CHAPTER 68 Laryngeal Reinnervation
In 1909, Horsley1 reported the first successful vocal cord reinnervation. He performed a neurorrhaphy of the RLN in a patient who had been shot in the neck, who had nearly complete recovery of laryngeal function. A number of writers have independently reported recurrent nerve repairs with return of function.2–4
In most cases vocal fold motion does not return after RLN anastomosis. Instead, a laryngeal synkinesis occurs with adductor and abductor nerve fibers nonselectively innervating the laryngeal muscles.5–8 The result of this neuromuscular mismatching is that counteracting forces are applied to the arytenoid by all the muscles innervated by the RLN and little or no functional movement occurs.9 An inspiratory medial bulging is seen that is thought to arise by the inappropriate activation of the thyroarytenoid muscle by abductor axons. The majority of RLN repairs, however, are incapable of producing abduction and adduction in functional coordination with the respiratory cycle.
The activity of the phrenic nerve during inspiration makes it an excellent candidate for reinnervation of the posterior cricoarytenoid (PCA) muscle. A similar muscle fiber–type profile between the diaphragm and the PCA also exists.10 Abductor mobility of the vocal fold had been obtained in multiple animal models.11–15 Experimental reinnervation with the phrenic nerve after a 9-month delay is successful but the functional recovery is reduced.16 Attempts at creating vocal fold motion with phrenic nerve reinnervation in humans, however, were unsuccessful.17
Because of the inability to consistently obtain cyclical motion of the vocal cords in coordination with respiration, reinnervation at the level of the nerve trunk has not gained acceptance as a clinical modality for bilateral vocal cord paralysis.18 Selective innervation of individual laryngeal muscles (in particular the PCA muscle) by neuromuscular transfer technique,19 selective anastomosis,20,21 or direct nerve implantation22,23 is required.
The NMP procedure, developed in the 1970s, involves placement of the distal portion of the ansa cervicalis branch to the omohyoid muscle along with a small block of muscle into a denervated laryngeal muscle. The procedure is indicated in patients with either unilateral or bilateral vocal fold paralysis, with different muscles being treated in each case. Depending on the success of reinnervation and the nature of the stimulus, there is a potential for recovery of vocal fold motion. In patients with unilateral vocal fold paralysis, the technique can be combined with a thyroplasty.24
The ansa-RLN anastomosis as an attempt to restore vocal fold movement was first reported by Frazier25 in 1924 and Frazier and Mosser26 in 1926. The current use of the technique was reported in 1986.27 An end-to-end anastomosis of the proximal ansa cervicalis nerve to the distal stump of the RLN is performed. The procedure is indicated for unilateral paralysis because vocal fold motion is not restored. Instead, muscle tone is restored to the entire hemilarynx, providing appropriate vocal fold position, bulk, and tone. The result is the potential for a near normal vocal ability.
Physiologic and Anatomic Issues in Laryngeal Reinnervation
There is substantial evidence that reinnervated muscle takes on the characteristics of the donor nerve (see Talmadge28 for review). This process appears to happen as the reinnervating nerve imposes a pattern of activity on the muscle fibers it contacts. Thus the selection of a donor nerve should ideally take into account the fiber type and contraction characteristics of the muscle to be reinnervated.
Nerve Characteristics
The RLN contains 1000 to 4000 motor axons, efferent axons, and sympathetic and parasympathetic secretomotor fibers, depending on the level at which the count is made. The RLN gives off branches to the cricopharyngeus and upper esophageal muscles29 as well as a sensory branch that communicates with the superior laryngeal nerve prior to entering the larynx. Five hundred to a thousand fibers are in the motor branches of the RLN.30 Before branching within the laryngeal framework, the motor fibers to the various muscles are intermixed throughout the RLN nerve trunk, making selective reinnervation at this level impractical.31 The anterior motor branch of the RLN enters the larynx posterior to the cricothyroid joint. The first branches that emerge from the RLN innervate the PCA muscle and often have connections to the midline interarytenoid muscle. The branch to the PCA muscle has characteristics of a slow-twitch motor nerve, with axons containing 200 to 250 muscle fibers in each motor unit.32 The interarytenoid muscle also receives a separate branch from each RLN, resulting in bilateral innervation. The terminal branches of the RLN innervate the lateral cricoarytenoid (LCA) and thyroarytenoid (TA) muscles. The axons in this branch are more characteristic of fast-twitch fibers, with motor unit sizes of 2 to 20 muscle fibers.33 In some larynges a connection between the superior laryngeal and the terminal fibers of the RLN can be seen within the TA.34
Except for proprioceptive fibers carried within the nerve, the ansa cervicalis is a purely motor derivative of the ventral rami of the cervical plexus. Fibers from the first cervical nerve join the hypoglossal nerve until it curves anteroinferiorly. At this point the first cervical nerve fibers leave the hypoglossal nerve to form the superior root of the ansa cervicalis. The geniohyoid and thyrohyoid are supplied entirely by these fibers. The branch to the superior belly of the omohyoid muscle typically originates from the superior loop of the ansa cervicalis. Because of its proximity to the thyroid ala and infrahyoid position, this branch has been frequently used as a donor for reinnervation using the NMP technique. The other strap muscles receive motor fibers from second and third cervical nerves primarily via the inferior root of the ansa cervicalis. The lower portions of the sternothyroid and sternohyoid muscles receive terminal nerves that branch off the loop of the ansa cervicalis as it passes deep to the omohyoid muscle.35
Muscle Fibers in General
The performance of a particular muscle depends on the character of the individual muscle fibers it contains. On the basis of their histochemical and contraction properties, the fibers of skeletal muscle can be classified as slow fatigue-resistant (type 1), fast fatigue-resistant (type 2A), or fast fatigable (type 2B). Several other classifications and subtypes exist (see Staron36 for review). Type 1 fibers are characterized by a low peak tension, slow contraction time, a low threshold of recruitment, fatigue resistance, and a dependence on aerobic metabolism. Muscles that are required to produce low tension over a prolonged period generally have a high proportion of type 1 fibers. Type 2 fibers are found to play a prominent role in muscles that can generate high tension for short periods. Type 2B fibers have a fast contraction time and can generate a higher tension. They are more easily fatigued, have a higher threshold of activation, and depend on anaerobic metabolism. Type 2A fibers are intermediate in tension, fatigability, and threshold, and they make use of both aerobic and anaerobic metabolism. The laryngeal muscles also have a high-velocity, low-tension fiber types (2L or mixed fiber type) that may be similar to those found in extraocular and jaw-closing muscles.37–40
Laryngeal Muscles
Activity of the PCA muscle is synchronous with inspiration and precedes activation of the diaphragm by 40 to 100 msec.41 The amount of activity varies directly with ventilatory resistance. Activity of the PCA produces lateral turning of the arytenoid, resulting in abduction of the vocal fold. Loss of PCA muscle tone results in instability of the arytenoid cartilage. On the basis of gross anatomy42 and innervations,43 the PCA muscle in humans can be divided into a horizontal compartment and a vertical/oblique compartment.43 Some researchers have reported that the PCA in laryngectomy specimens consists of nearly equal percentages of type 1 and type 2 fibers.44 A similar ratio is seen in the primate PCA.45 Other writers have found the PCA to have a higher percentage of type 1 fibers.10 The remainder of the muscle fibers appears to be mostly of the intermediate (type 2A) variety. These findings are consistent with the peak contraction time of the PCA, which is approximately 40 msec.
