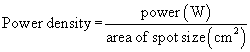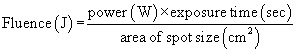Chapter 24 Laser Revision Stapedectomy
 Videos corresponding to this chapter are available online at www.expertconsult.com.
Videos corresponding to this chapter are available online at www.expertconsult.com.
LASER PHYSICS AND PRINCIPLES
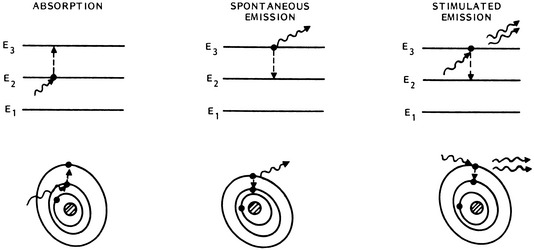
Laser energy is derived from the release of energy (photons) occurring when stimulated electrons return to their resting orbital. As proposed by Einstein in 1917, when photons of the appropriate wavelength strike excited atoms, a second additional photon is released as the electron returns to its ground state (Fig. 24-1). In this stimulated emission situation, the photon that is emitted from the excited atom has exactly the same frequency, direction, and phase as the incident photon, providing laser energy that is collimated, coherent, and monochromatic.
Lasers are typically named by the active medium, or the source of atoms that are excited and undergo stimulated emission of photons. The active medium can be a liquid, solid, or gas. Common gas lasers include CO2, argon, and helium-neon. An example of solid state lasers are the neodymium:yttrium-aluminum-garnet (Nd:YAG) and the potassium titanyl phosphate crystal (KTP). The KTP laser is simply a Nd:YAG laser beam that passes through a KTP crystal, which halves the wavelength and doubles the frequency of the laser beam (Table 24-1).
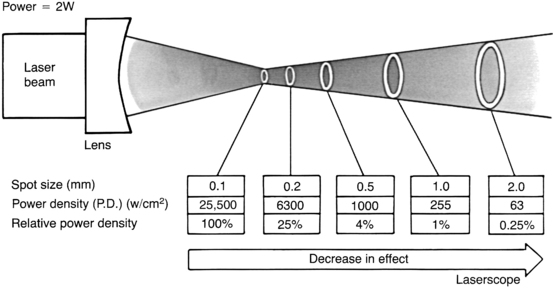
For any laser, the magnitude of the laser-tissue interaction can be regulated by the laser’s power output, the power density at the point of impact, and the energy fluence. Every surgeon who uses a laser should thoroughly understand these fundamental concepts. Power is the time rate at which energy is emitted, and is expressed as watts. The power output is directly adjusted by the control panel on the laser console. Laser energy is delivered through a focusing lens. Power density is a measure of the intensity, or concentration, of the laser beam spot size (Fig. 24-2). It is the ratio of power to surface area of the spot size, and is expressed in terms of watts per square centimeter:
where area of spot size is πr2, and where r = spot size radius in centimeters.
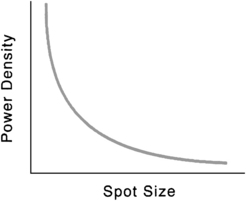
Power density is inversely proportional to the square of the radius of the spot size. Consequently, for any specific power output, changes in the spot size can have a tremendous effect on power density (Fig. 24-3).
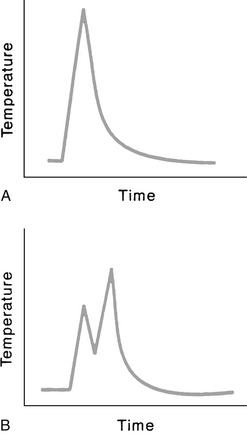
As fluence increases, the volume of affected tissue also increases. The thermal energy having an impact on tissue increases dramatically as the time of exposure increases. If power density (W/cm2) is held constant, and exposure time is doubled, the energy delivered is doubled. The thermal effect of the tissue increases significantly, however, because the increase in temperature is continuous. For example, assume the laser power is set at 2 W, the spot size is 0.2 mm2, and exposure time is 0.2 second. Is this the same as delivering two separate impulses at 0.1 second each? Yes and no. Yes, it is the same in terms of energy delivered from the laser. But no, it is not the same in terms of thermal energy imparted to the tissue because during the time between the two separate 0.1 second pulses, no matter how brief, the tissue is cooling (Fig. 24-4). Other factors come into play, such as the absorption characteristics of the damaged tissue in the center of the laser spot and dissipation of heat, but the important concept is that of the increase and decrease of the temperature.
