LARGE BLOOD VESSELS
Introduction
It is often assumed that the heart propels blood around the body but this is only true to a certain extent. When the heart goes into its refilling phase, diastole, and no more blood is entering the arterial tree, the peripheral circulation does not stop and the diastolic pressure does not fall to zero. Flow is maintained by the pressure in the large arteries which pushes blood through the small vessels. During systole, as the stroke volume of blood (about 70 mL in the resting ‘textbook’ person), enters the large arteries, the vessels are stretched. During diastole, the elastic recoil of the arteries helps to maintain arterial pressure and, hence, keep tissue perfusion going (see Chapter 1). The role of the heart, therefore, is to keep the arterial pressure reservoir ‘topped up’.
An important functional characteristic of the cardiovascular system is that each part of the body needs to be provided with a blood flow which is appropriate to the metabolic and functional needs of that tissue. The driving force to perfuse tissues is provided by the pressure in the arteries (see Chapter 10). How much blood passes from the arterial system into the blood vessels serving a particular tissue will depend on the relative resistance to flow in the tissue. Thus, local dilatation of blood vessels will reduce resistance and increase flow, whereas constriction of vessels will increase resistance and decrease flow locally but will also serve to divert blood flow to other tissues. As this occurs throughout the microvasculature, we are provided with a precise and effective system for matching blood flow to metabolic demand. The factors regulating the microcirculation (resistance blood vessels) will be considered in Chapter 9.
In this chapter we will first consider the flow characteristics of blood as a fluid and then some of the characteristics of the large blood vessels. The common pathological changes affecting large arteries and veins will be reviewed. The case history of a man who is suffering the consequences of pathological changes in the arterial blood supply to his legs is summarized in Case 8.1:1.
Haemorheology: the physical characteristics of blood flow
Blood flows down a pressure gradient
Blood, like any other fluid, will only flow from an area of relatively high pressure to somewhere where the pressure is lower. This is equivalent to pointing out that rivers only flow downhill and the water in a pond, with no outlet to a lower point, does not flow anywhere. Jamshed Patel (Case 8.1:1) has used this principle by putting his leg out of bed in order to try to increase blood flow.
Viscosity of blood
The viscosity of blood is mainly determined by the haematocrit, the percentage of blood volume which is occupied by the red blood cells. The viscosity of blood is frequently expressed as a relative viscosity, i.e. the viscosity compared to pure water taken as unity. On this basis, the relative viscosity of blood plasma, which contains protein but no red cells, is about 1.3. At a normal haematocrit, of the order of 45%, the relative viscosity of blood flowing through tissues is about 2.4 (Fig. 8.1). This assumes that the blood flow is fast enough to keep the red cells apart as, if blood flow is sluggish, the red cells tend to stick together. This is because the large plasma proteins (globulins and fibrinogen) form cross-bridges between slowly moving red cells. These bonds are disrupted in faster moving blood. Sometimes red cells pile up in a similar fashion to a pile of dinner plates and this is called rouleaux formation. Aggregation of red cells becomes an important factor increasing the resistance to blood flow in circulatory shock. It will tend to occur in the postcapillary blood vessels, the venules, when the velocity of flow is low. This may be part of the explanation for the swollen appearance (localized oedema) of Jamshed’s foot (see Case 8.1:1). Blood flow may cease completely if the resistance is too high and this contributes to the localized tissue oedema which sometimes develops during circulatory shock (see Chapter 14).
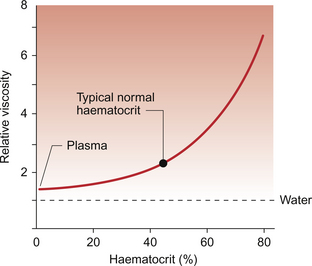
Fig. 8.1 Effect of haematocrit on the relative viscosity of blood. ‘Relative’ viscosity is compared to water = 1. The figure shown applies to a typical vascular bed which includes vessels of a mixture of different sizes but predominantly contains small vessels. Relative viscosity of blood is much higher when measured in a glass viscometer which has a wide bore tube (see Fig. 8.3).
