Laparoscopic Adnexal Surgery
Ovarian Cystectomy
Ovarian cystectomy is the treatment of choice for the conservative management of ovarian cysts presumed to be benign. Simple aspiration is associated with a high recurrence rate, and cyst fluid cytology is unreliable. Transvaginal ultrasonography is used to evaluate an ovarian cyst. High-risk criteria on ultrasonography for predicting the pathologic diagnosis, such as a cystic-solid mass or ascites, are a contraindication to laparoscopic surgery unless a dermoid or endometriosis is suspected. A preoperative CA-125 level is useful in postmenopausal, but not in premenopausal, women. The same principle applies to Doppler flow assessment. Magnetic resonance imaging (MRI) does not help in distinguishing a malignant from a benign mass.
An attempt should be made to remove the cyst without rupture. Equivalent rates of rupture are found with laparotomy and laparoscopy. Rupture of a dermoid cyst does not appear to be associated with any short-term complications if copious irrigation is used. Intraoperative rupture of stage I ovarian carcinomas does not appear to affect prognosis.
The patient should have consented to a laparotomy if a cancer is found. Pneumatic compression stockings are placed on the calves. An orogastric tube is used if stomach distention is suspected. An examination is carried out with the patient under anesthesia, and a Foley catheter is inserted.
To perform ovarian cystectomy, the standard three-puncture technique is used. The peritoneal cavity is systematically assessed as described in Chapter 116. Peritoneal fluid should be obtained for cytology. If no excrescences or peritoneal signs of malignancy are present, the surgeon can proceed with the cystectomy.
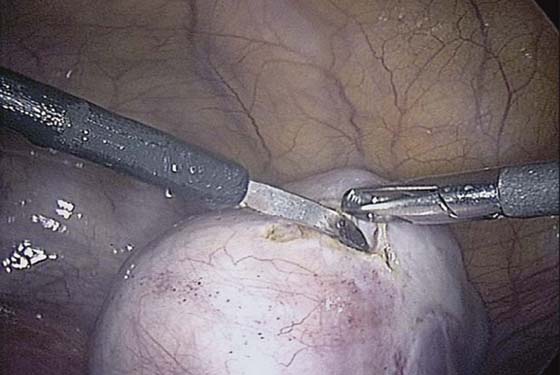
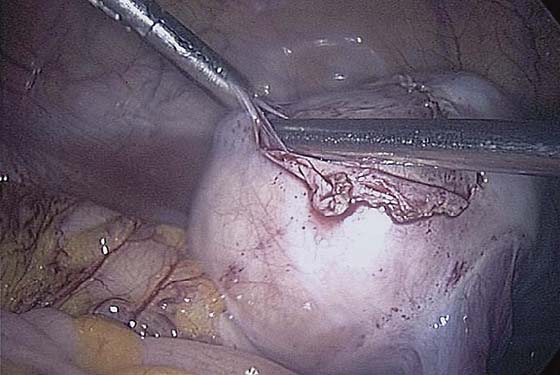
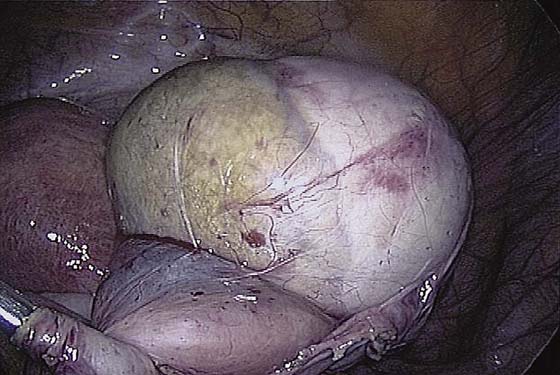
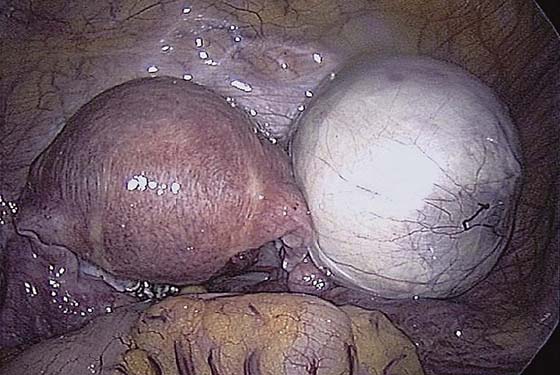
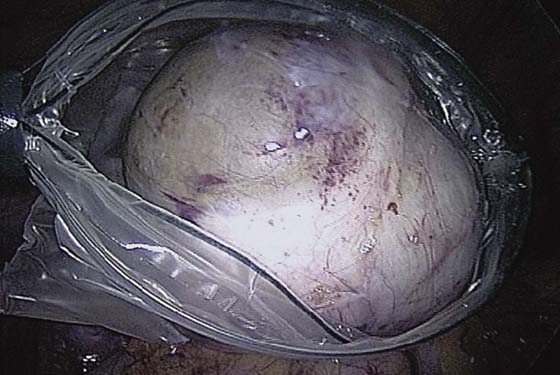
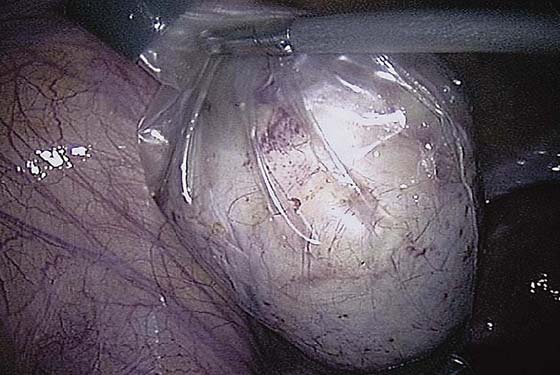
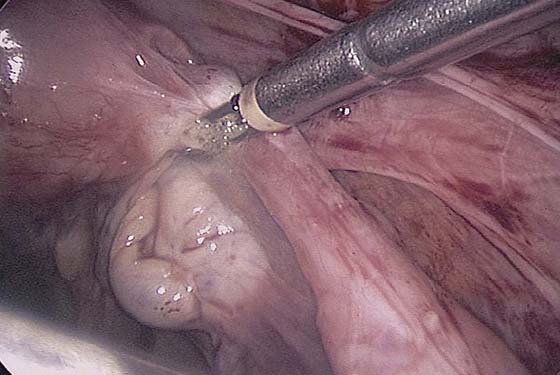
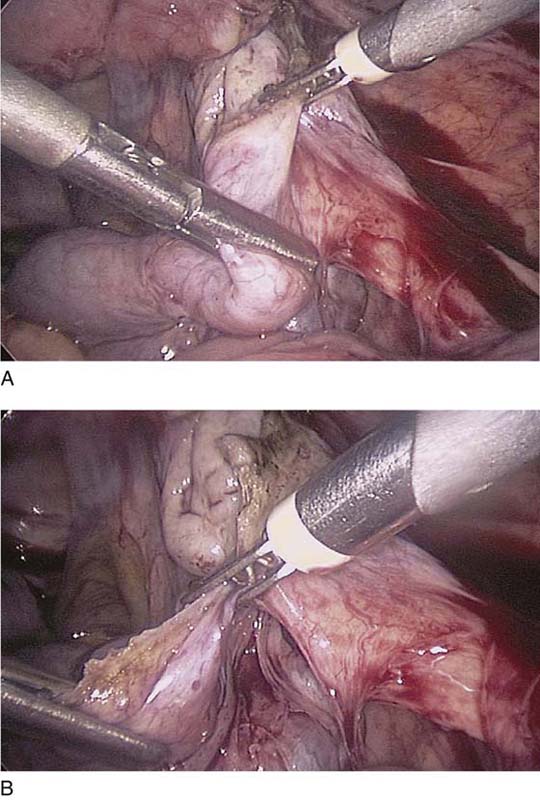
The ovarian cortex is coagulated, and an incision is made (Fig. 118–1). The edge of the cortex is grasped with an Allis forceps and dissected from the cyst. The cyst can be separated from the cortex with blunt dissection using the suction-irrigation device (Fig. 118–2). The cyst is then enucleated from the ovary (Fig. 118–3) and is placed into the anterior cul-de-sac (Fig. 118–4). Bipolar cautery is used to achieve hemostasis from any blood vessels encountered. Once the cyst is ready to be removed from the peritoneal cavity, it is placed in a bag (Fig. 118–5). The bag is then brought up to the skin, and the cyst decompressed within it (Fig. 118–6). The cyst can then be morcellated out of the bag. The ovarian cortex is not usually closed, but a simple suture can be applied to close a deep defect.
