CHAPTER 21 Large to Massive Rotator Cuff Tears
PREOPERATIVE CONSIDERATIONS
During the past 10 to 15 years, there has been a dramatic evolution in the arthroscopic treatment of massive rotator cuff tears. In the past, many massive rotator cuff tears (more than 5 cm in greatest dimension, as defined by DeOrio and Cofield1) were regarded as irreparable. Today, we have gone from being unable to repair any rotator cuff tears arthroscopically to being able to repair almost all rotator cuff tears, even massive ones, arthroscopically.
Massive rotator cuff tears make up approximately 20% of all cuff tears2 and a considerably greater percentage (80%) of recurrent rotator cuff tears.3 In the early 1990s, the recommendation often included simple débridement of the massive tears.4 Although débridement of massive tears has been relatively successful in terms of pain relief, overhead function is often not an attainable goal, particularly for patients who do not have balanced force couples.5,6
Advancement in arthroscopic shoulder surgery has now improved our understanding of tear patterns and has offered new opportunities and capabilities in terms of rotator cuff repair techniques.2 Understanding tear patterns is particularly important. Without a comprehensive understanding of tear patterns, the traditional repair techniques have focused on covering the hole. This was usually accomplished via an open or miniopen approach and subsequent extensive release of the cuff muscles to achieve enough mobilization to allow a repair to the bone. This medial to lateral technique did not respect the true pattern of pathology. Also, the extensive mobilization required can result in overtensioning of the rotator cuff and its subsequent failure.7 This can lead to damage to the suprascapular nerve, thereby leaving a repaired cuff completely nonfunctional.8 This may explain the traditionally poor results repairing primary and recurrent massive rotator cuff tears via an open approach1,9,10 or an arthroscopic approach based on a purely medial to lateral mobilization and repair.11
More recently, some have proposed the use of orthobiologic tissue patches to cover the hole in massive rotator cuff tears.12 Again, this approach does not respect the biomechanics of the rotator cuff and is therefore doomed to failure. Unless the rotator cuff tendons can be appropriately tensioned to achieve an optimal position along the muscle length-tension curve, ultimate improvement in function is unlikely.
Although truly irreparable rotator cuff tears do exist (tears with 75% or greater fatty infiltration),13 most patients with massive rotator cuff tears can obtain a good cuff closure and clinical result. The key to repairing the traditional irreparable tear is understanding the pattern of the tear and then knowing how to deal with each pattern. With a suitable knowledge of tear patterns and with adequate and appropriate mobilization and repair techniques (see later), most massive rotator cuff tears can and should be repaired. This leads not only to improved pain relief but also a return of a significant amount of function, usually including overhead function.2,3
PRINCIPLES AND ANATOMY
Force Couples
A primary function of the rotator cuff is to balance the force couples about the glenohumeral joint. A force couple is a pair of forces that act on an object and cause it to rotate. For any object to be in equilibrium, the forces must create moments about a center of rotation that are equal in magnitude and opposite in direction. In the situation in which the center of rotation is equidistant from the point of application of each force, the forces must be equal and opposite in direction for equilibrium to exist.
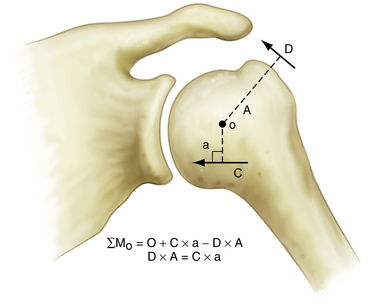
In the shoulder, the coronal plane force couple has been described by Inman and colleagues.14 This force couple is a result of the balance of moments created by the deltoid versus those created by the intact portions of the inferior rotator cuff (Fig. 21-1). During abduction, the coronal plane force couple will only be balanced if the line of action of the rotator cuff force is below the center of rotation of the humeral head, so that it can oppose the moment created by the deltoid. Balancing the coronal plane force couple is essential because this maintains a stable fulcrum for glenohumeral joint motion.
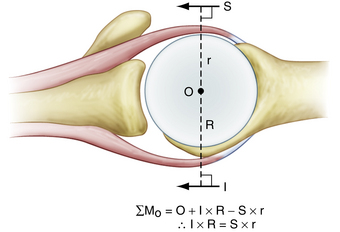
An equally important force couple is the transverse plane force couple. This force couple consists of the subscapularis anteriorly, balanced against the posterior rotator cuff (Fig. 21-2). This force couple is relevant in massive rotator cuff tears in which the tear may extend more posteriorly, leaving only a remnant of intact posterior cuff. In many cases, the posterior rotator cuff is so weak that it cannot balance the anterior moment created by the large subscapularis tendon. Furthermore, a large posterior rotator cuff tear can result in such a deficiency that the moment created by the inferior rotator cuff is also insufficient to maintain equilibrium in the coronal plane. This can then result in anterior and superior translation of the humeral head and the inability to maintain a stable fulcrum of motion. Therefore, when faced with a rotator cuff tear, the primary goal of surgery is to balance the force couples in the transverse and coronal planes and not necessarily to cover the hole. Again, the goal of rotator cuff repair is not to bring a medialized tendon stump laterally to bone, but to restore the balance of forces that control shoulder motion.