HISTORY OF REVISION STAPEDECTOMY
Background
After the introduction of the stapedectomy procedure by Shea1 in 1958, the surgical treatment of otosclerosis was revolutionized. During the 1960s and 1970s, otologic surgeons performed hundreds of thousands of stapedectomies. An otologic surgeon commonly performed thousands of stapes procedures during his or her peak professional years. The accepted success rate (as defined by closure of the air-bone gap to ≤10 dB) was 90% or greater, with a 1% or less incidence of significant sensorineural hearing loss, including deafness. The most common technique during this time was the total stapedectomy, with removal of the entire stapes footplate. The small fenestra technique began gaining some acceptance, although not universal, in the early 1980s.
As with any surgical procedure, the success rate of stapedectomy was not 100%; with even a small percentage of failures (i.e., air-bone gap closure of >10 dB) in such a large pool of patients, there was a significant number of patients who were candidates for revision stapes procedures. Stapes surgeons soon discovered two important facts: The success rate of revision stapedectomy was not nearly as high as that of primary stapedectomy, and the incidence of significant sensorineural hearing loss, including dead ears, was significantly higher than the incidence associated with primary stapedectomy. Prominent otologists obtained air-bone gap closure within 10 dB in 50% or less of revision cases.2–7 The incidence of significant postoperative sensorineural hearing loss ranged from 3% to 20%, with 14% having profound loss.2,3,6,8
Glasscock2 and Sheehy3 and their associates and Lippy and Schuring8,9 advocated leaving the oval window neomembrane intact and undisturbed, if possible, in revision cases to reduce the risk of severe sensorineural hearing loss, even though it may result in fewer patients with postoperative hearing improvement. Feldman and Schuknecht,4 Pearman and Dawes,10 and Derlacki5 reported opening the neomembrane to identify the vestibule and ensure correct prosthesis placement.
Laser
Concomitant with the realization that revision stapedectomy surgery was not as successful as primary stapedectomy was the introduction of lasers in temporal bone surgery. The use of the laser in temporal bone surgery was the object of experiments in 1967 by Sataloff11 and 1972 by Stahle and colleagues.12 The evolution of laser otologic surgery has been based on a mixture of clinical, experimental animal, and laboratory observations. In 1977, Wilpizeski13 examined argon and CO2 lasers on monkeys by performing myringotomy, ossicular amputation, stapes fenestrations, lysis of stapedial tendon, and crurotomy. He noted damage to the organ of Corti in monkeys after using “excessive power.”
Escudero and associates14 were the first to use a laser in human otologic surgery in 1977. They used the argon laser with a fiberoptic handpiece to tack temporalis fascia to tympanic membrane perforations. In 1979, Perkins15 presented a preliminary report of argon laser stapedotomy with excellent initial results in 11 patients. In 1980, DiBartolomeo and Ellis16 expanded argon laser applications in 30 patients for middle ear and external ear soft tissue and bony problems. In 10 patients, otosclerosis was corrected, including one revision case. In 1983, McGee17 reported on the use of argon laser in more than 500 otologic cases, 100 of which were primary stapedectomies. There were no laser-related complications in his study. In 1989, McGee18 reported an update on 2500 tympanomastoid procedures, of which 510 were primary stapedectomies. By comparing 100 consecutive laser stapedectomies with a previous 139 small fenestra stapedectomies using instruments, McGee found that the laser technique permitted much shorter hospital stay, less vertigo, and excellent hearing results (93% air-bone gap closure ≤10 dB at 6 months). This large study indicated the safety of argon laser use for stapedectomy, and yielded comparable hearing results and less vertigo. This clinical evidence is inconsistent with experimental animal studies, in which temporary changes of cochlear microphonics and saccular perforations were noted.17,19,20 Clinical experience from several centers has illustrated the safe use of lasers in ear surgery; however, arguments and opinions persist regarding the best type of laser.21–25
Comparison of Lasers
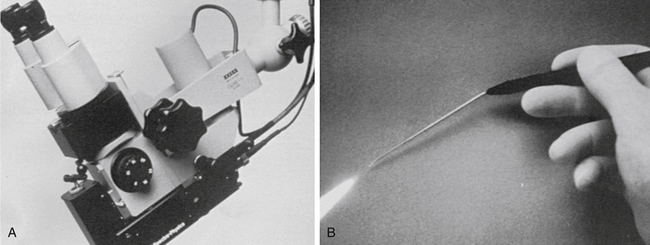
The visible-wavelength laser beam can be carried by thin fiberoptic cables, which allow two modes of delivery: a micromanipulator attached to the microscope or a hand-held probe (Fig. 24-5). The hand-held probe provides greater angle of divergence of the laser beam (i.e., rapid deterioration of power density) and must be placed very close to the tissue. This instrument is directly in the operative field and can obstruct a portion of the visual operative field. With the micromanipulator, the angle of the laser beam is less divergent. It does not require an instrument in the field (except a suction for smoke plume removal), but does require the use of a “joystick” control mechanism to direct the beam. The CO2 laser energy cannot be carried by a standard fiberoptic cable; use of a micromanipulator system is the only choice. Older CO2 lasers have a system of articulated arms and mirrors that significantly reduces accuracy. Newer models allow the optical chamber to be attached to the side of the microscope, however, with fewer arms and mirrors, a significant technologic advance.
