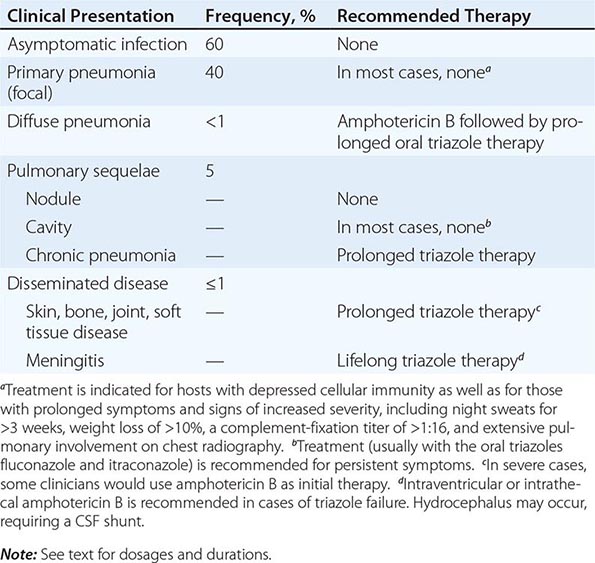
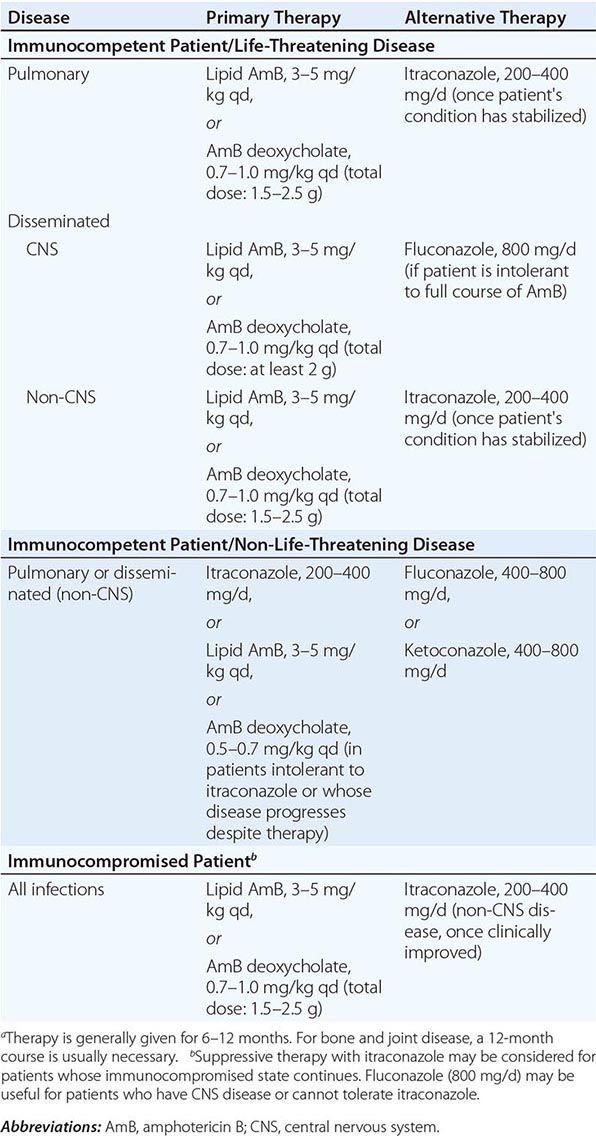
236 |
Histoplasmosis |
ETIOLOGY
![]() Histoplasma capsulatum, a thermal dimorphic fungus, is the etiologic agent of histoplasmosis. In most endemic areas, H. capsulatum var. capsulatum is the causative agent. In Africa, H. capsulatum var. duboisii also is found; var. duboisii can be differentiated from var. capsulatum as the duboisii yeasts are larger. In Central and South America, histoplasmosis is common and is caused by clades of H. capsulatum var. capsulatum that differ genetically from those involved elsewhere.
Histoplasma capsulatum, a thermal dimorphic fungus, is the etiologic agent of histoplasmosis. In most endemic areas, H. capsulatum var. capsulatum is the causative agent. In Africa, H. capsulatum var. duboisii also is found; var. duboisii can be differentiated from var. capsulatum as the duboisii yeasts are larger. In Central and South America, histoplasmosis is common and is caused by clades of H. capsulatum var. capsulatum that differ genetically from those involved elsewhere.
Mycelia—the naturally infectious form of Histoplasma—have a characteristic appearance, with microconidial and macroconidial forms. Microconidia are oval and are small enough (2–4 μm) to reach the terminal bronchioles and alveoli. Shortly after infecting the host, mycelia transform into the yeasts that are found inside macrophages and other phagocytes. The yeast forms are characteristically small (2–5 μm), with occasional narrow budding. In the laboratory, mycelia are best grown at room temperature, whereas yeasts are grown at 37°C on enriched media.
EPIDEMIOLOGY
![]() Histoplasmosis is the most prevalent endemic mycosis in North America. Although this fungal disease has been reported throughout the world, its endemicity is particularly notable in certain parts of North, Central, and South America; Africa; and Asia. In Europe, histoplasmosis is diagnosed fairly often, mostly in emigrants from or travelers to endemic areas on other continents. In the United States, the endemic areas spread over the Ohio and Mississippi river valleys. This pattern is related to the humid and acidic nature of the soil in these areas. Soil enriched with bird or bat droppings promotes the growth and sporulation of Histoplasma. Disruption of soil containing the organism leads to aerosolization of the microconidia and exposure of humans nearby. Activities associated with high-level exposure include spelunking, excavation, cleaning of chicken coops, demolition and remodeling of old buildings, and cutting of dead trees. Most cases seen outside of highly endemic areas represent imported disease—e.g., cases reported in Europe after travel to the Americas, Africa, or Asia.
Histoplasmosis is the most prevalent endemic mycosis in North America. Although this fungal disease has been reported throughout the world, its endemicity is particularly notable in certain parts of North, Central, and South America; Africa; and Asia. In Europe, histoplasmosis is diagnosed fairly often, mostly in emigrants from or travelers to endemic areas on other continents. In the United States, the endemic areas spread over the Ohio and Mississippi river valleys. This pattern is related to the humid and acidic nature of the soil in these areas. Soil enriched with bird or bat droppings promotes the growth and sporulation of Histoplasma. Disruption of soil containing the organism leads to aerosolization of the microconidia and exposure of humans nearby. Activities associated with high-level exposure include spelunking, excavation, cleaning of chicken coops, demolition and remodeling of old buildings, and cutting of dead trees. Most cases seen outside of highly endemic areas represent imported disease—e.g., cases reported in Europe after travel to the Americas, Africa, or Asia.