Reinnervation occurs most easily at the sites of the original motor end plates. It is possible that placement of a reinnervating NMP near a large population of motor end plates could improve the end result.46 The motor end plates of the human PCA muscle are distributed in a loosely arranged arc in the midportion of the muscle. The density of end plates is higher in the deeper portions of the PCA.
The TA and LCA are faster muscles. Their peak contraction times of 14 msec (TA) and 19 msec (LCA) make them some of the more rapid muscles in the body.45,47 The composition of muscle fibers in the TA is approximately 40% type 1, 55% type 2A, and 5% type 2B. The higher frequency of oxidative fibers characterizes the muscle as having aerobic metabolism, resistance to fatigue, and fast contraction.48 The faster response characteristics of these muscles are appropriate for their phonatory and protective functions.
The pattern of motor end plate distribution in the TA and cricothyroid (CT) muscles is more diffuse than that in the PCA. In the CT muscle the majority of the motor end plates are in the medial two thirds of the muscle49 while in the TA the end plates are scattered throughout the muscle.50 Placement of a NMP, or a direct nerve implant in precise locations, therefore, may offer a limited advantage in reinnervating the PCA and CT muscles. These diffuse patterns differ from skeletal muscles elsewhere in the body where they form a narrow band at the midpoint of the muscle.49
Both adductor and abductor laryngeal muscles contribute to the position and stability of the arytenoid cartilage.51 Restoration of adductor and abductor muscle tone should allow appropriate arytenoid position and stability.
Infrahyoid Muscles
The ansa cervicalis provides motor supply to the infrahyoid muscles and is the most commonly used donor nerve in laryngeal reinnervation. The peak contraction times of the thyrohyoid and sternothyroid muscles are approximately 50 msec.52 Approximately two thirds of the muscle fibers are type 1.53 The infrahyoid muscles have enzyme profiles similar to those of limb muscles and lack the faster mixed fibers found in the laryngeal muscles.54
In the hope of obtaining vocal fold movement, the activity of the various infrahyoid muscles has been examined. The strap muscles extending below the larynx function as accessory muscles of inspiration in varying degrees. The sternohyoid muscle has consistently shown little to no consistent activity with respiration. In the canine, a regular contraction of the sternothyroid muscle is seen with quiet respiration, but all strap muscles lack phasic activity in the primate. With greater airway resistance and hypoxia, inspiratory activity in the primate is greatest in the omohyoid, followed by the sternothyroid muscle .55 The sternothyroid in labored respiration can result in vocal cord lateralization even if the larynx is paralyzed, by means of the sternothyroid muscle’s effect on the laryngeal skeleton.56
Effects of Denervation
With denervation, the area of sensitivity to acetylcholine, which is limited to the end plate region in the intact muscle, spreads out over most of the external membrane.57 Denervation also eliminates both the trophic and activity-related influences on the muscle, resulting in muscle atrophy. Without reinnervation there is progressive atrophy and eventual destruction of the muscle despite an adequate supply of all nutrients.
Reinnervation becomes less effective the longer a muscle is denervated. The different laryngeal muscles appear to atrophy at different rates that vary with the muscles’ fiber composition. In a study on primate larynges, the faster TA muscle showed a far greater reduction in fiber size than the slower CT or PCA muscle. By 8 weeks of denervation, however, all laryngeal muscles were fibrosed.58 Muscle atrophy in human skeletal muscles apparently proceeds at a slower pace, with muscles surviving at least 3 years after denervation. Successful laryngeal reinnervation has been claimed as much as 50 years after onset of laryngeal paralysis.59 It is possible that some nerve sprouting occurs, resulting in a low-grade but functionless innervation that preserves the muscle’s ability to be reinnervated.60 Innervation of laryngeal muscles can also come from misdirected reinnervation from autonomic nerves.61
Restoration of Function
Regeneration is pointless unless the regenerating axons grow back to reinnervate the original targets. Motor axons preferentially reinnervate distal motor branches of a severed nerve. The specificity is gained primarily through pruning of misdirected fibers.62 Some evidence for a neurotrophic mechanism of directional nerve regrowth exists,63 but the usual outcome of nerve injury suggests the specificity is low. A major problem is that after division of a nerve, a functional specificity in motoneuron regeneration for the original muscle does not exist.64–66 The result, in a regenerating nerve supplying several muscles such as the RLN, is synkinesis.
Muscle fiber types can change under the influence of activation pattern67 and altered innervation.68 The changes following reinnervation with a different donor nerve may be related to a different pattern of activity imposed by the new nerve supply. Fiber type grouping is thought to be a result of this process.69 Mechanisms for directing the regenerating motor axons within a single muscle leading slow- and fast-twitch axons to the appropriate muscle fibers have some influence on reinnervation.70 The effect, however, is not enough to prevent changes in muscle fiber type. Reinnervation with a new donor nerve would be expected to change the fiber type composition of the reinnervated muscle and thus its contraction characteristics.
The Neuromuscular Pedicle
Reinnervation with the Neuromuscular Pedicle
Successful reinnervation with an NMP depends partly on the ability of the transplanted axons to reach receptive sites on the recipient muscle fibers and partly on the ability of the muscle fibers to accept innervation from foreign nerves. With partial denervation of the transferred nerve, the remaining intact motor neurons develop sprouts that provide reinnervation preferentially at the original end plate sites of the denervated muscle fibers.71,72 This phenomenon is important to successful transfer of the NMP, because a portion of the nerve fibers in an NMP may have an intact motor unit.73 Axon sprouting results in a larger number of motor fibers innervated by each nerve. Mature nerve terminals appear to be limited in functional expansion to three to five times the normal terminal field.74
Laboratory Investigations
In early experiments a block of muscle containing the terminal branches of the recurrent laryngeal nerve was removed and reimplanted into the larynx.73,75 A small portion of muscle was incorporated at the distal end of the transferred nerve to transfer intact nerve fibers. The short time to functional recovery (2 to 6 weeks) was thought to be secondary to the avoidance of degeneration and regeneration before effective reinnervation.
In 1973, reinnervation of the canine larynx with an NMP derived from the ansa cervicalis nerve and its insertion into the sternohyoid muscle was described.76 The technique was modified because of the unavailability of the RLN in most cases of laryngeal paralysis and the unsuitability of preserving a portion of the larynx in the presence of cancer.77 Return of function was seen at approximately 6 weeks after surgery. Reinnervation could be successful up to 6 months after denervation.78
Evidence for reinnervation with the NMP has included muscle response to pedicle stimulation, demonstration of neural tracer in the central motor nuclei of the donor nerve,79 and glycogen depletion in fiber of reinnervated muscles.80 Successful use of the NMP technique in animal models has been reported by multiple researchers.19,81–85 In their studies, electrical stimulation of the transferred nerve or induction of greater respiratory effort was required to produce gross motion.