Laminar and turbulent flow
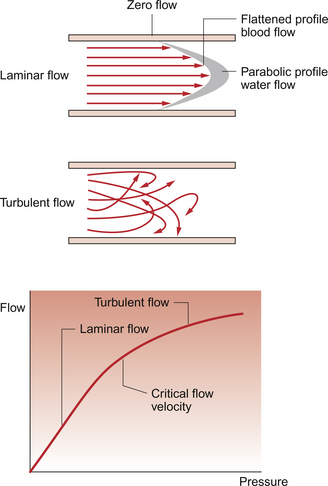
Blood flow may be either laminar or turbulent. ‘Laminar’, otherwise known as ‘streamline’, fluid flow means that all the particles in the fluid are flowing parallel to the wall of the tube (Fig. 8.2). However, they are not all moving at the same velocity. Those fluid particles in contact with the wall of the tube are theoretically stationary whilst those at the centre (axis) of the tube are flowing fastest (Fig. 8.2). The opposite of laminar flow is turbulent flow. In this case the fluid particles follow a much more irregular pathway and may develop vortices (whirlpools) in the blood vessel.
The conditions which result in the transition from laminar to turbulent flow were described mathematically by Osborne Reynolds in 1883. The essence of Reynolds’ law is that turbulence is more likely to occur in large tubes than in small tubes. Turbulence is more likely when the velocity of flow is high and when the viscosity of the fluid is low. The blood flow velocity at which there is a transition from laminar to turbulent flow is called the ‘critical velocity’ (Fig. 8.2). Laminar flow is essentially silent but turbulent flow sets up vibrations in the blood vessel wall which can be heard using a stethoscope. Turbulent flow in the circulation produces the noises which are called murmurs.
As the viscosity of blood depends primarily on the haematocrit and a low viscosity makes the development of turbulence more likely, anaemic patients are more likely to have murmurs in their circulation than those with a normal haematocrit. A murmur caused in this way would disappear once the anaemia was corrected. An example of this is during pregnancy. Maternal haematocrit decreases in pregnancy because the plasma volume expands by more than the red cell volume. Flow murmurs are therefore more likely to be heard in pregnant women and may cause temporary alarm, but they normally disappear when the baby is delivered and haematocrit returns to normal. We also use the development of artificially induced flow murmurs, produced by compressing an artery with a sphygmomanometer cuff so that flow velocity increases as the basis for non-invasive measurement of arterial blood pressure (see Chapter 10).
Within the normal circulation, blood flow can be thought of as approximating to a laminar flow pattern in most large vessels. The site where turbulence is most likely to occur in a normal person is in the first segment of the aorta because here the velocity of flow is high in a relatively large tube. During exercise, when the cardiac output, and therefore the velocity of flow, increases, turbulence will extend further down the aorta than at rest. Local changes in blood flow dynamics leading to turbulence are contributory factors in determining the location of endothelial cell damage which is a precursor to the development of atherosclerotic plaques in the circulation (see p. 91). Thrombus formation is more likely when blood flow is slow and there is no turbulence, i.e. in the veins.
In Jamshed’s case (see Box 8.1:1) a loud bruit (flow murmur produced by turbulent blood flow) was heard over the femoral artery. The probable explanation is that a narrowing of the vessel led to locally increased blood flow velocity through the constriction and hence the development of turbulence on the downstream side.
Relationship between blood vessel radius and blood flow
The physiological consequence of the fourth power relationship is that small changes in the diameter of resistance blood vessels lead to relatively big changes in flow. Blood can, therefore, be diverted to match metabolic needs by constricting and dilating small blood vessels. These are known as the resistance vessels (see Chapter 9). In pathological terms, small changes in blood vessel diameter produced by, for example, atherosclerosis may result in large reductions in blood flow (see p. 90).