PATHOGENESIS AND PATHOLOGY
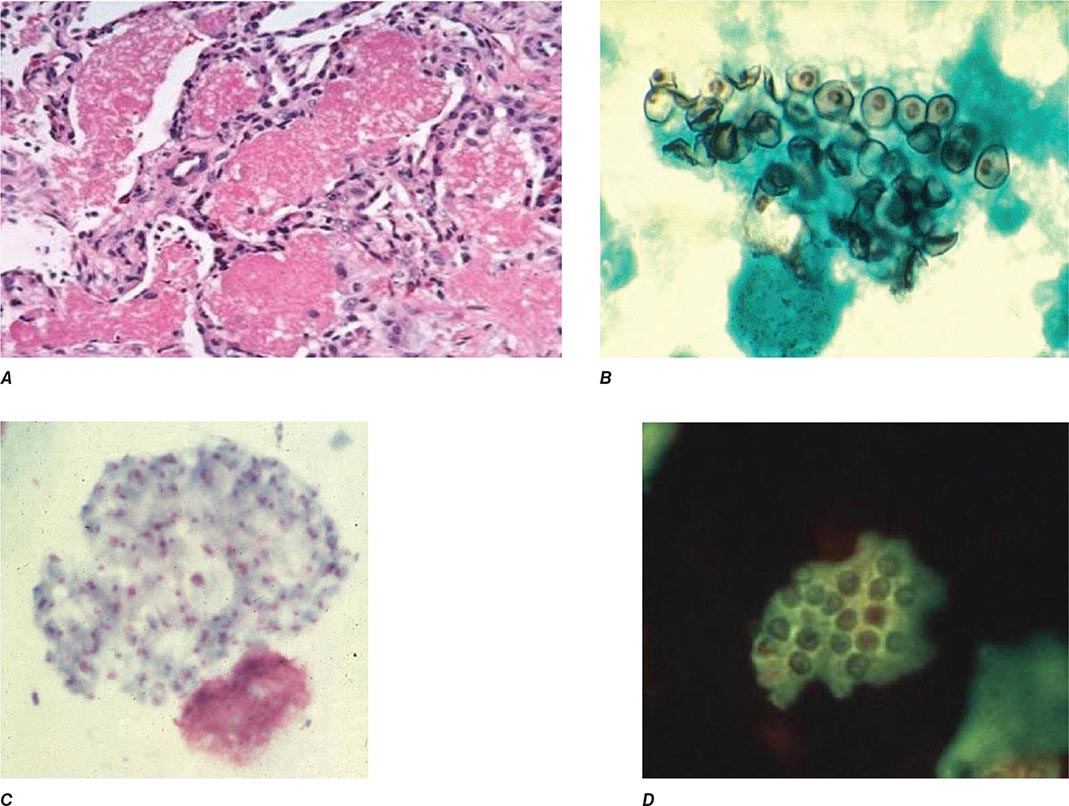
Infection follows inhalation of microconidia (Fig. 236-1). Once they reach the alveolar spaces, microconidia are rapidly recognized and engulfed by alveolar macrophages. At this point, the microconidia transform into budding yeasts (Fig. 236-2), a process that is integral to the pathogenesis of histoplasmosis and is dependent on the availability of calcium and iron inside the phagocytes. The yeasts are capable of growing and multiplying inside resting macrophages. Neutrophils and then lymphocytes are attracted to the site of infection. Before the development of cellular immunity, yeasts use the phagosomes as a vehicle for translocation to local draining lymph nodes, whence they spread hematogenously throughout the reticuloendothelial system. Adequate cellular immunity develops ~2 weeks after infection. T cells produce interferon γ to assist the macrophages in killing the organism and controlling the progression of disease. Interleukin 12 and tumor necrosis factor α (TNF-α) play an essential role in cellular immunity to H. capsulatum. In the immunocompetent host, macrophages, lymphocytes, and epithelial cells eventually organize and form granulomas that contain the organisms. These granulomas typically fibrose and calcify; calcified mediastinal lymph nodes and hepatosplenic calcifications are frequently found in healthy individuals from endemic areas. In immunocompetent hosts, infection with H. capsulatum confers some immunity to reinfection. In patients with impaired cellular immunity, the infection is not contained and can disseminate. Progressive disseminated histoplasmosis (PDH) can involve multiple organs, most commonly the bone marrow, spleen, liver (Fig. 236-3), adrenal glands, and mucocutaneous membranes. Unlike latent tuberculosis, latent histoplasmosis rarely reactivates.
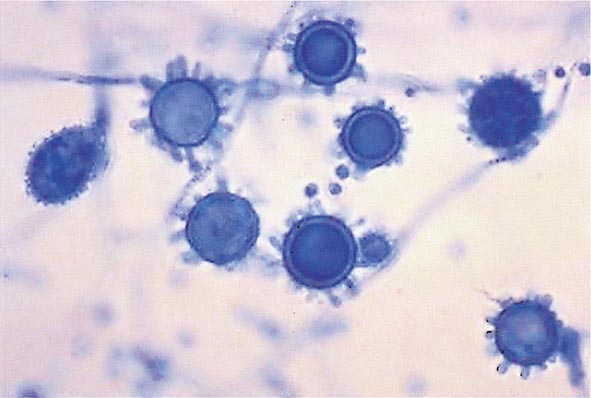
FIGURE 236-1 Spiked spherical conidia of H. capsulatum (lactophenol cotton blue stain).
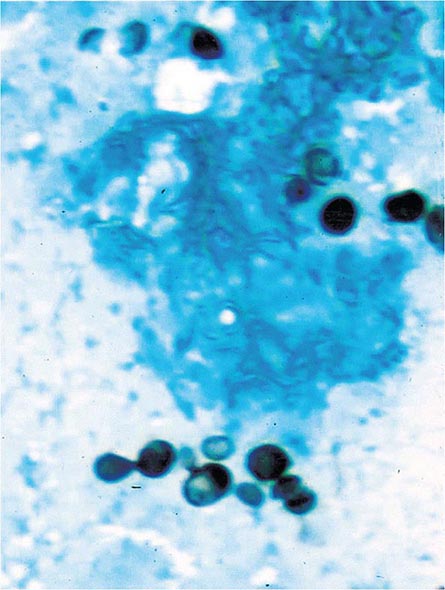
FIGURE 236-2 Small (2–5 μm) narrow budding yeasts of H. capsulatum from bronchoalveolar lavage fluid (Grocott’s methenamine silver stain).
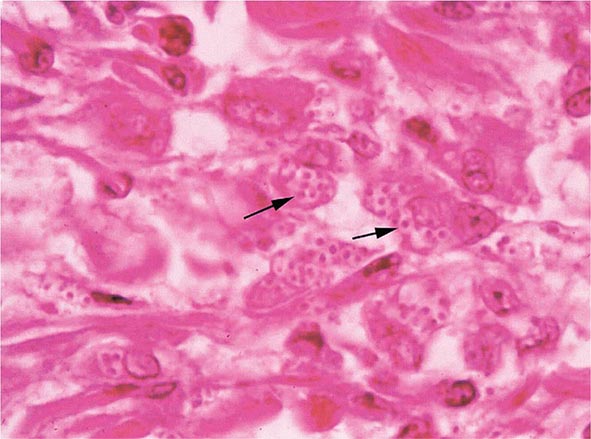
FIGURE 236-3 Intracellular yeasts (arrows) of H. capsulatum in a liver biopsy specimen (hematoxylin and eosin stain).
Structural lung disease (e.g., emphysema) impairs the clearance of pulmonary histoplasmosis, and chronic pulmonary disease can result. This chronic process is characterized by progressive inflammation, tissue necrosis, and fibrosis mimicking cavitary tuberculosis.