The delivery of CO2 laser energy has potentially advanced with the introduction of an omnidirectional photonic bandgap reflector system developed by researchers at Massachusetts Institute of Technology in 1998. This technology allows for the CO2 laser beam to be transported within a hollow-core, flexible fiber, eliminating the need for articulating arms and mirrors.26 A commercial organization (OmniGuide, Inc.) was established in 2004, and the first otology device, OtoBeam, was used in June 2007. This optical fiber has an outer diameter of 0.9 mm, with a spot size of 0.25 mm. The angle of divergence when the CO2 beam leaves the end of the optical fiber is 7 degrees; the tip of the optical fiber must be relatively close to the tissue to avoid significant power loss.
In recent years, questions have arisen regarding the safety of visible-spectrum lasers in stapedectomy and revision stapedectomy. The issue revolves around depth of penetration of laser energy into an open vestibule with potential injury to inner ear structures, such as the saccule or utricle. No laser surgeon advocates firing any type of laser beam into an open vestibule. These concerns have not been borne out, as experience with hundreds of patients and multiple authors has indicated.15,16,21–23,25,27
Serious theoretical safety issues related to visible spectrum lasers arose when Lesinski23 pointed out that the shorter wavelength of the visible wavelength (argon and KTP) lasers penetrates tissue more deeply than the longer wavelength (CO2) lasers. By constructing a model of the vestibule and placing a black painted thermocouple in it at a depth comparable to the saccule or utricle, he measured very high temperatures when the visible laser was allowed to strike the thermocouple directly. This effect was not noticeable with the CO2 laser. He concluded there was the potential for the visible lasers to traumatize the saccule or the utricle or both, risking sensorineural hearing loss.
Laser Use: 1990s to 2007
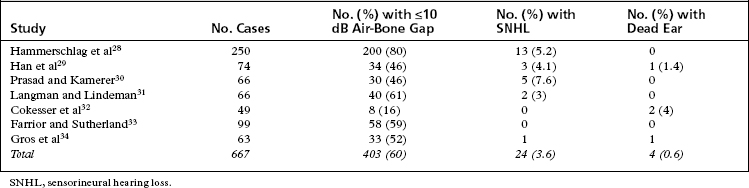
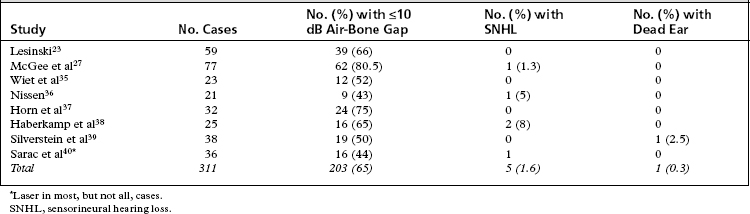
The renewed interest in laser use for revision stapes surgery combined with the theoretical issues of “best” type of laser (visible versus invisible) sparked a series of self-appraisals by stapes surgeons. The two major concerns of revision stapes surgery—successful outcome (air-bone gap ≤10 dB) versus sensorineural hearing loss—continue to be important, regardless of use of the laser or not. In essence, revision stapes surgery has improved as surgeons have employed better selection criteria. A recurring theme throughout the literature is that patient selection for revision stapes affects the outcome. If a patient never had a good result to begin with, the chances are less that a good result would be obtained by revision. Likewise, patients with vertigo and reaccumulation of the air-bone gap do not fare as well. The results of revision stapes surgery without the laser are better than historical controls of the 1970s and 1980s (Table 24-2).28–34 The results of revision stapes surgery using the laser are also better than the historical controls, and show a lesser incidence of sensorineural hearing loss (Table 24-3).23,27,35–40
Analysis of Failed Stapes Surgery
Nonetheless, several factors responsible for the reaccumulation of the air-bone gap have been repeatedly identified in almost all studies. The most common intraoperative finding associated with recurrence of conductive hearing loss is a displaced prosthesis.29,44,45 Findings at the time of revision stapes surgery generally can be classified as common or uncommon (Table 24-4).
