Chapter 33 Larynx and Hypopharynx Cancer
Epidemiology and Etiology
The median age of patients presenting with larynx cancer is 65 years. Less than 4% of patients are younger than 45 years old. Laryngeal cancer remains predominantly a disease affecting men. The most recent data from the Surveillance, Epidemiology, and End Results (SEER) program (2003–2007)1 estimated an overall incidence of 3.4 cases per 100,000 people per year, a slight decrease of 0.4 cases per 100,000 compared with the previous 5 years of data.2 Comparisons reveal case incidences of 6.1 versus 1.3 in males and females, respectively. There is a higher incidence among blacks than among whites for both men and women.
Tobacco use is strongly associated with the development of cancer of the larynx, with the highest risk among active heavy smokers and an intermediate risk among ex-smokers.3,4 More than 95% of patients with laryngeal cancer have a history of tobacco use.5 Cigar and pipe smoking have also been associated with cancer of the larynx,4 but studies on this issue have been more controversial.3,6,7
Alcohol use is also associated with laryngeal cancer but is believed to act synergistically with tobacco rather than independently. It is unusual to see laryngeal cancer in nonsmoking patients with alcohol abuse histories.7 A history of heavy alcohol use is more strongly associated with supraglottic and hypopharyngeal cancers.4,8 Likewise, occupational exposures to asbestos,4,9,10 mustard gas, nickel, soot, and tars have been linked to laryngeal cancer, but generally a tobacco use history is also present.11 Several authors have evaluated the influence of diet on the development of larynx cancer and have found, while controlling for tobacco and alcohol use, a higher incidence among patients with vitamin- and nutrient-deficient diets.6,12,13
Attention has been directed at the influence of gastroesophageal reflux disease (GERD) on laryngeal diseases, including carcinomas. Three separate studies14,15,16 have described cohorts of nonsmoking patients with GERD and larynx cancer. Bacciu and colleagues17 compared 36 consecutive patients with no history of tobacco and alcohol consumption who developed laryngeal carcinoma to a group of 125 lifetime nonsmokers who were cancer free. They found a much higher prevalence of GERD among the patients with laryngeal cancer. It is believed that chronic irritation on the larynx from acid may predispose these patients to cancer. It is thought that if this cancer is seen in nonsmokers, the influence of GERD on the development of laryngeal cancer in smokers and alcohol users (who are at higher risk for GERD) may be very significant.
Human papillomavirus (HPV) has been causally linked to multiple cancers, including head and neck cancers,18,19 particularly cancers of the tonsil. HPV has also been demonstrated to be associated with laryngeal cancers, although studies are, in general, retrospective and the reported prevalence rates vary widely. The evidence for HPV having a role in laryngeal cancer is less obvious than in other malignancies, and its interaction with other carcinogens such as tobacco is unclear. As in cervical cancer in women and tonsillar cancer, HPV-16 is the most common type noted.
Hypopharyngeal cancers are less common than laryngeal tumors. The estimated incidence in the United States is 2500 cases per year. Etiologic risk factors are similar to those for laryngeal tumors,12 with a predominance among men and older individuals. This type of cancer is closely linked to tobacco and alcohol use, and the ties to heavy alcohol use seem stronger than those for laryngeal cancer.
Prevention and Early Detection
Prevention
The primary preventive methods taken to eliminate malignancies of the upper aerodigestive tract have come from public awareness that tobacco and alcohol are the major causative agents of these cancers. National public health measures have been directed at diminishing the prevalence of smoking and drinking. A byproduct of these policies may be a decline in the incidence of laryngeal and hypopharyngeal carcinomas. The most recent SEER data has identified a decrease in the incidence of larynx cancer over the past two decades (1988–2007).1 The annual percentage decrease over this time period is 2.6%.
Although government policies have been directed at diminishing carcinogenic etiologic agents for the general population, investigators have tried to identify high-risk groups in whom more direct measures can be taken. The major group identified consists of patients who have been cured of a cancer of the upper aerodigestive tract,20,21–23 particularly patients who have smoking or alcohol use histories.24 The incidence of second primary cancers of the upper aerodigestive tract ranges from 10% to 30%. The locations of these second cancers are evenly divided between the lungs, esophagus, and head and neck mucosal sites, including the larynx and hypopharynx.24
The high incidence of new cancers in this patient population has led investigators to develop programs designed to diminish the occurrence of second primary tumors. Hong and associates25 studied 13-cis-retinoic acid as a possible agent to prevent new cancers in patients with a history of head and neck malignancy. A randomized trial showed a 14% versus 31% incidence of second primary tumors in patients who received a relatively high dose of 13-cis-retinoic acid versus placebo.26 The Radiation Therapy Oncology Group (RTOG), in 2002, completed accrual to a trial testing chemoprevention with 13-cis-retinoic acid in a multi-institutional setting.27 Nearly 1400 patients with stage I or II cancer were accrued. The dose of 13-cis-retinoic acid was lower than that used by Hong and associates to ensure more compliance and less side effects. Unfortunately, the RTOG trial was negative and did not show any benefit to low dose isotretinoin in the prevention of second primary cancers. The RTOG study did show that the continuation of smoking had adverse effects on outcome and those who smoked were more likely to develop second cancers.
Papadimitralopoulou and colleagues28,29 investigated α-tocopherol, interferon-alfa, and isotretinoin in patients with laryngeal dysplasia and reported a 50% complete response rate at 12 months. The current thinking is that these agents may delay or prevent subclinical cancer from manifesting as clinical disease but are less likely to prevent cellular transformation. This three-drug combination is being tested in a phase III trial, but accrual has not been robust.
Early Detection
Similar to the issues surrounding prevention, early detection of laryngeal and hypopharyngeal cancers centers on targeting the population at highest risk for developing these carcinomas. Cancers of the larynx and hypopharynx affect roughly 15,000 Americans a year and therefore is not a large enough health problem to warrant screening of the general population. Some investigators have studied the role of screening a more focused population such as tobacco users who work at high-risk occupations and question the value of screening even a more limited population.30 However, Prout and colleagues31 argue that a primary care practitioner can, as part of a general evaluation, inquire about hoarseness in a patient from an at-risk population. If a positive response is obtained, the patient can be referred to an otolaryngologist for appropriate evaluations. The laryngeal carcinoma detection rate in this situation ranges between 3% and 4%.31,32
Pathology and Pathways of Spread
Pathology
Less frequently, the larynx and hypopharynx can give rise to variants of squamous cell carcinoma. The most common of these cancers is verrucous carcinoma, accounting for approximately 4% of all larynx cancers.33 They are classically slow-growing tumors with a gross warty appearance. A less common variant with numerous nomenclatures is squamous cell carcinoma with spindle cell features. As its name implies, along with typical squamous cells, carcinoma cells are spindle cells. The significance of these spindle cells is the subject of debate, because theories range from these cells being a benign reactionary process with little clinical significance to highly malignant elements with adverse outcome.34 Molecular evidence suggests the sarcomatoid carcinoma evolves from the conventional epithelium-type component and the sarcomatoid component has a malignant nature.35 Grossly, they can often present as large polypoid lesions that sometimes act as ball valves in the larynx. Basaloid squamous cell carcinoma and lymphoepithelioma of non-nasopharyngeal origin are rare tumors seen in numerous head and neck mucosal sites, including the larynx and hypopharynx.
The remaining 5% of larynx cancers are composed of neoplasms more commonly found in other locations. Salivary gland cancers, neuroendocrine tumors36 (including small cell carcinomas), sarcomas, and lymphomas have all been reported in the literature.37
Pathways of Spread
Primary Site and Regional Lymphatics
Larynx
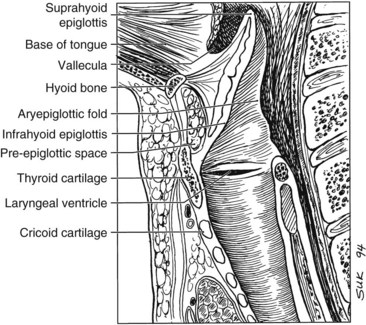
The larynx is divided into three regions: the supraglottis, glottis, and subglottis. The supraglottic larynx lies above the level where the mucosa of the upper surface of the true vocal cords turns upward to form the lateral wall of the ventricle. It consists of the false vocal cords, arytenoids, aryepiglottic folds, and infrahyoid and suprahyoid epiglottis. The glottic region by definition includes the true vocal cords and extends 0.5 cm inferiorly, and the subglottic region extends from there to the superior aspect of the trachea (Fig. 33-1).
Glottis
The mucosa of the true vocal cords has a sparse lymphatic supply. Thus, glottic carcinomas have a low propensity for lymphatic spread. The incidence of lymphadenopathy at diagnosis is approximately 5% for T1 and T2 lesions and approximately 20% for T3 and T4 tumors.38 The frequency of occult nodal involvement is also low. Byers and colleagues39 found microscopic nodal involvement in 9 of 57 patients who underwent elective nodal dissection for T3 or T4 vocal cord lesions; most frequently involved nodes were the upper jugular (level II), midjugular (level III), and paratracheal groups (level VI).
Supraglottis
The primary difference between supraglottic cancers and true glottic cancers is the likelihood of developing cervical nodal metastases. At diagnosis, 55% of patients with supraglottic cancers have clinically involved lymph nodes. Lymphatic vessels in the supraglottic larynx collect in channels that pass through the piriform sinuses to drain to nodes along the jugular chain, particularly the upper (level II) and midjugular (level III) lymph nodes. Lee and associates40 reported on the data of a subgroup of patients with intermediate-stage disease who underwent supraglottic laryngectomy with neck dissections. One third of patients had palpable nodes on presentation, and nearly an additional third of patients had pathologic nodal involvement.
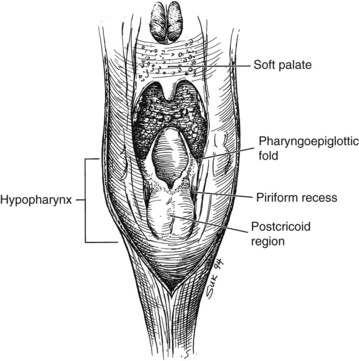
Hypopharynx
The hypopharynx is the inferior portion of the three divisions of the pharynx (Fig. 33-2). It extends from the hyoid bone superiorly to the cricoid inferiorly. Valleculae, pharyngoepiglottic fold, and lateral projections of aryepiglottic folds are considered the superior border separating the hypopharynx from oropharynx. Inferiorly, the hypopharynx ends at the cervical esophageal inlet. The hypopharynx is subdivided into three components: the pharyngeal walls, the piriform sinus, and the postcricoid pharynx. The hypopharyngeal walls are a continuation of the lateral and posterior oropharyngeal walls. The pair of piriform sinuses is created by the invagination of the larynx into the hypopharynx. They are conical (more truly pear shaped, hence the derivation of its name). Each sinus (or recess) consists of three walls. The medial wall is essentially the lateral aspect of the larynx; superiorly, it becomes the aryepiglottic fold. The lateral wall is a continuation of the lateral wall of the oropharynx. Anteriorly, the medial and lateral walls converge to form the narrow anterior wall. Superior is the base or vestibule formed by the rim of the three walls. Inferiorly, the three walls merge to form the apex.
Hypopharyngeal cancers also commonly present as nodal metastases. Lindberg41 reported a 75% incidence of nodal metastases in patients presenting to the M.D. Anderson Cancer Center (MDACC) with hypopharyngeal tumors. Level II and III nodes were most frequently involved, and bilateral lymphadenopathy was seen in 15% of patients. These tumors also have access to deep jugular and retropharyngeal lymph nodes.
Distant Metastases
The incidence of distant metastases from cancers of the head and neck and specifically laryngeal and hypopharyngeal cancers is low and is generally thought to be less than 10%. An often-referenced study by Crile in 190642 reported an incidence of 1% distant metastases in 4500 patients with epidermoid cancers of the head and neck. In subsets of patients with head and neck squamous cell cancers, patients with glottic tumors usually have the lowest rates of distant metastases whereas patients with hypopharyngeal carcinomas often have the highest rates.
Merino and colleagues43 analyzed the incidence of distant disease in over 5000 patients with head and neck squamous cell carcinomas treated from 1948 through 1967. Among patients with control of disease above the clavicle, the incidence of distant failure was 1%, 13%, and 23% for patients with carcinomas of the true vocal cords, supraglottic larynx, and hypopharynx, respectively. Similarly, Marks and associates44 found that 23% of patients with piriform sinus cancers developed distant metastases, with a higher rate among patients who underwent total laryngectomies as part of their therapy. The incidence of metastases is associated with stage of disease, because the clinical incidence of metastases is approximately 20% in patients with stage IV disease.
The sites of presentation of distant metastases are similar for cancers of the larynx and hypopharynx.43 The lungs are the most common site and are the first site of presentation in nearly 60% of patients. Bones are the next most common site, because 20% of patients with distant disease develop osseous metastases. Liver metastases are common in autopsy studies, but clinical liver metastases develop in only 10% of patients with hematogenous spread of disease from the larynx and hypopharynx.45 Spread to mediastinal lymph nodes, the brain, or other organs is very uncommon.
Molecular Biology
A general view is that tumorigenesis of head and neck malignancies occurs because of a combination of factors. Carcinogen exposure (primarily tobacco) results in genetic damage. However, not all smokers develop cancer. The genetic damage in individuals varies based on the degree of exposure of the offending agent as well as the individual’s inherent sensitivity to genetic damage. The latter can be tested indirectly in individuals using an assay quantifying chromosomal breakage induced by in-vitro exposure to bleomycin. Patients with upper aerodigestive tract malignancies were compared with healthy controls. Mutagen-sensitive individuals had a higher likelihood of having a cancer, and mutagen-sensitive smokers were at the highest risk.46
A field of mucosa is placed at risk for developing carcinoma by the exposure and sensitivity described earlier, but it requires a number of events to develop a frank cancer. The concept of field cancerization was first described in 1953 by Slaughter and associates.47 These researchers found widespread microscopic abnormalities in “normal” mucosa adjacent to tumor obtained from resected specimens. These abnormalities ranged from hyperplasia to areas of carcinoma both invasive and in situ away from the known resected cancer. Califano and colleagues48 proposed a genetic progression model in which the local clinical phenomenon of field cancerization involves the expansion and migration of clonally related preneoplastic cells.