Narrowing of the femoral artery and hence reduction of blood flow has limited the delivery of blood to Jamshed’s calf muscle (see Box 8.1:1), hence causing hypoxic pain in the muscle. This became worse during exercise because the increased oxygen demand could not be satisfied by an adequate increase in blood flow.
Red cell distribution over the cross-section of a blood vessel
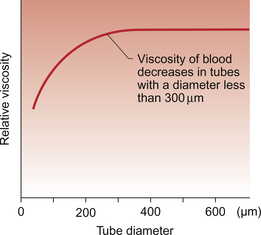
‘Axial accumulation’ of red cells is a consequence of laminar blood flow. Red cells are dragged into the part of the blood vessel which has the fastest flow, i.e. down the middle of the blood vessel. The blood flowing slowly near the wall of the blood vessel, therefore, has a lower haematocrit than the fast flowing blood at the centre of the vessel. Small branches from a large vessel hence receive blood which has a lower haematocrit than the average for the large vessel. Because the viscosity of blood is dependent on the haematocrit, the relative viscosity of blood in small vessels will be lower than that in large vessels. Experimentally, this was shown to become significant in blood vessels with a diameter of 300 μm or less and is known as the Fåhraeus–Lindqvist effect (Fig. 8.3). It provides an explanation for the fact that the relative viscosity of blood flowing through a vascular bed (which has lots of small-diameter vessels) is lower than the relative viscosity measured in a glass viscometer (which has a large tube diameter). A key point in understanding this complex phenomenon is to recognize that axial accumulation means that the red cells flow faster than the plasma in blood vessels.
Capillary blood flow
The smallest blood vessels which have the thinnest walls behave effectively as rigid tubes. This, at first, may seem surprising. In very small blood vessels concepts such as laminar flow have little meaning as capillaries have a diameter in the range of 3–8 μm. Red blood cells have a diameter of about 8 μm which means that they are a very tight fit passing through many capillaries. Clearly, either the capillary or the red cell has to deform and it is, in fact, the red cell which changes shape (see Chapter 11). As red cells are such a tight fit in capillaries, there is a region of trapped plasma in between successive cells. This is referred to as bolus flow.
Elasticity of blood vessel walls
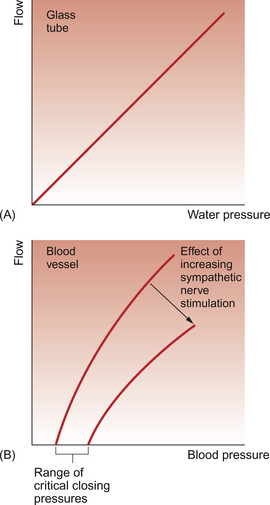
Large blood vessels are distensible. For a rigid (e.g. glass) tube with a laminar flow pattern, there would be a linear relationship between pressure gradient across the ends of the tube and flow down the tube (Fig. 8.4A). In a large blood vessel, Figure 8.4B shows that the relationship between flow and pressure gradient is different in two ways:
1. there is an intercept on the pressure axis (critical closing pressure)
2. there is a curvilinear relationship between driving pressure and flow.
The critical closing pressure means that there has to be a certain pressure inside the vessel in order to keep it inflated, i.e. if the pressure gradient across the wall of a blood vessel (transmural pressure) falls below a certain limiting value, then the vessel will collapse and flow will cease. Stimulation of sympathetic vasoconstrictor nerves to a blood vessel will increase the critical closing pressure and so a higher pressure will be needed inside the vessel in order to keep it open. This concept is important in understanding the shutdown of some blood vessels in circulatory shock. The fall in arterial blood pressure in shock leads, via the baroreceptor reflex, to sympathetically induced vasoconstriction in the arterioles (see Chapter 10). The pressure of blood in the vessels after this increased resistance will fall and, hence, there is a tendency for vessels to collapse (see Chapter 14).