FIGURE 118–1 The ovarian cortex is coagulated and incised.
FIGURE 118–2 A suction-irrigation device is used to get a plane of dissection between the cortex of the ovary and the cyst.
FIGURE 118–3 The cyst is dissected from the ovary with a traction-countertraction technique.
FIGURE 118–4 The cyst is placed in the cul-de-sac so that the ovary can be inspected for bleeding.
FIGURE 118–5 The cyst is placed in the bag prior to removal.
FIGURE 118–6 The bag is brought through a port site. Most of the bag remains within the peritoneal cavity. The cyst is ruptured within the bag, the contents suctioned, and the solid parts removed in pieces.
Salpingo-oophorectomy
A salpingo-oophorectomy is the treatment of choice for ovarian cysts found in perimenopausal and postmenopausal women because the chance of rupture is reduced substantially. The same preoperative and intraoperative preparation as for the cystectomy is performed prior to a salpingo-oophorectomy. A retroperitoneal approach is recommended.
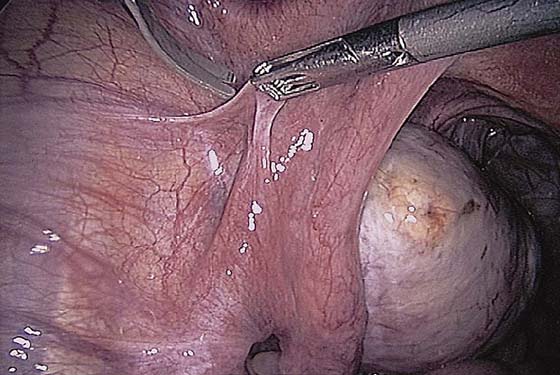
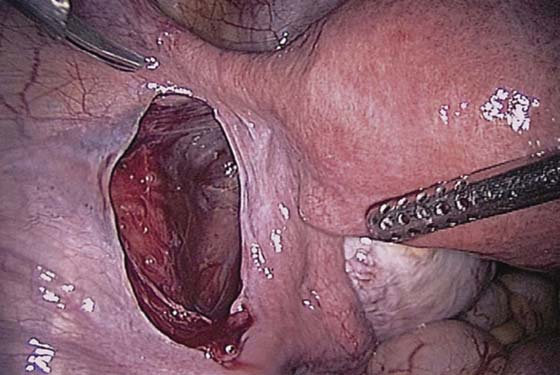
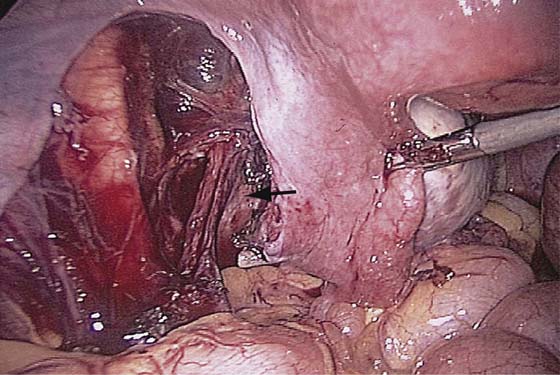
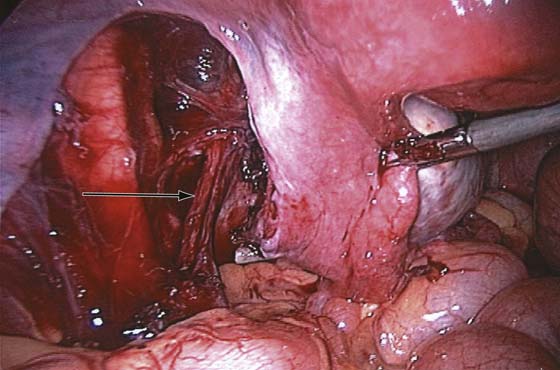