In other studies, the presence of vocal fold movement under hypercarbic conditions was not associated with evidence of reinnervation by either direct stimulation86–88 or histologic evaluation.88 Other maneuvers, such as division of the inferior pharyngeal constrictor86 or of the superior laryngeal nerve or the strap muscles,87 were effective in eliminating the observed vocal fold motion. Postoperative scarring, which could stabilize the arytenoid and lateral tethering of the arytenoid to the thyroid cartilage, is a potential explanation for vocal fold motion not related to reinnervation.89
A modification of the NMP technique involves using a transferred NMP as a means of providing access to a denervated muscle for long-term electrical stimulation.90–92 A cuffed electrode can be placed around the nerve component of an implanted NMP. This should avoid deterioration at the electrode-muscle interface. Successful pacing was accomplished for the sternothyroid90 and PCA91 muscles with the use of tracheal motion with respiration as the triggering signal.
Indications
Bilateral Paralysis
The NMP technique can be considered for any patient with bilateral vocal cord paralysis that has persisted for 6 months59 to a year.93 In practice, however, only half of such patients are suitable candidates.94 Fixation or limitation of the cricoarytenoid joint is the most common contraindication, affecting approximately one third of patients.93 Direct laryngoscopy and palpation of the arytenoids prior to proceeding with the NMP procedure is recommended.
The ansa cervicalis nerve and its insertion into the appropriate strap muscle must be available for this technique. Any prior surgery or trauma that may have injured the ansa cervicalis should be carefully reviewed. Other causes of airway obstruction should be resolved before reinnervation.93
Central nervous system disease resulting in vocal cord paralysis is a relative contraindication. Tucker93 has reported only a 40% to 50% success rate in patients with such diseases, which may also disrupt the function of the ansa cervicalis nerve.
Finally, the laryngeal muscles must be able to accept reinnervation. Although successful reinnervation has been reported after 22 and 50 years of denervation, muscle atrophy probably plays a role in hampering the outcome. The incidence of cricoarytenoid fixation may increase with time95 but this finding has not been confirmed hstologically.96
Unilateral Paralysis
Consideration of reinnervation is usually delayed in patients with unilateral paralysis until enough time has passed to make spontaneous recovery unlikely. In patients who have difficulty with aspiration, vocal fold injection has been recommended. Prior injection of polytetrafluoroethylene (Teflon) into the vocal cord, however, has had a poor vocal result.97 The procedure has also been limited to patients who require above-average vocal quality.
Like patients with bilateral vocal cord paralysis who are being considered for an NMP procedure, the patient with unilateral paralysis must be able to tolerate the procedure, and the ansa cervicalis nerve must be intact, the selected adductor muscle must be in suitable condition for reinnervation, and there must be a mobile arytenoid muscle on the paralyzed side. Inability to visualize the larynx by normal endoscopic methods, which precludes transoral injection, is an additional indication for NMP reinnervation.97
Technique
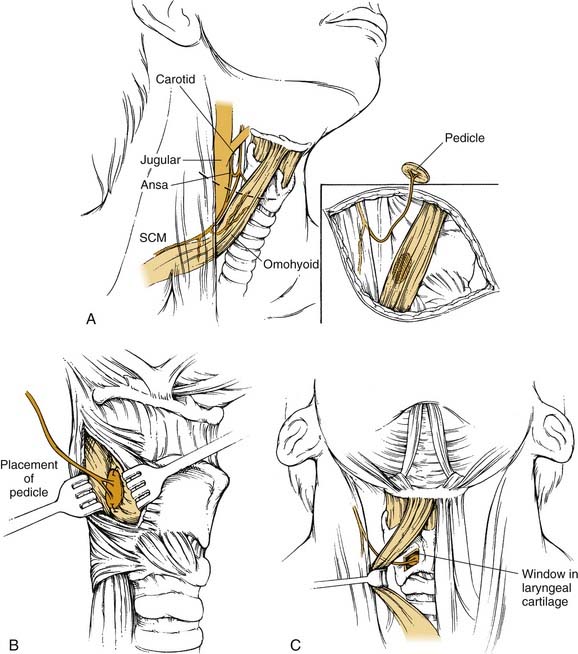
The description of the NMP procedure below is that first described by Tucker59with modifications by other writers as noted (Fig. 68-1).77,93,95,98
In Bilateral Paralysis
A horizontal incision is made near the lower border of the thyroid cartilage. The anterior border of the sternocleidomastoid muscle is exposed and retracted laterally. Dissection of the anterior belly of the omohyoid muscle and the fascia overlying the internal jugular vein is completed. The branch of the ansa cervicalis supplying the anterior belly of the omohyoid muscle can be identified in either of the following two ways: (1) by finding the main trunk of the ansa cervicalis as it crosses the internal jugular vein and tracing it proximally and distally until the appropriate branch is recognized or (2) by mobilizing the medial border of the omohyoid near its attachment to the hyoid and dissecting in a medial to lateral direction along the muscle. The nerve is usually accompanied by a small arterial vessel, which is preserved. If the nerve is injured, the branch to the sternothyroid is also acceptable.89,99
Unilateral Paralysis
The thyroid cartilage on the denervated side is exposed, and the lower half of the perichondrium of the thyroid cartilage is incised in the midline. A posteriorly based perichondrial flap exposing the lower half of the thyroid ala is created. A block of thyroid cartilage is removed from this area, leaving the underlying perichondrium and the inferior margin of the thyroid cartilage intact. The inner perichondrium is incised to expose the denervated LCA muscle, and the NMP is sutured in place with two or three sutures of 5-0 nylon. The outer perichondrial flap may be sutured back in place, and the wound is closed in layers with a drain. One modification consists of placement of two pedicles (each from a separate strap muscle) into the recipient muscle.100
Intravenous dexamethasone has been given to reduce laryngeal edema in the early recovery period after NMP in unilateral vocal paralysis.99 Voice rest and postoperative antibiotics are not necessary.
Reported Results
In 1976, Tucker77 reported using the NMP in humans for bilateral vocal fold paralysis. All five patients he studied demonstrated vocal cord abduction with inspiration within 6 to 8 weeks after the procedure. In two patients with a marked improvement in exercise tolerance, vocal cord motion was not seen without an increased respiratory effort. In 1978, Tucker59 reviewed 45 cases of NMP laryngeal reinnervation for bilateral vocal cord paralysis, reporting an 88.8% (40/45) success rate. Duration of follow-up ranged from 3 months to 4 years, and duration of paralysis ranged from 6 months to 50 years. The time to return of function varied from 6 to 12 weeks.
In 1982, Tucker93 divided results of NMP reinnervation for bilateral vocal cord paralysis into three categories. In approximately 40% of his cases, airway improvement was accompanied by visible motion of the vocal cord with inspiratory effort; 20% of patients had no airway improvement. The remaining patients had airway improvement but little vocal cord motion. Most of the patients in this last group demonstrated vocal cord motion or tonic abduction only with increased respiratory demand. A second NMP procedure to the opposite PCA muscle has been performed successfully in patients in whom the first procedure had an inadequate result.93
A review of 214 patients with bilateral vocal cord paralysis demonstrated a 74% long-term success rate.94 In 48% of patients, tracheotomy was performed at the time of reinnervation. Success was defined as decannulation with improved airway that did not limit regular daily activities or exacerbate acute upper respiratory disease. Minimum follow-up was 2 years. Six-month results showed an 89% success rate, but 31 patients had subsequent deterioration of the airway. In a majority of patients with late failure who were evaluated, fixation of the cricoarytenoid joint was identified.