Rotator Cable-Crescent Complex: Suspension Bridge Analogy
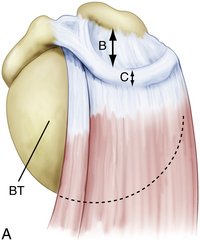
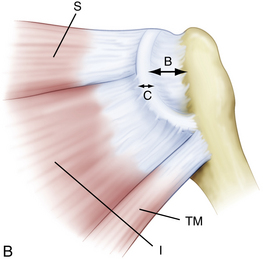
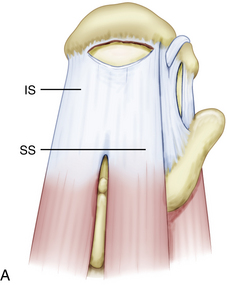
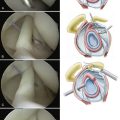
When arthroscopically viewed from the glenohumeral joint, the articular surface of the intact rotator cuff demonstrates an arching, cable-like thickening of the capsule surrounding a thinner crescent of tissue that inserts into the greater tuberosity of the humerus.15 This cable-like structure, which represents a thickening of the coracohumeral ligament, is consistently located at the avascular zone margin of the rotator cuff (Fig. 21-3). It extends from its anterior attachment into the greater tuberosity of the humerus, just posterior to the biceps tendon, to its posterior attachment near the inferior border of the infraspinatus. This rotator cable potentially serves a protective role by stress-shielding the thinner, avascular crescent tissue, analogous to a load-bearing suspension bridge.
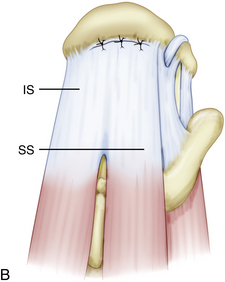
Similar to the intact rotator cuff, a rotator cuff tear can also be modeled after a suspension bridge, in which the free margin of the tear corresponds to the cable and the anterior and posterior attachments of the tear correspond to the supports at each end of the cable’s span. This model would predict, and subsequent studies have shown, that despite a tear in the avascular zone of the supraspinatus tendon, the supraspinatus muscle could still exert a compressive effect on the glenohumeral joint by means of its distributed load along the span of the suspension bridge configuration. The study by Halder and associates16 supports the concept that in small and medium-sized rotator cuff tears, the rotator cuff muscle forces are effectively transmitted along the rotator cuff cable, bypassing the torn supraspinatus tendon. That is why certain rotator cuff tears, even massive ones, can demonstrate functional kinematics and why after rotator cuff repair, good results can be achieved, even when a water-tight closure is not obtained.11,17
Furthermore, the implication of the cable-crescent concept contradicts the historic principle of covering the hole, particularly when using techniques such as tendon transfers to obtain closure of massive, so-called irreparable, rotator cuff tears. Others have advocated the use of tendon transfers, including the infraspinatus and subscapularis, to cover rotator cuff defects in massive tears.17 Transferring these muscles superiorly, however, significantly alters the normal mechanics of the rotator cuff.
Tear Patterns
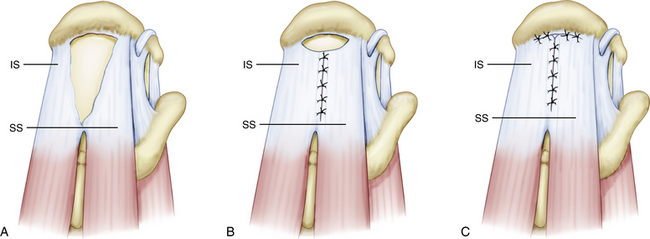
Crescent-shaped tears are the simplest of all tears and, although they can be massive, these tears do not typically retract medially to a significant degree. They demonstrate excellent mobility from a medial to lateral direction and can be repaired directly to bone, with minimal tension to the anatomic bone bed (Fig. 21-4).
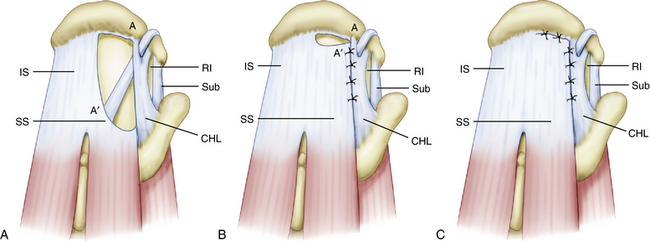
In contrast, U-shaped rotator cuff tears extend much farther medially than crescent-shaped tears, with the apex of the tear adjacent to or medial to the glenoid rim. Recognizing this tear pattern is critical, because attempting to mobilize and repair the apex of the tear to a lateral bone bed will result in overtensioning of the rotator cuff margin and subsequent failure. These tears demonstrate significant mobility from an anterior to posterior direction and should be initially repaired in a side to side fashion using the biomechanical principle of margin convergence (Fig. 21-5).
L-shaped and reverse L-shaped tears are similar to U-shaped tears. These are usually massive tears, with a longitudinal component in the direction of the fibers of the rotator cuff and a transverse component along the lateral cuff insertion (Fig. 21-6). One of the leaves is more mobile than the other leaf and can be easily brought to the bone bed.
These first three tear patterns represent about 90% of posterosuperior rotator cuff tears and can be repaired using the principles outlined here. Thus, most rotator cuff tears, even massive tears, can be repaired without extensive mobilization with an understanding and recognition of these tear patterns. Repairing tears according to their tear pattern can lead to excellent results.2
In the fourth tear pattern, the massive, contracted, immobile rotator cuff tear, other techniques must be used.