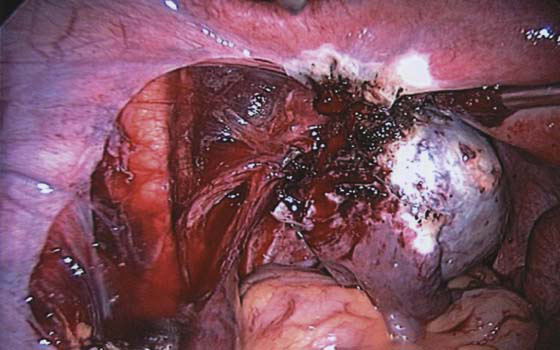
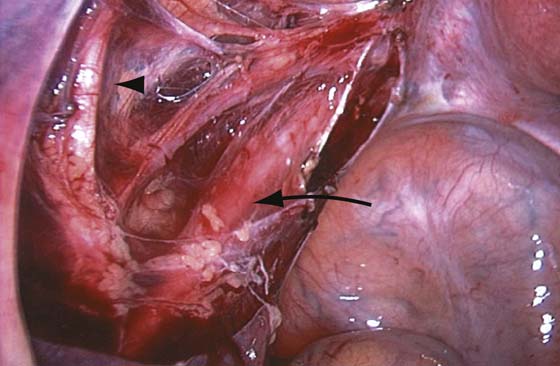
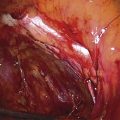
The technique of ovarian salpingo-oophorectomy is as follows. The peritoneum is incised lateral to the ovarian vessels, and the retroperitoneal space is identified (Fig. 118–7). Blunt dissection is used to identify the ureter that is attached to the medial leaf of the broad ligament (Fig. 118–8). A window is then made in the broad ligament above the ureter. The ovarian vessels are coagulated and cut (Fig. 118–9). The ureter is always identified before any coagulation is performed (Fig. 118–10). The utero-ovarian ligament is then coagulated and cut (Fig. 118–11). The specimen is placed in a bag. The anatomy of the retroperitoneal space is clearly seen at the end of the dissection (Fig. 118–12).
Ectopic Pregnancy
Prospective randomized clinical trials have demonstrated an advantage of laparoscopy over laparotomy for treatment of ectopic pregnancy. Tubal rupture can make surgery for salvage of the tube more difficult; however, no preoperative criteria can predict tubal rupture, and therefore the surgeon should be prepared to proceed appropriately.