The molecular events leading to the development of carcinomas are believed to occur by a multistep process and have been described for other cancers.49 This theory is applicable to head and neck cancers and appears consistent with the pathologic findings of field cancerization. Genotypic alterations in histologically normal epithelium adjacent to invasive carcinomas have been described. Voravud and associates50 found increased chromosomal polysomies in normal mucosa of smokers with invasive carcinomas but not in mucosa of healthy nonsmoking volunteers. The most frequent findings in premalignant tissue are deletions of one of the two alleles at chromosomes 3p and 9p21.51 These regions harbor tumor suppressor genes and thus may be involved with malignant transformation. It has also been observed that telomerase is activated in head and neck squamous carcinoma and dysplasia and may be an early event in the tumorigenic process.51
TP53 is a tumor suppressor gene. A mutation in TP53 is regarded as the most common related genetic change in human cancers.52 The normal TP53 protein has a short half-life, so its detection is thought to represent TP53 mutation. Shin and colleagues53 found TP53 expression in normal tissue and premalignant lesions adjacent to head and neck tumors but not in normal tissues from normal control patients. Numerous investigators have found overexpression of TP53 protein in laryngeal carcinomas, typically in approximately half the tumors studied.53,54 Alterations in TP53 are thought to be an important step early in the carcinogenic process but not a necessary step for all laryngeal and hypopharyngeal tumors. The finding of this protein in laryngeal specimens is also unclear, because, although there are diverging study results, most investigators have not found a prognostic value to the finding of TP53 protein in larynx tumor specimens.54–57
Other cellular changes in laryngeal carcinogenesis involve epidermal growth factor receptor (EGFR), CCND1 (formerly cyclin D1), and CDKN2A. Weichselbaum and colleagues58 described elevations in EGFR expression in head and neck tumor cell lines. In an analysis of premalignant and malignant head and neck tumor specimens, EGFR levels correlated with increasing severity of dysplasia.59 Furthermore, Shin and colleagues60 found elevated EGFR levels in premalignant tissues but dramatic up-regulation in invasive carcinoma specimens. EGFR was amplified in DNA from 29% of sampled hypopharyngeal cancers, and it is overexpressed in at least 80% of head and neck cancers. This overexpression has been demonstrated to be an independent prognosticator in head and neck cancer.59
Clinical Manifestations, Patient Evaluation, and Staging
Patient Evaluation
Laryngeal Cancer
Clinical evaluation of laryngeal cancer includes indirect laryngoscopy with a mirror that is frequently supplemented by fiberoptic endoscopy (Table 33-1). Similar to other tumors in the head and neck, the examiner assesses the tumor size, morphology, infiltration (defect, distortion) of adjacent structures, and vocal cord mobility. It is important to palpate the base of the tongue to determine direct invasion from the supraglottic larynx and to look for indirect signs of pre-epiglottic space invasion such as fullness of the vallecula or ulceration of the infrahyoid epiglottis. A direct laryngoscopy is often the final step in evaluation. It is needed to further outline the disease extent and, in particular, to obtain biopsy specimens for tissue diagnosis.
TABLE 33-1 Evaluation of Patients with Suspected Laryngeal or Hypopharyngeal Cancer
* In patients with more advanced disease, these tests may augment the workup for metastatic disease; PET and barium studies may help better define the inferior extent of advanced hypopharyngeal cancers.
Radiologic studies are indicated when there is the suggestion of deep infiltration. CT (Fig. 33-3) or MRI is useful for the assessment of the pre-epiglottic space, tongue base, paraglottic region, and subglottis (which is sometimes difficult to evaluate from above with the mirror or fiberoptic scope). These images may help differentiate direct primary tumor extension to the soft tissue of the neck from nodal involvement. Anterior commissure lesions may have subtle thyroid cartilage invasion only detectable by CT.
Hypopharyngeal Cancer
Evaluation of hypopharyngeal tumors is similar to that of larynx cancers. Because these lesions are typically at an advanced stage, it is not uncommon to palpate disease in the neck by direct extension. The thyroid click may be lost owing to anterior displacement of the larynx by a posteriorly located (particularly postcricoid) lesion. Phonation may provide better visualization of the piriform sinus on indirect mirror and fiberoptic examination. If unsuccessful, a Valsalva maneuver may open the sinus. Deep infiltrative lesions in the apex may be hard to see but are suspected by either pooling of secretions or arytenoid edema. Assessment of laryngeal mobility is important in medial wall lesions because they can invade directly into the laryngeal framework. In addition to CT (Fig. 33-4), combined 18F-fluorodeoxyglucose-labeled positron emission tomography and CT (FDG-PET/CT) may be helpful in defining tumor extent, particularly the inferior border where subtle changes in the inferior hypopharynx and superior cervical esophagus may be hard to identify solely on anatomic images.
Staging
Larynx
Tumors are staged using the American Joint Committee for Cancer (AJCC) TNM system27 (Table 33-2). The system is currently in its seventh iteration, most recently revised in 2010. The system classifies/categorizes larynx cancer primary tumors (T) by sites of extension and cord mobility but not size. For glottic tumors, stage T1 is disease limited to the vocal cord(s). The AJCC staging system further divides T1 glottic tumors into T1a for tumors confined to one true vocal cord and T1b for tumors involving both vocal cords. In general, this subdivision is more useful for communication and may influence the treatment decision (i.e., limited surgery vs. RT) but does not have a strong impact on prognosis. T2 glottic tumors have either supraglottic or subglottic extension and/or impaired mobility. Although not defined by the AJCC, T2 glottic lesions can be subdivided into T2a for normal mobility and T2b for impaired mobility. This subdivision is not uniformly used, however, because there is no general agreement that treatment outcomes differ among the substages.
TABLE 33-2 American Joint Committee on Cancer Staging of Laryngeal and Hypopharyngeal Cancers
| Glottis | |
| Tis | Carcinoma in situ |
| T1 | Tumor limited to the vocal cord(s) with normal mobility |
| T2 | Tumor extends to supraglottis and/or subglottis and/or with impaired cord mobility |
| T3 | Tumor limited to the larynx with vocal cord fixation and/or invades paraglottic space and/or minor thyroid cartilage erosion |
| T4a | Tumor invades through the thyroid cartilage and/or invades tissues beyond the larynx |
| T4b | Tumor invades prevertebral space, encases carotid artery, or invades mediastinal structures |
| Supraglottis | |
| Tis | Carcinoma in situ |
| T1 | Tumor limited to one subsite of the supraglottis with normal vocal cord mobility |
| T2 | Tumor invades mucosa of more than one subsite of the supraglottis or glottis or adjacent site outside the glottis, without fixation of the larynx |
| T3 | Tumor limited to the larynx with vocal cord fixation and/or invades any of the following: the pre-epiglottic space, the postcricoid area, and/or minor thyroid cartilage erosion |
| T4a | Tumor invades through the thyroid cartilage and/or invades tissues beyond the larynx |
| T4b | Tumor invades prevertebral space, encases carotid artery, or invades mediastinal structures |
| Hypopharynx | |
| T1 | Tumor limited to one subsite of hypopharynx and 2 cm or less in greatest dimension |
| T2 | Tumor invades more than one subsite of hypopharynx or an adjacent site or measures between 2 and 4 cm in greatest dimension, without fixation of hemilarynx |
| T3 | Tumor measures greater than 4 cm or with fixation of hemilarynx |
| T4a | Tumor invades thyroid/cricoid cartilage, hyoid bone, thyroid gland, esophagus, or central compartment soft tissue |
| T4b | Tumor invades prevertebral fascia, encases carotid artery, or involves mediastinal structures |
| Nodal Involvement (N) | |
| Nx | Nodes cannot be assessed |
| N0 | No clinically positive node |
| N1 | Single clinically positive ipsilateral node 3 cm or less in diameter |
| N2 | Single clinically positive ipsilateral node more than 3 cm, but not more than 6 cm in diameter or multiple clinically positive ipsilateral or bilateral or contralateral nodes, none more than 6 cm in diameter |
| N2a | Single clinically positive ipsilateral node more than 3 cm, but not more than 6 cm in diameter |
| N2b | Multiple clinically positive ipsilateral nodes, none more than 6 cm in diameter |
| N2c | Bilateral or contralateral lymph node, none more than 6 cm in greatest dimension |
| N3 | Metastases in a lymph node more than 6 cm in greatest dimension |
| Stage Groupings | |
| Stage I | T1N0M0 |
| Stage II | T2N0M0 |
| Stage III | T3N0M0 |
| T1-3N1 | |
| Stage IVA | T4aN0-1M0 |
| T1-4aN2M0 | |
| Stage IVB | T4bN0-3M0 |
| T1-4bN3M0 | |
| Stage IVC | T1-4N0-3M1 |
From Edge SB, Byrd DR, Compton C, et al, editors: AJCC Cancer Staging Handbook, ed 7. New York, 2010, Springer, pp 33-57. Used with permission of the American Joint Committee on Cancer.
Two significant revisions were made to the sixth edition of the staging system.61 Historically, the definition of T3 glottic disease has been fixation of the vocal cord. The sixth edition expanded on this definition and included paraglottic space invasion or minor thyroid cartilage invasion. Previous editions had defined T4 disease as invasion through the thyroid cartilage, but a common misinterpretation was to include any thyroid cartilage invasion into the T4 category. The new definition was made to clarify this point. The significance of this distinction is in the era of larynx preservation (see later); many still think that the presentation of invasion through the cartilage into the extralaryngeal tissues precludes a nonsurgical approach, but patients presenting with minimal invasion into the inner cortex may have disease suitable for larynx preservation.
Hypopharynx
T category for hypopharyngeal tumors is also based on sites of involvement and larynx motion (an indirect measurement of disease extension). The fifth edition of this staging classification had a significant modification to also include the size of the tumor in staging.62 This change addressed a major deficiency in prior systems, because the older systems tended to understage hypopharynx tumors relative to other head and neck mucosal cancers. The hypopharyngeal T category still does not reflect morphology, which remains an important criterion for selection of local therapies, because exophytic tumors in this site are usually selected more frequently than infiltrative lesions for organ-preserving therapies. The sixth edition made one additional modification. As described for laryngeal cancers, resectable T4a and unresectable T4b categories were defined. T4a hypopharyngeal disease can invade thyroid or cricoid cartilages, hyoid bone, thyroid gland esophagus, or central compartment, while T4b disease invades prevertebral fascia, encases carotid artery, or involves mediastinal structures. The newest edition now defines T4a and T4b as moderately advanced and very advanced.
Regional Classification/Categorizaton
The nodal (N) category is uniform for all head and neck cancer, including laryngeal and hypopharyngeal tumors. No changes were made in the seventh edition. The details of T and N categories for laryngeal (glottic and supraglottic) and hypopharyngeal cancers are summarized in Table 33-2.
Primary Therapy
Early Carcinomas
T1 and T2 Glottic Tumors
There are several difficulties in comparing the results of various treatment options. Nearly all series are retrospective single-institutional studies. It is thus difficult to ascertain if small differences in control rates between series are real. Mendenhall and colleagues63 address this issue by defining the surgical procedure that would have been recommended in their irradiated patients. They found that the surgical treatment option would have required total laryngectomy, rather than partial voice-conserving procedures, in 10% of patients with T1 disease and 55% of those with T2 disease. However, the supracricoid laryngectomy, a procedure that removes an entire circumferential portion of the larynx and subsequently reconstitutes the larynx, has largely been developed during the past decade.64 Many patients described by Mendenhall and colleagues in 1993 deemed suitable only for total laryngectomy may now be managed with this voice-preserving surgery. Olsen65 states that it is very unusual for disease that does not fix the arytenoids to require total laryngectomy. Although this is possibly true from a technical standpoint, many larynx cancer patients are older, have long histories of tobacco exposure, and medically may not be suitable for partial laryngeal surgery other than excisions of small T1 lesions on the midcord.
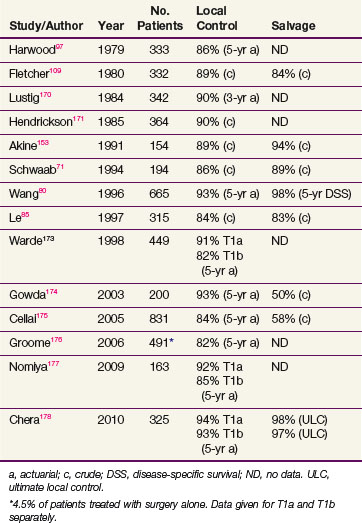
In general, T1 tumors can be treated effectively with laser excision,66 laryngofissure,67 partial laryngectomy,68,69 or irradiation. Table 33-3 summarizes the results of RT. Local control rates of RT range from 85% to 95%, and ultimate control rates after salvage surgery for recurrences are greater than 95%. Surgical salvage for the uncommon local relapse often requires a total laryngectomy, but an occasional patient can have a lesion amenable to a voice-conserving partial laryngectomy.70–74
An important factor in selecting therapy for individual patients is the anticipated voice quality after therapy. Unfortunately, studies comparing voice quality between laser excision and RT have yielded conflicting conclusions. Hirano and associates75 concluded in their analysis that hoarseness and incomplete glottic closure were more frequently seen in patients treated with laser. McGuirt and associates76 had physicians, patients, and speech pathologists compare voice quality of patients who received RT or laser excision for early glottic cancer. They concluded that patients treated with limited excisions of tumors of the midcord had equivalent quality compared with those receiving RT. Using computerized-assisted voice analysis, Harrison and associates77 demonstrated that most of their patients irradiated for early vocal cord cancer maintained excellent voices. Mittal and colleagues78 reported a cost savings with radiation compared with hemilaryngectomy and thought this should be an important component in treatment decisions if other important criteria are equivalent. A recent meta-analysis performed by Higgins and associates79 of over 7600 patients suggested a trend toward improved voice quality with RT as compared with transoral laser excision.
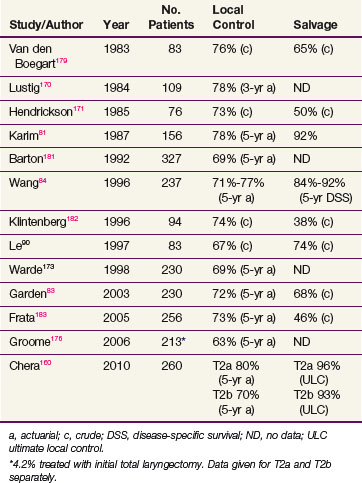
The outcome of RT for T2 cancers is, as expected, less favorable than that for T1 tumors. Local control rates range from 65% to 80%, and ultimate control rate is about 90% (Table 33-4). The wider range in the reported local control rates can, at least in part, be ascribed to a considerable heterogeneity of presentations within this stage, that is, varying from a superficial tumor spilling into the ventricle to a more bulky lesion transgressing the paraglottic space and impairing motion.