HISTORY AND PHYSICAL EXAMINATION
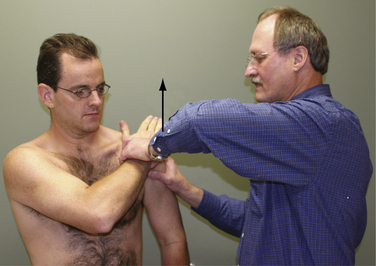
The subscapularis examination is a key element to the shoulder examination that is frequently untested. The lift-off test is not positive until at least 75% of the subscapularis tendon is detached and therefore is not a good test for upper subscapularis tears.18 The bear hug test is the most sensitive test for a torn subscapularis, particularly a partial tear involving only the upper subscapularis.19 In this test, the patient places the hand of the affected side on the opposite shoulder with the fingers extended and the elbow elevated into a forward position. The examiner then tries to pull the patient’s hand off the shoulder perpendicular to the plane of the forearm as the patient resists (Fig. 21-7). If the examiner is able to lift the patient’s hand off the shoulder, this is a positive finding and is suggestive of at least a partially torn subscapularis tendon. Other tests for the subscapularis include the belly press and Napoleon tests.20
INDICATIONS AND CONTRAINDICATIONS
Traditionally, fatty infiltration of more than 50% on preoperative MRI scans has been considered a poor prognostic indicator for rotator cuff repairs.22–24 These patients have been discouraged from undergoing cuff repair with the assumption that surgical repair would not improve their outcome. In the senior author’s practice (SB), assessment of fatty infiltration within the muscle belly of the infraspinatus is critical, because it affects outcome. Muscle imparts elasticity to the rotator cuff and, without adequate muscle tissue, it would be difficult, if not impossible, to repair the tendon to the greater tuberosity. Lo and Burkhart have reported the results of repair of massive immobile rotator cuff tears that required the interval slide technique.21 Their patients demonstrated significant improvements in pain, active forward elevation, active external rotation, strength, and function. More recently, Burkhart and coworkers13 have evaluated the outcomes of massive cuff repairs and correlated these outcomes to preoperative MRI findings. The data suggest that patients who have greater than 75% fatty infiltration have a guarded prognosis for significant improvement because only 40% of these patients demonstrated clinical improvement. This is essential to determine prior to surgery because these patients can then be informed of their less favorable prognosis. They may choose not to proceed with surgical intervention, given their limited probability of functional improvement. However, patients with 50% to 75% fatty infiltration actually improved significantly after rotator cuff repair. In fact, 100% (17/17) of patients with less than 75% fatty infiltration had significant clinical and functional improvement after rotator cuff repair. Thus, in contradistinction to Goutallier’s cutoff of 50%, we consider all patients with less than 75% fatty infiltration on MRI scans to be potential surgical candidates.13
One area in which massive contracted rotator cuff tears are frequently encountered is during revision rotator cuff repair. Many authors have reported the results of revision rotator cuff surgery done through an open approach.1,9,10,22 Overall, these studies have demonstrated that although frequently effective in terms of pain relief, functional improvements have been slight. Recently, Lo and colleagues3 have reported on the arthroscopic revision of failed rotator cuff tears and demonstrated significant functional improvement in 93% of these patients, with a mean increase in the UCLA score of 15.5.
Some authors have also suggested proximal migration as a poor prognostic sign for rotator cuff surgery.23,24 Burkhart and associates18 have demonstrated results to the contrary. In their study of patients who had proximal migration of the humeral head associated with anterosuperior cuff tears (subscapularis plus supraspinatus ± infraspinatus), 80% of patients demonstrated lasting reversal of the superior humeral head migration and a return of overhead function after repair. Proximal migration of the humeral head is not a contraindication to rotator cuff repair. Instead, one could argue that patients with proximal migration of the humeral head have the most to gain from rotator cuff repair. It is important to note, however, in the senior author’s (SB) experience, that proximal migration of the humerus combined with radiographic findings of a rounded greater tuberosity and acetabularization of the acromion suggest a long-standing tear that may be irreparable. In these situations, arthroscopic surgery may have limited applications.
TREATMENT OPTIONS
Conservative Management
Nonoperative treatment for massive rotator cuff tears includes lifestyle changes, exercise programs, and steroid injections. These methods are preferred in dealing with older low-demand patients with massive rotator cuff tears before consideration of surgery. Because these patients are often disabled at the time of their initial office visit, the temptation is to rush into surgical management. This can be a mistake. These patients should initially undergo nonoperative treatment with an exercise program. If the primary complaint includes pain, a series of steroid injections may dramatically benefit the patient. The senior author (SB) prefers a series of 3 monthly injections to control the inflammation and then limit the injections to once yearly. After these nonoperative measures have been used, a decision regarding surgical management can be considered. The surgeon may accelerate the surgical timetable somewhat if the involved extremity is on the patients’ dominant arm, if the patient is extremely disabled by his or her condition, and if the patient is physiologically younger than his or her chronologic age.
Arthroscopic Technique
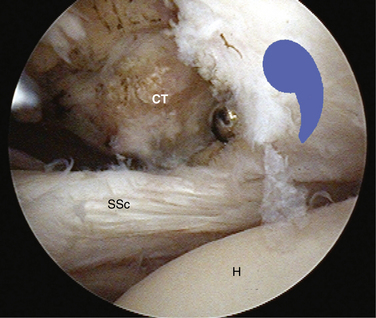
The arthroscopic examination should begin with the anterior structures. A 30-degree arthroscope is used and the biceps tendon, anterior labrum, glenohumeral ligaments, and subscapularis tendons are examined. Particular attention must be paid to the insertion of the subscapularis tendon and the medial sling of the biceps. The junction of the medial sling with the superior border of the subscapularis tendon make up what has been called the comma sign (Fig. 21-8 ).25
At this point, the 70-degree arthroscope should be inserted to provide an aerial view of the subscapularis insertion as well as the medial sling of the biceps tendon. To visualize the subscapularis footprint better, the assistant can hold the arm in internal rotation and perform a posterior lever push.26 The relationship between the anterior surface of the subscapularis and the biceps tendon is extremely important. At no time during rotation of the humerus should the biceps cut posterior to the plane of the subscapularis. If this occurs, it is suggestive of an incompetent medial sling of the biceps and early biceps tendon instability. This will require a biceps tenotomy or tenodesis.