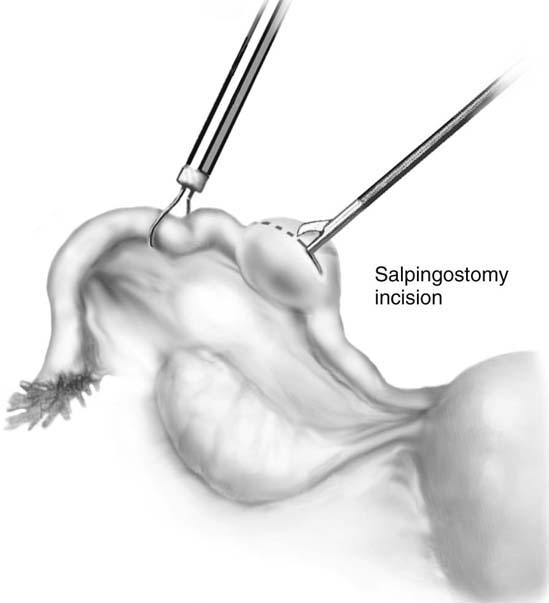
The use of dilute vasopressin helps ensure hemostasis and reduces the need for electrocautery; however, it should not be used in hypertensive patients. Salpingostomy can be performed for ampullary ectopic pregnancies. Closure of the tube is not required. An exclusively isthmic ectopic pregnancy is usually managed with partial salpingectomy and anastomosis. A total or partial salpingectomy is performed if the tube is damaged beyond repair, if the patient has had prior tubal surgery or previous ectopic pregnancy within the ipsilateral tube, or if fertility is no longer desired.
The technique of salpingostomy is as follows. A standard laparoscopy with three ports is used. If a large quantity of blood is present in the peritoneal cavity, a larger port (10 mm) should be inserted to aspirate with a 10-mm suction cannula.
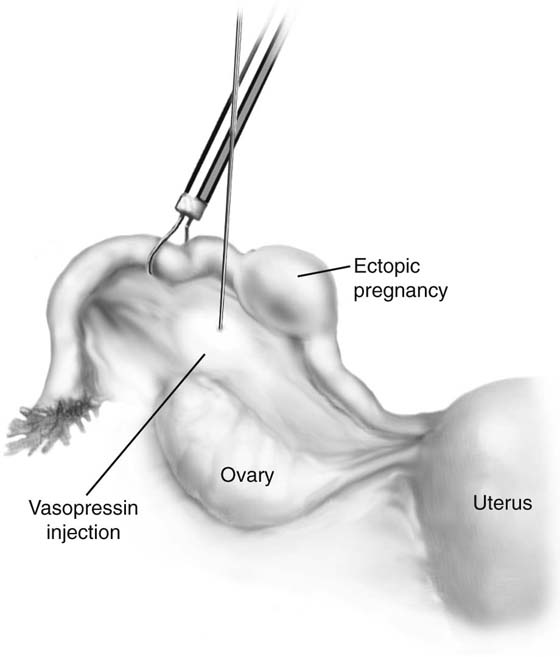
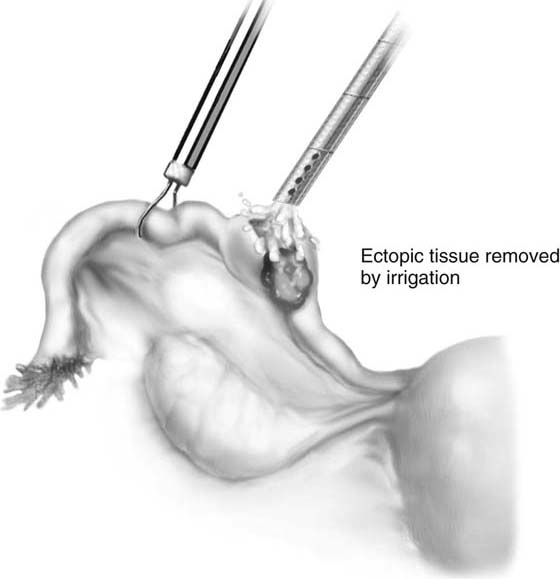
If it is an unruptured ectopic pregnancy, dilute vasopressin (10 IU in 100 mL of saline solution) is injected subserosally into the mesosalpinx beneath the mass, as well as where the incision will be made (Fig. 118–13). The serosa on the antimesenteric side is coagulated. The tubal wall is incised with scissors (Fig. 118–14). Hydrodissection is used to facilitate removal of the products of conception (Fig. 118–15). The implantation site is irrigated and observed for hemostasis. Bleeding is controlled with bipolar cautery. The specimen is extracted through the 10-mm port (usually umbilical) with a 5-mm laparoscope inserted through a lower port.