| Common | Uncommon |
|---|---|
| Displaced prosthesis | Dislocated incus |
| Incus erosion | Prosthesis too long |
| Fibrosis of oval window | Fixation of malleus/incus |
| New bone growth | Depressed footplate fragment |
| Prosthesis too short | Reparative granuloma |
| Residual footplate | Perilymph fistula |
Although there are no excellent long-term follow-up data to support or implicate any one technique because of the previously mentioned social factors, certain conclusions can still be reached. Most of the primary stapes surgery involved total or near-total removal of the footplate during the years of frequent stapes surgery. Regardless of the prosthesis used, the oval window tissue seal is much larger than the prosthesis. All of the soft tissue seals (lobule fat, areolar fascia, temporalis fascia, vein, tragal perichondrium) have to occlude the oval window to prevent perilymph leak. Subsequently, a snug fit of this tissue results in prolapse of the tissue into the vestibule to some degree, or a relative heaping up of the tissue in the oval window niche, or both. As this tissue heals, portions within the vestibule could easily band to the saccule or utricle41,42 because the vestibule is only a few millimeters deep. When this tissue in the oval window is removed mechanically during revision surgery, tears and avulsions of the saccule or utricle or both could easily occur, resulting in vertigo or significant sensorineural hearing loss, or both. This potential for inner ear damage can be estimated only because there is no good way to assess this possible condition preoperatively or intraoperatively.
Incus erosion at the lenticular process at the attachment of the prosthesis is another common finding in revision cases.43–46 One theory of causation holds that a tightly crimped shepherd’s crook results in ischemia and subsequent necrosis of the lenticular process.7,47,48 Another theory holds that a poorly performed crimping initially or owing to the “spring-back” nature of stainless steel used in the shepherd’s crook results in a loose-fitting shepherd’s crook.49 This laxity results in differential vibration of the incus and wire, ultimately eroding a notch in the lenticular process. Several prostheses are available with platinum ribbons for the shepherd’s crook, supposedly for easier and better fitting crimping. Also, as noted earlier, migration of the distal end of the prosthesis, particularly with previous total stapedectomy, can result in fixation of the distal end of the prosthesis. Subsequent normal vibration of the incus within the prosthesis crook or bucket handle would likely result in erosion of the incus at its attachment. The appearance of the lenticular process is that it has been “sawn” through from repeated vibration.
Case for Use of Lasers in Revision Stapes Surgery
During the 1990s through 2007, several studies analyzed the success and complication rates of laser revision stapes surgery (see Table 24-3). The results are consistent regardless of the type of laser used or the method of delivery of the laser energy. The average success rate in closing the air-bone gap to within 10 dB is 65%; the incidence of significant sensorineural hearing loss is 1.6%, and the incidence of dead ears is 0.3%. There is no significant difference in outcomes using the visible spectrum or CO2 laser. Although these results do not equal the results of primary stapes surgery, they do represent an improvement of the historical controls.