Next, it is important to determine the space available for the subscapularis. The phenomenon of the roller-wringer effect has been described, and subcoracoid impingement is important to diagnose and treat appropriately.27 The best portal for approaching the subscapularis and coracoid is an anterosuperolateral portal, adjacent to the anterolateral corner of the acromion. The coracoid tip should be identified. If the subscapularis is not retracted, the tip of the coracoid should be at the level of the superior border of the subscapularis. The surgeon should make a window in the rotator interval to identify the coracoid tip and determine whether there is subcoracoid stenosis. If the distance from the coracoid tip to the anterior aspect of the subscapularis is less than 7 mm, an arthroscopic coracoplasty is necessary. The posterolateral aspect of the coracoid can be skeletonized with a combination of the shaver and electrocautery through the anterosuperolateral portal (Fig. 21-9). In exposing the coracoid, one must preserve the attachment of the conjoined tendon. The plane of the coracoplasty is parallel to the plane of the subscapularis tendon. The goal is to create a 7- to 8-mm space between the reconfigured coracoid tip and the anterior plane of the subscapularis tendon. The 30-degree arthroscope should be used initially to be sure that one does not get disoriented and wander inferior to the coracoid.
To allow complete visualization and classification of the rotator cuff tear, all fibrofatty and bursal tissue must be removed from the margins of the tear. The tear is then identified and assessed for mobility. For tear pattern recognition, it is helpful to expose the scapular spine because it serves as a landmark to separate the supraspinatus tendon anteriorly and the infraspinatus located posteriorly (Fig. 21-10). Then, with the arthroscope posterior, the medial to lateral mobility of the tear can be assessed using a soft tissue grasper introduced through the lateral portal. Noncontracted crescent-shaped tears have excellent medial to lateral mobility and reduce easily to the bone bed. These tears are repaired directly to bone. Tears with poor medial to lateral mobility are then further assessed for anterior and posterior mobility, with the arthroscope in the lateral position. Posterior leaf mobility is assessed by pulling the leaf anteriorly with the grasper placed through the anterior portal; the anterior leaf is assessed by pulling the anterior leaf posteriorly with the grasper placed through the posterior portal. If the anterior and posterior leaves are mobile enough to allow side to side closure, then the tear is classified as a U-shaped massive tear and the technique of margin convergence is used. The converged rotator cuff margin is then repaired to bone as described here.
U-Shaped Tears
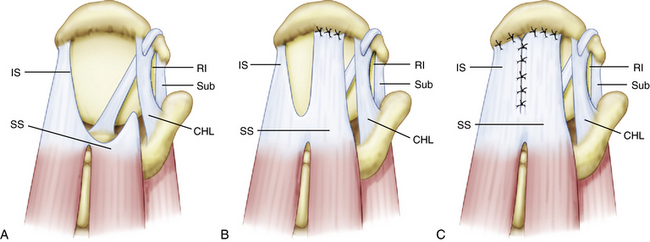
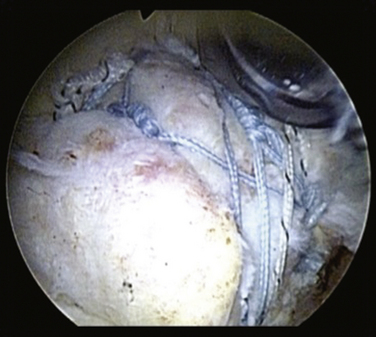
Repair of massive U-shaped tears can be gratifying, because many of these tears appear irreparable on first view. U-shaped tears demonstrate significant mobility from an anterior to posterior direction and should be initially repaired in a side to side fashion using the biomechanical principle of margin convergence (see later). One or two side to side sutures often will achieve such dramatic margin convergence that repair to bone can be simple (see Fig. 21-5). We typically begin side to side closure with a combination of the Penetrator and BirdBeak suture passers (Arthrex, Naples, Fla) that are used to pass sutures through the posterior and anterior leaves of the cuff tear, while using the arthroscope in a lateral subacromial position. Most of the large and massive tears require three to four side to side sutures. These sutures are tied sequentially, the most medial suture first, to achieve margin convergence of the cuff margin over the prepared bone bed. Two suture anchors are then placed, one for each leaf of the cuff tear. The anchor for the posterior leaf is placed approximately 1 cm anterolateral to the edge of the posterior leaf of the cuff so that the posterior leaf can be shifted proximally and anteriorly. This maneuver maximizes the moment produced by the posterior cuff in the transverse and coronal planes, thereby creating balanced force couples in both planes. The achievement of balanced force couples allows the shoulder to establish a stable fulcrum of glenohumeral motion. The anterior leaf typically is not as mobile as the posterior leaf in a U-shaped tear, and a significant shift of the anterior leaf usually is not possible or necessary, so the anterior anchor is placed adjacent to the edge of the anterior leaf. The sutures from the anchor are then passed through the tissue and tied. The free margin of the rotator cuff can then be easily repaired to the bone bed in a tension-free manner. The technique of margin convergence allows repair of seemingly irreparable tears and minimizes strain at the repair site.28 Theoretically, this decreases the risk of rupture and subsequent failure of the rotator cuff repair.