CLINICAL MANIFESTATIONS
The clinical spectrum of histoplasmosis ranges from asymptomatic infection to life-threatening illness. The attack rate and the extent and severity of the disease depend on the intensity of exposure, the immune status of the exposed individual, and the underlying lung architecture of the host.
In immunocompetent individuals with low-level exposure, most Histoplasma infections are either asymptomatic or mild and self-limited. Of adults residing in endemic areas, 50–80% have skin-test and/or radiographic evidence of previous infection without clinical manifestations. When symptoms do develop, they usually appear 1–4 weeks after exposure. Heavy exposure leads to a flulike illness with fever, chills, sweats, headache, myalgia, anorexia, cough, dyspnea, and chest pain. Chest radiographs usually show signs of pneumonitis with prominent hilar or mediastinal adenopathy. Pulmonary infiltrates may be focal with light exposure or diffuse with heavy exposure. Rheumatologic symptoms of arthralgia or arthritis, often associated with erythema nodosum, occur in 5–10% of patients with acute histoplasmosis. Pericarditis may also develop. These manifestations represent inflammatory responses to the acute infection rather than its direct effects. Hilar or mediastinal lymph nodes may undergo necrosis and coalesce to form large mediastinal masses that can cause compression of great vessels, proximal airways, and the esophagus. These necrotic lymph nodes may also rupture and create fistulas between mediastinal structures (e.g., bronchoesophageal fistulas).
PDH is typically seen in immunocompromised individuals, who account for ~70% of cases. Common risk factors include AIDS (CD4+ T cell count, <200/μL), extremes of age, immunosuppressive medications administered for prevention or treatment of rejection following transplantation (e.g., prednisone, mycophenolate, calcineurin inhibitors, and biologic response modifiers), and methotrexate, anti-TNF-α agents, or other biologic response modifiers given for inflammatory arthritis or Crohn’s disease.
The spectrum of PDH ranges from an acute, rapidly fatal course—with diffuse interstitial or reticulonodular lung infiltrates causing respiratory failure, shock, coagulopathy, and multiorgan failure—to a more subacute course with a focal organ distribution. Common manifestations include fever and weight loss. Hepatosplenomegaly also is common. Other findings may include meningitis or focal brain lesions, ulcerations of the oral mucosa, gastrointestinal ulcerations, and adrenal insufficiency. Prompt recognition of this devastating illness is of paramount importance in patients with more severe manifestations or with underlying immunosuppression, especially AIDS (Chap. 226).
Chronic cavitary histoplasmosis is seen in smokers who have structural lung disease (e.g., bullous emphysema). This chronic illness is characterized by productive cough, dyspnea, low-grade fever, night sweats, and weight loss. Chest radiographs usually show upper-lobe infiltrates, cavitation, and pleural thickening—findings resembling those of tuberculosis. Without treatment, the course is slowly progressive.
Fibrosing mediastinitis is an uncommon and serious complication of histoplasmosis. In certain patients, acute infection is followed for unknown reasons by progressive fibrosis around the hilar and mediastinal lymph nodes. Involvement may be unilateral or bilateral; bilateral involvement carries a worse prognosis. Major manifestations include superior vena cava syndrome, obstruction of pulmonary vessels, and airway obstruction. Patients may experience recurrent pneumonia, hemoptysis, or respiratory failure. Fibrosing mediastinitis is fatal in up to one-third of cases.
In healed histoplasmosis, calcified mediastinal nodes or lung parenchyma may erode through the walls of the airways and cause hemoptysis. This condition is called broncholithiasis.
![]() The clinical features and management of histoplasmosis caused by the genetically different clades in Central and South America are similar to those of the disease in North America. African histoplasmosis caused by var. duboisii is clinically distinct and is characterized by frequent skin and bone involvement.
The clinical features and management of histoplasmosis caused by the genetically different clades in Central and South America are similar to those of the disease in North America. African histoplasmosis caused by var. duboisii is clinically distinct and is characterized by frequent skin and bone involvement.
DIAGNOSIS
Fungal culture remains the gold standard diagnostic test for histoplasmosis. However, culture results may not be known for up to 1 month, and cultures are often negative in less severe cases. Cultures are positive in ~75% of cases of PDH and chronic pulmonary histoplasmosis. Cultures of bronchoalveolar lavage (BAL) fluid are positive in about half of cases that include acute pulmonary histoplasmosis causing diffuse infiltrates with hypoxemia. In PDH, the culture yield is highest for BAL fluid, bone marrow aspirate, and blood. Cultures of sputum or bronchial washings are usually positive in chronic pulmonary histoplasmosis. Cultures are typically negative, however, in other forms of histoplasmosis.
Fungal stains of cytopathology or biopsy materials showing structures resembling Histoplasma yeasts are helpful in the diagnosis of PDH, yielding positive results in about half of cases. Yeasts can be seen in BAL fluid (Fig. 236-2) from patients with diffuse pulmonary infiltrates, in bone marrow biopsy samples, and in biopsy specimens of other involved organs (e.g., the adrenal glands). Occasionally, yeasts are seen in blood smears from patients with severe PDH. However, artifacts and other fungal elements sometimes stain positive and may be misidentified as Histoplasma yeasts.
The detection of Histoplasma antigen in body fluids is extremely useful in the diagnosis of PDH and acute diffuse pulmonary histoplasmosis. The sensitivity of this technique is >95% in patients with PDH and >80% in patients with acute pulmonary histoplasmosis if both urine and serum are tested. Antigen level correlates with severity of illness in PDH and can be used to follow disease progression as levels predictably decrease with effective therapy. An increase in antigen levels also predicts relapse. Antigen can be detected in cerebrospinal fluid from patients with meningitis and in BAL fluid from those with pneumonia. Cross-reactivity occurs with African histoplasmosis, blastomycosis, coccidioidomycosis, paracoccidioidomycosis, and Penicillium marneffei infection.
Serologic tests, including immunodiffusion and complement fixation, are useful for the diagnosis of histoplasmosis in immunocompetent patients. Serum antibody titers may rise fourfold in patients with acute histoplasmosis. Serologic tests are especially useful for the diagnosis of chronic pulmonary histoplasmosis. The limitations of serology, however, include insensitivity early in the course of infection (at least 1 month is required for the production of antibodies), insensitivity in immunosuppressed patients, and persistence of detectable antibody for several years after infection. Positive results from past infection may lead to a misdiagnosis of active histoplasmosis in a patient with another disease process.
237 |
Coccidioidomycosis |
DEFINITION AND ETIOLOGY
Coccidioidomycosis, commonly known as Valley fever (see “Epidemiology,” below), is caused by dimorphic soil-dwelling fungi of the genus Coccidioides. Genetic analysis has demonstrated the existence of two species, C. immitis and C. posadasii. These species are indistinguishable with regard to the clinical disease they cause and their appearance on routine laboratory media. Thus, the organisms will be referred to simply as Coccidioides for the remainder of this chapter.