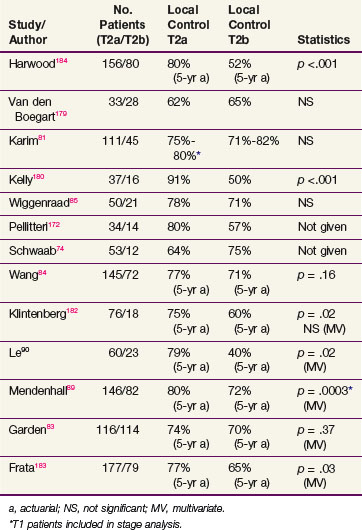
Oncologists have searched for prognostic determinants in this group of patients. Several reports showed that impairment in cord mobility adversely affects tumor control by RT (Table 33-5). Some physicians favor a hemilaryngectomy, if feasible, for T2b tumors.68,69,80 However, Karim and colleagues81 did not find impaired cord mobility to result in a worse outcome and attributed this observation to prescribing higher radiation doses to patients with such a tumor feature. Similarly, a report from MDACC did not show a relationship between mobility and outcome but some patients were treated with altered fractionated schedules that were theorized to have a therapeutic advantage.82 An update of this report reinforced the lack of impact of mobility on prognosis, independent of fractionation.83 This was contrary to the finding of Wang,84 who found that twice-daily radiation improved local control of T2 tumors with normal mobility but not those with impaired mobility.
Several studies were also undertaken to assess the influence of impaired cord mobility on the success rate of salvage surgery for patients who developed recurrences. Schwaab and associates74 noted difficulties in attempting salvage on a small cohort of patients presenting with T2b tumors (although, surprisingly, the initial control rate of T2b tumors in this series was better than that of T2a lesions). Wiggenraad and colleagues85 reported ultimate control rates of 98% for T2a and 76% for T2b tumors (p <.05). Other groups, however, have reported equivalent ultimate local control rates for tumors with normal or impaired cord mobility.74,83,86 Ang and Peters87 suggested that the timeliness of diagnosis and the extent of surgery rather than the initial disease presentation might account for the difference in the salvage results.
Anterior commissure involvement was considered a potential prognostic factor because it may carry an increased risk for unsuspected thyroid cartilage invasion. However, this feature was not found to be a prognostic determinant in most recent series. Sessions and associates88 found that anterior commissure89 involvement was more frequently associated with larger tumors, and it was the extent of disease rather than commissure involvement per se that impacted on outcome. Le and associates90 found, in a multivariate analysis, that patients with anterior commissure involvement had a poorer local control rate but concluded that these patients also had a larger tumor burden and sometimes unsuspected subglottic disease. Modern high-resolution imaging techniques can detect clinically unsuspected thyroid cartilage invasion and eliminate the clinical significance of anterior commissure involvement.
Glottic Carcinoma in Situ
Controversies of management even exist for carcinoma in situ of true vocal cords. Although not all lesions progress to invasive cancer, Hintz and colleagues91 reported that with a watchful waiting policy nearly two thirds of patients developed invasive cancer that was not always suitable to voice-preserving therapies. Ferlito and associates92 polled otolaryngologists and found practices of management ranging from postbiopsy observation, vocal cord stripping, cordectomy, open partial laryngectomy, to primary irradiation.
Primary RT is an effective treatment for carcinoma in situ of the larynx. However, conservative surgical approaches such as microexcision, laser ablation,93,94 or vocal cord stripping95 are considered sufficient for initial treatment. Repeated biopsies, strippings, or laser excisions may be counterproductive and lead to worsening of quality of voice. RT is usually reserved for recurrences or for diffuse lesions that are not suitable for limited surgery as the first therapy.
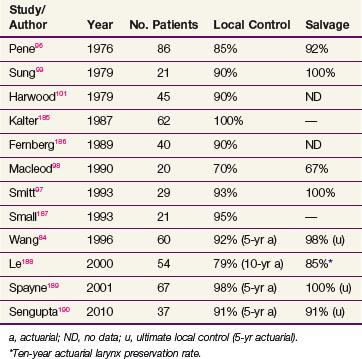
The local control rates reported in modern series of patients with carcinoma in situ treated with radiation ranges from 70% to 100% and are similar to rates described for invasive T1 disease (Table 33-6). It should be recognized that, owing to limited biopsy sampling, many radiation series may include patients with unrecognized invasive disease. For example, nearly one third of the patients in the series of Pene and Fletcher96 diagnosed with carcinoma in situ had clinical T2 disease. Most patients developing recurrent laryngeal carcinoma in situ undergo salvage surgery, most often with total laryngectomy, although some series have reported salvage with voice-preserving surgery.97,98 Although control rates after irradiation are similar to those reported for early invasive disease, recurrences in patients with carcinoma in situ take longer to manifest. Most series report median times to failure of greater than 2 years, but the majority of tumors recur within 5 years of treatment.91,96,98–100
T1, T2, and Selected T3 Supraglottic Tumors
Similar to glottic cancer, the optimal management of supraglottic carcinomas is still being debated. Confounding the issue even more is the necessity to manage the neck lymphatics in addition to the primary tumor. As mentioned earlier, nearly one third of patients with early- to intermediate-stage supraglottic neoplasms present with palpable cervical lymphadenopathy41,101 and another third have subclinical nodal involvement detected by elective neck dissections.102
Voice-preserving treatment options for early- and intermediate-stage tumors are supraglottic laryngectomy or primary RT.40,68,71,103–107 Local control rates in most surgical series range from 80% to 90%. Bocca and colleagues105 from Milan reported a local control rate of 85% in one of the largest series of over 400 patients with predominantly T2 tumors. Of note is that postoperative RT is often given to many patients undergoing partial laryngectomy. Common indications are presence of multiple nodes, extracapsular spread of nodal disease, and close or positive surgical margins. For example, 50 of 60 (83%) patients who underwent supraglottic laryngectomy at MDACC from 1974 to 1987 had indications for postoperative RT.40 The leading complication of supraglottic laryngectomy is aspiration, which can be morbid in heavy smokers (the majority of patients) with chronic pulmonary disease. Although preliminary data of endoscopic removal of early supraglottic lesions have been reported recently,108,109 more experience is needed to determine its relative value.
Most surgical series selected middle-aged men who are medically fit for supraglottic laryngectomy. Hinerman and colleagues110 analyzed 274 patients who underwent primary RT at the University of Florida and retrospectively grouped these patients into three groups: (1) suitable for conservation surgery, (2) anatomically suitable but medically unsuitable, and (3) anatomically unsuitable. Using 1998 AJCC criteria, they reported that 45% of T1 patients, 36% of T2 patients, and 14% of T3 patients were suitable for laryngectomy. Only 14% of T1 patients were anatomically unsuitable, compared with 46% and 68% of T2 and T3 patients, respectively. Of note, results after RT for all their patients were dependent on T stage but were not dependent on suitability for conservation surgery. Similar to the situation in patients with glottic carcinomas, the development of the supracricoid laryngectomy would alter the balance of patients with T2 and T3 patients thought to be anatomically unsuitable for a conservative procedure. In a small series, Laccourreye and associates111 reported on 19 patients treated with supracricoid laryngectomy. This cohort had disease in the infrahyoid epiglottis and was not suitable for more traditional conservative procedures.
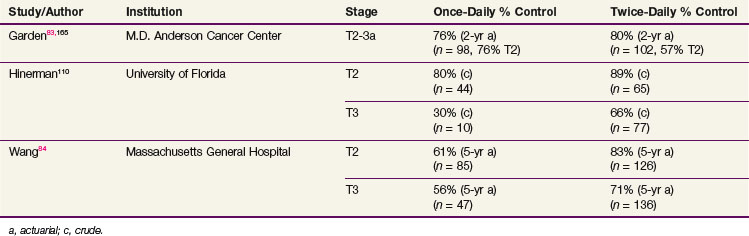
In 1974, Bataini and colleagues112 published an overall locoregional control rate of 76% for a relatively large series of patients with T1-2, N0-2 supraglottic carcinomas treated by RT alone. Subsequent series reported control rates of 84% to 100% for T1 tumors.84,113,114 Table 33-7 summarizes the outcome of RT in T2 and favorable T3 lesions. Fletcher and Goepfert113 obtained a local control rate of 76% in this subset of patients by escalating the total dose of the conventional daily irradiation to 70 Gy. Application of an altered fractionation regimen during the past decade appears to have resulted in a trend toward further improvement in the local control.84,102,114 In the Danish Head and Neck Cancer Group (DAHANCA) randomized fractionation trials, the benefit of accelerated treatment for head and neck cancer in general was most noticeable in the subgroup of patients with supraglottic cancers.115
TABLE 33-7 Comparison of Once-Daily versus Twice-Daily Fractionated Radiation for T2-3 Supraglottic Carcinomas
The impact of clinically positive nodal disease on local control has been evaluated in several series. Wall and colleagues116 found the presence of lymphadenopathy was associated with a significantly lower local control rate. Similarly, Wang and associates117 describe a marked worsening of local control in node-positive patients. The 5-year actuarial local control rate obtained with accelerated fractionation was 86% in patients with no adenopathy as opposed to 46% in patients with N2-3 disease. In contrast, Freeman and colleagues118 did not find an association between local control and the presence of lymph nodes.
The potential impact of tumor volume, estimated by CT measurements, on the results of RT was addressed by Freeman and associates119 in patients with T3 supraglottic tumors. They found a control rate of 83% for tumors of less than 6 cm3 compared with 46% for tumors of greater than 6 cm3. This latter group may be more suitable for surgery, particularly if voice-conserving surgery is feasible. Kraas and associates120 also studied tumor volume measurements, and although their threshold was 8 cm3, the concept of increasing tumor volume predicting worse outcome with RT was validated in a separate cohort.
Comparative studies have been undertaken in an attempt to delineate therapy recommendations for different subsets of patients. Fein and colleagues107 analyzed the University of Florida experience and concluded that RT yielded a local control rate equivalent to that obtained by conservative surgery but was associated with a lower complication rate. Robbins and colleagues104 from MDACC compared the outcome of patients who underwent supraglottic laryngectomy with that of patients who received RT for tumors judged suitable for partial laryngectomy. They found a better tumor control rate in the surgically treated group, but the incidence of morbidity due to chronic aspiration was rather high. This finding contributed to the development of the current treatment policy at this institution. Briefly, supraglottic laryngectomy, with postoperative RT when indicated, is recommended for medically fit patients with bulky, infiltrative T2 and T3 tumors (e.g., invasion into the false cords or infrahyoid epiglottis). RT is selected for patients with either smaller or exophytic lesions (T2 or favorable T3) or patients with bulky lesions who are medically unfit for voice-conserving surgery.
T1 and T2 Hypopharyngeal Tumors
Although uncommon, these tumors can be treated with RT. Because of the high propensity for neck metastases, it is not unusual to see patients with small-volume primary disease suitable for RT but with large neck disease that requires multimodality treatment. In this situation, the options are to irradiate the primary tumor and neck nodes to high doses and dissect the residual neck mass about 6 weeks after RT or to perform a neck dissection followed by definitive RT to the primary tumor and with adjunctive RT to the neck. The latter approach is frequently recommended to patients who need to undergo dental extractions, requiring a delay in initiation of irradiation.121
Some results with primary RT for early-stage hypopharynx lesions are shown in Table 33-8. Compared with historical control data, local control rates seem to have improved with the use of hyperfractionated regimens. For example, analysis of MDACC data revealed actuarial local control rates of 86% for hyperfractionation (35 patients) and 63% for conventional fractionation (23 patients).122 Similarly, for stage T2 piriform sinus primary tumors, Amdur and colleagues123 reported local control in 89% of patients treated with twice-a-day irradiation compared with 73% for those treated only once per day (p = .09). Although it is believed that hyperfractionation has improved the results, it should be realized that refinements in RT technique, including customized Cerrobend portal shaping, more generous coverage of the posterior route of spread, and the use of 6-MV x rays with smaller penumbra, may all have contributed to the improvement of outcome. In addition, it is difficult to discern whether the rather subjective patient selection criteria have changed over the years and what impact this has on the results. Superficial and exophytic tumors, particularly in the vestibule, tend to be selected for primary RT, whereas infiltrative tumors of similar stage, especially in the apex, are chosen for multimodality treatment.
Management of Squamous Carcinoma Variants
Verrucous Carcinoma
Verrucous carcinoma is generally a well-differentiated, slow-growing, wartlike lesion. This tumor has a reputation for its relative radioresistance and a tendency for converting to a highly anaplastic neoplasm after RT.33 However, no firm data are available to support this belief. A 5-year control rate of 59% was achieved in a series of 43 patients treated with RT at the Princess Margaret Hospital.124 The majority of these patients had early-stage disease, although in over half the disease was stage T2. The surgical salvage rate for radiation recurrences was high, and only 1 patient died of the disease. Therefore it is reasonable to treat patients with early verrucous carcinoma with radiation if the surgical option is a total laryngectomy. However, those with well-lateralized early tumors may be better suited for conservative surgery.
Sarcomatoid Carcinoma
Sarcomatoid carcinoma of the larynx has a similar reputation for radioresistance and for dedifferentiation after RT. In a review of 28 irradiated patients with T1-2 glottic sarcomatoid carcinoma, we found a 5-year actuarial control rate of 89%, which is similar to that obtained for ordinary squamous cell carcinoma.125 Although the control rate for 7 patients with T2 tumor was only 57%, all patients with recurrences could undergo salvage surgery and only 1 patient died of the disease.
Neuroendocrine Carcinoma
In a report126 describing the results of patients presenting with nonsinonasal neuroendocrine cancer, we found that the majority of patients (65%) presented with laryngeal or hypopharyngeal disease. RT was very effective for locoregional control, but most patients succumb to distant metastases. The addition of chemotherapy appeared to decrease the risk of distant failure and translated into improved survival. Our current policy is to recommend neoadjuvant chemotherapy, typically with cisplatin and etoposide followed by RT to the primary tumor and lymphatics. Concurrent chemoradiation is offered to patients who have initial large primary tumors or have a suboptimal response to chemotherapy alone.