Limitations of Lasers
The laser can also be useful in managing the incus because it can be used to vaporize the necrotic tip of the lenticular process. A prosthesis using nitinol for the shepherd’s crook has been introduced for primary stapes surgery.50 Nitinol is an alloy with shape memory made of nickel (55.3% by weight) and titanium (44.7% by weight).51 The shepherd’s crook “shrink wraps” around the incus or malleus with the introduction of heat at 45° C (113° F). This heat can easily be obtained with a single KTP laser shot at 0.5 W at 0.1 second duration or use of a battery-powered cautery unit (eye cautery). Current published literature is limited to primary stapedotomy with this prosthesis,50–54 but the author has used it on revision cases successfully with its attachment to the incus and the malleus. The use of a stapes prosthesis with a nitinol self-crimping shepherd’s crook eliminates the need for manual crimping, greatly simplifying this step. When this prosthesis is used for malleus attachment, care must be taken not to place the heat source too close to the tympanic membrane because a perforation could result. With the KTP laser, this possibility is greatly reduced because of the ability to place the laser spot accurately on the shepherd’s crook.
TECHNIQUE
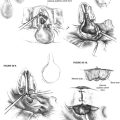
A transcanal tympanomeatal-stapedectomy flap is raised with the patient under local anesthesia with vasoconstriction and intravenous sedation. On entry into the middle ear, the cause of the conductive hearing loss is assessed (Fig. 24-6). The malleus and incus are inspected and gently palpated to rule out fixation. Any obstructing middle ear fibrous adhesions are lysed with the laser. The chorda tympani nerve, if present, is frequently adhered to the tympanomeatal flap and can be sharply dissected with the laser. At the appropriate setting, the obliterating tissue of the oval window surrounding the prosthesis is vaporized until the exact oval window margins and depth are identified. For the KTP laser, the spot size is 0.15 mm, the power setting is 1.2 to 1.4 W, and the pulse duration is 0.1 second. For the CO2 laser on the superpulse mode, the spot size is also 0.15 mm, the power setting is 0.8 to 1 W, and the pulse duration is 0.1 second.
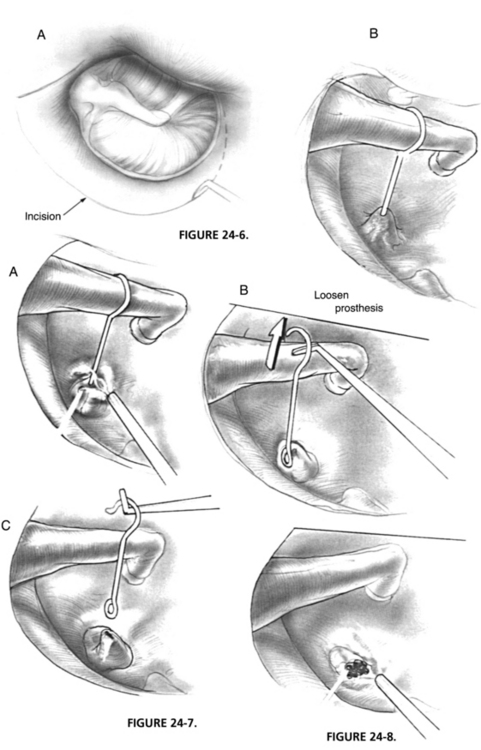
FIGURE 24-6 A, Elevation of tympanomeatal flap. B, Migrated prosthesis with oval window obliteration.
The attachment of the prosthesis at the incus is freed or loosened with a right angle hook. The prosthesis may be removed at this point or may require further lysis at its base (Fig. 24-7). A series of laser hits are applied to the oval window neomembrane in a nonoverlapping rosette pattern in the center of the oval window (Fig. 24-8). A minimum of 2 second intervals between bursts is necessary to minimize heat buildup of the neomembrane and perilymph. A 0.6 mm stapedotomy is created and is incomplete until the vestibule and clear perilymph are identified. The stapedotomy size is confirmed by use of a 0.4 mm and 0.7 mm McGee rasp. If the incus long process is satisfactory, a nitinol prosthesis with a fluoroplastic piston of 0.5 mm diameter is used. A length of 4.25 mm is used in 90% to 95% of cases. If the lenticular process is unsatisfactory, the same type of prosthesis is used from the malleus to the fenestra. The most commonly used length for this prosthesis is 4.50 to 4.75 mm. After laser crimping, the ossicular chain is gently palpated to ensure freedom of movement and appropriateness of prosthesis length. The oval window is sealed with areolar fascia. The tympanomeatal flap is returned to its anatomic position and secured with saline-moistened absorbable gelatin sponges or antibiotic ointment.