For salpingectomy, a standard three-port technique is used. Adhesiolysis is performed and the tube freed. The proximal tube is electrocoagulated and cut (Figs. 118–16 and 118–17). The mesosalpinx is then serially coagulated and cut, staying close to the tube to avoid compromising the blood supply to the ovary (Fig. 118–18A, B). The specimen is removed through the 10-mm port.
Follow-up serum human chorionic gonadotropin (hCG) levels should be obtained weekly until the level is less than the threshold for the lab. Rh-negative patients should receive RhoGAM.
FIGURE 118–7 The peritoneum lateral to the ovarian vessels and cephalad to the round ligament is grasped and incised.
FIGURE 118–8 The retroperitoneal space is dissected, and the ureter is identified.
FIGURE 118–9 The ovarian vessels are cauterized with bipolar cautery.
FIGURE 118–10 The ureter (arrow) is always in view throughout the procedure.
FIGURE 118–11 The utero-ovarian ligament is coagulated.
FIGURE 118–12 The ureter is clearly seen (arrow). The internal iliac artery giving off the umbilical artery (arrowhead) and the uterine artery are seen. The uterine artery runs parallel to the ureter before crossing over it.
FIGURE 118–13 Dilute vasopressin is injected into the mesosalpinx.
FIGURE 118–14 An incision is made with scissors.
FIGURE 118–15 An irrigation cannula is inserted into the ectopic area, and using hydrodissection, the pregnancy is delivered through the incision.
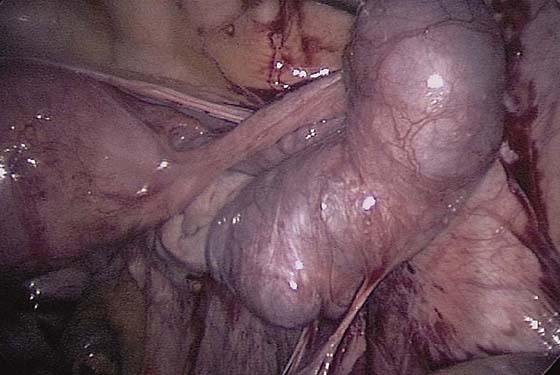
FIGURE 118–16 A large tube is distended with an ectopic pregnancy.
FIGURE 118–17 The proximal tube is coagulated.
FIGURE 118–18 A and B. The mesosalpinx is serially coagulated and cut.
Tubal Ligation
A tubal ligation can be performed with electrocautery, with the application of Silastic, Hulka, or Filshie clips. The fallopian tube is identified at the fimbriated end before proceeding with the tubal ligation.
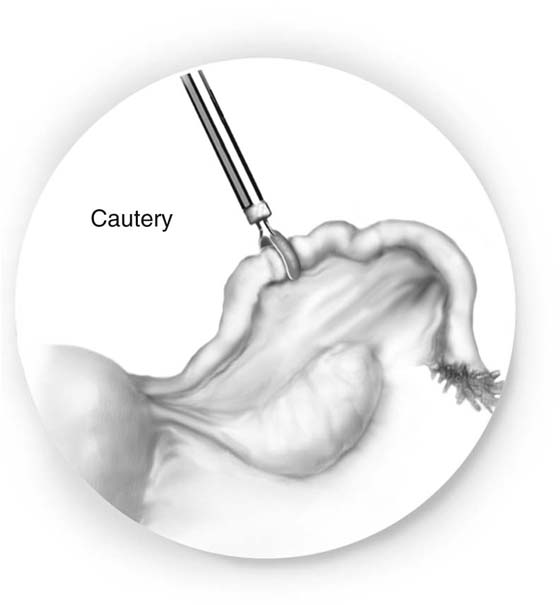
For the electrocautery technique, the fallopian tube is cauterized 2 cm from the junction with a 40W cutting current. Two or three contiguous areas are cauterized (Fig. 118–19).