EPIDEMIOLOGY
![]() Coccidioidomycosis is confined to the Western Hemisphere between the latitudes of 40°N and 40°S. In the United States, areas of high endemicity include the southern portion of the San Joaquin Valley of California and the south-central region of Arizona. However, infection may be acquired in other areas of the southwestern United States, including the southern coastal counties in California, southern Nevada, southwestern Utah, southern New Mexico, and western Texas, including the Rio Grande Valley. Outside the United States, coccidioidomycosis is endemic to northern Mexico as well as to localized regions of Central America. In South America, there are endemic foci in Colombia, Venezuela, northeastern Brazil, Paraguay, Bolivia, and north-central Argentina.
Coccidioidomycosis is confined to the Western Hemisphere between the latitudes of 40°N and 40°S. In the United States, areas of high endemicity include the southern portion of the San Joaquin Valley of California and the south-central region of Arizona. However, infection may be acquired in other areas of the southwestern United States, including the southern coastal counties in California, southern Nevada, southwestern Utah, southern New Mexico, and western Texas, including the Rio Grande Valley. Outside the United States, coccidioidomycosis is endemic to northern Mexico as well as to localized regions of Central America. In South America, there are endemic foci in Colombia, Venezuela, northeastern Brazil, Paraguay, Bolivia, and north-central Argentina.
The risk of infection is increased by direct exposure to soil harboring Coccidioides. Because of difficulty in isolating Coccidioides from the soil, the precise characteristics of potentially infectious soil are not known. In the United States, several outbreaks of coccidioidomycosis have been associated with soil from archaeologic excavations of Amerindian sites both within and outside of the recognized endemic region. These cases often involved alluvial soils in regions of relative aridity with moderate temperature ranges. Coccidioides was isolated at depths of 2–20 cm below the surface. The recent identification of three cases of coccidioidomycosis in eastern Washington State may suggest that the endemic region is expanding.
In endemic areas, many cases of Coccidioides infection occur without obvious soil or dust exposure. Climatic factors appear to increase the infection rate in these regions. In particular, periods of aridity following rainy seasons have been associated with marked increases in the number of symptomatic cases. Overall, the incidence within the United States has increased substantially over the past decade, with nearly 43 cases per 100,000 residents of the endemic region in 2011. Most of that increase has occurred in south-central Arizona, where most of that state’s population resides, and in the southern San Joaquin Valley of California, a much less populated region. The factors causing this increase have not been fully elucidated; however, an influx of older individuals without prior coccidioidal infection appears to be involved. Other variables, such as climate change, construction activity, and increased awareness and reporting, may also be factors. Health care providers should consider coccidioidomycosis when evaluating persons with pneumonia who live in or have traveled to endemic areas.
PATHOGENESIS, PATHOLOGY, AND IMMUNE RESPONSE
On agar media and in the soil, Coccidioides organisms exist as filamentous molds. Within this mycelial structure, individual filaments (hyphae) elongate and branch, some growing upward. Alternating cells within the hyphae degenerate, leaving barrel-shaped viable elements called arthroconidia. Measuring ∼2 by 5 μm, arthroconidia may become airborne for extended periods. Their small size allows them to evade initial mechanical mucosal defenses and reach deep into the bronchial tree, where infection is initiated in the nonimmune host.
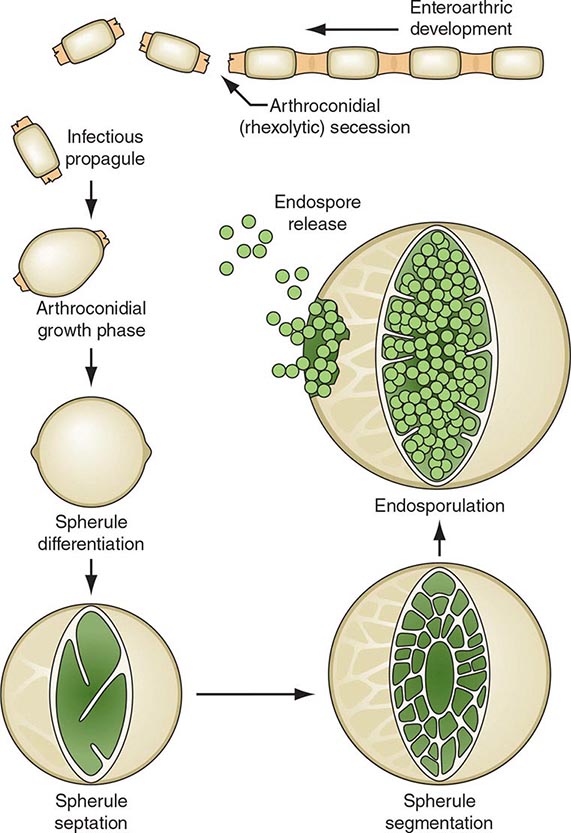
Once in a susceptible host, the arthroconidia enlarge, become rounded, and develop internal septations. The resulting structures, called spherules (Fig. 237-1), may attain sizes of 200 μm and are unique to Coccidioides. The septations encompass uninuclear elements called endospores. Spherules may rupture and release packets of endospores that can themselves develop into spherules, thus propagating infection locally. If returned to artificial media or the soil, the fungus reverts to its mycelial stage.
FIGURE 237-1 Life cycle of Coccidioides. (From TN Kirkland, J Fierer: Emerg Infect Dis 2:192, 1996.)
Clinical observations and data from studies of animals strongly support the critical role of a robust cellular immune response in the host’s control of coccidioidomycosis. Necrotizing granulomas containing spherules are typically identified in patients with resolved pulmonary infection. In disseminated disease, granulomas are generally poorly formed or do not develop at all, and a polymorphonuclear leukocyte response occurs frequently. In patients who are asymptomatic or in whom the initial pulmonary infection resolves, delayed-type hypersensitivity to coccidioidal antigens has been routinely documented.
CLINICAL AND LABORATORY MANIFESTATIONS
Of infected individuals, 60% are completely asymptomatic, and the remaining 40% have symptoms that are related principally to pulmonary infection, including fever, cough, and pleuritic chest pain. The risk of symptomatic illness increases with age. Coccidioidomycosis is commonly misdiagnosed as community-acquired bacterial pneumonia.
There are several cutaneous manifestations of primary pulmonary coccidioidomycosis. Toxic erythema consisting of a maculopapular rash has been noted in some cases. Erythema nodosum (see Fig. 25e-40)—typically over the lower extremities—or erythema multiforme (see Fig. 25e-25)—usually in a necklace distribution—may occur; these manifestations are seen particularly often in women. Arthralgias and arthritis may develop. The diagnosis of primary pulmonary coccidioidomycosis is suggested by a history of night sweats or profound fatigue as well as by peripheral-blood eosinophilia and hilar or mediastinal lymphadenopathy on chest radiography. While pleuritic chest pain is common, pleural effusions occur in fewer than 10% of cases. Such effusions are invariably associated with a pulmonary infiltrate on the same side. The cellular content of these effusions is mononuclear in nature; Coccidioides is rarely grown from effusions.
