At this point, 3 mL of blood is drawn and transferred to the scrub nurse.
Surgical Technique
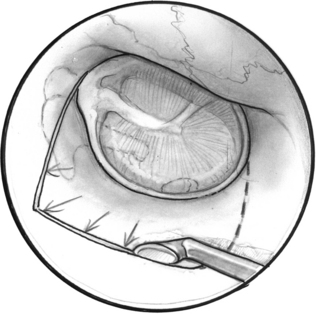
A tympanomeatal flap is incised using disposable tympanoplasty blades (7200 and 7210 BD Beaver). These disposable blades result in a sharp incision and rapid healing of the ear canal. Two incisions are made tangential to the annulus, one beginning adjacent to the short process of the malleus and following the tympanosquamous suture line. The second is adjacent to the tympanic annulus starting at approximately the 6 o’clock position and extending posterosuperiorly in the canal. These two incisions (Fig. 22-2) are connected with a transverse incision approximately 5 mm posterior to the tympanic annulus. The flap length is approximately the width of two Sheehy weapons (3 mm round knife). An excessively long flap would result in more bleeding, and can be difficult to fold forward in a narrow ear canal because of the bulk of the flap.
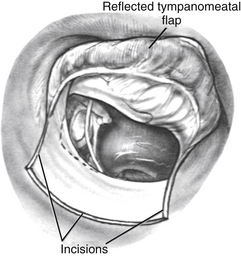
The flap is elevated inferiorly to allow visualization of the round window (Fig. 22-3). It is elevated superiorly until the neck of the malleus is visualized. The chorda tympani nerve is adherent to the posterosuperior bony tympanic annulus. It is separated from this bone, and separated from the undersurface of the malleus. Separating the chorda tympani allows mobilization of the nerve inferiorly for good visualization of the stapes, and prevents injury during curetting.
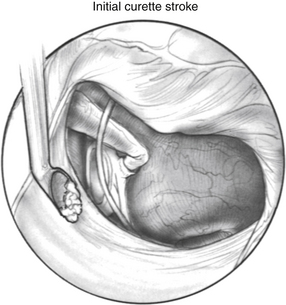
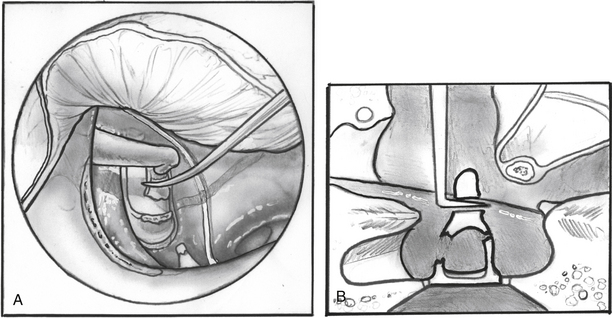
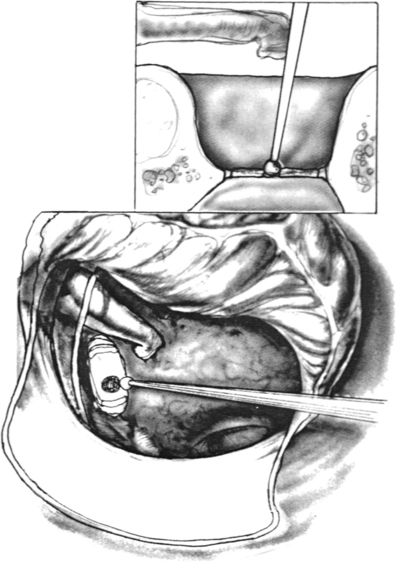
Posterior external auditory canal bone is removed with a curette to visualize the oval window. Initial curetting occurs several millimeters posterior to the edge of the tympanic annulus (Fig. 22-4). Initial curetting on the edge can result in displacement of the curette into the middle ear with dislocation of the incus. The posterior curetting allows for the bone to be weakened by creating a trough in this area. When the bone is weakened, the edge of the bone can be cracked off with the curette and little force is needed. The bone should be removed posteriorly to allow visualization of the stapedial tendon and pyramidal process (Fig. 22-5). For a right-handed surgeon working on a right ear, the bone should be removed superiorly so that half of the diameter of the horizontal fallopian canal can be visualized. For a right-handed surgeon working on a left ear, more curetting is necessary to introduce instruments in the middle ear, and bone should be removed so that the entire diameter of the horizontal fallopian canal can be visualized. At the conclusion of the curetting, all of the resultant bone chips should be removed from the ear canal to prevent postoperative healing problems.
