Laboratory haematology II – Coagulation and the acute phase response
Simple tests of blood coagulation
Quantitation of plasma fibrinogen
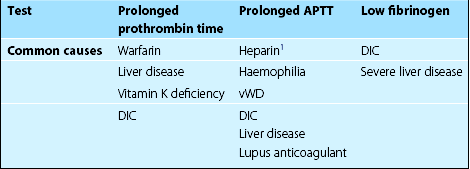
Common clinical causes of abnormal first-line coagulation tests are shown in Table 10.1. Second-line tests may be needed for more precise diagnosis. In mixing experiments (or correction tests) patient plasma is mixed with normal or factor-deficient plasma prior to repeating first-line tests. If a particular coagulation factor is thought to be lacking, a quantitative assay can then be performed. A circulating inhibitor of coagulation is suggested by failure of the coagulation abnormality to be corrected by the addition of normal plasma. Many routine tests are now automated. Most coagulation instruments rely on measurement of changes in optical density to detect clot formation.
Measurement of the acute phase response
ESR
In this simple and inexpensive test venous blood (in citrate anticoagulant) is drawn up into a vertical tube (Fig 10.1) and allowed to stand for 1 hour. The red cells settle out of suspension and the length of plasma cleared after the hour is measured. The normal values are less than 5 mm/hour in men and less than 7 mm/hour in women, although values of up to 15 mm/hour are not infrequent in those over 60 years old. The test mainly reflects fibrinogen levels but is also influenced by α2-macroglobulin, immunoglobulins and albumin. These proteins buffer the electrostatic repellent forces on the red cell membrane and allow the cells to come together and form reversible aggregates or rouleaux which fall more quickly through the plasma. The ESR result is affected by the haemoglobin concentration with high values seen in anaemia and low values in polycythaemia. A fresh sample must be processed as the result also changes over time.
Plasma viscosity
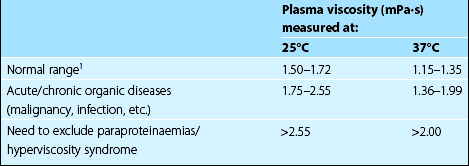
This test also measures the acute phase response indirectly, the result correlating with fibrinogen and immunoglobulin levels. The plasma viscosity has some advantages over the ESR. The normal range is the same in males and females and the result is independent of haemoglobin concentration. The sample can be taken from the EDTA anticoagulated blood count bottle and the test does not need to be performed immediately. The normal range, which is temperature dependent, is detailed in Table 10.2. Plasma viscosity measurement has direct pathophysiological relevance in myeloma where very high values are seen in the hyperviscosity syndrome.
Electrophoresis
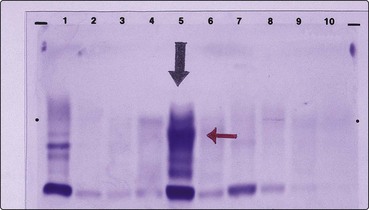
Electrophoresis has two routine applications in haematology. In the diagnosis of haemoglobinopathies (e.g. thalassaemia), cellulose acetate electrophoresis at alkaline pH is used to separate the abnormal haemoglobins. Citrate agar electrophoresis at a lower pH may be helpful in selected cases. In the investigation of myeloma, serum and urine electrophoresis is performed to detect the monoclonal immunoglobulin or light chains characteristic of the disease (Fig 10.2).
Flow cytometry
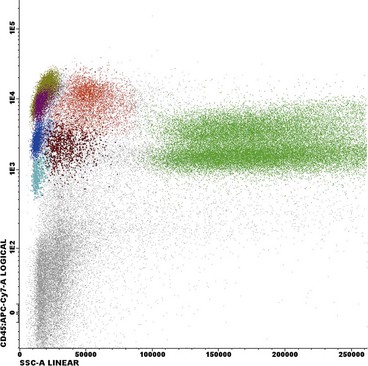
Flow cytometry is essentially the measurement of the characteristics of cells passing in a fluid stream through a detection apparatus. The automated cell counters described in the previous section are the major application of the flow cytometry principle in haematology but the technique also plays a key role in the diagnosis of haematological malignancy. Leukaemic cells often have a particular ‘immunophenotype’ – a characteristic pattern of detectable antigens on the cell surface and in the cell cytoplasm (see also relevant disease sections). The antigens are identified by cluster differentiation (CD) numbers (e.g. CD13 is a myeloid antigen; see Appendix II). Cells from peripheral blood or a bone marrow aspirate sample are incubated with specific CD monoclonal antibodies which are conjugated with a fluorochrome, a molecule which emits light at a specific wavelength when excited by a laser. The flow cytometer is then used to detect populations of cells labelled by the fluorescent marker (Fig 10.3). Flow cytometry may be used in conjunction with molecular methods for the detection of minimal residual disease in leukaemia.