Locally Advanced Carcinomas
These tumors have been traditionally managed with total laryngectomy with or without postoperative RT. It had been believed that although primary RT can preserve the larynx for some patients, this approach generally results in a lower survival rate than does surgery. Series describing RT alone tended to be small, retrospective, and influenced by patient selection bias, so they were not generally accepted despite reporting results similar to surgery. Additionally, data from large national databases on patient outcomes suggest that patients treated with RT alone have worse survival outcome compared with those who undergo total laryngectomy.127 However, as opposed to phase III data, these patients are possibly not truly comparable, and it is likely that patients treated by RT alone often have worse performance status and increased comorbidities.
Although it remains debatable whether induction chemotherapy has a role in the management of head and neck cancer, it was randomized trials using induction therapy that popularized the concept of larynx preservation. This led to the RTOG/Intergroup phase III trial 91-11, which randomized patients between three approaches: RT alone, induction chemotherapy and local treatment based on response, and concurrent chemoradiation128 (see later discussion). This study added to our knowledge of the role of combined-modality treatment (concurrent chemotherapy and RT) and gave further validity to nonsurgical approaches for locally controlled advanced larynx cancers.
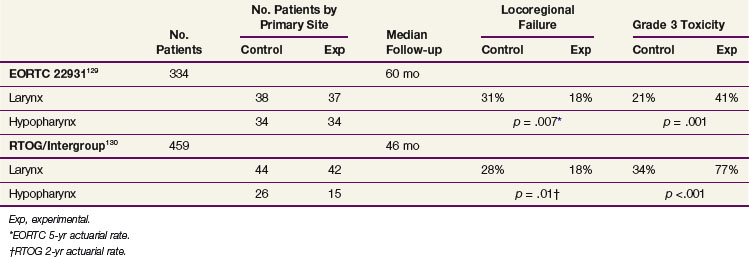
Randomized trials evaluating concurrent chemoradiation in the post–head and neck surgical setting have recently been reported. For patients deemed to be at high risk of locoregional recurrence, both the EORTC and RTOG/ Intergroup129,130 demonstrated that RT plus concurrent chemotherapy improve locoregional control compared with RT alone. The definitions of high risk were different in the two studies. Thirty percent to 40% of patients in both studies had laryngeal or hypopharyngeal cancers. The findings of these two important trials are summarized and contrasted in Table 33-9.
TABLE 33-9 Comparison of the EORTC and RTOG/Intergroup “High-Risk” Postoperative Radiation With or Without Concurrent High-Dose Cisplatin Trials
T3 Glottic Cancer
Most series of primary RT for T3 glottic tumors are relatively small. The available data are summarized in Table 33-10. Initial control rates range from 44% to 70%. Various twice-a-day fractionation schedules were found to control disease in approximately two thirds of patients.131,132 In a comparative study, Bryant and colleagues133 found that the disease-specific survival of patients treated with RT and surgical salvage was not significantly different from that of patients who underwent surgery and, occasionally, postoperative RT. They noted, however, that the control rate of patients requiring an emergency tracheotomy before RT was very low (18%). Mendenhall and colleagues131 also found similar disease-free and overall survival (OS) between patients treated with RT and those treated with total laryngectomy (occasionally with postoperative RT). The success rate of salvage surgery for radiation recurrences is on average 50% but decreases with increasing initial disease extent.
Bulky Supraglottic Cancer
Most patients with bulky, infiltrative T3 supraglottic primary tumors (often with vocal cord fixation) have been treated with total laryngectomy with or without postoperative RT. Clinical trials addressing the role of neoadjuvant chemotherapy have enrolled predominantly this subset of patients.134,135,136 The Veterans Affairs study,134 for example, randomized patients to either surgery at the outset (standard therapy arm) or neoadjuvant chemotherapy, with the responders receiving radiation and the nonresponders undergoing surgery. This trial showed that no difference in survival and larynx preservation was achieved in a substantial number of patients. However, this trial did not answer the important question of whether induction chemotherapy was more beneficial than RT alone.
Subsequently, the RTOG/Intergroup trial 91-11 tested differing larynx preservation approaches.128 Induction chemotherapy was the control arm, and RT alone or combined with concurrent cisplatin were two experimental arms. The results of this trial addressed several issues. Survival rates were equivalent for all three approaches. Locoregional control and larynx preservation were superior with concurrent chemoradiation. Induction chemotherapy was not significantly better than RT alone.
The addition of chemotherapy did increase the toxicity of treatment, principally acute toxicity. The suspected grade 5 toxicity rate in patients receiving concurrent chemoradiation was 5%, and the total rate of severe toxic effects was 20% in patients receiving chemotherapy. However, preliminary assessment of late dysphagia did not reveal an increased rate of swallowing difficulties in any treatment approach at 2 years, with all three trial arms reporting dysphagia rates ranging from 14% to 16%.128
In Europe, investigators have looked at other methods of larynx preservation. One group followed up on data demonstrating that a taxane added to the induction regimen of cisplatin and 5-fluorouracil improved outcomes in head and neck cancer patients137 by testing this approach for larynx preservation. Pointreau and colleagues reported a phase III trial for advanced laryngeal and hypopharyngeal cancers comparing the three-drug combination to the standard two-drug combination of cisplatin and 5-fluorouracil.138 Responders received RT (with or without concurrent chemotherapy) and nonresponders underwent laryngectomies followed by RT (with or without chemotherapy). Patients receiving the three-drug combination had more infections, but, despite this, these patients had a better response rate. In addition, those randomized to receive docetaxel had a 70% larynx preservation rate compared with 58% for those patients who did not receive the taxane.
A second approach investigated in Europe was to compare sequential chemoradiation to alternating chemoradiation.139 It was hoped that the alternating chemotherapy approach, which used lower doses of chemotherapy and RT (60 Gy compared with 70 Gy in the sequential arm) would mimic results seem with concurrent chemotherapy as in RTOG 91-11. Ultimately there was no difference in either toxicity or outcomes between the two approaches.
T4 Larynx Cancer
Patients with T4 glottic and supraglottic tumors are best treated with total laryngectomy and irradiation. The larynx preservation clinical trials testing neoadjuvant chemotherapy have not yielded encouraging results for patients with T4 disease.134,136 Only 10% of the RTOG-Intergroup trial cohort had “T4” disease, most likely with minimal thyroid cartilage invasion that has been downstaged by the criteria of the current AJCC classification. In addition, many patients with large bulky disease often will be left with dysfunctional larynges, leading to aspiration if the therapy is successful in eradicating the tumors.
In addition to general indications of postoperative RT in head and neck cancers (i.e., close or positive margins, multiple nodes, extracapsular spread of nodal disease, or extension of the primary lesion into the soft tissues of the neck), post–total laryngectomy RT is also recommended when thyroid/cricoid cartilage invasion and extensive subglottic disease are present or emergency tracheotomy is performed before RT. In a series by Klein and Fletcher,140 stomal recurrence occurred in 13 of 54 patients (24%) with radiologic evidence of subglottic disease who did not receive postoperative RT. None of these patients was salvaged. Yuen and coworkers141 analyzed the data of patients with T3 and T4 glottic lesions and found that surgery alone achieved locoregional control in 90% of patients without the adverse features listed earlier compared with 73% of those with some of the adverse features; with surgery and postoperative RT, locoregional control was obtained in 46 of 50 patients (92%). In patients with stage IV supraglottic cancer, Goepfert and colleagues142 reported a 2-year determinate control rate of 37% for surgery alone as opposed to 63% for combined surgery and postoperative RT.
Several groups have investigated chemoradiation for T4 disease. Clinicians from the University of Chicago used an aggressive concurrent regimen of paclitaxel, 5-fluorouracil, hydroxyurea, and twice-daily RT and reported 4-year locoregional control and laryngectomy-free survival rates of 71% and 87%, respectively.143 They suggested that patients who received induction chemotherapy in addition to concurrent chemoradiation fared best. At the University of Michigan, investigators are using a strategy of chemoselection.144 Patients with T4 disease are treated with one cycle of neoadjuvant chemotherapy. Those with a greater than 50% response are treated with chemoradiation, whereas the remaining patients go immediately to laryngectomy. Using this approach in 36 patients, 27 underwent chemoradiation, with a 58% laryngeal preservation rate. In both the Chicago and Michigan series there were modest rates of permanent tracheostomies and gastrostomies.143,144
Locally Advanced Hypopharynx Cancer
Surgery is the treatment of choice for patients with T3-4 hypopharyngeal cancer, but adjuvant irradiation is almost always indicated. Vandenbrouck and colleagues145 performed a randomized trial comparing preoperative and postoperative RT in the treatment of hypopharyngeal tumors. They reported a statistically better 5-year OS in patients treated postoperatively (56%) compared with those treated preoperatively (20%). Table 33-11 lists the results of postoperative RT. Analyzing a series of patients treated between 1949 and 1976, El Badawi and colleagues found that patients who received surgery and RT had significantly higher local control and 5-year OS than those who underwent surgery alone.146 Frank and colleagues147 found that, despite having higher stages, patients who received postoperative irradiation had only a 14% locoregional failure rate compared with 57% in those treated with surgery only. When adjusted for confounding variables, particularly the stage discrepancy, they also found that patients receiving postoperative irradiation had an improvement in OS.
The issue of larynx preservation in hypopharyngeal cancer has been addressed by the European Organization for Research and Treatment of Cancer (EORTC) in a multi-institutional phase III trial.148 Similar to the trial of the U.S. Department of Veterans Affairs for larynx cancer, the EORTC compared induction chemotherapy, followed by RT or surgery depending on the response, with immediate total laryngectomy and partial pharyngectomy. The 5-year actuarial voice-preservation rate was 35% in patients receiving chemotherapy, and there was no difference in survival among the two arms.
Patients with locally advanced hypopharynx cancer may be suitable for therapy with concurrent chemoradiation. These patients have often been included in trials in which the eligibility has been broad and included patients with locally advanced cancers of multiple head and neck sites.149,150 In most studies, as a subgroup, hypopharyngeal cancers represent a minority of patients. These studies have demonstrated an advantage to concurrent chemoradiation, and none of the studies has suggested that this general conclusion does not apply to locally advanced hypopharyngeal cancers. With regard to organ preservation for patients with locally advanced hypopharynx cancer, the same principles used for patient selection of larynx cancer should be applied.151,152
Irradiation Techniques
Primary Radiation Therapy
T1-2N0 Glottic Carcinomas
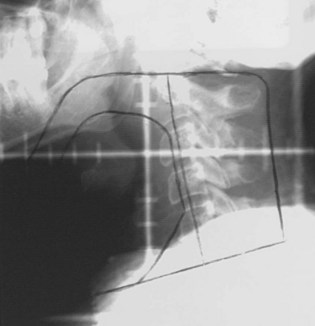
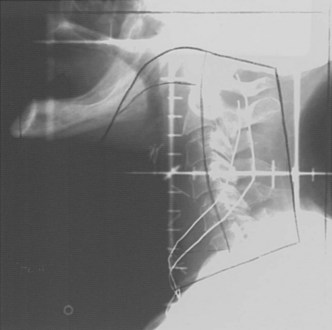
T1-2N0 glottic carcinomas are treated with a pair of small, lateral, opposed photon fields that encompass only the larynx proper (Fig. 33-5). In patients with a short neck, a 5- to 10-degree inferior tilt may be necessary to avoid irradiation through the shoulders. Other techniques include anterior wedged pairs or a four-field technique as described by Wang.84 For T1 lesions the field is centered on the true vocal cord and extends at or above the top of the thyroid notch superiorly to the lower edge of the cricoid cartilage inferiorly and from the anterior margin of the vertebral bodies posteriorly to approximately 1-cm falloff anteriorly. The superior and inferior borders are adjusted for T2 lesions that extend out of the glottis. When there is subglottic disease, at least one tracheal ring must be included in the fields. Typical field sizes range from 25 cm2 (5 × 5 cm) to 36 cm2 (6 × 6 cm). Harwood and associates101 reported better results with larger fields but acknowledged that at the time they used a free setup that likely accounted for some cases of geographic misses.
It is proper to use cobalt-60 gamma rays for treating glottic cancer, because higher-energy beams (6 MV or greater) may underdose the anterior commissure region, particularly in thin patients. Most radiation oncology centers nowadays no longer have cobalt-60 units and thus rely on linear accelerators. Local control rates of over 90% have been obtained for T1 lesions treated with 6-MV photons.153,154 Sombeck and colleagues155 performed a dosimetric comparison between cobalt-60 and 6-MV beams and found a similar dose distribution except for the most anterior tissues (3 mm below the surface) where underdosing occurred with the 6-MV beam. Consequently, these authors cautioned against using the 6-MV beam for anterior commissure lesions. However, advocates of 6-MV photons for glottic cancer recommend either using tissue-equivalent bolus material for buildup or using a beam spoiler for thin patients or patients with anterior lesions.154
A contour or CT plan is obtained to evaluate the dose distribution and determine the need for wedges (Fig. 33-6). Fifteen- or 30-degree wedges are often used and improve the dose distribution to the vocal cord, particularly for mid and posterior lesions. Treating without wedges results in a less homogeneous distribution, but this may be advantageous for anterior lesions when the built-in dose differential (5% to 10%) is desirable. Unilateral lesions can be treated by unequally weighting (e.g., 3 : 2) the fields, favoring the side of the lesion.
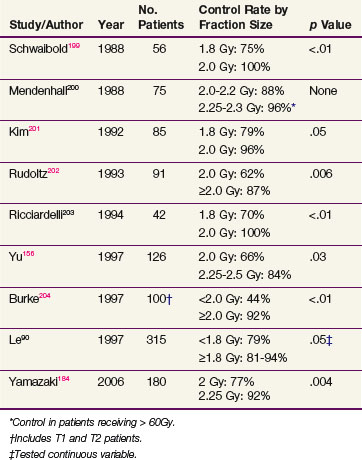
Radiation doses range from 60 to 70 Gy given in 2-Gy fractions. In general, a dose of 60 Gy is given to patients with no obvious clinical disease after stripping, 66 Gy to those with bulky T1, and 68 to 70 Gy to patients with T2 lesions. Most centers deliver 2 Gy per day, five fractions a week. Several studies evaluating the importance of dose per fraction principally for T1 disease show that 2 Gy per fraction yields better control rates than 1.8 Gy per treatment (Table 33-12). Some centers even advocate the use of larger fraction sizes.156 Investigators from Osaka randomized patients to either 2 Gy or 2.25 Gy per fraction and demonstrated an improvement in control in patients receiving the higher dose per fraction.157 Many groups now treat patients with T1 glottic disease with more hypofractionated regimens, and 63 Gy in 28 fractions is a very common regimen.