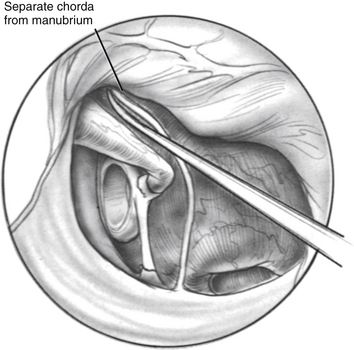
FIGURE 22-5 Completed curetting exposes stapedial tendon, pyramidal process, and horizontal fallopian canal.
The incudostapedial joint is separated with a joint knife (Fig. 22-6). The joint is typically 1 mm medial to the undersurface of the incus and can be identified by pushing the incus slightly forward and laterally. The joint is separated with a side-to-side motion by the knife. When the joint is separated, the malleus mobility is tested to rule out idiopathic malleus head fixation. The stapes is tested for motion, and the anterior oval window is inspected visually for evidence of otosclerosis. Otosclerosis can reliably be seen in this area and appears to be bright white bone with prominent vessels on it. The adjacent normal otic capsule bone looks slightly gray compared with the otosclerotic bone. If otosclerosis is not visualized, one must be quite confident that the stapes is not mobile before proceeding (see the section on pitfalls).
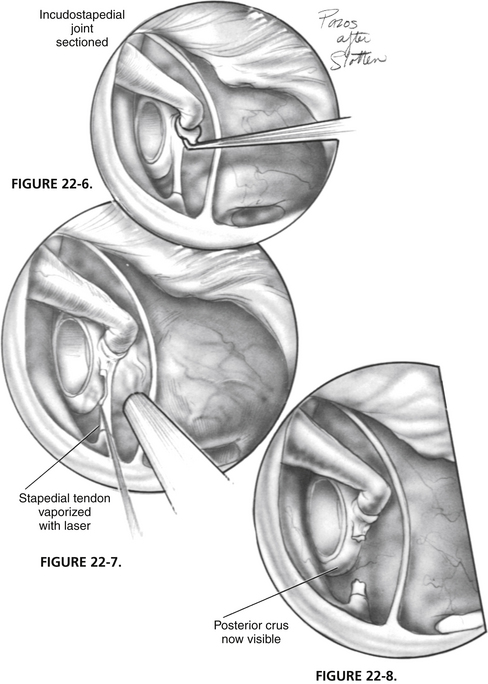
FIGURE 22-6 Incudostapedial joint is separated with joint knife.
Using the laser, the stapedial tendon and posterior crus are removed (Figs. 22-7 through 22-12). A typical setting for the KTP or argon laser is 1.5 W, at 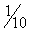 of a second duration. The author prefers a fiberoptic probe for delivery of the laser. Any mucosal adhesions in the oval window area are also removed with the laser to prevent bleeding from the mucosa. Care should be taken not to use the laser directly on the tympanic facial nerve, particularly if dehiscent. In some cases, the posterior stapes crus has a three-dimensional shape like the letter C. It is important to char not only the posterior aspects of the crus, but also the superior and inferior aspects. The charred area is picked through with a Rosen needle (see Fig. 22-10), and the superstructure is down fractured toward the promontory with a Rosen needle (see Fig. 22-12A). A quick sharp jerk on the superstructure is important to fracture the anterior crus (see Fig. 22-12B). A slow down fracture movement may result in mobilization of the footplate.