L-Shaped Tears
In L-shaped and reverse L-shaped tears, one of the leaves is more mobile than the other leaf and can be easily brought to the bone bed and to the other leaf. In these cases, side to side suturing is first performed along the longitudinal split and then the converged margin is repaired to bone (see Fig. 21-6). In more chronic cases, the physiologic pull of the rotator muscle posteriorly will cause a reversed L-shaped tear to assume a more U-shaped configuration.
Specific Techniques
A category of great interest is that of the immobile contracted rotator cuff tear. These are rotator cuff tears in which the tear margins will not reach the bone bed by standard repair and mobilization techniques. In the past, when such tears were encountered, only rudimentary mobilization techniques were available and frequently partial repairs were all that could be performed. With recent advances in arthroscopic shoulder surgery, we now have multiple tools in our arsenal to tackle these difficult tears. We will discuss some of them here.
Excavation of the Rotator Cuff.
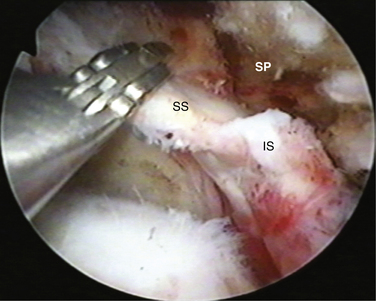
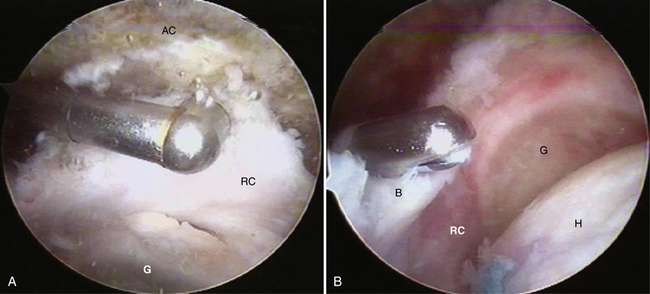
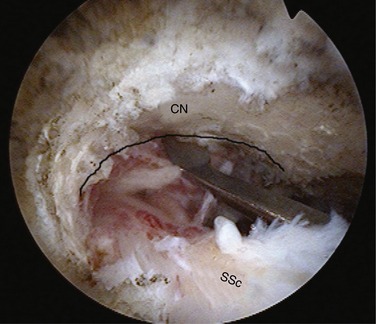
In massive rotator cuff tears, the cuff is often scarred to the deltoid and the acromion. In such cases, one must excavate the cuff from the bone and soft tissue to which it has scarred. Identifying the fat pad posterior to the acromioclavicular (AC) joint and then developing the fatty layer between the rotator cuff below and the acromion above is the best way to achieve this goal (Fig. 21-11). One then proceeds laterally until the rotator cuff margin is found. In performing this dissection, we commonly view through a lateral portal and introduce our instruments through a posterior portal. By staying in the fatty layer, one can be sure to stay above any viable rotator cuff tissue. It is helpful to identify the spine of the scapula prior to doing this to delineate the border between the supraspinatus and infraspinatus. The skeletonized spine of the scapula looks much like the keel of a boat. The tissue anterior to the keel is the supraspinatus and the tissue posterior is the infraspinatus.
Margin Convergence.
The term margin convergence refers to the phenomenon that occurs with side to side closure of large rotator cuff tears, in which the free margin of the tear converges toward the greater tuberosity as side to side repair progresses (see Fig. 21-5). This technique reduces the strain at the free edge of the cuff dramatically, leaving an almost tension-free converged cuff margin overlying the humeral bone bed for repair. For example, side to side closure of two thirds of a U-shaped tear will reduce the strain at the cuff margin to one sixth the strain that existed at the preconverged cuff margin. This mechanical strain reduction creates an added safety factor for the repair of tendon to bone because decreased strain means that there will be a lower likelihood of failure of fixation to bone. Strain reduction should also be effective in protecting against suture failure and propagation of the tear, even if the cuff margin is not repaired to bone. It is possible that the reduced strain can enhance function indirectly by decreased mechanical stimulation of pain receptors in the rotator cuff because deformation of these receptors should decrease as the strain decreases. Margin convergence is a powerful tool in the surgeon’s armamentarium for repairing massive rotator cuff tears.
Interval Slides.
In the orthopedic literature, the use of the open interval slide has been introduced. Cordasco and Bigliani29 have described the open anterior interval slide, whereas Codd and Flatow30 have suggested an open posterior interval slide as well.
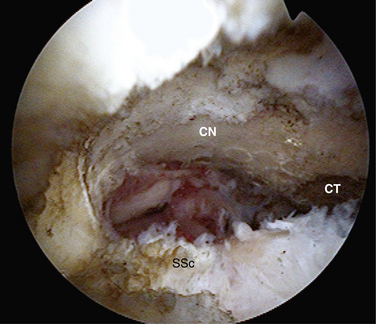
Tauro31 was the first to describe an arthroscopic interval slide for release of the rotator interval from the supraspinatus tendon. An arthroscopic adaptation of the Bigliani technique was used to release the coracohumeral ligament and contracted immobile adhesed tears. This technique involved arthroscopic division of the rotator interval just anterior to the leading edge of the supraspinatus tendon. The line of this cut went just above the root of the biceps and aimed toward the base of the coracoid. This release improved the mobility of the supraspinatus, allowing repair of retracted tears.