In 1977, Tucker reported the use of the NMP procedure in 9 patients with unilateral vocal cord paralysis.98 Recovery of adductive function was seen 2 to 12 weeks after surgery in all of the patients. Only 6 patients had a satisfactory improvement in vocal quality, and a subsequent polytetrafluoroethylene injection was required in the other 3. In a subsequent report on the use of the NMP in unilateral vocal cord paralysis, improvement was found in 25 of 27 patients undergoing a NMP procedure to the LCA.99
In a 1989 study, NMP procedures for unilateral paralysis were reported to have a success rate of 88%.94 Success was established by voice improvement, vocal cord adduction, and change in cord tension with elevated pitch. Voice results were noted to improve for several months and were aided by the addition of speech therapy.
May and colleagues101 reported their results with the NMP procedure in 1980. Of 8 patients undergoing NMP reinnervation of the PCA for airway obstruction only 3 experienced improvement; 13 of 15 patients with disordered phonation and/or deglutition were improved by NMP reinnervation of the LCA. Applebaum and associates100 reported successful results in all 6 of their patients undergoing the NMP technique. Four of the patients had bilateral vocal cord paralysis, with 1 requiring a tracheostomy. Dyspnea and stridor were relieved in all 4 patients, in whom vocal cord movement was seen only after physical exertion.
For isolated unilateral recurrent laryngeal paralysis, May and Beery97 reported improvement in the voice in 19 of 20 (95%) patients treated with NMP implantation into the LCA muscle. Observable movement of the reinnervated cord was seen in only 1 patient, suggesting that enhanced muscle tone or a slight change in position was responsible for voice improvement. The degree of voice improvement was substantially less in patients with multiple cranial nerve deficits or vocal cord fixation.
Complications
The main complication of the NMP technique is failure to obtain a satisfactory result. Many of the patient selection factors listed previously as contraindications are a result of these experiences. Relatively few operative complications associated with the NMP procedure are described. In 214 patients undergoing an NMP procedure for bilateral vocal cord paralysis, Tucker99 noted only four complications. Three were wound infections, and the fourth was related to the previously placed tracheotomy. No complications were reported in 73 patients undergoing NMP for unilateral paralysis. No operative complications were reported in other series.97,100
Future of the Neuromuscular Pedicle Procedure
The NMP can provide a mechanism to access a denervated muscle for long-term pacing.90,91 With electrical pacing, the options for a potential triggering source and the stimulus for muscle contraction are increased, providing a potentially more effective treatment of airway impairment secondary to bilateral vocal fold paralysis.
Ansa Cervicalis–Recurrent Laryngeal Nerve Anastomosis
The current use of the ansa-RLN anastomosis is in unilateral vocal fold paralysis. No motion of the reinnervated vocal fold with respiration or phonation is expected. A variation of this technique involves a hypoglossal nerve–RLN anastomosis.102,103 Use of the ansa cervicalis as a donor nerve has gained the widest acceptance because of the lower morbidity associated with the sacrifice of this nerve.
Advantages
The sacrifice of the sternothyroid muscle is clinically insignificant and may theoretically improve vocal function. The resting tone of the sternothyroid muscle slightly lateralizes the normal vocal cord.56 Loss of this tone may result in a slight medialization of the paralyzed vocal cord and an improvement in vocal quality early after surgery.60
Disadvantage
The ansa-RLN transfer procedure requires a deeper level of dissection in the neck and typically takes longer to perform than thyroplasty or vocal fold injection. The time for completion of the ansa-RLN is estimated at 2 hours.104 Although thyroplasty is typically performed with the patient under sedation and vocal fold injection can be performed in the clinic, ansa-RLN anastomosis is performed under general anesthesia.
Surgical Technique
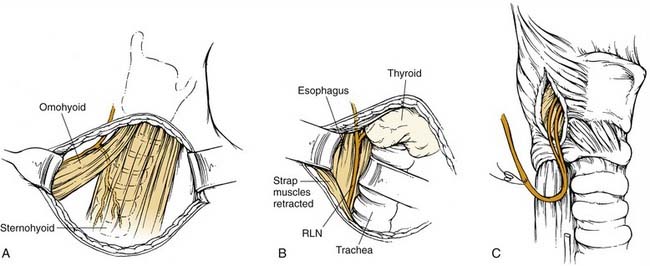
An incision in a natural crease of the neck is made below the level of the cricoid cartilage, often at the level of a thyroidectomy incision (Fig. 68-2). The incision need not cross the midline, and 4 cm is usually adequate. The strap muscles are separated vertically in the midline, and the RLN is identified in the tracheoesophageal groove. The nerve is dissected to its entrance into the larynx. In the presence of local scar tissue, the RLN can be found behind the cricothyroid joint. A vessel loop can be placed around the nerve. The anterior border of the sternocleidomastoid muscle is identified. The ansa cervicalis nerve can be identified along the lateral border of the sternothyroid muscle, at the level of the omohyoid muscle, or along the internal jugular vein.
Clinical Results
Ansa-RLN anastomosis has produced near-normal vocal quality in most reports. The initial two reports by Crumley and colleagues27,105 described success in two patients and four patients. In one of these patients the contralateral ansa cervicalis nerve served as the donor nerve. No motion of the reinnervated vocal fold was seen in reinnervated patients. The vocal improvement was believed to be secondary to precise positioning of the reinnervated vocal fold. Reinnervation of the of PCA, LCA, and TA muscles appears to provide the needed tone to stabilize and position the arytenoid properly.51
A series of 20 patients undergoing ansa-RLN anastomosis was reviewed by Crumley60 in 1991. Vocal quality was judged to be normal or near normal in 18 of 20 cases. In the one failure, identification of the RLN was in doubt secondary to local scar formation from multiple prior procedures. A second patient demonstrated improvement but to a lesser extent. Lateralization of the arytenoid and the patient’s age (65 years) were cited as possible factors contributing to the lesser result. Several patients in whom the delay between injury and reinnervation exceeded 8 years had successful results.
Ansa-RLN anastomosis was successful in two elderly patients (66 and 71 years old) following resection of mediastinal tumors with early reinnervation.106 Zheng and coworkers107 presented eight cases with one disappointing result. They used an anterior branch of the RLN. Slight improvement was seen early after surgery, with additional improvement seen at 2 to 3 months. Electromyographic confirmation was obtained in five patients. Passive arytenoid mobility was important in achieving good results and was related to the one substandard result.
Similar results in terms of mean phonation time and vocal fold position are seen after a direct anastomosis of the RLN, a free nerve graft reconstructing the RLN, a vagus nerve–RLN anastomosis, and an ansa-RLN anastomosis performed during the course of thyroid surgery.108 The ansa-RLN anastomosis has several advantages over other means of reconstruction of a divided RLN during the course of thyroid or other neck surgery. These include the requirement of only one anastomosis performed at a relatively accessible level in the neck, a relatively short time to recovery because the anastomosis is close to the larynx, and availability when the RLN is injured within the mediastinum. Performing the anastomosis behind the thyroid cartilage108 as well as using the contralateral ansa cervicalis as a donor nerve109 improves the chances of successful reconstruction.