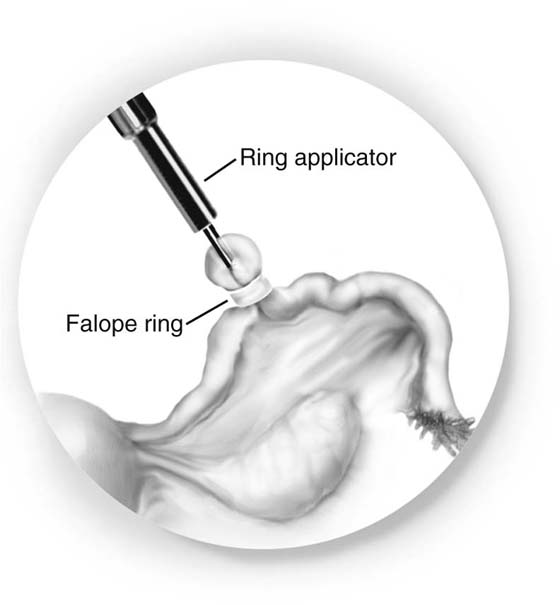
For the Falope ring technique, the tube is grasped 2 cm from the uterus with the applicator. The tube is drawn into the cylinder, and the band applied across a loop of it (Fig. 118–20). The applicator should be moved forward as the tube is brought into it, or the tube could be transected. At the end of the procedure, the loop of tube is inspected to ensure that two complete lumina are distal to the band.
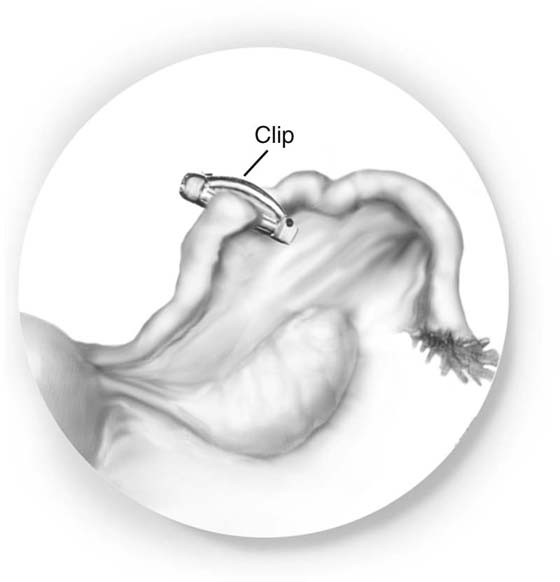
With the clip technique, the tube is grasped 2 cm from the uterus with the applicator that has a clip already in place (Fig. 118–21). Once it is clear that the clip is applied across the entire tube, it is clamped down firmly. One clip per tube is sufficient.
Tuboplasty
Patients with moderate to severe tubal disease by the American Fertility Society classification should usually be treated with in vitro fertilization rather than surgery. Infertility patients who are candidates for tubal surgery should have an assessment of the tube during hysterosalpingography or at the time of surgery. These criteria should be met:
 Thin-walled hydrosalpinges with mild dilation
Thin-walled hydrosalpinges with mild dilation
 Minimal peritubal adhesions
Minimal peritubal adhesions
 Preservation of mucosal folds
Preservation of mucosal folds
A fimbrioplasty is performed when fimbrial phimosis, a constriction of the distal tube, is present. A tuft of fimbriae may be extruding from the distal lumen. A neosalpingostomy is performed when the distal end of the tube is totally occluded.
Scissors should be used with no energy. Bipolar and microbipolar electrocautery should be used.