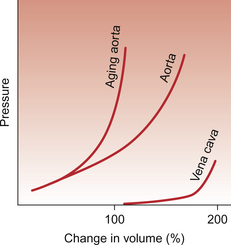
The curvilinear relationship between blood pressure and flow (Figure 8.4B) is attributed to the fact that a blood vessel will be distended as the pressure inside it increases. Blood flow will also therefore increase. The extent to which a blood vessel can be distended by increasing the internal pressure inside it will depend on the blood vessel size and wall structure. Figure 8.5 shows a comparison between an ‘old’ and a ‘young’ aorta and a vein. Firstly, it can be seen that the vein is more distensible than the arteries. Second, Figure 8.5 shows that an aorta from a young person is easier to distend than an aorta from an older individual. Distensibility is the increase in volume of a blood vessel per unit increase of pressure inside it. Veins have relatively thin walls which contain only small amounts of collagen compared to arteries (see Chapter 1). Veins are easily distended and are, therefore, referred to as capacitance vessels. Normally, the veins contain approximately two thirds of the body’s total blood volume at any one time. Extra blood transfused into a person would primarily be accommodated in the veins and, correspondingly, blood loss would particularly result in a decrease in the volume held in the veins. Distribution of blood volume within the circulatory system is shown in Figure 1.7.
Pathology of arteries and veins
Congenital defects
Congenital abnormalities of arteries and veins are often related to an abnormal course, pattern of branching or anatomical relations of a vessel. This may be particularly important during surgery. An abnormally positioned coronary artery may predispose the patient to cardiac arrhythmias or even sudden death (see Chapter 3).
Atherosclerosis
Atherosclerosis is the hardening and narrowing of arteries due to atheroma. This term is derived from a Greek word meaning porridge, so atherosclerosis may be thought of as hardened porridge in the artery wall. It is a problem which occurs in large blood vessels (>2 mm internal diameter) which are exposed to high blood pressures. The vessels most commonly affected are the aorta, carotid, coronary, iliac and femoral arteries. The pulmonary arteries are only affected after the development of pulmonary hypertension and veins do not develop atherosclerotic lesions unless they are transplanted to the high pressure arterial side of the circulation. This point is of particular relevance to coronary artery bypass grafting (CABG) when veins from the legs are often used to bypass diseased coronary arteries (p. 59).
Pathogenesis of atherosclerosis
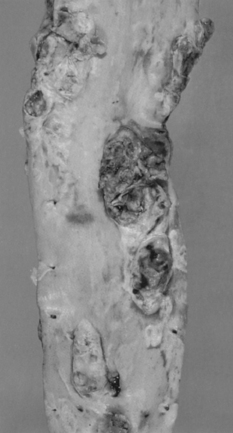
Final development of the plaque into a hard, white fibrolipid plaque (Fig. 8.6) follows further production of collagen and sometimes calcification of accumulated extracellular lipid. The damaged endothelial layer may ulcerate providing a site for thrombosis to form.
The process of atherosclerosis described above has many of the characteristics of an inflammatory response. These include invasion of monocytes which become macrophages, the involvement of T lymphocytes, the production of cytokines and growth factors and, in the later stages, focal necrosis in the blood vessel wall. Answering the question: ‘An inflammatory response to what?’ may prove productive in the future.
Pathological consequences of atheroma
Major blood vessels may become narrowed by atheroma hence reducing blood flow. This may range from a fairly modest change which is insufficient to cause symptoms to almost complete interruption of flow (Fig. 8.7). This frequently results in ischaemic heart disease, or inadequacy of cerebral blood flow or peripheral vascular disease as described in the history of Jamshed in Case 8.1:1.