Olsen and colleagues110 performed a quantitative analysis of 12 patients with ansa-RLN anastomosis for unilateral vocal fold paralysis. Improvement in vocal quality was demonstrated. Results of acoustic analyses in postoperative patients were similar to those in a control group of normal subjects. Patients with isolated vocal fold paralysis had the best results. Improvement in overall severity, roughness, and breathiness of the patient’s voice more than 6 months following ansa-RLN anastomosis has also been demonstrated.111 The greatest improvement was seen with breathiness measures. Postoperative electromyographic studies of patients undergoing reinnervation with an ansa-RLN anastomosis as well as NMP implantation into the TA have shown activation of the reinnervated muscle with speech and head elevation.112 Head elevation maneuvers that directly activate the strap muscles were routinely found to result in greater activation of the TA and LCA muscles. Activation of the TA muscles with speech was found to increase with time.
Chhetri and colleagues113 reported that the addition of ansa-RLN anastomosis to arytenoid adduction did not result in better acoustic results than arytenoid adduction alone. All patients in their series had significant perceptual improvement in voice, but these investigators questioned the relationship of surgical repositioning of the arytenoid and reinnervation in unilateral paralysis.
Direct Nerve Implant
Early in the 20th century, direct implantation of a nerve into a denervated muscle was shown to produce successful reinnervation in experimental models.114–116 Insertion of a nerve ending into skeletal muscle results in the establishment of neuromuscular junctions.117,118 Using multiple implants distributed over a wide area increases the effectiveness of reinnervation with the direct nerve implantation technique.119 Direct nerve implants have been successfully used in 10 patients, with active muscle movement occurring in all 10 patients.120 A short period of denervation, a healthy donor nerve, and a wide distribution of nerve fibers entering the muscle had a positive effect on the outcome.
Early experiments investigating direct nerve implantation also examined the value of the technique in laryngeal reinnervation.121,122 Direct implantation of the RLN into the PCA muscle was successfully performed in the canine and proposed as a promising method for reinnervation.4,123
Comparisons of the NMP procedure with direct implantation of the ansa cervicalis nerve into denervated muscle have shown mixed results. Meikle and associates82 showed no advantage of one method over the other, whereas Hall and colleagues83 found the NMP procedure to produce stronger evoked contractions and to involve a shorter time to return of function. Both groups judged that their results were consistent with the action of the NMP as a source for multiple direct nerve implants rather than a predominant reliance on transfer of intact motor units. Because of the need to support a distal muscle pedicle, the NMP procedure was inferior to the direct nerve implantation when the reinnervation procedure was extended with a cable graft.84
Direct nerve implantation of the TA muscle was performed in ten patients with unilateral paralysis.124 Improvement of voice was seen in eight, with a return to “normal” or “great improvement” in 6 patients. Reinnervation was supported by electromyography in all four patients tested.
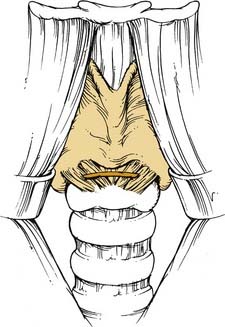
A variant of this technique, in which a nerve acts as a conduit from an innervated muscle to the contralateral, denervated muscle (muscle-nerve-muscle), has been successfully used.125 Selective cricothyroid reinnervation with a muscle-nerve-muscle graft from the contralateral cricothyroid was used in combination with ansa-RLN anastomosis in three patients (Fig. 68-3). All patients had improved voice and electromyographic evidence of cricothyroid muscle reinnervation.
Chhetri DK, Gerratt BR, Kreiman J, et al. Combined arytenoid adduction and laryngeal reinnervation in the treatment of vocal fold paralysis. Laryngoscope. 1999;109:1928-1936.
Crumley RL, Izdebski K, McMicken B. Nerve transfer versus Teflon injection for vocal cord paralysis: a comparison. Laryngoscope. 1988;98:1200-1204.
Crumley RL. Update: ansa cervicalis to recurrent laryngeal nerve anastomosis for unilateral laryngeal paralysis. Laryngoscope. 1991;101:384-388.
Crumley RL, Izdebski K. Voice quality following laryngeal reinnervation by ansa hypoglossi transfer. Laryngoscope. 1986;96:611-616.
El-Kashlan HK, Carroll WR, Hogikyan ND, et al. Selective cricothyroid muscle reinnervation by muscle-nerve-muscle neurotization. Arch Otolaryngol Head Neck Surg. 2001;127:1211-1215.
Lee WT, Milstein C, Hicks D, et al. Results of ansa to recurrent laryngeal nerve reinnervation. Otolaryngol Head Neck Surg. 2007;136:450-454.
Maronian N, Waugh P, Robinson L, et al. Electromyographic findings in recurrent laryngeal nerve reinnervation. Ann Otol Rhinol Laryngol. 2003;112:314-323.
Miyauchi A, Matsusaka K, Kawaguchi H, et al. Ansa-recurrent nerve anastomosis for vocal cord paralysis due to mediastinal lesions. Ann Thorac Surg. 1994;57:1020-1021.
Miyauchi A, Matsusaka K, Kihara M, et al. The role of ansa-to-recurrent-laryngeal nerve anastomosis in operations for thyroid cancer. Eur J Surg. 1998;164:927-933.
Miyauchi A, Yokozawa T, Kobayashi K, et al. Opposite ansa cervicalis to recurrent laryngeal nerve anastomosis to restore phonation in patients with advanced thyroid cancer. Eur J Surg. 2001;167:540-541.
Olson DE, Goding GS, Michael DD. Acoustic and perceptual evaluation of laryngeal reinnervation by ansa cervicalis transfer. Laryngoscope. 1998;108:1767-1772.
Su WF, Hsu YD, Chen HC, et al. Laryngeal reinnervation by ansa cervicalis nerve implantation for unilateral vocal cord paralysis in humans. J Am Coll Surg. 2007;204:64-72.
Tucker HM. Reinnervation of the paralyzed larynx: a review. Head Neck Surg. 1979;1:235-242.
Tucker HM. Long-term results of nerve-muscle pedicle reinnervation for laryngeal paralysis. Ann Otol Rhinol Laryngol. 1989;98:674-676.
Zheng H, Li Z, Zhou S, et al. Update: laryngeal reinnervation for unilateral vocal cord paralysis with the ansa cervicalis. Laryngoscope. 1996;106:1522-1527.
1. Horsley JA. Suture of the recurrent laryngeal nerve with report of a case. Trans South Surg Gynecol Assoc. 1909;22:161-170.
2. Lahey FH. Successful suture of recurrent laryngeal nerve for bilateral abductor paralysis, with restoration of function. Ann Surg. 1928;87:481-484.
3. McCall JW, Hoerr NL. Reinnervation of a paralyzed vocal cord. Laryngoscope. 1946;56:527.
4. Doyle PJ, Brummett RE, Everts EC. Results of surgical section and repair of the recurrent laryngeal nerve. Laryngoscope. 1967;77:1245-1254.
5. Kelly JD. Surgical treatment of bilateral paralysis of the abductor muscles. Arch Otolaryngol. 1941;33:293-304.
6. Edwards TM. Progress in the surgical treatment of bilateral laryngeal paralysis. Ann Otol Rhinol Laryngol. 1952;61:159-178.