of a second duration. The author prefers a fiberoptic probe for delivery of the laser. Any mucosal adhesions in the oval window area are also removed with the laser to prevent bleeding from the mucosa. Care should be taken not to use the laser directly on the tympanic facial nerve, particularly if dehiscent. In some cases, the posterior stapes crus has a three-dimensional shape like the letter C. It is important to char not only the posterior aspects of the crus, but also the superior and inferior aspects. The charred area is picked through with a Rosen needle (see Fig. 22-10), and the superstructure is down fractured toward the promontory with a Rosen needle (see Fig. 22-12A). A quick sharp jerk on the superstructure is important to fracture the anterior crus (see Fig. 22-12B). A slow down fracture movement may result in mobilization of the footplate.
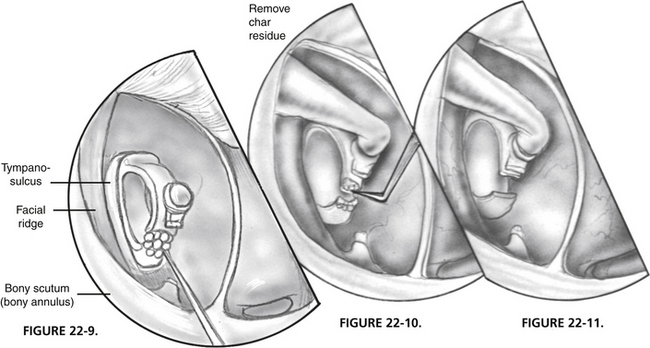
FIGURE 22-9 Posterior crus is vaporized with laser.
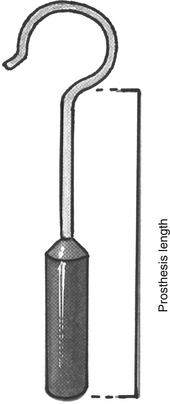
The distance between the surface of the footplate and the lateral aspect of the incus is measured (Fig. 22-13). In most cases, this measurement is 4.5 mm. If it is shorter, one should suspect a thick footplate. Measurements can also be routinely taken to the undersurface of the incus. The surgeon should always measure in a consistent fashion, however, and know how the prosthesis he or she uses is sized to determine the correct prosthesis length (Fig. 22-14). Optimally, the prosthesis should extend 0.5 mm into the vestibule.8 The fenestra in the footplate is usually placed in the middle or posterior half.
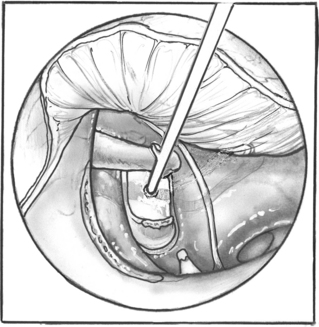
FIGURE 22-13 Measurement is taken between the surface of the footplate and the lateral aspect of the incus.
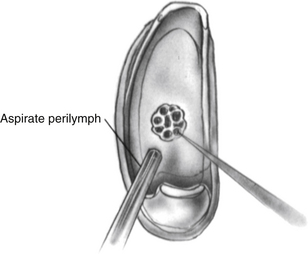
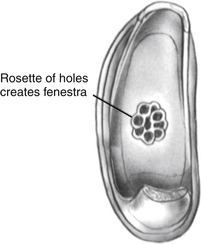
Using the laser, a footplate vessel is the initial target. With a visible wavelength laser, the energy is absorbed by pigment. The white footplate is a poor absorber of this energy; however, if an initial char is developed by using a laser on a red vessel, the char can be overlapped with each subsequent laser blast (Fig. 22-15) creating a rosette of chars. Perilymph seeps through the charred footplate and can be aspirated with a 24 gauge suction tip (Fig. 22-16). The charred area is created larger than the intended fenestra (Fig. 22-17). It is easier to remove charred footplate than normal footplate if the fenestra needs to be enlarged.
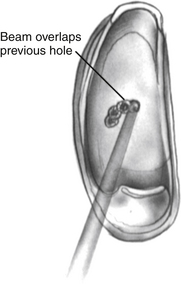
FIGURE 22-15 Char is created on the footplate with the laser. Subsequent chars overlap creating a rosette.