Based on biologic rationale, it has been attempted to improve the control rate of T2 glottic carcinoma by increasing the total dose through hyperfractionation.82 Two large retrospective studies have since been reported and both have suggested a trend (p >.05 but <.1) toward improved control with twice-daily treatments.83,89 Mendenhall and colleagues89 compared 182 patients treated with once-daily or twice-daily treatment and reported the following 5-year actuarial local control rates: T2a, 82% and 83%, respectively, and T2b, 71% and 69%, respectively. The authors contemplated that the advantages of hyperfractionation were mitigated by selection bias. Garden and colleagues83 analyzed 230 patients, of whom 89 were treated with twice-daily fractionation. Local control rates were 79% and 67%, respectively, for patients treated with twice-daily or once-daily schedules. The authors hypothesized that although there may be a benefit to twice-daily treatment the data more strongly favored a higher daily dose as a more important treatment factor, because those patients treated with less than 2 Gy per day had control rates of 59% compared with an 80% control rate for those treated with more than 2 Gy per day.
The RTOG has completed accrual to a randomized trial designed to address this issue, and the results were presented in 2006. Hyperfractionation was associated with an increase in local control of approximately 9% compared with conventional RT at 5 years. Although this result was not statistically significant, it was noted that this small improvement was consistent with results seen in other hyperfractionation studies.158
RT of early glottic cancers with small fields rarely induces severe complications. Fletcher and Goepfert113 described a 1% crude incidence of late edema in patients treated from 1962 through 1979. An analysis of late complications in 230 patients with T2 tumors revealed a 4% incidence of severe complications.83 The complications were five cases of dysfunctional larynx and/or chondronecrosis requiring tracheotomy, one case of reversible laryngeal edema, and four cases of postoperative complications related to salvage.
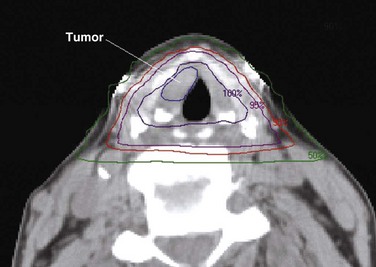
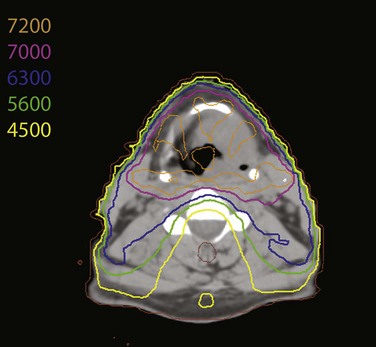
Despite the absence of obvious complications, questions have arisen as to whether irradiation to the neck can lead to an increase in the incidence of stroke.159 This has led several groups to assess the role of intensity-modulated radiation therapy (IMRT) in irradiating the larynx while sparing the carotid vessels. Two recent reports160,161 have demonstrated that IMRT can reduce the dose to the carotid arteries (Fig. 33-7). A study by MDACC investigators also describes a preliminary experience using IMRT to treat 12 patients, and early data suggest local control rates equivalent to those expected for patients with stage I and II glottic cancer.161
T3N0 Glottic, T1-3 Supraglottic, T Any N Glottic, or Hypopharyngeal Carcinomas
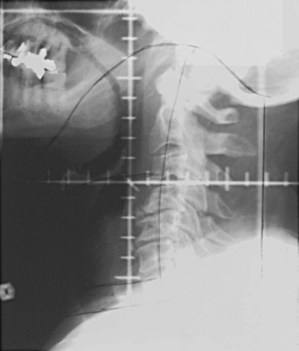
T3N0 glottic and T1-3N0 supraglottic carcinomas are treated with parallel, opposed, lateral portals covering the primary tumor and the subdigastric (level II) and midjugular (level III) lymph nodes. A matching anterior portal (with superior midline block to prevent overlap on the spinal cord) encompassing the lower jugular (level IV) and supraclavicular lymph nodes is also used (Figs. 33-8 and 33-9). Hypopharyngeal cancers and larynx carcinomas with nodal involvement are treated with larger lateral portals covering the primary disease and the upper (level II) and midjugular (level III) nodes (under the insertion of the sternocleidomastoid muscle) and a matching anterior portal encompassing lower jugular (level IV) and supraclavicular lymph nodes. For hypopharyngeal tumors the inferior border must cover the entire piriform sinus including the apex (Figs. 33-10 and 33-11). Because of the posterior location of the majority of these tumors, the off-spinal cord fields extend slightly more posteriorly than for other head and neck primary tumors. This necessitates more frequent portal verification. It is important to encompass the retropharyngeal lymph nodes generously in patients with hypopharyngeal tumors. However, supraglottic lesions invading the medial wall of the piriform sinus only do not necessitate wide coverage of the retropharyngeal nodes.
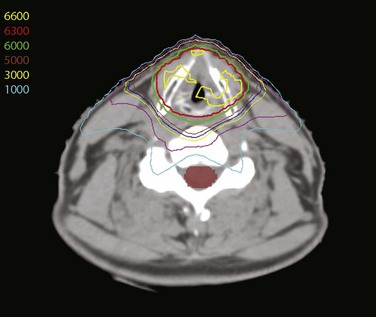
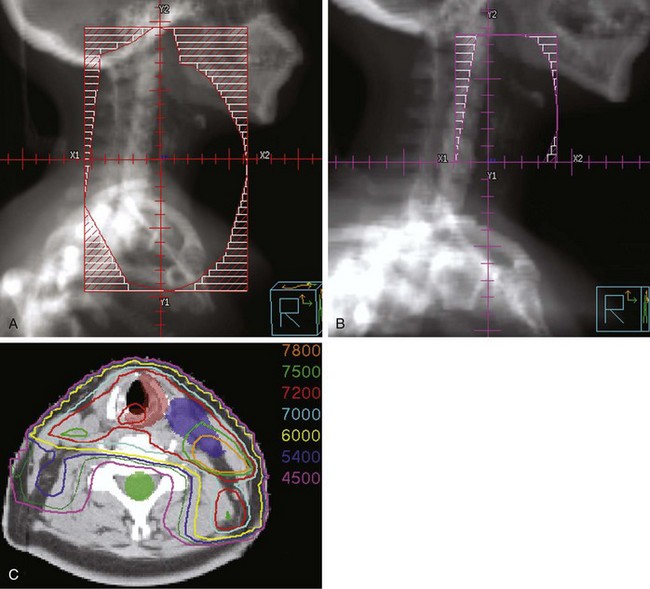
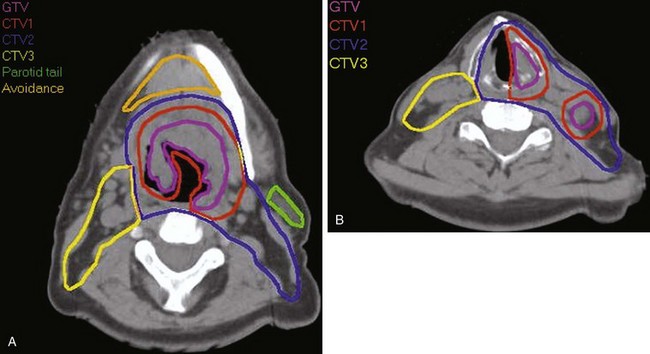
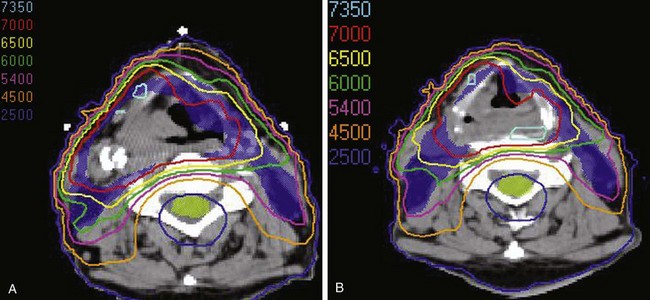
Treatment of head and neck cancer with IMRT has rapidly increased since the early 2000s. However, preliminary reports of IMRT have focused on treatment of nasopharynx and oropharynx cancer. In trials reporting more general head and neck results, the percentage of patients with laryngeal or hypopharyngeal cancers is extremely low.162,163 However, particularly for hypopharyngeal cancers or patients with extensive nodal disease, the appeal of IMRT for potentially reducing xerostomia exists. Examples of selected target delineationand isodose distributions for patients with advanced supraglottic cancer and a patient with hypopharyngeal cancer are shown in Figures 33-12 to 33-14.
Patients with T1 disease usually receive daily 2-Gy fractions to 64 to 68 Gy. Patients with T2 to T3 lesions receive either daily 2-Gy fractions to 70 Gy, although preferably if they are to be treated with RT and no chemotherapy they should be treated with either hyperfractionated twice-daily RT with fraction size of 1.2 Gy to 76.8 Gy or, alternatively, are treated with an accelerated schedule such as concomitant boost or the DAHANCA schedule.115 The concomitant boost schedule164 treats patients to their large fields with 1.8-Gy fractions to 54 Gy and delivers the boost dose with fraction size of 1.5 Gy per fraction during the last 2.5 weeks as a second daily fraction to bring the total dose to gross disease to 72 Gy delivered in 6 weeks. The DAHANCA regimen also treats patients in 6 weeks, but in 35 fractions. This is accomplished by delivering the boost as a second daily fraction once per week (for 5 weeks). Portal size is reduced to encompass the gross primary disease with 1- to 2-cm margins after reaching an elective dose of 50 Gy (2-Gy fractions) to 54 (1.8-Gy fractions) or 55.2 Gy (1.2-Gy fractions).
Because of larger radiation volume, RT of supraglottic and hypopharyngeal tumors induces a higher complication rate than treatment of early-stage glottic carcinomas. Mendenhall and associates114 have reported a 6% severe acute and late complication rate in 211 patients receiving primary RT for supraglottic carcinomas. In a series of 236 patients treated with hyperfractionation at MDACC, 21 patients developed grade 3 late complications; this includes 2 patients who died of massive hemoptysis 2 months post therapy and tracheal necrosis after a tracheotomy, respectively, and 2 patients who underwent laryngectomy for necrosis.165 However, the 3-year actuarial complication rate has decreased from 14% to 8% since increasing the interfraction interval from 4 to 6 hours. We currently maintain the 6-hour interfraction interval for twice-daily irradiation.
Postoperative Radiation Therapy
The technique for postoperative RT is similar to that for primary RT. Briefly, initial fields cover the operative and tumor bed. Patients with adverse features requiring postoperative irradiation are at increased risk for stomal failure. Patients are typically treated with a three-field technique: two lateral-opposed fields and an anterior low-neck portal (Fig. 33-15). The inferior borders of the lateral fields are in close proximity or even include the superior aspect of the stoma. The stoma itself is included in the anterior low-neck field. Occasionally it is complicated to find the ideal location for the match because of the stomal location. Caudally oriented fields often provide a simple solution for this problem and obviate concerns regarding matching through the stoma or needing to block the stoma for safety reasons.166
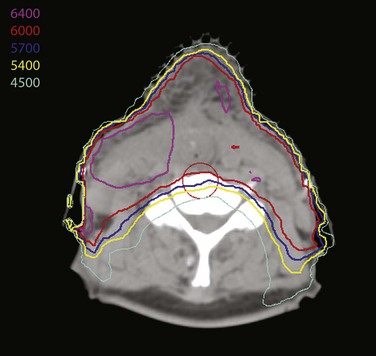
IMRT is being used more often to treat these patients. This allows tailoring of the dose to high- and intermediate-risk regions, avoids a match because the entire volume can be encompassed by the fields, and, particularly in node-positive patients, may allow for parotid sparing (Fig. 33-16).
The operative bed is treated to 57.6 Gy, and areas of increased risk are boosted to 63 Gy given in 1.8-Gy fractions167 or 56 Gy and 60 Gy, respectively, given in 2-Gy fractions. These dose prescriptions are also applicable to targets delineated with IMRT. No dose reduction is applied for patients who have had free jejunal autograft reconstruction, because no significant complications have been observed in a series of 29 patients treated to these doses.168 The dose delivered to surgically undisturbed elective sites is 50 to 54 Gy. The stoma is treated to 50 to 54 Gy and boosted only if there was extensive paratracheal or soft tissue extension in the region of the stoma.
Treatment Algorithm, Controversies, Challenges, and Future Possibilities
Treatment Algorithm
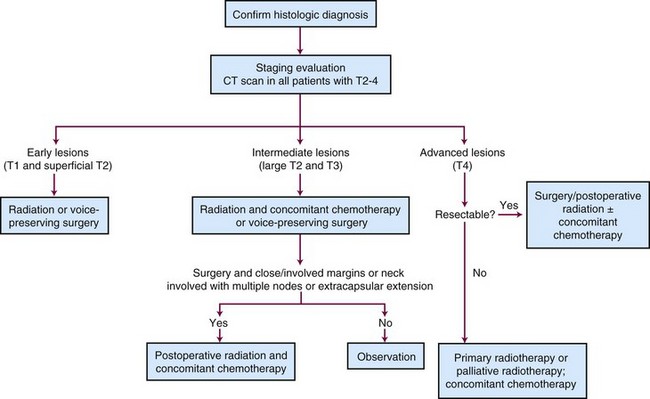
A treatment algorithm for laryngeal and hypopharyngeal cancer is shown in Figure 33-17.
Controversies, Challenges, and Future Possibilities
The results of RTOG 91-11 led us to conclude that most patients with laryngeal cancer can be managed without surgery except for those with T4 disease.128 This conclusion has come under more scrutiny as SEER data have suggested that survival rates for larynx cancer have decreased in recent years.127 However, whether this change in survival can be attributed to a greater use of larynx preservation remains moot.169
1 Altekruse S, Kosary C, Krapcho M, et al. SEER Cancer Statistics Review 1975-2007. Bethesda, MD: National Cancer Institute; 2009.