1. Shea J.J. Fenestration of the oval window. Ann Otol Rhinol Laryngol. 1958;67:932-951.
2. Glasscock M.E.III, McKennan K.X., Levine S.C. Revision stapedectomy surgery. Otolaryngol Head Neck Surg. 1987;96:141-148.
3. Sheehy J.L., Nelson R.A., House H.P. Revision stapedectomy: A review of 258 cases. Laryngoscope. 1981;91:43-51.
4. Feldman B.A., Schuknecht H.F. Experiences with revision stapedectomy procedures. Laryngoscope. 1970;80:1281-1291.
5. Derlacki E.L. Revision stapes surgery: Problems with some solutions. Laryngoscope. 1985;95:1047-1053.
6. Crabtree J. An evaluation of revision stapes surgery. Laryngoscope. 1980;90:224-227.
7. Lippy W.H. Stapedectomy revision. Am J Otol. 1980;2:15-21.
8. Lippy W.H., Schuring A.G. Stapedectomy revision of the wire-Gelfoam prosthesis. Otolaryngol Head Neck Surg. 1983;91:9-13.
9. Lippy W.H., Schuring A.G. Stapedectomy revision following sensorineural hearing loss. Otolaryngol Head Neck Surg. 1984;92:580-582.
10. Pearman K., Dawes J.D.K. Post-stapedectomy conductive deafness and results of revision surgery. J Laryngol Otol. 1982;96:405-410.
11. Sataloff J. Experimental use of the laser in otosclerotic stapes. Arch Otolaryngol Head Neck Surg. 1967;85:58-60.
12. Stahle J., Hoegberg L., Engstrom B. The laser as a tool in inner ear surgery. Acta Otolaryngol (Stockh). 1972;73:27-37.
13. Wilpizeski C.R. Otological applications of laser. In: Wolbarsht M.K., editor. Laser Applications in Medicine and Biology. New York: Plenum; 1977:289-328. vol. 3
14. Escudero L.H., Castro A.O., Drummond M., et al. Argon laser in human tympanoplasty. Arch Otolaryngol Head Neck Surg. 1979;105:252-253.
15. Perkins R.C. Laser stapedotomy for otosclerosis. Laryngoscope. 1980;90:228-241.
16. DiBartolomeo J.R., Ellis M. The argon laser in otology. Laryngoscope. 1980;90:1786-1796.
17. McGee T.M. Argon laser in chronic ear and otosclerosis. Laryngoscope. 1983;93:1177-1182.
18. McGee T.M. Lasers in otology. Otolaryngol Clin North Am. 1989;22:233-238.
19. Gantz B.J., Kischimoto S., Jenkins H.A., et al. Argon laser stapedotomy. Ann Otol Rhinol Laryngol. 1982;91:25-26.
20. Vollrath M., Schreiner C. Influence of argon laser stapedotomy on cochlear potentials. Acta Otolaryngol (Stockh). 1982;385(Suppl):1-31.
21. Bartels L. KTP laser stapedotomy: Is it safe? Otolaryngol Head Neck Surg. 1990;103:685-692.
22. Horn K., Gherini S., Griffin G. Argon laser stapedotomy using an endo-otoprobe system. Otolaryngol Head Neck Surg. 1990;102:193-198.
23. Lesinski S. Lasers for otosclerosis. Laryngoscope. 1989;99(Suppl):1-24.
24. McGee T.M., Kartush J.M. Laser-stapes surgery. Laryngoscope. 1990;100:106-108.
25. Vernick D.M. CO2 laser safety. Laryngoscope. 1990;100:108-109.
26. Jacobs S.A., Temelkuran B., Weisberg O., et al. Hollow-core fibers. In: Mendez A., Morse T.E., editors. Specialty Optical Fibers. Amsterdam: Academic Press, 2007.
27. McGee T.M., Diaz-Ordaz E.A., Kartush J.M. The role of KTP laser in revision stapedectomy. Otolaryngol Head Neck Surg. 1993;109:839-843.
28. Hammerschlag P.E., Fishman A., Scheer A.A. A review of 308 cases of revision stapedectomy. Laryngoscope. 1998;108:1794-1800.
29. Han W.W., Incesulu A., McKenna M.J., et al. Revision stapedectomy: Intraoperative findings, results, and review of the literature. Laryngoscope. 1997;107:1185-1192.