In most patients, primary pulmonary coccidioidomycosis usually resolves without sequelae in weeks. However, several pneumonic complications may arise. Pulmonary nodules are residua of primary pneumonia. Generally single, frequently located in the upper lobes, and ≤4 cm in diameter, nodules are often discovered on a routine chest radiograph in an asymptomatic patient. Calcification is uncommon. Coccidioidal pulmonary nodules can be difficult to distinguish radiographically from pulmonary malignancies. Like malignancies, coccidioidal nodules often enhance on positron emission tomography. However, routine CT often demonstrates multiple nodules in coccidioidomycosis. Biopsy is often required to distinguish between these two conditions.
Pulmonary cavities occur when a nodule extrudes its contents into the bronchus, resulting in a thin-walled shell. These cavities can be associated with persistent cough, hemoptysis, and pleuritic chest pain. Rarely, a cavity may rupture into the pleural space, causing pyopneumothorax. In such cases, patients present with acute dyspnea, and the chest radiograph reveals a collapsed lung with a pleural air-fluid level. Chronic or persistent pulmonary coccidioidomycosis manifests with prolonged symptoms of fever, cough, and weight loss and is radiographically associated with pulmonary scarring, fibrosis, and cavities. It occurs most commonly in patients who already have chronic lung disease due to other etiologies.
In some cases, primary pneumonia presents as a diffuse reticulonodular pulmonary process (detected by plain chest radiography) in association with dyspnea and fever. Primary diffuse coccidioidal pneumonia may occur in settings of intense environmental exposure or profoundly suppressed cellular immunity (e.g., in patients with AIDS), with unrestrained fungal growth that is frequently associated with fungemia.
Clinical dissemination outside the thoracic cavity occurs in fewer than 1% of infected individuals. Dissemination is more likely to occur in male patients, particularly those of African-American or Filipino ancestry, and in persons with depressed cellular immunity, including patients with HIV infection and peripheral-blood CD4+ T cell counts of <250/μL; those receiving chronic glucocorticoid therapy; those with allogeneic solid-organ transplants; and those being treated with tumor necrosis factor α antagonists. Women who acquire infection during the second or third trimester of pregnancy also are at risk for disseminated disease. Common sites for dissemination include the skin, bone, joints, soft tissues, and meninges. Dissemination may follow symptomatic or asymptomatic pulmonary infection and may involve only one site or multiple anatomic foci. When it occurs, clinical dissemination is usually evident within the first few months after primary pulmonary infection.
Coccidioidal meningitis, if untreated, is uniformly fatal. Patients usually present with a persistent headache, which is sometimes accompanied by lethargy and confusion. Nuchal rigidity, if present, is not severe. Examination of cerebrospinal fluid (CSF) demonstrates lymphocytic pleocytosis with profound hypoglycorrhachia and elevated protein levels. CSF eosinophilia is occasionally documented. With or without appropriate therapy, patients may develop hydrocephalus, which presents clinically as a marked decline in mental status, often with gait disturbances.
DIAGNOSIS
As mentioned above, coccidioidomycosis is often misdiagnosed as community-acquired bacterial pneumonia. Clues that suggest a diagnosis of coccidioidomycosis include peripheral-blood eosinophilia, hilar or mediastinal adenopathy on radiographic imaging, marked fatigue, and failure to improve with antibiotic therapy.
Serology plays an important role in establishing a diagnosis of coccidioidomycosis. Several techniques are available, including the traditional tube-precipitin (TP) and complement-fixation (CF) assays, immunodiffusion (IDTP and IDCF), and enzyme immunoassay (EIA) to detect IgM and IgG antibodies. TP and IgM antibodies are found in serum soon after infection and persist for weeks. They are not useful for gauging disease progression and are not found in the CSF. The CF and IgG antibodies occur later in the course of the disease and persist longer than TP and IgM antibodies. Rising CF titers are associated with clinical progression, and the presence of CF antibody in CSF is indicative of coccidioidal meningitis. Antibodies disappear over time in persons whose clinical illness resolves.
Because of its commercial availability, the coccidioidal EIA is frequently used as a screening tool for coccidioidal serology. There has been concern that the IgM EIA is occasionally falsely positive, particularly in asymptomatic individuals. In addition, while the sensitivity and specificity of the IgG EIA appear to be higher than those of the CF and IDCF assays, the optical density obtained in the EIA does not correlate with the serologic titer of either of the latter tests.
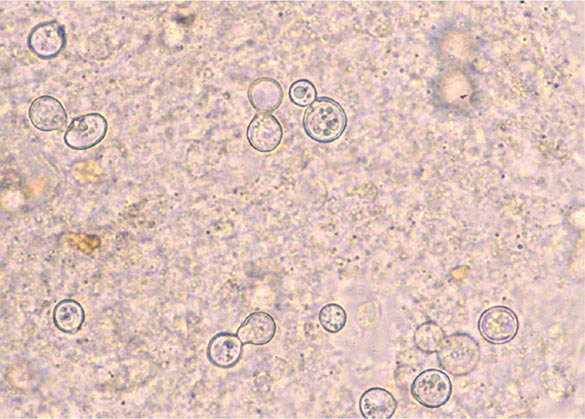
Coccidioides grows within 3–7 days at 37°C on a variety of artificial media, including blood agar. Therefore, it is always useful to obtain samples of sputum or other respiratory fluids and tissues for culture in suspected cases of coccidioidomycosis. The clinical laboratory should be alerted to the possibility of this diagnosis, since Coccidioides poses a significant laboratory hazard if it is inadvertently inhaled. The organism can also be identified directly. While treatment of samples with potassium hydroxide is rarely fruitful in establishing the diagnosis, examination of sputum or other respiratory fluids after Papanicolaou or Gomori methenamine silver staining reveals spherules in a significant proportion of patients with pulmonary coccidioidomycosis. For fixed tissues (e.g., those obtained from biopsy specimens), spherules with surrounding inflammation can be demonstrated with hematoxylin-eosin or Gomori methenamine silver staining.
A commercially available test for coccidioidal antigenuria and antigenemia has been developed and appears to be particularly useful in immunosuppressed patients with severe or disseminated disease. False-positive results may occur in cases of histoplasmosis or blastomycosis. Some laboratories offer genomic detection by polymerase chain reaction.
PREVENTION
There are no proven methods to reduce the risk of acquiring coccidioidomycosis among residents of an endemic region, but avoidance of direct contact with uncultivated soil or with visible dust containing soil is reasonable. For individuals with suppressed cellular immunity, the risk of developing symptomatic coccidioidomycosis is greater than that in the general population. Among those about to undergo allogeneic solid-organ transplantation, antifungal therapy is appropriate when there is evidence of active or recent coccidioidomycosis. Several cases of donor-transmitted coccidioidomycosis have occurred during transplantation. If possible, donors from an endemic region should be screened for coccidioidomycosis before transplantation. Data on the use of antifungal agents for prophylaxis in other situations are limited. The administration of an antifungal drug to prevent symptomatic coccidioidomycosis is not recommended for HIV-1-infected patients who live in an endemic region. Most experts would administer a triazole to patients with a history of active coccidioidomycosis or a positive coccidioidal serology in whom therapy with tumor necrosis factor α antagonists is being initiated.
238 |
Blastomycosis |
Blastomycosis is a systemic pyogranulomatous infection, involving primarily the lungs, that follows inhalation of the conidia of Blastomyces dermatitidis. Pulmonary blastomycosis varies from an asymptomatic infection to acute or chronic pneumonia. Hematogenous dissemination to skin, bones, and the genitourinary system is common; however, almost any organ can be involved.