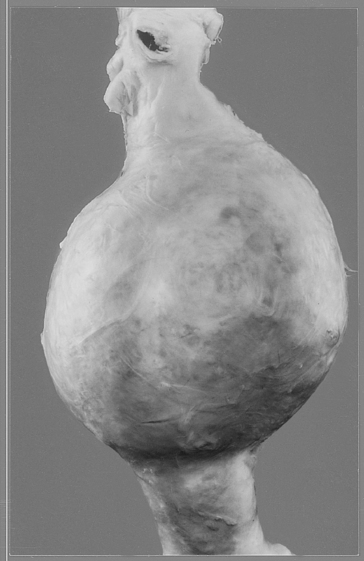
Weakening of the blood vessel wall as a consequence of atheroma may lead to the formation of an aneurysm, a region where the weakened wall balloons out (see p. 94). The most common site for this to occur is in the abdominal aorta (Fig. 8.8).
Vasculitis
Giant cell arteritis—occurs in elderly patients as a granulomatous inflammation of the aorta and its branches, often the temporal artery.
Takayasu’s arteritis—occurs in younger patients as a granulomatous inflammation of the aorta and its branches.
• Medium sized vessel vasculitis:
Polyarteritis nodosa—often called classical polyarteritis which occurs in 40–50 year olds, mainly men. Often occurs at branch points of arteries.
Kawasaki’s disease—occurs in children. Any size vessel may be involved but there is a predilection for coronary arteries.
Wegener’s granulomatosis—usually occurs in adults. It is a granulomatous inflammation of the respiratory tract with medium/small blood vessels also involved. Renal glomerular necrosis is common. Blood ANCA (anti-neutrophil cytoplasmic antibodies) levels are a disease marker.
Churg–Strauss syndrome—occurs in adults and the inflammation typically includes numerous eosinophils. Respiratory tract involvement may occur and the patient may suffer from asthma.
Microscopic polyangiitis/polyarteritis—occurs in adults and may cause glomerular necrosis. It is associated with ANCA.
Henoch–Schönlein purpura—occurs in children and adults and is relatively common compared to other types of vasculitis. IgA is seen in vascular deposits. It may involve joints, glomeruli and the bowel.
Essential cryoglobulinaemic vasculitis—cryoglobulins are present in the blood with skin and glomerular capillaries usually involved.
Cutaneous leukocytoclastic angiitis—occurs in all age groups; skin vasculitis.
Vascular pathology of diabetes mellitus
Broadly speaking, the vascular effects of diabetes can be divided into:
Microvasculopathy
Hyaline arteriolosclerosis is a frequent finding in the arteriolar (and capillary) circulation of diabetic patients. This thickening of the vessel wall, which looks very pink in a section stained with haematoxylin and eosin, may be due to increased flow of plasma proteins across the vessel wall leading to deposition of high relative molecular mass proteins such as fibrinogen and LDL cholesterol. It is important to realize that hyaline arteriolosclerosis is also seen in amyloidosis, benign hypertension and in the arterioles of the elderly. This microvasculopathic process causes ischaemic lesions in the retina associated with haemorrhage and vessel proliferation and in peripheral nerves with the resulting neuropathy leading to skin insensitivity to pain and ulceration.
Aneurysms
By far the commonest type of aneurysm is that caused by atheroma (Fig. 8.8). These aneurysms are most often located in the distal part of the abdominal aorta close to the bifurcation into the common iliac arteries. The build up of intimal atheroma weakens the arterial wall, mural fibrosis occurs and there is thrombus formation on the luminal surface. With the pulsatile arterial pressure, the artery gradually dilates and may erode on the posterior side of the aorta into the vertebral bodies giving the patient back pain. An aneurysm on the anterior side of the aorta may present as a palpable, pulsatile abdominal mass.
• Rupture: this may lead to torrential and often fatal haemorrhage into the peritoneal cavity (haemoperitoneum).
• Embolus formation: variable sized fragments may split off from the intimal thrombus and cause occlusion of the small arteries in the legs and feet leading to gangrenous necrosis.