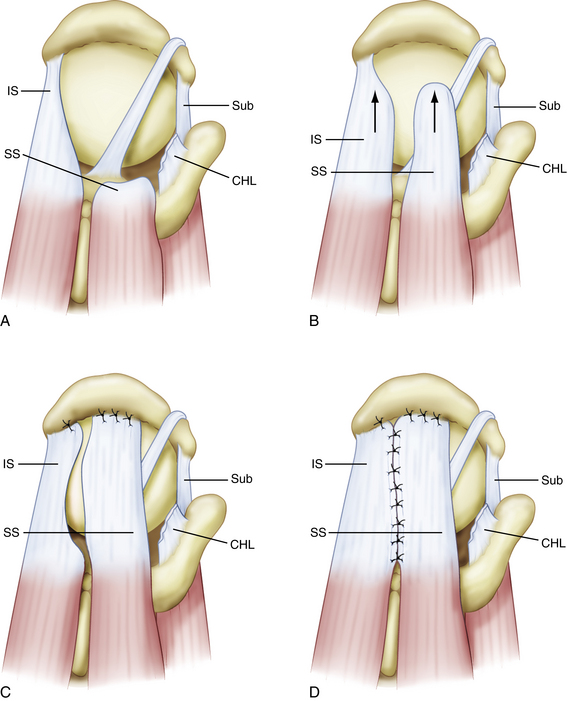
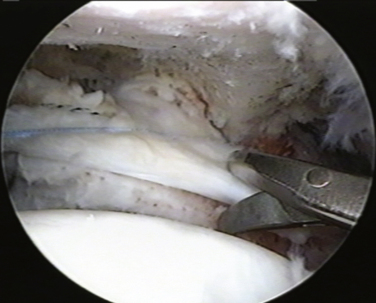
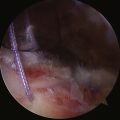
Our procedure for the anterior interval slide is similar to the release of the subscapularis. The base of the coracoid is skeletonized and, in so doing, adhesions securing the anterior edge of the supraspinatus to the coracoid are released. The supraspinatus tendon is then identified and a traction stitch is placed at the anterolateral corner of the tendon and pulled out through a lateral portal. When performing an anterior interval slide, it is easiest to view from the lateral or posterior subacromial portal. These portals allow the correct orientation and visualization of the release. A dissecting scissor is introduced and the supraspinatus tendon is released from lateral to medial, starting at the free margin of the tendon tear and progressing to the base of the coracoid. This release also incises the coracohumeral ligament (Fig. 21-12). Alternatively, an anterior interval slide in continuity can be done by bluntly releasing the coracohumeral ligament from the coracoid base.32
In general, with an isolated anterior interval slide, only 1 to 2 cm of lateral excursion of the supraspinatus is gained. With massive adhesed retracted tears, this is often not enough lateral excursion to allow apposition of the tendon to its bone bed. In this case, the addition of a second, more posteriorly located interval slide is necessary. When done in combination, the procedure is known as a double-interval slide.21
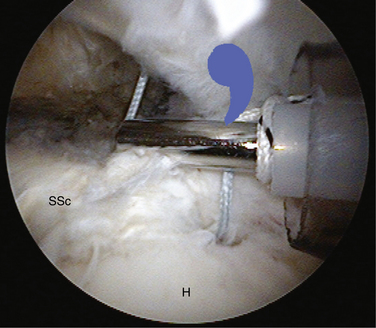
The posterior interval slide releases the interval between the supraspinatus and infraspinatus (Fig. 21-13). Arthroscopically exposing the scapular spine, which marks the interval between the supraspinatus and infraspinatus tendon, is imperative. This can be done by resecting the medial fibrofatty tissue until the arching column of the scapular spine is visible. Traction sutures are then placed on the supraspinatus and infraspinatus tendons. The posterior release is performed by cutting between the supraspinatus and infraspinatus tendons with an arthroscopic scissor, aiming toward the base of the scapular spine (Fig. 21-14).
Footprint Reconstruction.
A double-row repair using a row of medial anchors at the articular margin and a row of lateral anchors restores the footprint of the rotator cuff more adequately and increases the area of contact for healing. Furthermore, by providing a second row of fixation, the number of points of fixation is increased, thereby increasing the strength of the initial repair construct and decreasing the load that each anchor must resist. All these factors can potentially improve the mechanical strength and function of the repaired rotator cuff by providing more complete healing across the anatomic footprint.33 Cadaveric studies have highlighted the superiority of a double-row repair at restoring the supraspinatus footprint compared with a single-row repair.37–39 In our experience, a single lateral row reconstituted 47% of the footprint in 26 patients whereas the double-row repair reconstituted the remaining 53% in these patients.34
Today, in addition to the double-row, we now have a transosseous-equivalent or suture bridge technique in which compression is applied to the tendon against the prepared bone bed (Fig. 21-15). In this technique, two rows of fixation with sutures from the medial row draping over the tendon laterally provide increased tendon contact and compression over the anatomic footprint to improve and promote healing.35,36 Using this technique, Frank and associates37 have demonstrated a high healing rate on imaging studies at 1 year. Of the first 25 patients repaired with this technique, 88% had an intact rotator cuff repair on MRI evaluation. This indicates excellent cuff healing, as judged by the intact repair sites, compared with most standard arthroscopic rotator cuff repair series. Although this is a small sample size, continuing advancements in arthroscopic rotator cuff repair hold much promise for the future.
Subscapularis Mobilization and Repair.
When there is a complete tear of the subscapularis with retraction, finding the retracted subscapularis tendon can be difficult. The key to finding the subscapularis and differentiating it from the conjoined tendon and the coracoacromial ligament is to locate the comma sign25 (see Fig. 21-8). The comma sign is a comma-shaped arc of tissue located at the superolateral border of the subscapularis, which always will lead the surgeon to the superolateral border of the subscapularis. The comma is actually the remnant of the medial sling of the biceps and is composed of fibers of the medial head of the coracohumeral ligament as well as a portion of the superior glenohumeral ligament. Typically, when the subscapularis fails completely, the medial sling of the biceps will pull away from the lesser tuberosity so that it remains confluent with the superolateral subscapularis tendon. This is convenient because the comma then allows the surgeon to be able to delineate the superolateral aspect of the subscapularis fully.