ETIOLOGIC AGENT
B. dermatitidis is the asexual state of Ajellomyces dermatitidis. Two serotypes have been identified on the basis of the presence or absence of the A antigen. Distinct genotypic groups have been differentiated by rDNA polymerase chain reaction restriction fragment length polymorphisms and microsatellite markers. B. dermatitidis exhibits thermal dimorphism, growing as the mycelial phase at room temperature and as the yeast phase at 37°C. Primary isolation in the laboratory is most dependable for the mycelial phase incubated at 30°C. Definitive identification usually requires conversion to the yeast phase at 37°C or—now more commonly—the use of nucleic acid amplification techniques that detect mycelial-phase growth. Under the microscope, the yeast cells are usually 8–15 μm in diameter, have thick refractile cell walls, are multinucleate, and exhibit a single, large, broad-based bud (Fig. 238-1).
FIGURE 238-1 Blastomyces dermatitidis broad-based budding yeast in the aspirate of a chest wall abscess. Note the presence of multiple nuclei, the thickened cell wall, and the broad-based bud.
EPIDEMIOLOGY
Most cases of blastomycosis have been reported in North America. Endemic areas include the southeastern and south-central states bordering the Mississippi and Ohio river basins, the midwestern states, and the Canadian provinces bordering the Great Lakes. A small endemic area exists in New York and Canada along the St. Lawrence River. Acute blastomycosis is typically found only in North America, and the clinical presentation of blastomycosis in nonendemic areas is as a chronic disease.
![]() Outside North America, blastomycosis occurs sporadically in Nigeria, Zimbabwe, Tunisia, Saudi Arabia, Israel, Lebanon, and India. The disease has been reported most frequently in Africa.
Outside North America, blastomycosis occurs sporadically in Nigeria, Zimbabwe, Tunisia, Saudi Arabia, Israel, Lebanon, and India. The disease has been reported most frequently in Africa.
Early studies indicated that middle-aged men with outdoor occupations were at greatest risk. Reported outbreaks, however, do not suggest a predilection according to sex, age, race, occupation, or season. The specific niche in nature in which the organism resides remains uncertain; B. dermatitidis probably grows as microfoci in the warm, moist soil of wooded areas rich in organic debris. Inhalation of conidia following exposure to soil, whether related to work or recreation, appears to be the common factor associated with infection. Outbreaks of human disease may be preceded by the occurrence of disease in simultaneously exposed dogs. Zoonotic transmission is rare but has been reported in association with dog bites, pet kinkajou bites, cat scratches, and animal necropsies.
PATHOGENESIS
Alveolar macrophages and polymorphonuclear leukocytes are critical for phagocytosis and killing of the inhaled conidia of B. dermatitidis. The interaction of these mediators of the innate immune response with local host factors, such as lung surfactant, plays a significant role in inhibiting conversion to the pathogenic yeast form. This inhibition prevents the establishment of symptomatic disease and may account for the high frequency of asymptomatic infections in outbreaks. Once conversion to the thick-walled yeast form has occurred, phagocytosis and killing are much more difficult, and the development of clinically apparent infection is much more likely. Ultimately, the T lymphocyte response—specifically, a TH1 response—is the primary factor in limiting infection and dissemination. Moreover, yeast-phase conversion results in the expression of yeast phase–specific proteins such as the 120-kDa glycoprotein adhesin BAD-1 and the Blastomyces yeast phase–specific protein 1 (BYS1). BAD-1 has been well characterized as a virulence factor and is the major epitope for humoral and cellular immunity. The role of BYS1, putatively identified as a signal peptide, has not been determined.
CLINICAL MANIFESTATIONS
Acute pulmonary infection is often diagnosed in association with point-source outbreaks. Typical symptoms include the abrupt onset of fever, chills, pleuritic chest pain, arthralgias, and myalgias. Cough is initially nonproductive but frequently becomes purulent as disease progresses. Chest radiographs usually reveal alveolar infiltrates with consolidation. Pleural effusions and hilar adenopathy are uncommon. Most patients diagnosed with pulmonary blastomycosis have chronic indolent pneumonia with signs and symptoms of fever, weight loss, productive cough, and hemoptysis. The most common radiologic findings are alveolar infiltrates with or without cavitation, mass lesions that mimic bronchogenic carcinoma, and fibronodular infiltrates. Hematogenous dissemination to the skin, bones, and genitourinary tract occurs most often in association with chronic pulmonary disease. Although blastomycosis is not considered an opportunistic infection, immunosuppression has been recognized as a risk factor for more serious pulmonary involvement, including respiratory failure (adult respiratory distress syndrome) associated with miliary disease or diffuse pulmonary infiltrates. In the late stages of AIDS, mortality rates of ≥50% have been documented. Most deaths occur within the first few days of therapy. Solid-organ transplant recipients with endemic fungal infections, including both histoplasmosis and blastomycosis, frequently have more severe pulmonary disease as well as dissemination. Blastomycosis has been associated with a mortality rate of 36% in these patients.
![]() In Africa, pulmonary cases typically include bony involvement (frequently of the vertebrae), with subcutaneous abscesses of the chest wall or legs. All of the manifestations seen in African patients fall within the spectrum of blastomycosis observed in North America. The increased prevalence of chronic and disseminated bone disease in these patients may reflect a delay in diagnosis in regions where spinal disease is often treated empirically as tuberculosis.
In Africa, pulmonary cases typically include bony involvement (frequently of the vertebrae), with subcutaneous abscesses of the chest wall or legs. All of the manifestations seen in African patients fall within the spectrum of blastomycosis observed in North America. The increased prevalence of chronic and disseminated bone disease in these patients may reflect a delay in diagnosis in regions where spinal disease is often treated empirically as tuberculosis.
Skin disease is the most common extrapulmonary manifestation of blastomycosis. Two types of skin lesions occur: verrucous (more common) and ulcerative. Osteomyelitis occurs in as many as one-fourth of B. dermatitidis infections. The vertebrae, pelvis, sacrum, skull, ribs, and long bones are most frequently involved. Patients with B. dermatitidis osteomyelitis often present with contiguous soft-tissue abscesses or chronic draining sinuses. In men, blastomycosis may involve the prostate and epididymis. Central nervous system (CNS) disease occurs in fewer than 5% of immunocompetent patients with blastomycosis. A recent multicenter review identified 22 patients with CNS disease, of whom 12 (54%) met at least one criterion for immunosuppression; although most cases of CNS blastomycosis are associated with infection at other sites, 22.7% of the reviewed cases had only CNS involvement. CNS disease, usually presenting as a brain abscess, has been reported in ~40% of cases in patients with AIDS. Less common forms of CNS disease are cranial or spinal epidural abscess and meningitis.