Other forms of aneurysm include the following:
• Infective aneurysm: also known as a mycotic aneurysm, this rare condition occurs when an infected embolus lodges in an artery and allows seeding of the vessel wall by the microbes. This classically occurs as a consequence of an embolus from an infected vegetation on a heart valve as in infective endocarditis (see Chapter 3). The vessel wall can become inflamed, soft and rupture. It is also possible for infective aneurysms to occur in septicaemia.
• Syphilitic aneurysms occur in the tertiary stage of syphilis, typically in the ascending thoracic (rather than abdominal) aorta. The disease process, which starts as an inflammatory process around the vasa vasorum of the adventitia, may lead to a thick-walled, dilated aorta which may extend back as far as the aortic root at the heart causing valvular incompetence.
• Vasculitic aneurysms. Macroscopic polyarteritis nodosa may lead to aneurysm formation by causing inflammation, necrosis, thrombosis, fibrosis and weakening of the vessel wall. With fragmentation of the elastic lamina, aneurysm formation can occur.
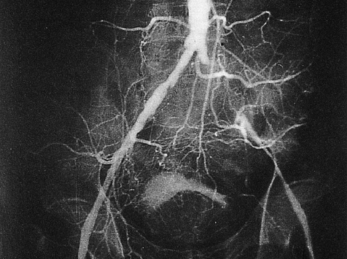
Non-invasive techniques for the assessment of arteries and veins
Magnetic resonance (MR) angiography is increasingly used for non-invasive imaging of blood flow and vessel structure. Modern systems can produce high resolution three dimensional reconstructions of vascular trees. These are of enormous value to the surgeon or interventional radiologist.
These modalities are gradually replacing invasive angiography in which a radio-opaque medium is injected into the vessel of interest under X-ray screening. The application of all these techniques to cardiac investigations is described more fully in Chapter 3.
Assmann, G., Nofer, J-R. Atheroprotective effects of high density lipoproteins. Annu. Rev. Med.. 2003; 54:321–341.
Bass, P., Burroughs, S., Carr, N., Way, C. Master Medicine: General and Systematic Pathology, third ed. Churchill Livingstone: Edinburgh; 2009.
Becker, A. E., de Boer, O. J., van der Wal, A. C. The role of inflammation and infection in coronary artery disease. Annu. Rev. Med.. 2001; 52:289–297.
Donnelly, R., London, N. J. M. ABC of Arterial and Venous Disease. London: BMJ Books; 2000.
Forbes, C. D., Jackson, W. F. Colour atlas and text of clinical medicine, third ed. Edinburgh: Mosby; 2002.
Gallagher, P. J. Cardiovascular system. In Underwood J. C. E., ed. : General and Systematic Pathology, fourth ed., Edinburgh: Churchill Livingstone, 2004.
Hamilton, C. A., Miller, W. H., Al-Benna, S., et al. Strategies to reduce oxidative stress in cardiovascular disease. Clin. Sci.. 2004; 106:219–234.
Hansson, G. K., Robertson, A-K. L., Soderberg-Naucler, C. Inflammation and atherosclerosis. Annu. Rev. Pathol. Mech. Dis.. 2006; 1:297–329.
Hunt, B. J., Poston, L., Schachter, M., Halliday, A. W. Introduction to Vascular Biology: From Basic Science to Clinical Practice, second ed. Cambridge: Cambridge University Press; 2002.
Jennette, J. C., Falk, R. J. Medical progress: small vessel vasculitis. N. Engl. J. Med.. 1997; 337:1512–1523.
Levick, J. R. An introduction to cardiovascular physiology, fifth ed. London: Arnold; 2009.
Smith, J. J., Kampine, J. P. Circulatory Physiology—The Essentials, third ed. Baltimore: Williams and Wilkins; 1990.
Stevens, A., Lowe, J. Pathology, second ed. Edinburgh: Mosby; 2000.
Underwood, J. C. E. General and Systematic Pathology, fourth ed. Edinburgh: Churchill Livingstone; 2004.