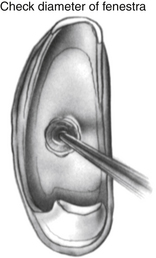
When a char of sufficient size has been fashioned, a sizing instrument “disk on a stick” (N1685-spec; Storz Instruments) is used to create the fenestra. The disk is 0.6 mm in diameter and creates a fenestra the same size as a 0.6 mm diameter piston. The disk is gently pushed through the char (Fig. 22-18) to create the hole. It can be used to rasp the hole, resulting in a hole slightly larger than the disk. When the fenestra is open, it is important to avoid suctioning directly into the oval window. A No. 24 suction is used at the margins of the oval window with the surgeon’s finger off the control hole. If a blood clot accumulates in the oval window obscuring the view, it can be lifted out using a Rosen needle rather than suction.
As an alternative to the laser, a small fenestra can be easily created using a microdrill. To make a footplate opening, a 0.7 mm diamond burr is used, which allows sufficient clearance for a 0.6 mm piston prosthesis. After the superstructure has been removed, the burr is lightly placed on the footplate (Fig. 22-19). Pressure is not exerted against the footplate, and a light touch is used. The drill motor is activated, and when the fenestra is completed, the surgeon senses a subtle resistance change at his or her fingertips. The drilling can be quite noisy and can startle a patient who is under local anesthesia. In these cases, it is helpful to place the drill against the promontory and activate it, alerting the patient to the noise of the drill and avoiding unexpected patient movement. The microdrill provides a uniformly sized footplate opening.
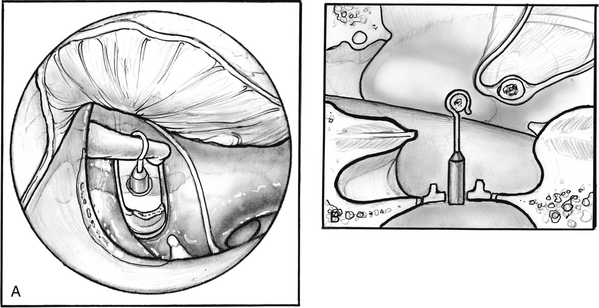
The prosthesis is introduced into the oval window using nonserrated alligator forceps. After placement in the oval window, the prosthesis is maneuvered into position with a strut guide. If using a platinum wire prosthesis, the wire is quite malleable, and care must be taken not to bend the wire using excessive force while manipulating the prosthesis into position. In some cases, the diameter of the incus is wider than the opening of the shepherd’s crook. Gentle downward pressure on the shoulder of the piston, pushing it into the open fenestra, allows the crook opening to enlarge and slip over the incus. When the piston is in the fenestra, and the wire is around the incus, it is crimped with crimping forceps. Allowing both jaws of the forceps to contact the prosthesis simultaneously prevents displacement during this maneuver. The crimp should be firm and not oval in shape. An oval crimp can lead to excessive movement of the wire on the incus and ultimately lead to incus necrosis. The prosthesis is crimped over the narrowest portion of the incus (Fig. 22-20), and then slid inferiorly toward the lenticular process. The incus typically widens at this point, and this results in a very snug connection.
INTRAOPERATIVE COMPLICATIONS
RESULTS
Success Rate
Small fenestra stapedectomy provides reliable hearing improvement for most patients. Reported closure of the air-bone gap to within 10 dB (when measured by comparing the postoperative bone conduction with the postoperative air conduction) ranges from 56% to 94%.9–12 Some authors have found an advantage of small fenestra stapedectomy performed with a laser compared with procedures done with a nonlaser technique; others have not found a difference. Sedgwick and coworkers13 compared small fenestra hearing results done by drill or laser, and found no difference for the closure of the air-bone gap within 10 dB or postoperative sensorineural hearing loss. When comparing small fenestra techniques with partial or total stapedectomy, there was better preservation of the high-frequency sensorineural hearing with a small fenestra technique, but the difference was small.