DIAGNOSIS
Definitive diagnosis of blastomycosis requires growth of the organism from sputum, bronchial washings, pus, or biopsy material. Specimens should be inoculated onto a fungal medium such as Sabouraud dextrose agar, with or without chloramphenicol. B. dermatitidis is generally visible in 5–10 days but may require incubation for up to 30 days if only a few organisms are present in the specimen. A presumptive diagnosis may be based on demonstration of the characteristic broad-based budding yeast by microscopic examination of wet preps of sputum in pneumonia or of skin-lesion scrapings. Serologic testing for antibodies to B. dermatitidis by complement fixation, immunodiffusion, or enzyme immunoassay is of little value for diagnosis because of limited sensitivity and specificity as well as cross-reactivity with other fungal antigens.
A Blastomyces antigen assay that detects antigen in urine and serum is commercially available and is reasonably sensitive and specific (MiraVista Diagnostics, Indianapolis, IN). Antigen detection appears to be more sensitive in urine than in serum. This antigen test may be useful for monitoring of patients during therapy or for early detection of relapse. Chemiluminescent DNA probes (AccuProbe; GenProbe Inc., San Diego, CA) are commonly used to confirm identification of B. dermatitidis once growth has been detected in culture. Repetitive sequence–based PCR is available (DiversiLab System; bioMérieux, Durham, NC). Molecular identification techniques are currently used only to supplement traditional diagnostic methods.
PROGNOSIS
Cure rates are 90–95% among compliant immunocompetent patients given itraconazole for mild to moderate pulmonary and extrapulmonary disease without CNS involvement. Bone and joint disease usually requires 12 months of therapy. The fewer than 5% of infections that relapse after an initial course of itraconazole usually respond well to a second treatment course.
ACKNOWLEDGMENT
The authors thank Dr. Stanley W. Chapman, Professor Emeritus, University of Mississippi, for his continued help and support and for his contributions to this chapter in an earlier edition.
239 |
Cryptococcosis |
DEFINITION AND ETIOLOGY
Cryptococcus, a genus of yeast-like fungi, is the etiologic agent of cryptococcosis. Both species, C. neoformans and C. gattii, can cause cryptococcosis in humans. The two varieties of C. neoformans—grubii and neoformans—correlate with serotypes A and D, respectively. C. gattii, although not divided into varieties, also is antigenically diverse, encompassing serotypes B and C. Most clinical microbiology laboratories do not routinely distinguish between C. neoformans and C. gattii, or among varieties, but rather identify and report all isolates simply as C. neoformans.
EPIDEMIOLOGY
Cryptococcosis was first described in the 1890s but remained relatively rare until the mid-twentieth century, when advances in diagnosis and increases in the number of immunosuppressed individuals markedly raised its reported prevalence. Although serologic evidence of cryptococcal infection is common among immunocompetent individuals, cryptococcal disease (cryptococcosis) is relatively rare in the absence of impaired immunity. Individuals at high risk for disease due to C. neoformans include patients with hematologic malignancies, recipients of solid organ transplants who require ongoing immunosuppressive therapy, persons whose medical conditions necessitate glucocorticoid therapy, and patients with advanced HIV infection and CD4+ T lymphocyte counts of <200/μL. In contrast, C. gattii–related disease is not associated with specific immune deficits and often occurs in immunocompetent individuals.
![]() Cryptococcal infection is acquired from the environment. C. neoformans and C. gattii inhabit different ecologic niches. C. neoformans is frequently found in soils contaminated with avian excreta and can easily be recovered from shaded and humid soils contaminated with pigeon droppings. In contrast, C. gattii is not found in bird feces. Instead, it inhabits a variety of arboreal species, including several types of eucalyptus tree. C. neoformans strains are found throughout the world; however, var. grubii (serotype A) strains are far more common than var. neoformans (serotype D) strains among both clinical and environmental isolates. The geographic distribution of C. gattii was thought to be largely limited to tropical regions until an outbreak of cryptococcosis caused by a new serotype B strain began in Vancouver in 1999. This outbreak has extended into the United States, and C. gattii infections are being encountered increasingly in several states in the Pacific Northwest.
Cryptococcal infection is acquired from the environment. C. neoformans and C. gattii inhabit different ecologic niches. C. neoformans is frequently found in soils contaminated with avian excreta and can easily be recovered from shaded and humid soils contaminated with pigeon droppings. In contrast, C. gattii is not found in bird feces. Instead, it inhabits a variety of arboreal species, including several types of eucalyptus tree. C. neoformans strains are found throughout the world; however, var. grubii (serotype A) strains are far more common than var. neoformans (serotype D) strains among both clinical and environmental isolates. The geographic distribution of C. gattii was thought to be largely limited to tropical regions until an outbreak of cryptococcosis caused by a new serotype B strain began in Vancouver in 1999. This outbreak has extended into the United States, and C. gattii infections are being encountered increasingly in several states in the Pacific Northwest.
The global burden of cryptococcosis was recently estimated at ~1 million cases, with >600,000 deaths annually. Thus cryptococci are important human pathogens. Since the onset of the HIV pandemic in the early 1980s, the overwhelming majority of cryptococcosis cases have occurred in patients with AIDS (Chap. 226). To comprehend the impact of HIV infection on the epidemiology of cryptococcosis, it is instructive to note that in the early 1990s there were >1000 cases of cryptococcal meningitis each year in New York City—a figure far exceeding that for all cases of bacterial meningitis. With the advent of effective antiretroviral therapy, the incidence of AIDS-related cryptococcosis has been sharply reduced among treated individuals. Thus most cases of cryptococcosis now occur in resource-limited regions of the world. The disease remains distressingly common in regions where antiretroviral therapy is not readily available (e.g., parts of Africa and Asia); in these regions, up to one-third of patients with AIDS have cryptococcosis. Among HIV-infected persons, those with a decreased percentage of memory B cells expressing IgM may be at greater risk for cryptococcosis.
PATHOGENESIS
Cryptococcal infection is acquired by inhalation of aerosolized infectious particles. The exact nature of these particles is not known; the two leading candidate forms are small desiccated yeast cells and basidiospores. Little is known about the pathogenesis of initial infection. Serologic studies have shown that cryptococcal infection is acquired in childhood, but it is not known whether the initial infection is symptomatic. Given that cryptococcal infection is common while disease is rare, the consensus is that pulmonary defense mechanisms in immunologically intact individuals are highly effective at containing this fungus. It is not clear whether initial infection leads to a state of immunity or whether most individuals are subject throughout life to frequent and recurrent infections that resolve without clinical disease. However, evidence indicates that some human cryptococcal infections lead to a state of latency in which viable organisms are harbored for prolonged periods, possibly in granulomas. Thus the inhalation of cryptococcal cells and/or spores can be followed by either clearance or establishment of the latent state. The consequences of prolonged harboring of cryptococcal cells in the lung are not known, but evidence from animal studies indicates that the organisms’ prolonged presence could alter the immunologic milieu in the lung and predispose to allergic airway disease.