30. Prasad S., Kamerer D.B. Results of revision stapedectomy for conductive hearing loss. Otolaryngol Head Neck Surg. 1993;109:742-747.
31. Langman A.W., Lindeman R.C. Revision stapedectomy. Laryngoscope. 1993;103:954-958.
32. Cokesser Y., Naguib M., Aristegui M. Revision stapes surgery: A critical evaluation. Otolaryngol Head Neck Surg. 1994;111:473-477.
33. Farrior J., Sutherland A. Revision stapes surgery. Laryngoscope. 1991;101:1155-1161.
34. Gros A., Vatovec J., Zargi M., Jenko K. Success rate in revision stapes surgery for otosclerosis. Otol Neurotol. 2005;26:1143-1148.
35. Wiet R.J., Kubek D.C., Lemberg P., Byskosh A.T. A meta-analysis review of revision stapes surgery with argon laser: Effectiveness and safety. Am J Otol. 1997;18:166-171.
36. Nissen R.L. Argon laser in difficult stapedotomy cases. Laryngoscope. 1998;108:1669-1673.
37. Horn K.L., Gherini S.G., Franz D.C. Argon laser revision stapedectomy. Am J Otol. 1994;15:383-388.
38. Haberkamp T.J., Harvey S.A., Khafagy Y. Revision stapedectomy with and without CO2 laser: Analysis of results. Am J Otol. 1996;17:225-229.
39. Silverstein H., Bendet E., Rosenberg S., Nichols M. Revision stapes surgery with and without laser: A comparison. Laryngoscope. 1994;104:1431-1434.
40. Sarac S., Mckenna M.J., Mikulec A.A. Results after revision stapedectomy with malleus grip prosthesis. Ann Otol Rhinol Laryngol. 2006;115:317-322.
41. Hohmann A. Inner ear reaction to stapes surgery (animal experiments). In: Schuknecht H.F., editor. Otosclerosis. Boston: Little Brown; 1982:305-317.
42. Linthicum F. Histologic evidence of the cause of failure in stapes surgery. Ann Otol Rhinol Laryngol. 1971;80:67-68.
43. Krieger L.W., Lippy W.H., Schuring A.G., Rizer F.M. Revision stapedectomy for incus erosion: Long-term hearing. Otolaryngol Head Neck Surg. 1998;119:370-373.
44. Lippy W.H., Battista R.A., Berenholz L., et al. Twenty year review of revision stapedectomy. Otol Neurotol. 2003;24:560-566.
45. Lesinski S.G. Causes of conductive hearing loss after stapedectomy or stapedotomy: A prospective study of 279 consecutive surgical revisions. Otol Neurotol. 2002;23:281-288.
46. Lippy W.H., Wingate J., Burkey J.M., et al. Stapedectomy revision in elderly patients. Laryngoscope. 2002;112:1100-1103.
47. Morganstein K.M., Manace E.D. Incus necrosis following stapedectomy. Laryngoscope. 1968;78:600-619.
48. Alberti P.W. The blood supply of the incus long process and the head and neck of the malleus. J Laryngol Otol. 1965;79:966-970.
49. McGee T.M. The loose wire syndrome. Laryngoscope. 1981;91:1478-1483.
50. Rajan G.P., Atlas M.D., Subramaniam K., Eikelboom R.H. Eliminating the limitations of manual crimping in stapes surgery: A preliminary trial with the shape memory Nitinol stapes piston. Laryngoscope. 2005;115:366-369.
51. Knox G.W., Reitan H. Shape-memory stapes prosthesis for otosclerosis surgery. Laryngoscope. 2005;115:1340-1346.
52. Sorom A.J., Driscoll C.L., Beatty C.W., Lundy L.B. Retrospective analysis of outcomes after stapedectomy with implantation of a self-crimping nitinol prosthesis. Otolaryngol Head Neck Surg. 2007;137:65-69.
53. Rajan G.P., Diaz J., Blackham R., et al. Eliminating the limitations of manual crimping in stapes surgery: Mid-term results of 90 patients in the Nitinol stapes piston multicenter trial. Laryngoscope. 2007;117:1236-1239.
54. Brown K.D., Gantz B.J. Hearing results after stapedotomy with a nitinol piston prosthesis. Arch Otolaryngol Head Neck Surg. 2007;133:758-762.