5 Wydner EL, Bross IJ, Day E. Epidemiological approach to the etiology of cancer of the larynx. JAMA. 1956;160:1384.
15 Morrison MD. Is chronic gastroesophageal reflux a causative factor in glottic carcinoma? Otolaryngol Head Neck Surg. 1988;99:370.
20 Cooper JS, Pajak TF, Rubin P, et al. Second malignancies in patients who have head and neck cancer. Incidence, effect on survival and implication based on the RTOG experience. Int J Radiat Oncol Biol Phys. 1989;17:449.
38 Million RR, Cassisi NJ, Mancuso AA. Larynx. In Cassisi NJ, et al, editors: Management of Head and Neck Cancer: A Multidisciplinary Approach, ed 2, Philadelphia: JB Lippincott, 1994.
39 Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg. 1988;10:160.
41 Lindberg RD. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446.
43 Merino OR, Lindberg RL, Fletcher GH. An analysis of distant metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1977;40:145.
44 Marks JE, Kurnik B, Powers WE, Ogura JH. Carcinoma of the pyriform sinus. An analysis of treatment results and patterns of failure. Cancer. 1978;41:1008.
79 Higgins KM, Shah MD, Ogaick MJ, et al. Treatment of early-stage glottic cancer. Meta-analysis comparison of laser excision versus radiotherapy. J Otolaryngol Head Neck Surg. 2009;38:603-612.
90 Le QX, Fu KK, Kroll S, et al. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:115.
110 Hinerman R, Mendenhall W, Amdur R, et al. Carcinoma of the supraglottic larynx. Treatment results with radiotherapy alone or with planned neck dissection. Head Neck. 2002;24:456.
111 Laccourreye O, Brasnu D, Merite-Drancy A, et al. Cricohyoidopexy in selected infrahyoid epiglottic carcinomas presenting with pathological preepiglottic space invasion. Arch Otolaryngol Head Neck Surg. 1993;119:881.
115 Overgaard J, Hansen H, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:1588.
122 Garden AS, Morrison WH, Clayman GL, et al. Early squamous cell carcinoma of the hypopharynx. Outcomes of treatment with radiation alone to the primary disease. Head Neck. 1996;18:317.
124 O’Sullivan B, Warde P, Keane T, et al. Outcome following radiotherapy in verrucous carcinoma of the larynx. Int J Radiat Oncol Biol Phys. 1995;32:611.
127 Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States. Changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116:1-13.
128 Forastiere A, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091.
129 Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945.
130 Cooper J, Pajak T, Forastiere A, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937.
131 Mendenhall WM, Parsons JT, Stringer SP, et al. Stage T3 squamous cell carcinoma of the glottic larynx. A comparison of laryngectomy and irradiation. Int J Radiat Oncol Biol Phys. 1992;23:725.
133 Bryant GP, Poulsen MG, Tripcony L, Dickie GJ. Treatment decision in T3N0M0 glottic carcinoma. Int J Radiat Oncol Biol Phys. 1995;31:285.
134 The Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685.
138 Pointreau Y, Garaud P, Chapet S, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst. 2009;101:498-506.
139 Lefebvre JL, Rolland F, Tesselaar M, et al. Phase 3 randomized trial on larynx preservation comparing sequential vs alternating chemotherapy and radiotherapy. J Natl Cancer Inst. 2009;101:142-152.
141 Yuen A, Medina J, Goepfert H, Fletcher G. Management of stage T3 and T4 glottic carcinomas. Am J Surg. 1984;148:467.
145 Vandenbrouck C, Sancho H, Le Fur R, et al. Results of a randomized clinical trial of preoperative irradiation versus postoperative in treatment of tumors of the hypopharynx. Cancer. 1977;39:1445.
148 Lefebvre JL, Chevalier D, Luboinski B, et al. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88:890.
149 Brizel D, Albers M, Fisher S, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798.
150 Adelstein D, Li Y, Adams G, et al. An Intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92.
154 Foote RL, Grado GL, Buskirk SJ, et al. Radiation therapy for glottic cancer using 6-MV photons. Cancer. 1996;77:381.
155 Sombeck MD, Kalbaugh KJ, Mendenhall WM, et al. Radiotherapy for early vocal cord cancer. A dosimetric analysis of 60Co versus 6 MV photons. Head Neck. 1996;18:167.
159 Haynes JC, Machtay M, Weber RS, et al. Relative risk of stroke in head and neck carcinoma patients treated with external cervical irradiation. Laryngoscope. 2002;112:1883-1887.
161 Rosenthal DI, Fuller CD, Barker JLJr, et al. Simple carotid-sparing intensity-modulated radiotherapy technique and preliminary experience for T1-2 glottic cancer. Int J Radiat Oncol Biol Phys. 2010;77:455-461.
162 Chao KSC, Ozygit G, Tran BN, et al. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2003;55:312.
163 Dawson LA, Anzai Y, Marsh L, et al. Patterns of local-regional recurrence following parotid-sparing conformal and segmental intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2000;46:1117.
165 Garden AS, Morrison WH, Ang KK, Peters LJ. Hyperfractionated radiation in the treatment of squamous cell carcinomas of the head and neck. A comparison of two fractionation schedules. Int J Radiat Oncol Biol Phys. 1995;31:493.
167 Peters LJ, Goepfert H, Ang KK, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys. 1993;26:3.
173 Warde P, O’Sullivan B, Bristow R, et al. T1/T2 glottic cancer managed by external beam radiotherapy. The influence of pretreatment hemoglobin on local control. Int J Radiat Oncol Biol Phys. 1998;41:347.
191 Vandenbrouck C, Eschwege F, De La Rochefordiere A, et al. Squamous cell carcinoma of the pyriform sinus. Retrospective study of 351 cases treated at the Institut Gustave-Roussy. Head Neck Surg. 1987;10:4.
196 Terhaard CHF, Karim ABMF, Hoogenraad WJ, et al. Local control in T3 laryngeal cancer treated with radical radiotherapy time dose relationship. The concept of nominal standard dose and linear quadratic model. Int J Radiat Oncol Biol Phys. 1991;20:1207.
197 Mendenhall WM, Parsons JT, Devine JW, et al. Squamous cell carcinoma of the pyriform sinus treated with surgery and/or radiotherapy. Head Neck Surg. 1987;10:88.
200 Mendenhall WM, Parsons JT, Million RR, Fletcher GH. T1-T2 squamous cell carcinoma of the glottic larynx treated with radiation therapy. Relationship of dose-fractionation factors to local control and complications. Int J Radiat Oncol Biol Phys. 1988;15:1267.
203 Ricciardelli EJ, Weymuller EAJr, Koh WJ, et al. Effect of radiation fraction size on local control rates for early glottic carcinoma. Arch Otolaryngol Head Neck Surg. 1994;120:737.
1 Altekruse S, Kosary C, Krapcho M, et al. SEER Cancer Statistics Review 1975-2007. Bethesda, MD: National Cancer Institute; 2009.
2 Ries LAG, Eisner MP, Kosary CL, et al, editors. SEER Cancer Statistics Review, 1997-2002. Bethesda, MD: National Cancer Institute, 2005.
3 Wydner EL. Toward the prevention of laryngeal cancer. Laryngoscope. 1975;85:1190.
4 Muscat JE, Wydner EL. Tobacco, alcohol, asbestos, and occupational risk factors for laryngeal cancer. Cancer. 1992;69:2244.
5 Wydner EL, Bross IJ, Day E. Epidemiological approach to the etiology of cancer of the larynx. JAMA. 1956;160:1384.
6 Freudenheim JL, Graham S, Byers TE, et al. Diet, smoking and alcohol in cancer of the larynx. A case-control study. Nutr Cancer. 1992;17:33.
7 Rothman KJ, Cann CI, Flanders WD, Fried MP. Epidemiology of laryngeal cancer. Epidemiol Rev. 1980;2:195.
8 Wydner EL, Stellman SD. Comparative epidemiology of tobacco-related cancers. Cancer Res. 1977;37:4608.
9 Burch JD, Howe GR, Miller AB, Semenciw R. Tobacco, alcohol, asbestos, and nickel in the etiology of cancer of the larynx. A case-control study. J Natl Cancer Inst. 1981;67:1219.
10 Stell PM, McGill T. Asbestos and laryngeal cancer. Lancet. 1973;2:416.
11 Merletti F, Heseltine E, Saracci R, et al. Target organs for carcinogenicity of chemicals and industrial exposures in humans. A review of results in the IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. Cancer Res. 1984;44:2244.
12 Esteve J, Riboli E, Pequignot G, et al. Diet and cancers of the larynx and hypopharynx. The IARC multi-center study in southwestern Europe. Cancer Causes Control. 1996;7:240.
13 Graham S, Mettlin C, Marshall J, et al. Dietary factors in the epidemiology of cancer of the larynx. Am J Epidemiol. 1981;113:675.
14 Freije JE, Beatty TW, Campbell BH, et al. Carcinoma of the larynx in patients with gastroesophageal reflux. Am J Otolaryngol. 1996;17:386.
15 Morrison MD. Is chronic gastroesophageal reflux a causative factor in glottic carcinoma? Otolaryngol Head Neck Surg. 1988;99:370.
16 Ward PH, Hanson DG. Reflux as an etiologic factor of carcinoma of the laryngopharynx. Laryngoscope. 1988;98:1195.
17 Bacciu A, Mercante G, Ingegnoli A, et al. Effects of gastroesophageal reflux disease in laryngeal carcinoma. Clin Otolaryngol Allied Sci. 2004;29:545.
18 Syrjanen S. Human papillomavirus (HPV) in head and neck cancer. J Clin Virol. 2005;32(Suppl 1):S59.
19 Hobbs C, Birchall M. Human papillomavirus infection in the etiology of laryngeal carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2004;12:88.
20 Cooper JS, Pajak TF, Rubin P, et al. Second malignancies in patients who have head and neck cancer. Incidence, effect on survival and implication based on the RTOG experience. Int J Radiat Oncol Biol Phys. 1989;17:449.
21 Fijuth J, Mazeron JJ, Le Pechoux C, et al. Second head and neck cancers following radiation therapy of T1 and T2 cancers of the oral cavity and oropharynx. Int J Radiat Oncol Biol Phys. 1992;24:59.
22 Gluckman JL, Cissman JD, Donegan JO. Multicentric squamous cell carcinoma of the upper aerodigestive tract. Head Neck Surg. 1980;3:90.
23 Tepperman BS, Fitzpatrick PJ. Second respiratory and upper digestive tract cancers after oral cancer. Lancet. 1981;2:547.
24 Schwartz LH, Ozsahin M, Zhang GN, et al. Synchronous and metachronous head and neck carcinomas. Cancer. 1994;74:1933.
25 Hong WK, Lippman SM, Itri LM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323:795.
26 Benner SE, et al. Prevention of second primary tumors with isotretinoin in patients with squamous cell carcinoma of the head and neck. Long-term follow-up. J Natl Cancer Inst. 1994;86:140.
27 Edge SB, Byrd DR, Compton CC, et al, editors. AJCC Cancer Staging Manual, ed 7, New York: Springer, 2010.
28 Papadimitrakopoulou V, Clayman G, Shin D, et al. Biochemoprevention for dysplastic lesions of the upper aerodigestive tract. Arch Otolaryngol Head Neck Surg. 1999;125:1083.
29 Papadimitrakopoulou V, Liu D, Mao L, et al. Biologic correlates of a biochemoprevention trial in advanced upper aerodigestive tract premalignant lesions. Cancer Epidemiol Biomarkers Prev. 2002;11:1605.
30 Fishinger J, Mlacak B. The usefulness of screening in the early detection of laryngeal cancer. Acta Otolaryngol. 1997;527:150.
31 Prout MN, Sidari JN, Witzburg RA, et al. Head and neck cancer screening among 4611 tobacco users older than forty years. Otolaryngol Head Neck Surg. 1997;116:201.
32 Hoare T, Thomson HG, Proops DW. Detection of early laryngeal cancer. The case for early specialist assessment. J R Soc Med. 1993;86:390.
33 Ferlito A. Diagnosis and treatment of verrucous squamous cell carcinoma of the larynx. A critical review. Ann Otol Rhinol Laryngol. 1985;94:575.
34 Brodsky G. Carcino (pseudo)sarcoma of the larynx: the controversy continues. Otolaryngol Clin North Am. 1984;17:185.
35 Choi H, Sturgis E, Rosenthal D, et al. Sarcomatoid carcinoma of the head and neck. Molecular evidence for evolution and progression from conventional squamous cell carcinomas. Am J Surg Pathol. 2003;27:1216.
36 Gnepp DR. Small cell neuroendocrine carcinoma of the larynx. A critical review of the literature. J Otorhinolaryngol Relat Spec. 1991;53:210.
37 Hyams VJ, Batsakis JG, Michaels L. Atlas of Tumor Pathology: Tumors of the Upper Respiratory Tract and Ear. Washington, DC: Armed Forces Institute of Pathology; 1988.
38 Million RR, Cassisi NJ, Mancuso AA. Larynx. In Cassisi NJ, et al, editors: Management of Head and Neck Cancer: A Multidisciplinary Approach, ed 2, Philadelphia: JB Lippincott, 1994.
39 Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg. 1988;10:160.
40 Lee NK, Goepfert H, Wendt CD. Supraglottic laryngectomy for intermediate stage cancer: U. T. M. D. Anderson Cancer Center experience with combined therapy. Laryngoscope. 1990;100:831.
41 Lindberg RD. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446.
42 Crile CW. Excision of cancer of the head and neck. JAMA. 1906;47:1780.
43 Merino OR, Lindberg RL, Fletcher GH. An analysis of distant metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1977;40:145.
44 Marks JE, Kurnik B, Powers WE, Ogura JH. Carcinoma of the pyriform sinus. An analysis of treatment results and patterns of failure. Cancer. 1978;41:1008.
45 Hong WK, Weber RS. Distant metastases from head and neck squamous cancer. The role of adjuvant chemotherapy. editors. Head and Neck Cancer: Basic and Clinical Aspects. Kluwer Academic Publishers, Boston, 1995;243.
46 Spitz MR, Fueger JJ, Halabi S, et al. Mutagen sensitivity in upper aerodigestive tract cancer. A case-control analysis. Cancer Epidemiol Biomarkers Prev. 1993;2:329.
47 Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium. Clinical implications of multicentric origin. Cancer. 1953;6:963.