7. Siribodhi C, Sundmaker W, Atkins JP. Electromyographic studies of laryngeal paralysis and regeneration of laryngeal motor nerve in dogs. Laryngoscope. 1963;73:148-164.
8. Hiroto I, Hirano M, Tomita H. Electromyographic investigations of human vocal cord paralysis. Ann Otol Rhinol Laryngol. 1968;77:296-304.
9. Crumley RL. Laryngeal synkinesis: its significance to the laryngologist. Ann Otol Rhinol Laryngol. 1989;98:87-92.
10. Brondbo K, Dahl HA, Teig E, et al. The human posterior cricoarytenoid (PCA) muscle and diaphragm: a histochemical comparison as a basis for reinnervation attempts. Acta Otolaryngol. 1986;102:474-481.
11. Iwaurma S. Functioning remobilization of the paralyzed vocal cord in dogs. Arch Otolaryngol. 1974;100:122-129.
12. Brondbo K, Hall C, Teig E, et al. Functional results after experimental reinnervation of the posterior cricoarytenoid muscle in dogs. J Otolaryngol. 1986;15:259-264.
13. Baldissera F, Cantarella G, Marini G, et al. Recovery of inspiratory abduction of the paralyzed vocal cords after bilateral reinnervation of the cricoarytenoid muscles by one single branch of the phrenic nerve. Laryngoscope. 1989;99:1286-1292.
14. Baldissera F, Tredici G, Marini G, et al. Innervation of the paralyzed laryngeal muscles by phrenic motoneurons: a quantitative study by light and electron microscopy. Laryngoscope. 1992;102:907-916.
15. van Lith-Bijl JT, Stolk RJ, Tonnaer JA, et al. Selective laryngeal reinnervation with separate phrenic and ansa cervicalis nerve transfers. Arch Otolaryngol Head Neck Surg. 1997;123:406-411.
16. van Lith-Bijl JT, Stolk RJ, Tonnaer JA, et al. Laryngeal abductor reinnervation with a phrenic nerve transfer after a 9-month delay. Arch Otolaryngol Head Neck Surg. 1998;124:393-398.
17. Crumley RL. Phrenic nerve graft for bilateral vocal cord paralysis. Laryngoscope. 1983;93:425-428.
18. Gacek RR. Morphologic correlates for laryngeal reinnervation. Laryngoscope. 2001;111:1871-1877.
19. Chang SY. Studies of early laryngeal reinnervation. Laryngoscope. 1985;95:455-457.
20. Sercarz JA, Nguyen L, Nasri S, et al. Physiologic motion after laryngeal nerve reinnervation: a new method. Otolaryngol Head Neck Surg. 1997;116:466-474.
21. van Lith-Bijl JT, Mahieu HF. Reinnervation aspects of laryngeal transplantation. Eur Arch Otorhinolaryngol. 1998;255:515-520.
22. Brondbo K, Hall C, Teig E, et al. Experimental laryngeal reinnervation by phrenic nerve implantation into the posterior cricoarytenoid muscle. Acta Otolaryngol. 1987;103:339-344.
23. Jacobs IN, Sanders I, Wu BL, et al. Reinnervation of the canine posterior cricoarytenoid muscle with sympathetic preganglionic neurons. Ann Otol Rhinol Laryngol. 1990;99:167-174.
24. Tucker HM. Combined laryngeal framework medialization and reinnervation for unilateral vocal fold paralysis. Ann Otol Rhinol Laryngol. 1990;99:778-781.
25. Frazier CH. Anastomosis of the recurrent laryngeal nerve with the descendens noni. JAMA. 1924;83:1637-1641.
26. Frazier CH, Mosser WB. Treatment of recurrent laryngeal nerve paralysis by nerve anastomosis. Surg Gynecol Obstet. 1926;43:134-139.
27. Crumley RL, Izdebski K. Voice quality following laryngeal reinnervation by ansa hypoglossi transfer. Laryngoscope. 1986;96:611-616.
28. Talmadge RJ. Myosin heavy chain isoform expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle Nerve. 2000;23:661-679.
29. Mu L, Sanders I. Neuromuscular specializations within human pharyngeal constrictor muscles. Ann Otol Rhinol Laryngol. 2007;116:604-617.
30. Hayashi M, Isozaki E, Oda M, et al. Loss of large myelinated nerve fibres of the recurrent laryngeal nerve in patients with multiple system atrophy and vocal cord palsy. J Neurol Neurosurg Psychiatry. 1997;62:234-238.
31. Gacek RR, Malmgren LT, Lyon MJ. Localization of adductor and abductor motor fibers to the larynx. Ann Otol Rhinol Laryngol. 1977;86:770-776.
32. Hast MH. The respiratory muscles of the larynx. Ann Otol Rhinol Laryngol. 1967;76:489-497.
33. Hirose H, Ushijima T, Kobayashi T, et al. An experimental study of the contraction properties of the laryngeal muscles in the cat. Ann Otol Rhinol Laryngol. 1969;78:297-306.
34. Sanders I, Wu BL, Mu L, et al. The innervation of the human larynx. Arch Otolaryngol Head Neck Surg. 1993;119:934-939.
35. Chhetri DK, Berke GS. Ansa cervicalis nerve: review of the topographic anatomy and morphology. Laryngoscope. 1997;107:1366-1372.
36. Staron RS. Human skeletal muscle fiber types: delineation, development, and distribution. Can J Appl Physiol. 1997;22:307-327.
37. Shiotani A, Westra WH, Flint PW. Myosin heavy chain composition in human laryngeal muscles. Laryngoscope. 1999;109:1521-1524.
38. Wu YZ, Crumley RL, Armstrong WB, et al. New perspectives about human laryngeal muscle: single-fiber analyses and interspecies comparisons. Arch Otolaryngol Head Neck Surg. 2000;126:857-864.
39. Sciote JJ, Morris TJ, Brandon CA, et al. Unloaded shortening velocity and myosin heavy chain variations in human laryngeal muscle fibers. Ann Otol Rhinol Laryngol. 2002;111:120-127.
40. Shiotani A, Flint PW. Myosin heavy chain composition in rat laryngeal muscles after denervation. Laryngoscope. 1998;108:1225-1229.
41. Green JG, Neil E. The respiratory function of the laryngeal muscles. J Physiol (Lond). 1955;129:134-141.
42. Goding GSJr, Bierbaum RW. Relationship of the posterior cricoarytenoid muscle to the posterior cricoid lamina. Otolaryngol Head Neck Surg. 1999;120:493-498.
43. Sanders I, Wu BL, Mu L, et al. The innervation of the human posterior cricoarytenoid muscle: evidence for at least two neuromuscular compartments. Laryngoscope. 1994;104:880-884.
44. Malmgren LT, Gacek RR. Histochemical characteristics of muscle fiber types in the posterior cricoarytenoid muscle. Ann Otol Rhinol Laryngol. 1981;90:423-429.
45. Hast MH. The primate larynx: a comparative physiological study of intrinsic muscles. Acta Otolaryngol. 1969;67:84-92.
46. Gambino DR, Malmgren LT, Gacek RR. Three-dimensional computer reconstruction of the neuromuscular junction distribution in the human posterior cricoarytenoid muscle. Laryngoscope. 1985;95:556-560.