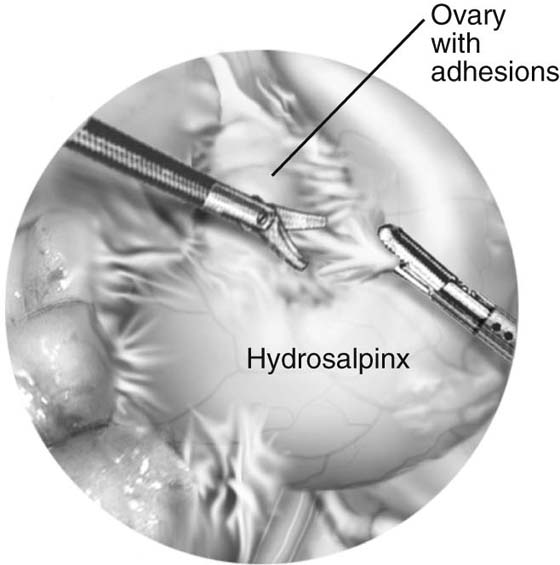
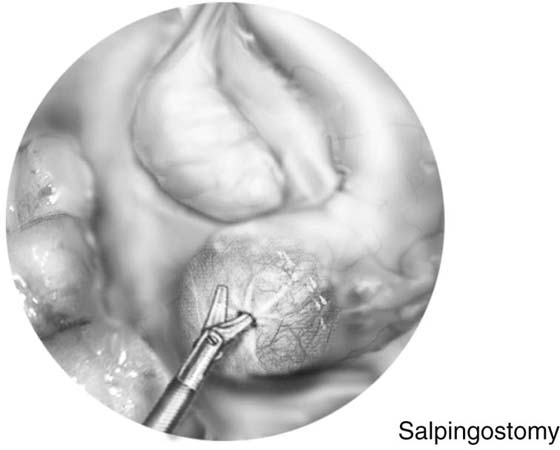
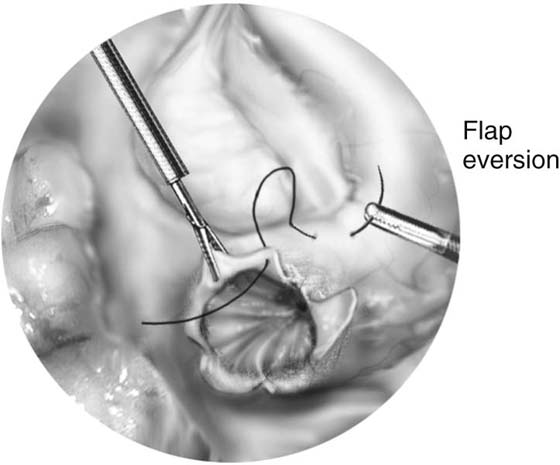
The technique for neosalpingostomy is as follows. The procedure is started with lysis of adhesions. The principles of traction and countertraction are used to develop tissue planes (Fig. 118–22). Chromotubation will dilate the distal tube. Dilute vasopressin is injected into the distal end. This will decrease the need for use of electrocautery. The tube is opened with scissors (Fig. 118–23). A cruciate incision is formed. A 4-0 to 5-0 reabsorbable suture is used to evert the edges. The suture goes through the mucosa from inside out and then through the serosa again distally (Fig. 118–24). It is tied intracorporeally. Usually two to three sutures are sufficient.
FIGURE 118–19 A bipolar cautery grasps the tube and cauterizes two or three contiguous areas.
FIGURE 118–20 A band is placed across a loop of tube.
FIGURE 118–21 A clip is placed across the entire tube.
FIGURE 118–22 Adhesions are excised with fine scissors using the principles of traction and countertraction.
FIGURE 118–23 The tube is distended with fluid, the vasopressin is injected, and a cruciate incision is made with scissors.
FIGURE 118–24 The stitch goes from inside the lumen and out through the serosa; then the needle is inserted through the serosa of the tube a few millimeters away.

 Mark D. Walters
Mark D. Walters