48 Califano J, van der Riet P, Westra W, et al. Genetic progression model for head and neck cancer. Implications for field cancerization. Cancer Res. 1996;56:2488.
49 Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal tumor development. N Engl J Med. 1988;319:523.
50 Voravud N, Shin DM, Ro JY, et al. Increased polysomies of chromosomes 7 and 17 during head and neck multistage tumorigenesis. Cancer Res. 1993;53:2874.
51 Mao L, Hong W, Papadimitrakopoulou V. Focus on head and neck cancer. Cancer Cell. 2004;5:311.
52 Hollstein ML, Sidransky D, Vogelstein B, Harris C. p53 mutations in human cancers. Science. 1991;253:49.
53 Shin DM, Kim J, Ro JY, et al. Activation of p53 gene expression in premalignant lesions during head and neck tumorigenesis. Cancer Res. 1994;54:321.
54 Salam MA, Crocker J, Morris A. Over-expression of tumour suppressor gene p53 in laryngeal squamous cell carcinomas and its prognostic significance. Clin Otolaryngol. 1995;20:49.
55 Shin DM, Lee JS, Lippman SM, et al. p53 Expressions. Predicting recurrence and second primary tumors in head and neck squamous cell carcinoma. J Natl Cancer Inst. 1996;88:519.
56 Bradford CR, Zhu S, Wolf GT, et al. Overexpression of p53 predicts organ preservation using induction chemotherapy and radiation in patients with advanced laryngeal cancer. Otolaryngol Head Neck Surg. 1995;113:408.
57 Nadal A, Campo E, Pinto J, et al. p53 expression in normal, dysplastic, and neoplastic laryngeal epithelium. Absence of a correlation with prognostic factors. J Pathol. 1995;175:181.
58 Weichselbaum R, Dunphy E, Beckett M, et al. Epidermal growth factor receptor gene amplification and expression in head and neck cancer cell lines. Head Neck. 1989;11:437.
59 Grandis J, Tweardy D. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579.
60 Shin DM, Ro JY, Hong WK, Hittelman WN. Dysregulation of epidermal growth factor receptor expression in premalignant lesions during head and neck tumorigenesis. Cancer Res. 1994;54:3153.
61 Greene F, Page D, Fleming I, et al, editors. AJCC Cancer Staging Manual, ed 6, New York: Springer-Verlag, 2002.
62 Fleming I, Cooper J, Henson D, et al, editors. AJCC Cancer Staging Manual, ed 5, Philadelphia: Lippincott-Raven, 1997.
63 Mendenhall WM, Parsons JT, Stringer SP, et al. Radiotherapy alone or combined with neck dissection for T1-T2 carcinoma of the pyriform sinus. An alternative to conservation surgery. Int J Radiat Oncol Biol Phys. 1993;27:1017.
64 Ferlito A, Silver C, Howard D, et al. The role of partial laryngeal resection in current management of laryngeal cancer. A collective review. Acta Otolaryngol. 2000;120:456.
65 Olsen KD. Reexamining the treatment of advanced laryngeal cancer. Head Neck. 2010;32:1-7.
66 Strong MS. Laser excision of carcinoma of the larynx. Laryngoscope. 1975;85:1286.
67 Sessions D, Maness G, McSwain B. Laryngofissure in the treatment of carcinoma of the vocal cord. Laryngoscope. 1964;75:490.
68 Soo KC, Shah JP, Gopinath KS, et al. Analysis of prognostic variables and results after supraglottic partial laryngectomy. Am J Surg. 1988;156:301.
69 Laccourreye O, Weinstein G, Brasnu D, et al. Vertical partial laryngectomy. A critical analysis of local recurrence. Ann Otol Rhinol Laryngol. 1991;100:68.
70 Biller HF, Barnhill FRJr, Ogura JH, Perez CA. Hemilaryngectomy following radiation failure for carcinoma of the vocal cords. Laryngoscope. 1970;80:249.
71 Laccourreye O, Weinstein G, Naudo P, et al. Supracricoid partial laryngectomy after failed laryngeal radiation therapy. Laryngoscope. 1996;106:495.
72 Lavey RS, Calcaterra TC. Partial laryngectomy for glottic cancer after high-dose radiotherapy. Am J Surg. 1991;162:341.
73 Rothfield RE, Johnson JT, Myers EN, Wagner RL. Hemilaryngectomy for salvage of radiation therapy failures. Otolaryngol Head Neck Surg. 1990;103:792.
74 Schwaab G, Mamelle G, Lartigau E, et al. Surgical salvage treatment of T1/T2 glottic carcinoma after failure of radiotherapy. Am J Surg. 1994;168:474.
75 Hirano M, Hirade Y, Kawasaki H. Vocal function following carbon dioxide surgery for glottic carcinoma. Ann Otol Rhinol Laryngol. 1985;94:232.
76 McGuirt WF, Blalock D, Koufman JA, et al. Comparative voice results after laser resection or irradiation of T1 vocal cord carcinoma. Arch Otolargol Head Neck Surg. 1994;120:951.
77 Harrison LB, Solomon B, Miller S, et al. Prospective computer-assisted voice analysis for patients with early stage glottic cancer. A preliminary report of the functional result of laryngeal irradiation. Int J Radiat Oncol Biol Phys. 1990;19:123.
78 Mittal B, Rao DV, Marks JE, Ogura JH. Comparative cost analysis of hemilaryngectomy and irradiation for early glottic carcinoma. Int J Radiat Oncol Biol Phys. 1983;9:407.
79 Higgins KM, Shah MD, Ogaick MJ, et al. Treatment of early-stage glottic cancer. Meta-analysis comparison of laser excision versus radiotherapy. J Otolaryngol Head Neck Surg. 2009;38:603-612.
80 Biller HF, Ogura JH, Pratt LL. Hemilaryngectomy for T2 glottic cancers. Arch Otolaryngol. 1971;93:238.
81 Karim ABMF, Kralendonk JH, Yap LY, et al. Heterogeneity of stage II glottic carcinoma and its therapeutic implications. Int J Radiat Oncol Biol Phys. 1987;13:313.
82 Howell-Burke D, Peters LJ, Goepfert H, Oswald MJ. T2 glottic cancer. Arch Otolargol Head Neck Surg. 1990;116:830.
83 Garden A, Forster K, Wong P, et al. Results of radiotherapy for T2N0 glottic carcinoma. Does the “2” stand for twice-daily treatment? Int J Radiat Oncol Biol Phys. 2003;55:322.
84 Wang CC. Carcinoma of the larynx. In: Radiation Therapy for Head and Neck Neoplasms. New York: Wiley-Liss; 1997:221.
85 Wiggenraad RG, Terhaard CH, Horidjik GJ, Ravasz LA. The importance of vocal cord mobility in T2 laryngeal cancer. Radiother Oncol. 1990;18:321.
86 Fein DA, Mendenhall WM, Parsons JT, Million RR. T1-T2 squamous cell carcinoma of the glottic larynx treated with radiotherapy. A multivariate analysis of variables potentially influencing local control. Int J Radiat Oncol Biol Phys. 1993;25:605.
87 Ang KK, Peters LJ. Vocal cord cancer. 2B worse than not 2B? Radiother Oncol. 1990;18:365.
88 Sessions D, Ogura J, Fried M. The anterior commissure in glottic carcinoma. Laryngoscope. 1974;85:1624.
89 Mendenhall W, Amdur R, Morris C, Hinerman R. T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J Clin Oncol. 2001;19:4029.
90 Le QX, Fu KK, Kroll S, et al. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:115.
91 Hintz BL, Kagan AR, Nussbaum H, et al. A watchful waiting policy for in situ carcinoma of the vocal cords. Arch Otolaryngol. 1981;107:746.
92 Ferlito A, Polidoro F, Rossi M. Pathological basis and clinical aspects of treatment policy in carcinoma in situ of the larynx. J Laryngol Otol. 1981;95:141.
93 McGuirt WF, Browne JD. Management decisions in laryngeal carcinoma in situ. Laryngoscope. 1991;101:125.
94 Wolfensberger M, Dort JC. Endoscopic laser surgery for early glottic carcinoma. A clinical and experimental study. Laryngoscope. 1990;100:1100.
95 Maran AG, Mackenzie IJ, Stanley RE. Carcinoma in situ of the larynx. Head Neck Surg. 1984;7:28.
96 Pene F, Fletcher GH. Results in irradiation of the in situ carcinoma of the vocal cord. Cancer. 1976;37:2586.
97 Smitt MC, Goffinet DR. Radiotherapy for carcinoma-in-situ of the glottic larynx. Int J Radiat Oncol Biol Phys. 1994;28:251.
98 MacLeod PM, Daniel F. The role of radiotherapy in in-situ carcinoma of the larynx. Int J Radiat Oncol Biol Phys. 1990;18:113.
99 Sung DI, Chang CH, Harisiadis L, Rosenstein LM. Primary radiotherapy for carcinoma in situ and early invasive carcinoma of the glottic larynx. Int J Radiat Oncol Biol Phys. 1979;15:467.
100 Elman AJ, Goodman M, Wang CC, et al. In situ carcinoma of the vocal cords. Cancer. 1979;43:2422.
101 Harwood AR, Hawkins NV, Rider WD, Bryce DP. Radiotherapy of early glottic cancer-I. Int J Radiat Oncol Biol Phys. 1979;5:473.
102 Wendt CW, Peters LJ, Ang KK, et al. Hyperfractionated radiotherapy in the treatment of squamous cell carcinomas of the supraglottic larynx. Int J Radiat Oncol Biol Phys. 1989;17:1057.
103 Levendag P, Vikram B, Sessions R. The problem of neck relapse in early stage supraglottic cancer. Results of different treatment modalities for the clinically negative neck. Int J Radiat Oncol Biol Phys. 1987;13:1621.
104 Robbins KT, Davidson W, Peters LJ, Goepfert H. Conservation surgery for T2 and T3 carcinomas of the supraglottic larynx. Arch Otolaryngol Head Neck Surg. 1988;114:421.
105 Bocca E, Pignataro O, Oldini C. Supraglottic laryngectomy. 30 years of experience. Ann Otol Rhinol Laryngol. 1983;92:14.
106 Burstein FD, Calcaterra TC. Supraglottic laryngectomy. Series report and analysis of results. Laryngoscope. 1985;95:833.
107 Fein DA, Nichols RCJr, Lee WR, et al. T2-T3 carcinoma of the supraglottic larynx. A comparison of surgery and radiotherapy. Radiat Oncol Investig. 1995;2:237.
108 Steiner W. Results of curative laser microsurgery of laryngeal carcinomas. Am J Otolaryngol. 1993;14:116.
109 Zeitels SM, Koufman JA, Davis RK, Vaughan CW. Endoscopic treatment of supraglottic and hypopharynx cancer. Laryngoscope. 1994;104:71.
110 Hinerman R, Mendenhall W, Amdur R, et al. Carcinoma of the supraglottic larynx. Treatment results with radiotherapy alone or with planned neck dissection. Head Neck. 2002;24:456.
111 Laccourreye O, Brasnu D, Merite-Drancy A, et al. Cricohyoidopexy in selected infrahyoid epiglottic carcinomas presenting with pathological preepiglottic space invasion. Arch Otolaryngol Head Neck Surg. 1993;119:881.
112 Bataini JP, Ennuyer A, Poncet P, Ghossein NA. Treatment of supraglottic cancer by radical high dose radiotherapy. Cancer. 1974;33:1253.
113 Fletcher GH, Goepfert H. Larynx and hypopharynx. In: Fletcher G, editor. Textbook of Radiotherapy. ed 3. Philadelphia: Lea & Febiger; 1980:330.
114 Mendenhall WM, Parsons JT, Mancuso AA, et al. Radiotherapy for squamous cell carcinoma of the supraglottic larynx. An alternative to surgery. Head Neck. 1996;18:24.
115 Overgaard J, Hansen H, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:1588.
116 Wall TJ, Peters LJ, Brown BW, et al. Relationship between lymph nodal status and primary tumor control probability in tumors of the supraglottic larynx. Int J Radiat Oncol Biol Phys. 1985;11:1895.
117 Wang CC, Nakfoor BM, Spiro IJ, Martins P. Role of accelerated fractionated irradiation for supraglottic carcinoma. Assessment of results. Cancer J Sci Am. 1997;3:88.
118 Freeman DE, Mendenhall WM, Parsons JT, Million RR. Does neck stage influence local control in squamous cell carcinomas of the head and neck? Int J Radiat Oncol Biol Phys. 1992;23:733.
119 Freeman DE, Mancuso AA, Parsons JT, et al. Irradiation alone for supraglottic larynx carcinoma. Can CT findings predict treatment results? Int J Radiat Oncol Biol Phys. 1990;19:485.
120 Kraas J, Underhill T, D’Agostino RJ, et al. Quantitative analysis from CT is prognostic for local control of supraglottic carcinoma. Head Neck. 2001;23:1031.
121 Byers RM, Clayman GK, Guillamondegui O, et al. Resection of advanced cervical metastasis prior to definitive radiotherapy for primary squamous carcinomas of the upper aerodigestive tract. Head Neck. 1992;14:133.
122 Garden AS, Morrison WH, Clayman GL, et al. Early squamous cell carcinoma of the hypopharynx. Outcomes of treatment with radiation alone to the primary disease. Head Neck. 1996;18:317.
123 Amdur R, Mendenhall W, Stringer S, et al. Organ preservation with radiotherapy for T1-T2 carcinoma of the pyriform sinus. Head Neck. 2001;23:353.
124 O’Sullivan B, Warde P, Keane T, et al. Outcome following radiotherapy in verrucous carcinoma of the larynx. Int J Radiat Oncol Biol Phys. 1995;32:611.
125 Ballo MT, Garden AS, El-Naggar AK, et al. Radiation therapy for early stage (T1-T2) sarcomatoid carcinoma of true vocal cords. Outcomes and patterns of failure. Laryngoscope. 1998;108:760.
126 Barker JLJr, Glisson BS, Garden AS, et al. Management of nonsinonasal neuroendocrine carcinomas of the head and neck. Cancer. 2003;98:2322.
127 Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States. Changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116:1-13.
128 Forastiere A, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091.
129 Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945.
130 Cooper J, Pajak T, Forastiere A, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937.
131 Mendenhall WM, Parsons JT, Stringer SP, et al. Stage T3 squamous cell carcinoma of the glottic larynx. A comparison of laryngectomy and irradiation. Int J Radiat Oncol Biol Phys. 1992;23:725.