Several prosthesis materials have been introduced more recently. Titanium has been advocated as a superior prosthesis material because of its light weight and ease of crimping. Some authors have reported better results14 with this prosthesis material, whereas others have found no advantage of it over a platinum and Teflon prosthesis.15
Nitinol is a bimetallic wire (nickel and titanium) that reforms to a preset shape on heating.16 It has been incorporated into a piston prosthesis (SMart) that allows for heat-activated crimping of the shepherd’s crook around the incus. Heat can be provided by laser or cautery. Early reports are encouraging, showing results comparable or superior to manually crimped prosthesis.17–19 The author has analyzed his own outcomes comparing the SMart piston with platinum-Teflon piston. Closure of the postoperative four-frequency air-bone gap to within 10 db occurred in 96% of the SMart prosthesis cases compared with 86% of the platinum-Teflon group. The SMart piston is now the author’s preferred prosthesis.
Postoperative Complications
The management of the stapedectomy patient with postoperative dizziness or sensorineural hearing loss is controversial. Some surgeons believe that these symptoms represent either a perilymphatic fistula or a reparative granuloma and advocate immediate surgical re-exploration.20 Other surgeons believe that these symptoms are caused by postoperative serous labyrinthitis, and no surgical intervention is needed. Blood entering the vestibule during surgery can irritate the labyrinth, particularly during its metabolism and degradation. These surgeons believe that early surgical exploration may be more harmful than helpful. Some authors have observed a transient decrease in the sensorineural hearing level after stapes surgery as a routine occurrence during the postoperative period.21
Patient Selection Pitfalls
Most patients with a conductive hearing loss and a normal tympanic membrane have otosclerosis. The term inner ear conductive hearing loss has been used to describe patients who had either a normal ossicular chain at the time of stapedectomy or a persistent conductive hearing loss after an uncomplicated stapedectomy.22 More recently, superior semicircular canal dehiscence23 and intralabyrinthine neuromas24 have been identified as possible causes of inner ear conductive hearing loss. In the case of an unchanged postoperative conductive loss after an uneventful stapedectomy, one should consider both entities, and further imaging may be indicated. Superior semicircular canal dehiscence typically has very good to supranormal bone thresholds and can be diagnosed on coronal CT scan. Intralabyrinthine neuromas have a mixed hearing loss and can be diagnosed on high-resolution MRI.
One must approach otosclerosis patients who have coexisting dizziness with caution. Most patients with otosclerotic inner ear syndrome25 have improvement in vertigo after stapedectomy, whereas patients with Meniere’s disease may have a profound postoperative sensorineural hearing loss. The dilated saccule caused by endolymphatic hydrops can be damaged during stapedectomy and lead to the sensorineural hearing loss. Issa and coworkers26 found stapedectomy to be safe in patients with Meniere’s disease if the bone conduction threshold at 500 Hz was 35 dB or better, and there was no high-frequency sensorineural hearing loss.
1. Bailey H.A.T., Pappas J.J., Graham S.S. Small fenestra stapedectomy technique: Reducing risk and improving hearing. Otolaryngol Head Neck Surg. 1983;91:516-520.
2. Rizer F.M., Lippy W.H. Evolution of techniques of stapedectomy from the total stapedectomy to the small fenestra stapedectomy. Otolaryngol Clin North Am. 1993;26:443-451.
3. Perkins R.C. Laser stapedotomy for otosclerosis. Laryngoscope. 1980;91:228-241.
4. Hodgson R.S., Wilson D.F. Argon laser stapedotomy. Laryngoscope. 1991;101:230-233.
5. Bartels L.J. KTP laser stapedotomy: Is it safe? Otolaryngol Head Neck Surg. 1990;103:685-692.
6. Lesinski S.G. Lasers for otosclerosis: CO2 versus argon and KTP-532. Laryngoscope. 1989;99(Suppl 46):1-8.
7. Gherini S.G., Horn K.L., Bowman C.A., Griffin G. Small fenestra stapedotomy using a fiberoptic hand-held argon laser in obliterative otosclerosis. Laryngoscope. 1990;100:1276-1282.