47. Sahgal V, Hast MH. Histochemistry of primate laryngeal muscles. Acta Otolaryngol. 1974;78:277-281.
48. Guida HL, Zorzetto NL. Morphometric and histochemical study of the human vocal muscle. Ann Otol Rhinol Laryngol. 2000;109:67-71.
49. De Vito MA, Malmgren LT, Gacek RR. Three-dimensional distribution of neuromuscular junctions in human cricothyroid. Arch Otolaryngol. 1985;111:110-113.
50. Rosen M, Malmgren LT, Gacek RR. Three-dimensional computer reconstruction of the distribution of neuromuscular junctions in the thyroarytenoid muscle. Ann Otol Rhinol Laryngol. 1983;92:424-429.
51. Crumley RL. Nerve transfer technique as it relates to phonatory surgery. In: Cummings CW, Fredrickson JM, Harker LA, et al, editors. Otolaryngology–Head and Neck Surgery: Update II. St. Louis: Mosby–Year Book; 1990:100-106.
52. Hast MH. Studies on the extrinsic laryngeal muscles. Arch Otolaryngol. 1968;88:273-278.
53. Hisa Y, Malmgren LT. Muscle fiber types in the human sternothyroid muscle: a correlated histochemical and ultrastructural morphometric study. In: Baer T, Sasaki C, Harris K, editors. Laryngeal Function in Phonation and Respiration. Boston: College-Hill; 1987:29-44.
54. Hisa Y, Malmgren LT, Lyon MJ. Quantitative histochemical studies on the cat infrahyoid muscles. Otolaryngol Head Neck Surg. 1990;103:723-732.
55. Ellenbogen BG, Gerber TG, Coon RL, et al. Accessory muscle activity and respiration. Otolaryngol Head Neck Surg. 1981;89:370-375.
56. Fink BR. Folding mechanism of the human larynx. Acta Otolaryngol. 1974;78:124-128.
57. Ochs S. Sensation and neuromuscular transmission. In: Selkurt EE, editor. Physiology. Boston: Little, Brown; 1984:46-61.
58. Sahgal V, Hast MH. Effect of denervation on primate laryngeal muscles: a morphologic and morphometric study. J Laryngol Otol. 1986;100:553-560.
59. Tucker HM. Human laryngeal reinnervation: long-term experience with the nerve-muscle pedicle technique. Laryngoscope. 1978;88:598-604.
60. Crumley RL. Update: ansa cervicalis to recurrent laryngeal nerve anastomosis for unilateral laryngeal paralysis. Laryngoscope. 1991;101:384-388.
61. Nomoto M, Yoshihara T, Kanda T, et al. Misdirected reinnervation in the feline intrinsic laryngeal muscles after long-term denervation. Acta Otolaryngol Suppl. 1993;506:71-74.
62. Brushart TM. Motor axons preferentially reinnervate motor pathways. J Neurosci. 1993;13:2730-2738.
63. Kuffler DP. Regeneration of muscle axons in the frog is directed by diffusible factors from denervated muscle and nerve tubes. J Comp Neurol. 1989;281:416-425.
64. Thomas CK, Stein RB, Gordon T, et al. Patterns of reinnervation and motor unit recruitment in human hand muscles after complete ulnar and median nerve section and resuture. J Neurol Neurosurg Psychiatry. 1987;50:259-268.
65. Bodine-Fowler SC, Meyer RS, Moskovitz A, et al. Inaccurate projection of rat soleus motoneurons: a comparison of nerve repair techniques. Muscle Nerve. 1997;20:29-37.
66. Brushart TM, Mesulam MM. Alteration in connections between muscle and anterior horn motoneurons after peripheral nerve repair. Science. 1980;208:603-605.
67. Kernell D, Wang LC. Simple methods for quantifying the spatial distribution of different categories of motoneuronal nerve endings, using measurements of muscle regionalization. J Neurosci Methods. 2000;100:79-83.
68. Romanul FC, Van der Meulen JP. Reversal of the enzyme profiles of muscle fibres in fast and slow muscles by cross-innervation. Nature. 1966;212:1369-1370.
69. Kugelberg E, Edstrom L, Abbruzzese M. Mapping of motor units in experimentally reinnervated rat muscle: interpretation of histochemical and atrophic fibre patterns in neurogenic lesions. J Neurol Neurosurg Psychiatry. 1970;33:319-329.
70. Wang L, Copray S, Brouwer N, et al. Regional distribution of slow-twitch muscle fibers after reinnervation in adult rat hindlimb muscles. Muscle Nerve. 2002;25:805-815.
71. Brown MC, Ironton R. Sprouting and regression of neuromuscular synapses in partially denervated mammalian muscles. J Physiol (Lond). 1978;278:325-348.
72. Slack JR, Hopkins WG. Neuromuscular transmission at terminals of sprouted mammalian motor neurons. Brain Res. 1982;237:121-135.
73. Tucker HM, Ogura JH. Vocal cord remobilization in the canine larynx: an histologic evaluation. Laryngoscope. 1971;81:1602-1606.
74. Rochel S, Robbins N. Effect of partial denervation and terminal field expansion on neuromuscular transmitter release and nerve terminal structure. J Neurosci. 1988;8:332-338.
75. Tucker HM, Harvey J, Ogura JH. Vocal cord remobilization in the canine larynx. Arch Otolaryngol. 1970;92:530-533.
76. Hengerer AS, Tucker HM. Restoration of abduction in the paralyzed canine vocal cord. Arch Otolaryngol. 1973;97:247-250.
77. Tucker HM. Human laryngeal reinnervation. Laryngoscope. 1976;86:769-779.
78. Lyons RM, Tucker HM. Delayed restoration of abduction in the paralyzed canine larynx. Arch Otolaryngol. 1974;100:176-179.
79. Anonsen CK, Patterson HC, Trachy RE, et al. Reinnervation of skeletal muscle with a neuromuscular pedicle. Otolaryngol Head Neck Surg. 1985;93:48-57.
80. Fata JJ, Malmgren LT, Gacek RR, et al. Histochemical study of posterior cricoarytenoid muscle reinnervation by a nerve-muscle pedicle in the cat. Ann Otol Rhinol Laryngol. 1987;96:479-487.
81. Sato F, Ogura JH. Functional restoration for recurrent laryngeal paralysis: an experimental study. Laryngoscope. 1978;88:855-871.
82. Meikle D, Trachy RE, Cummings CW. Reinnervation of skeletal muscle: a comparison of nerve implantation with neuromuscular pedicle transfer in an animal model. Ann Otol Rhinol Laryngol. 1987;96:152-157.
83. Hall SJ, Trachy RE, Cummings CW. Facial muscle reinnervation: a comparison of neuromuscular pedicle with direct nerve implant. Ann Otol Rhinol Laryngol. 1988;97:229-233.
84. Goding GS, Cummings CW, Bright DA. Extension of neuromuscular pedicles and direct nerve implants in the rabbit. Arch Otolaryngol. 1989;115:217-223.
85. Toth A, Szucs A, Harasztosi C, et al. Intrinsic laryngeal muscle reinnervation with nerve-muscle pedicle. Otolaryngol Head Neck Surg. 2005;132:701-706.