132 Wang CC. Carcinoma of the hypopharynx. In: Radiation Therapy for Head and Neck Neoplasms. New York: Wiley-Liss; 1997:205.
133 Bryant GP, Poulsen MG, Tripcony L, Dickie GJ. Treatment decision in T3N0M0 glottic carcinoma. Int J Radiat Oncol Biol Phys. 1995;31:285.
134 The Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685.
135 Pfister D, Strong E, Harrison L. Larynx preservation with combined chemo and radiotherapy in advanced head and neck cancer. J Clin Oncol. 1991;9:830.
136 Shirinian MH, Weber RS, Lippman SM, et al. Laryngeal preservation by induction chemotherapy plus radiotherapy in locally advanced head and neck cancer: the M. D. Anderson Cancer Center experience. Head Neck. 1994;16:39.
137 Posner MR, Norris CM, Wirth LJ, et al. Sequential therapy for the locally advanced larynx and hypopharynx cancer subgroup in TAX 324. Survival, surgery, and organ preservation. Ann Oncol. 2009;20:921-927.
138 Pointreau Y, Garaud P, Chapet S, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst. 2009;101:498-506.
139 Lefebvre JL, Rolland F, Tesselaar M, et al. Phase 3 randomized trial on larynx preservation comparing sequential vs alternating chemotherapy and radiotherapy. J Natl Cancer Inst. 2009;101:142-152.
140 Klein R, Fletcher GH. Evaluation of the clinical usefulness of radiological findings in squamous cell carcinomas of the larynx. AJR Am J Roentgenol. 1964;92:43.
141 Yuen A, Medina J, Goepfert H, Fletcher G. Management of stage T3 and T4 glottic carcinomas. Am J Surg. 1984;148:467.
142 Goepfert H, Jesse RH, Fletcher GH, Hamberger A. Optimal treatment for the technically resectable squamous cell carcinoma of the supraglottic larynx. Laryngoscope. 1975;85:14.
143 Knab BR, Salama JK, Solanki A, et al. Functional organ preservation with definitive chemoradiotherapy for T4 laryngeal squamous cell carcinoma. Ann Oncol. 2008;19:1650-1654.
144 Worden FP, Moyer J, Lee JS, et al. Chemoselection as a strategy for organ preservation in patients with T4 laryngeal squamous cell carcinoma with cartilage invasion. Laryngoscope. 2009;119:1510-1517.
145 Vandenbrouck C, Sancho H, Le Fur R, et al. Results of a randomized clinical trial of preoperative irradiation versus postoperative in treatment of tumors of the hypopharynx. Cancer. 1977;39:1445.
146 El Badawi SA, Goepfert H, Fletcher GH, et al. Squamous cell carcinoma of the pyriform sinus. Laryngoscope. 1982;92:357.
147 Frank JL, Garb JL, Kay S, et al. Postoperative radiotherapy improves survival in squamous cell carcinoma of the hypopharynx. Am J Surg. 1994;168:476.
148 Lefebvre JL, Chevalier D, Luboinski B, et al. Larynx preservation in pyriform sinus cancer: Preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88:890.
149 Brizel D, Albers M, Fisher S, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798.
150 Adelstein D, Li Y, Adams G, et al. An Intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92.
151 Beauvillain C, et al. Final results of a randomized trial comparing chemotherapy plus radiotherapy with chemotherapy plus surgery plus radiotherapy in locally advanced resectable hypopharyngeal carcinomas. editors]. Laryngoscope. 1997;107:648-653.
152 Jean-Michel Prades, et al. Randomized phase III trial comparing induction chemotherapy followed by radiotherapy to concomitant chemoradiotherapy for laryngeal preservation in T3Mo pyriform sinus carcinoma. editors]. Acta Otolaryngol. 2010;130:150-155.
153 Akine Y, Tokit N, Ogino T, et al. Radiotherapy of T1 glottic cancer with 6 MeV x-rays. Int J Radiat Oncol Biol Phys. 1991;20:1215.
154 Foote RL, Grado GL, Buskirk SJ, et al. Radiation therapy for glottic cancer using 6-MV photons. Cancer. 1996;77:381.
155 Sombeck MD, Kalbaugh KJ, Mendenhall WM, et al. Radiotherapy for early vocal cord cancer. A dosimetric analysis of 60Co versus 6 MV photons. Head Neck. 1996;18:167.
156 Yu E, Shenouda G, Beaudet MP, Black MJ. Impact of radiation therapy fraction size on local control of early glottic carcinoma. Int J Radiat Oncol Biol Phys. 1997;37:587.
157 Yamazaki H, Nishiyama K, Tanaka E, et al. Radiotherapy for early glottic carcinoma (T1N0M0). Results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006;64:77-82.
158 Trotti A, Pajak T, Emami B, et al. A randomized trial of hyperfractionation versus standard fractionation in T2 squamous cell carcinoma of the vocal cord. Int J Radiat Oncol Biol Phys. 2006;66(Suppl):s15.
159 Haynes JC, Machtay M, Weber RS, et al. Relative risk of stroke in head and neck carcinoma patients treated with external cervical irradiation. Laryngoscope. 2002;112:1883-1887.
160 Chera BS, Amdur RJ, Morris CG, et al. Carotid-sparing intensity-modulated radiotherapy for early-stage squamous cell carcinoma of the true vocal cord. Int J Radiat Oncol Biol Phys. 2010;77:1380-1385.
161 Rosenthal DI, Fuller CD, Barker JLJr, et al. Simple carotid-sparing intensity-modulated radiotherapy technique and preliminary experience for T1-2 glottic cancer. Int J Radiat Oncol Biol Phys. 2010;77:455-461.
162 Chao KSC, Ozygit G, Tran BN, et al. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2003;55:312.
163 Dawson LA, Anzai Y, Marsh L, et al. Patterns of local-regional recurrence following parotid-sparing conformal and segmental intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2000;46:1117.
164 Ang KK, Peters LJ, Weber RS, et al. Concomitant boost radiotherapy schedules in the treatment of carcinoma of the oropharynx and nasopharynx. Int J Radiat Oncol Biol Phys. 1990;19:1339-1345.
165 Garden AS, Morrison WH, Ang KK, Peters LJ. Hyperfractionated radiation in the treatment of squamous cell carcinomas of the head and neck. A comparison of two fractionation schedules. Int J Radiat Oncol Biol Phys. 1995;31:493.
166 Yom SS, Morrison WH, Ang KK, et al. Two-field versus three-field irradiation technique in the postoperative treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;66:469-476.
167 Peters LJ, Goepfert H, Ang KK, et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer. First report of a prospective randomized trial. Int J Radiat Oncol Biol Phys. 1993;26:3.
168 Cole CJ, Garden AS, Frankenthaler RA, et al. Postoperative radiation of free jejunal autografts in patients with advanced cancer of the head and neck. Cancer. 1995;75:2356.
169 Cosetti M, Yu GP, Schantz SP. Five-year survival rates and time trends of laryngeal cancer in the US population. Arch Otolaryngol Head Neck Surg. 2008;134:370-379.
170 Lustig RA, MacLean CJ, Hanks GE, Kramer S. The patterns of care outcome studies. Results of the national practice in carcinoma of the larynx. Int J Radiat Oncol Biol Phys. 1984;10:2357.
171 Hendrickson FR. Radiation therapy treatment of larynx cancers. Cancer. 1985;55:2058.
172 Pellitteri PK, Kennedy TL, Vrabec DP, et al. Radiotherapy—the mainstay in the treatment of early glottic carcinoma. Arch Otolaryngol Head Neck Surg. 1991;117:297.
173 Warde P, O’Sullivan B, Bristow R, et al. T1/T2 glottic cancer managed by external beam radiotherapy. The influence of pretreatment hemoglobin on local control. Int J Radiat Oncol Biol Phys. 1998;41:347.
174 Gowda RV, Henk JM, Mais KL, et al. Three weeks radiotherapy for T1 glottic cancer. The Christie and Royal Marsden Hospital Experience. Radiother Oncol. 2003;68:105-111.
175 Cellai E, Frata P, Magrini S, et al. Radical radiotherapy for early glottic cancer. Results in a series of 1087 patients from two Italian radiation oncology centers. I. The case of T1N0 disease. Int J Radiat Oncol Biol Phys. 2005;63:1378-1386.
176 Groome PA, O’Sullivan B, Mackillop WJ, et al. Compromised local control due to treatment interruptions and late treatment breaks in early glottic cancer. Population-based outcomes study supporting need for intensified treatment schedules. Int J Radiat Oncol Biol Phys. 2006;64:1002-1012.
177 Nomiya T, Nemoto K, Wada H, et al. Long-term results of radiotherapy for T1a and T1bN0M0 glottic carcinoma. Laryngoscope. 2009;118:1417-1421.
178 Chera BS, Amdur RJ, Morris CG, et al. T1N0 to T2N0 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:461-466.
179 Van den Bogaert W, Ostyn F, van der Schueren E. The significance of extension and impaired mobility in cancer of the vocal cord. Int J Radiat Oncol Biol Phys. 1983;9:181.
180 Kelly MD, Hahn SS, Spaulding CA, et al. Definitive radiotherapy in the management of stage I and II carcinomas of the glottis. Ann Otol Rhinol Laryngol. 1989;98:235.
181 Barton MB, Keane TJ, Gadalla T, Maki E. The effect of treatment time and treatment interruption on tumor control following radical radiotherapy of laryngeal cancer. Radiother Oncol. 1992;23:137.
182 Klintenberg C, Lundgren J, Adell G, et al. Primary radiotherapy of T1 and T2 glottic carcinoma. Analysis of treatment results and prognostic factors in 223 patients. Acta Oncol. 1996;35(Suppl 8):81.
183 Frata P, Cellai E, Magrini S, et al. Radical radiotherapy for early glottic cancer. Results in a series of 1087 patients from two Italian radiation oncology centers. II. The case of T2N0 disease. Int J Radiat Oncol Biol Phys. 2005;63:1387-1394.
184 Harwood AR, Beale FA, Cummings BJ, et al. T2 glottic cancer. An analysis of dose-time-volume factors. Int J Radiat Oncol Biol Phys. 1981;7:1501.
185 Kalter PO, Lubsen H, Delemarre JFM, Snow GB. Squamous hyperplasia of the larynx (a clinical follow-up study). J Laryngol Otol. 1987;101:579.
186 Fernberg JO, Ringborg U, Silfversward C, et al. Radiation therapy in early glottic cancer. Analysis of 177 consecutive cases. Acta Otolaryngol. 1989;108:478.
187 Small WJr, Mittal BB, Brand EN, et al. Role of radiation therapy in the management of carcinoma in situ of the larynx. Laryngoscope. 1993;103:663.
188 Le QT, Takamiya R, Shu HK, et al. Treatment results of carcinoma in situ of the glottis. An analysis of 82 cases. Arch Otolaryngol Head Neck Surg. 2000;126:1305-1312.
189 Spayne J, Warde P, O’Sullivan B, et al. Carcinoma-in-situ of the glottic larynx. Results of treatment with radiation therapy. Int J Radiat Oncol Biol Phys. 2001;49:1235.
190 Sengupta N, Morris CG, Kirwan JM, et al. Definitive radiotherapy for carcinoma in situ of the true vocal cords. Am J Clin Oncol. 2010;33:94-95.
191 Vandenbrouck C, Eschwege F, De La Rochefordiere A, et al. Squamous cell carcinoma of the pyriform sinus. Retrospective study of 351 cases treated at the Institut Gustave-Roussy. Head Neck Surg. 1987;10:4.
192 Rabbini A, Amdur RJ, Mancuso AA, et al. Definitive radiotherapy for T1-T2 squamous cell carcinoma of pyriform sinus. Int J Radiat Oncol Biol Phys. 2008;72:351-355.
193 Lundgren JAV, Gilbert RW, Van Nostrand AWP, et al. T3N0M0 glottic carcinoma. A failure analysis. Clin Otolaryngol. 1988;13:455.
194 Croll GA, Gerritsen GJ, Tiwari RM, Snow GB. Primary radiotherapy with surgery in reserve for advanced laryngeal carcinoma. Results and complications. Eur J Surg Oncol. 1989;15:350.
195 Wylie JP, Sen M, Swindell R, et al. Definitive radiotherapy for 114 cases of T3N0 glottic carcinoma. Influence of dose-volume parameters on outcome. Radiother Oncol. 1999;53:15-21.
196 Terhaard CHF, Karim ABMF, Hoogenraad WJ, et al. Local control in T3 laryngeal cancer treated with radical radiotherapy time dose relationship. The concept of nominal standard dose and linear quadratic model. Int J Radiat Oncol Biol Phys. 1991;20:1207.
197 Mendenhall WM, Parsons JT, Devine JW, et al. Squamous cell carcinoma of the pyriform sinus treated with surgery and/or radiotherapy. Head Neck Surg. 1987;10:88.
198 Slotman BJ, Kralendonk JH, Snow GB, et al. Surgery and postoperative radiotherapy and radiotherapy alone in T3-T4 cancers of the pyriform sinus. Treatment results and patterns of failure. Acta Oncol. 1994;33:55.
199 Schwaibold F, Scariato A, Nunno M, et al. The effect of fraction size on control of early glottic cancer. Int J Radiat Oncol Biol Phys. 1988;14:451.
200 Mendenhall WM, Parsons JT, Million RR, Fletcher GH. T1-T2 squamous cell carcinoma of the glottic larynx treated with radiation therapy. Relationship of dose-fractionation factors to local control and complications. Int J Radiat Oncol Biol Phys. 1988;15:1267.
201 Kim RY, Marks ME, Salter MM. Early-stage glottic cancer. Importance of dose fractionation in radiation therapy. Radiology. 1992;182:273.
202 Rudoltz MS, Benammar A, Mohiuddin M. Prognostic factors for local control and survival in T1 squamous cell carcinoma of the glottis. Int J Radiat Oncol Biol Phys. 1993;26:767.
203 Ricciardelli EJ, Weymuller EAJr, Koh WJ, et al. Effect of radiation fraction size on local control rates for early glottic carcinoma. Arch Otolaryngol Head Neck Surg. 1994;120:737.
204 Burke LS, Greven KM, McGuirt WT, et al. Definitive radiotherapy for early glottic carcinoma. Prognostic factors and implications for treatment. Int J Radiat Oncol Biol Phys. 1997;38:37.