Initially, two half- racking sutures are placed through the biceps tendon and the tendon is detached from the superior labrum with arthroscopic scissors (Fig. 21-16). The tendon is then exteriorized through the anterosuperolateral portal and a whipstitch is placed in the tendon (Fig. 21-17). The biceps tendon is allowed to retract down the bicipital groove prior to performing a tenodesis with a Biotenodesis interference screw (Arthrex) after the subscapularis repair. One must recognize that biceps tenotomy or tenodesis is important in the case of biceps subluxation or dislocation. If the biceps is not transferred out of the way of the subscapularis to a tenodesis site, the persistent force of the subluxed biceps over the top of the subscapularis repair will cause the repair to fail.
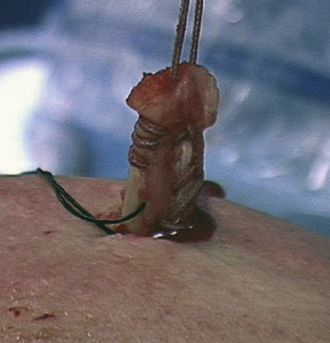
FIGURE 21-17 The biceps is pulled out through the anterosuperolateral portal and a whipstitch is placed into the tendon.
In mobilizing a retracted subscapularis, one must become comfortable dissecting around the coracoid. Remember that the posterolateral aspect of the coracoid is at least 15 mm away from all neurovascular structures.38
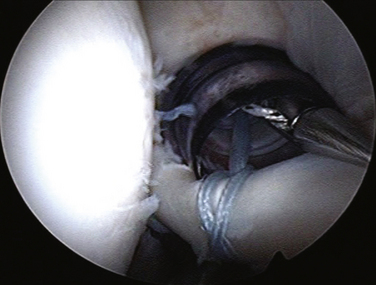
First, a traction suture is placed at the junction between the comma tissue and upper border of the subscapularis. Using the traction suture and pulling this suture through the anterosuperolateral portal makes the subscapularis release much easier, because it gives a counterforce for the dissecting instrument (e.g., scissors, elevator) to work against. In the case of a complete tear, the subscapularis will retract considerably, often to the level of the anterior labrum medially. For a retracted subscapularis, a three-sided release (anterior, superior, and posterior) is necessary, using a combination of a shaver and electrocautery to release the tendon from the coracoid neck and base (Fig. 21-18). We also use an arthroscopic elevator to release adhesions from the arch of the coracoid neck and anterior glenoid neck. In performing the release, one should be careful not to plunge inferomedially when working around the base of the coracoid.
In repairing the subscapularis, it is optimal to use one anchor per linear centimeter of detachment. In anatomic dissections, we have found that the superior to inferior length of the footprint of the normal subscapularis is 2.5 cm.39 Therefore, for a 50% tear, one anchor should be sufficient and, for a 100% tear, two or three anchors are optimal.
Anchors are placed through an accessory anterior portal to obtain an appropriate angle of approach to the lesser tuberosity. Once the anchors are placed, sutures are passed through the superolateral border of the subscapularis tendon using a Viper suture passer (Arthrex) and tied down through the anterosuperolateral portal (Fig. 21-19). We prefer using static knots here, rather than sliding knots, because the acute angle makes the use of sliding knots difficult.
Partial Rotator Cuff Repair: Balancing Force Couples.
PEARLS& PITFALLS
PEARLS
COMPLICATIONS AND TECHNICAL PITFALLS
Obviously, when dealing with a retracted, immobile, massive rotator cuff tear, the risk of intraoperative and postoperative complications escalates. Many of these complications can be minimized with meticulous surgical technique. Most importantly, the surgeon should understand the complexity and difficulty of these cases. They can easily take 2 to 3 hours to complete, even in the hands of an experienced arthroscopic shoulder surgeon.
1. DeOrio JK, Cofield RH. Results of a second attempt at surgical repair of a failed initial rotator-cuff repair. J Bone Joint Surg Am. 1984;66:563-567.
2. Burkhart SS, Danaceau SM, Pearce CEJr Arthroscopic rotator cuff repair: Analysis of results by tear size and by repair technique-margin convergence versus direct tendon-to-bone repair. Arthroscopy, 17; 2001:905-912.
3. Lo IK, Burkhart SS Arthroscopic revision of failed rotator cuff repairs: technique and results. Arthroscopy, 20; 2004:250-267.
4. Rockwood CAJr, Williams GRJr, Burkhead WZJr. Debridement of degenerative, irreparable lesions of the rotator cuff. J Bone Joint Surg Am. 1995;77:857-866.
5. Burkhart SS. Arthroscopic treatment of massive rotator cuff tears. Clin Orthop Relat Res. 2001;390:107-118.
6. Burkhart SS. Arthroscopic treatment of massive rotator cuff tears. Clinical results and biomechanical rationale. Clin Orthop Relat Res. 1991;267:45-56.
7. Gerber C, Meyer DC, Schneeberger AG, et al Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am, 86; 2004:1973-1982.
8. Greiner A, Golser K, Wambacher M, et al. The course of the suprascapular nerve in the supraspinatus fossa and its vulnerability in muscle advancement. J Shoulder Elbow Surg. 2003;12:256-259.