86. Neal GD, Cummings CW, Sutton D. Delayed reinnervation of unilateral vocal cord paralysis in dogs. Otolaryngol Head Neck Surg. 1981;89:608-612.
87. Crumley RL. Experiments in laryngeal reinnervation. Laryngoscope. 1982;92:1-27.
88. Rice DH, Owens O, Burstein F. The nerve-muscle pedicle. Arch Otolaryngol. 1983;109:233-234.
89. Crumley RL. Update of laryngeal reinnervation concepts and options. In: Bailey BJ, Biller HF, editors. Surgery of the Larynx. Philadelphia: W.B. Saunders Co; 1985:135-147.
90. Broniatowski M, Tucker HM, Jacobs G, et al. Laryngeal pacemaker part 1: electronic pacing of reinnervated strap muscles in the dog. Otolaryngol Head Neck Surg. 1986;94:41-44.
91. Broniatowski M, Kaneko S, Jacobs G, et al. Laryngeal pacemaker II: electronic pacing of reinnervated posterior cricoarytenoid muscles in the canine. Laryngoscope. 1985;95:1194-1198.
92. Broniatowski M, Dessoffy R, Strome M. Long-term excitability and fine tuning of nerve pedicles reinnervating strap muscles in the dog. Ann Otol Rhinol Laryngol. 1998;107:301-311.
93. Tucker HM. Nerve-muscle pedicle reinnervation of the larynx: avoiding pitfalls and complications. Ann Otol Rhinol Laryngol. 1982;91:440-444.
94. Tucker HM. Long-term results of nerve-muscle pedicle reinnervation for laryngeal paralysis. Ann Otol Rhinol Laryngol. 1989;98:674-676.
95. Tucker HM. Reinnervation of the paralyzed larynx: a review. Head Neck Surg. 1979;1:235-242.
96. Gacek M, Gacek RR. Cricoarytenoid joint mobility after chronic vocal cord paralysis. Laryngoscope. 1996;106:1528-1530.
97. May M, Beery Q. Muscle-nerve pedicle laryngeal reinnervation. Laryngoscope. 1986;96:1196-1200.
98. Tucker HM. Reinnervation of the unilaterally paralyzed larynx. Ann Otol Rhinol Laryngol. 1977;86:789-794.
99. Tucker HM, Rusnov M. Laryngeal reinnervation for unilateral vocal cord paralysis: long-term results. Ann Otol Rhinol Laryngol. 1981;90:457-459.
100. Applebaum EL, Allen GW, Sisson GA. Human laryngeal reinnervation: the Northwestern experience. Laryngoscope. 1979;89:1784-1787.
101. May M, Lavorato AS, Bleyaert AL. Rehabilitation of the crippled larynx: application of the Tucker technique for muscle-nerve reinnervation. Laryngoscope. 1980;90:1-18.
102. Paniello RC, Lee P, Dahm JD. Hypoglossal nerve transfer for laryngeal reinnervation: a preliminary study. Ann Otol Rhinol Laryngol. 1999;108:239-244.
103. Paniello RC, West SE. Laryngeal adductory pressure as a measure of post-reinnervation synkinesis. Ann Otol Rhinol Laryngol. 2000;109:447-451.
104. Crumley RL. Teflon versus thyroplasty versus nerve transfer: a comparison. Ann Otol Rhinol Laryngol. 1990;99:759-763.
105. Crumley RL, Izdebski K, McMicken B. Nerve transfer versus Teflon injection for vocal cord paralysis: a comparison. Laryngoscope. 1988;98:1200-1204.
106. Miyauchi A, Matsusaka K, Kawaguchi H, et al. Ansa-recurrent nerve anastomosis for vocal cord paralysis due to mediastinal lesions. Ann Thorac Surg. 1994;57:1020-1021.
107. Zheng H, Li Z, Zhou S, et al. Update: laryngeal reinnervation for unilateral vocal cord paralysis with the ansa cervicalis. Laryngoscope. 1996;106:1522-1527.
108. Miyauchi A, Matsusaka K, Kihara M, et al. The role of ansa-to-recurrent-laryngeal nerve anastomosis in operations for thyroid cancer. Eur J Surg. 1998;164:927-933.
109. Miyauchi A, Yokozawa T, Kobayashi K, et al. Opposite ansa cervicalis to recurrent laryngeal nerve anastomosis to restore phonation in patients with advanced thyroid cancer. Eur J Surg. 2001;167:540-541.
110. Olson DE, Goding GS, Michael DD. Acoustic and perceptual evaluation of laryngeal reinnervation by ansa cervicalis transfer. Laryngoscope. 1998;108:1767-1772.
111. Lee WT, Milstein C, Hicks D, et al. Results of ansa to recurrent laryngeal nerve reinnervation. Otolaryngol Head Neck Surg. 2007;136:450-454.
112. Maronian N, Waugh P, Robinson L, et al. Electromyographic findings in recurrent laryngeal nerve reinnervation. Ann Otol Rhinol Laryngol. 2003;112:314-323.
113. Chhetri DK, Gerratt BR, Kreiman J, et al. Combined arytenoid adduction and laryngeal reinnervation in the treatment of vocal fold paralysis. Laryngoscope. 1999;109:1928-1936.
114. Erlacher P. Direct and muscular neurotization of paralyzed muscles. Am J Orthop Surg. 1915;13:22-32.
115. Heineke H. Die direkte Einpflanzung der Nerven in den Muskel. Zentralbl Chir. 1914;41:465-466.
116. Steindler A. Direct neurotization of paralyzed muscles: further studies of the question of direct implantation. Am J Orthop Surg. 1916;14:707-719.
117. Park DM, Shon SK, Kim YJ. Direct muscle neurotization in rat soleus muscle. J Reconstr Microsurg. 2000;16:135-140.
118. Saito A, Zacks SI. Fine structure of neuromuscular junction after nerve section and implantation of nerve in denervated muscle. Exp Mol Pathol. 1969;10:256-273.
119. Brunelli GA, Brunelli GR. Direct muscle neurotization. J Reconstr Microsurg. 1993;9:81-90.
120. Becker M, Lassner F, Fansa H, et al. Refinements in nerve to muscle neurotization. Muscle Nerve. 2002;26:362-366.
121. Hoessly H. Uber Nervenimplantation bei Recurrenslahmungen eine experimentelle Studie. Beitr Klin Chir. 1916;99:186-192.
122. Nikolajew NA. Zur frage de Implantation von Nerven in Muskeln (Nervenuberpflanzung auf Kehlkopfmuskeln). Monatsschr Ohrenheilkd. 1927;61:1005-1031.
123. Doyle PJ, Everts EC, Brummett RE. Treatment of recurrent laryngeal nerve injury. Arch Surg. 1968;96:517-520.
124. Su WF, Hsu YD, Chen HC, et al. Laryngeal reinnervation by ansa cervicalis nerve implantation for unilateral vocal cord paralysis in humans. J Am Coll Surg. 2007;204:64-72.
125. El-Kashlan HK, Carroll WR, Hogikyan ND, et al. Selective cricothyroid muscle reinnervation by muscle-nerve-muscle neurotization. Arch Otolaryngol Head Neck Surg. 2001;127:1211-1215.