8. Pauw B.K.H., Pollack A.M., Fisch U. Utricle, saccule and cochlear duct in relation to stapedotomy. Ann Otol Rhinol Laryngol. 1991;100:966-970.
9. Grolman W., Tange R.A. First experience with a new stapes clip piston in stapedotomy. Otol Neurotol. 2005;26:595-598.
10. Quaranta N., Besozzi G., Fallacara R.A., Quaranta A. Air and bone conduction change after stapedotomy and partial stapedectomy for otosclerosis. Otolaryngol Head Neck Surg. 2005;133:116-120.
11. Jovanovic S., Schonfeld U., Scherer H. CO2 laser stapedotomy with the “one-shot” technique—clinical results. Otolaryngol Head Neck Surg. 2004;131:750-757.
12. Vincent R., Sperling N.M., Oates J., Jindal M. Surgical findings and long-term hearing results in 3,050 stapedotomies for primary otosclerosis: a prospective study with the otology-neurotology database. Otol Neurotol. 2006;27(Suppl 2):S25-S47.
13. Sedgwick J.D., Louden C.L., Shelton C. Stapedectomy vs stapedotomy: Do you really need a laser? Arch Otolaryngol Head Neck Surg. 1997;123:177-180.
14. Zuur C.L., de Bruijn A.J.G., Lindeboon R., Tange R.A. Retrospective analysis of early postoperative hearing results obtained after stapedotomy with implantation of a new titanium prosthesis. Otol Neurotol. 2003;24:863-867.
15. Massey B.L., Kennedy R.J., Shelton C. Stapedectomy outcomes: Titanium vs. Teflon wire prosthesis. Laryngoscope. 2005;115:249-252.
16. Knox G.W., Reitan H. Shape-memory stapes prosthesis for otosclerosis surgery. Laryngoscope. 2005;115:1340-1346.
17. Sorom A.J., Driscoll C.L.W., Beatty C.W., Lundy L. Retrospective analysis of outcomes after stapedectomy with implantation of a self-crimping Nitinol stapes prosthesis. Otolaryngol Head Neck Surg. 2007;137:65-69.
18. Harris J.P., Gong S. Comparison of hearing results of Nitinol SMART stapes piston prosthesis with conventional piston prostheses: Postoperative results of the Nitinol stapes prosthesis. Otol Neurotol. 2007;28:692-695.
19. Rajan G.P., Diaz J., Blackham R., et al. Eliminating the limitations of manual crimping in stapes surgery: Mid-term results of 90 patients in the Nitinol stapes piston multicenter trial. Laryngoscope. 2007;117:1236-1239.
20. Gacek R.R. The diagnosis and treatment of poststapedectomy granuloma. Ann Otol Rhinol Laryngol. 1970;79:970-975.
21. Somers T., Vercruysse J.P., Zarowski A., et al. Stapedotomy with microdrill or carbon dioxide laser: Influence on inner ear function. Ann Otol Rhinol Laryngol. 2006;115:880-885.
22. House J.W., Sheehy J.L., Antunez J.C. Stapedectomy in children. Laryngoscope. 1980;90:1804-1809.
23. Hillman T.A., Kertez T.R., Hadley K., Shelton C. Reversible peripheral neuropathy: The treatment of superior canal dehiscence. Otolaryngol Head Neck Surg. 2006;134:431-436.
24. Kennedy R.J., Shelton C., Saltzman K.L., et al. Intralabyrinthine schwannomas: Diagnosis, management and a new classification system. Otol Neurotol. 2004;25:160-167.
25. Linthicum F.H., Ghorayeb V.Y. Otosclerotic inner ear syndrome. Ann Otol Rhinol Laryngol. 1978;87:85-90.
26. Issa T.K., Bagguette M.A., Linthicum F.H., House H.P. The effect of stapedectomy on hearing of patients with otosclerosis and Ménière’s disease. Am J Otol. 1983;4:323-326.