9. Djurasovic M, Marra G, Arroyo JS, et al Revision rotator cuff repair: factors influencing results. J Bone Joint Surg Am, 83; 2001:1849-1855.
10. Bigliani LU, Cordasco FA, McIlveen SJ, Musso ES. Operative treatment of failed repairs of the rotator cuff. J Bone Joint Surg Am. 1992;74:1505-1515.
11. Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219-224.
12. Dejardin LM, Arnoczky SP, Ewers BJ, et al. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. Am J Sports Med. 2001;29:175-184.
13. Burkhart SS, Barth JR, Richards DP, et al. Arthroscopic repair of massive rotator cuff tears with stage 3 and 4 fatty degeneration. Arthroscopy. 2007;23:347-354.
14. Inman V, Saunder J, Abbott L. Observations on the function of the shoulder. J Bone Joint Surg Am. 1944;26:1-30.
15. Burkhart SS, Esch JC, Jolson RS The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge”. Arthroscopy, 9; 1993:611-616.
16. Halder AM, O’Driscoll SW, Heers G, et al. Biomechanical comparison of effects of supraspinatus tendon detachments, tendon defects, and muscle retractions. J Bone Joint Surg Am. 2002;84:780-785.
17. Cofield RH. Subscapular muscle transposition for repair of chronic rotator cuff tears. Surg Gynecol Obstet. 1982;154:667-672.
18. Burkhart SS, Tehrany AM Arthroscopic subscapularis tendon repair: technique and preliminary results. Arthroscopy, 18; 2002:454-463.
19. Barth JR, Burkhart SS, De Beer JF The bear-hug test: a new and sensitive test for diagnosing a subscapularis tear. Arthroscopy, 22; 2006:1076-1084.
20. Burkhart SS, Lo IK, Brady PC Burkhart’s View of the Shoulder: A Cowboy’s Guide to Advanced Shoulder Arthroscopy. Philadelphia: Lippincott Williams & Wilkins; 2006.
21. Lo IK, Burkhart SS Arthroscopic repair of massive, contracted, immobile rotator cuff tears using single and double interval slides: technique and preliminary results. Arthroscopy, 20; 2004:22-33.
22. Neviaser RJ. Evaluation and management of failed rotator cuff repairs. Orthop Clin North Am. 1997;28:215-224.
23. Klinger HM, Steckel H, Ernstberger T, Baums MH Arthroscopic debridement of massive rotator cuff tears: negative prognostic factors. Arch Orthop Trauma Surg, 125; 2005:261-266.
24. Vandenbussche E, Bensaida M, Mutschler C, et al. Massive tears of the rotator cuff treated with a deltoid flap. Int Orthop. 2004;28:226-230.
25. Lo IK, Burkhart SS The comma sign: an arthroscopic guide to the torn subscapularis tendon. Arthroscopy, 19; 2003:334-337.
26. Adams CR, Schoolfield JD, Burkhart SS. The results of arthroscopic subscapularis tendon repairs. Arthroscopy. 2008;24:1381-1389.
27. Lo IK, Burkhart SS The etiology and assessment of subscapularis tendon tears: a case for subcoracoid impingement, the roller-wringer effect, and TUFF lesions of the subscapularis. Arthroscopy., 19; 2003:1142-1150.
28. Burkhart SS, Athanasiou KA, Wirth MA Margin convergence: a method of reducing strain in massive rotator cuff tears. Arthroscopy, 12; 1996:335-338.
29. Cordasco FA, Bigliani LU. The rotator cuff. Large and massive tears. Technique of open repair. Orthop Clin North Am. 1997;28:179-193.
30. Codd T, Flatow EL. Anterior acromioplasty, tendon mobilization, and direct repair of massive rotator cuff tears. In: Burkhead WZJr, editor. Rotator Cuff Disorders. Baltimore: Williams and Wilkins; 1996:323-334.
31. Tauro JC. Arthroscopic “interval slide” in the repair of large rotator cuff tears. Arthroscopy. 1999;15:527-530.
32. Lo IK, Burkhart SS The interval slide in continuity: a method of mobilizing the anterosuperior rotator cuff without disrupting the tear margins. Arthroscopy, 20; 2004:435-441.
33. Lo IK, Burkhart SS Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy, 19; 2003:1035-1042.
34. Brady PC, Arrigoni P, Burkhart SS. Evaluation of residual rotator cuff defects after in vivo single- versus double-row rotator cuff repairs. Arthroscopy. 2006;22:1070-1075.
35. Park MC, Cadet ER, Levine WN, et al. Tendon-to-bone pressure distributions at a repaired rotator cuff footprint using transosseous suture and suture anchor fixation techniques. Am J Sports Med. 2005;33:1154-1159.
36. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89:953-960.
37. Frank JB, El Attrache NS, Dines JS, et al. Repair site integrity after arthroscopic transosseous-equivalent suture-bridge rotator cuff repair. Am J Sports Med. 2008;36:1496-1503.
38. Lo IK, Lind CC, Burkhart SS Glenohumeral arthroscopy portals established using an outside-in technique: neurovascular anatomy at risk. Arthroscopy, 20; 2004:596-602.
39. Richards DP, Burkhart SS, Tehrany AM, Wirth MA The subscapularis footprint: an anatomic description of its insertion site. Arthroscopy, 23; 2007:251-254.
40. Burkhart SS Partial repair of massive rotator cuff tears: the evolution of a concept. Orthop Clin North Am, 28; 1997:125-132.
41. Burkhart SS, Nottage WM, Ogilvie-Harris DJ, et al. Partial repair of irreparable rotator cuff tears. Arthroscopy. 1994;10:363-370.