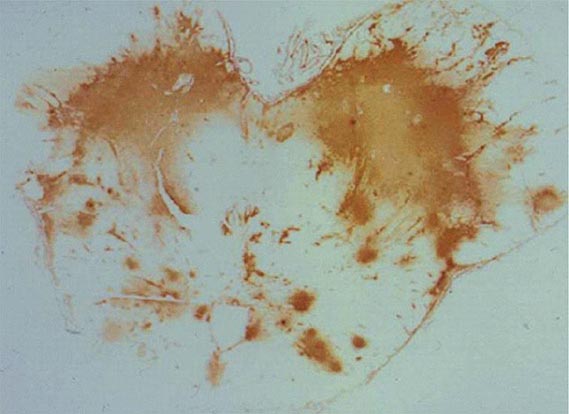
Cryptococcosis usually presents clinically as chronic meningoencephalitis. The mechanisms by which the fungus undergoes extrapulmonary dissemination and enters the central nervous system (CNS) remain poorly understood. The mechanism by which cryptococcal cells cross the blood–brain barrier is a subject of intensive study. Current evidence suggests that both direct fungal-cell migration across the endothelium and fungal-cell carriage inside macrophages as “Trojan horse” invaders can occur. Cryptococcus species have well-defined virulence factors that include the expression of the polysaccharide capsule, the ability to make melanin, and the elaboration of enzymes (e.g., phospholipase and urease) that enhance the survival of fungal cells in tissue. Among these virulence factors, the capsule and melanin production have been most extensively studied. The cryptococcal capsule is antiphagocytic, and the capsular polysaccharide has been associated with numerous deleterious effects on host immune function. Cryptococcal infections can elicit little or no tissue inflammatory response. The immune dysfunction seen in cryptococcosis has been attributed to the release of copious amounts of capsular polysaccharide into tissues, where it probably interferes with local immune responses (Fig. 239-1). In clinical practice, the capsular polysaccharide is the antigen that is measured as a diagnostic marker of cryptococcal infection.
FIGURE 239-1 Cryptococcal antigen in human brain tissue, as revealed by immunohistochemical staining. Brown areas show polysaccharide deposits in the midbrain of a patient who died of cryptococcal meningitis. (Reprinted with permission from SC Lee et al: Hum Pathol 27:839, 1996.)
CLINICAL MANIFESTATIONS
The clinical manifestations of cryptococcosis reflect the site of fungal infection. The spectrum of disease caused by Cryptococcus species consists predominantly of meningoencephalitis and pneumonia, but skin and soft tissue infections also occur; in fact, cryptococcosis can affect any tissue or organ. CNS involvement usually presents as signs and symptoms of chronic meningitis, such as headache, fever, lethargy, sensory deficits, memory deficits, cranial nerve paresis, vision deficits, and meningismus. Cryptococcal meningitis differs from bacterial meningitis in that many Cryptococcus-infected patients present with symptoms of several weeks’ duration. In addition, classic characteristics of meningeal irritation, such as meningismus, may be absent in cryptococcal meningitis. Indolent cases can present as subacute dementia. Meningeal cryptococcosis can lead to sudden catastrophic vision loss.
Pulmonary cryptococcosis usually presents as cough, increased sputum production, and chest pain. Patients infected with C. gattii can present with granulomatous pulmonary masses known as cryptococcomas. Fever develops in a minority of cases. Like CNS disease, pulmonary cryptococcosis can follow an indolent course, and the majority of cases probably do not come to clinical attention. In fact, many cases are discovered incidentally during the workup of an abnormal chest radiograph obtained for other diagnostic purposes. Pulmonary cryptococcosis can be associated with antecedent diseases such as malignancy, diabetes, and tuberculosis.
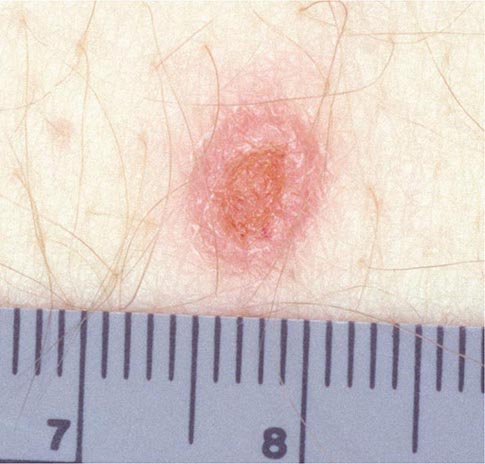
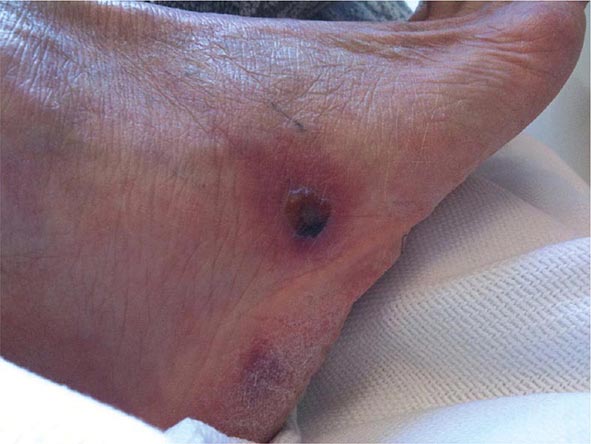
Skin lesions are common in patients with disseminated cryptococcosis and can be highly variable, including papules, plaques, purpura, vesicles, tumor-like lesions, and rashes. The spectrum of cryptococcosis in HIV-infected patients is so varied and has changed so much since the advent of antiretroviral therapy that a distinction between HIV-related and HIV-unrelated cryptococcosis is no longer pertinent. In patients with AIDS and solid organ transplant recipients, the lesions of cutaneous cryptococcosis often resemble those of molluscum contagiosum (Fig. 239-2; Chap. 220e).
FIGURE 239-2 Disseminated fungal infection. A liver transplant recipient developed six cutaneous lesions similar to the one shown. Biopsy and serum antigen testing demonstrated Cryptococcus. Important features of the lesion include a benign-appearing fleshy papule with central umbilication resembling molluscum contagiosum. (Photo courtesy of Dr. Lindsey Baden; with permission.)
DIAGNOSIS
A diagnosis of cryptococcosis requires the demonstration of yeast cells in normally sterile tissues. Visualization of the capsule of fungal cells in cerebrospinal fluid (CSF) mixed with India ink is a useful rapid diagnostic technique. Cryptococcal cells in India ink have a distinctive appearance because their capsules exclude ink particles. However, the CSF India ink examination may yield negative results in patients with a low fungal burden. This examination should be performed by a trained individual, since leukocytes and fat globules can sometimes be mistaken for fungal cells. Cultures of CSF and blood that are positive for cryptococcal cells are diagnostic for cryptococcosis. In cryptococcal meningitis, CSF examination usually reveals evidence of chronic meningitis with mononuclear cell pleocytosis and increased protein levels. A particularly useful test is cryptococcal antigen (CRAg) detection in CSF and blood. The assay is based on serologic detection of cryptococcal polysaccharide and is both sensitive and specific. A positive CRAg test provides strong presumptive evidence for cryptococcosis; however, because the result is often negative in pulmonary cryptococcosis, the test is less useful in the diagnosis of pulmonary disease and is of only limited usefulness in monitoring the response to therapy.
![]() In areas of Africa where there is a high prevalence of HIV infection, routine screening of blood for CRAg in HIV-infected patients with low CD4+ T lymphocyte counts may identify individuals at high risk of cryptococcal disease who are candidates for antifungal therapy. Similarly, CRAg screening has shown that a significant proportion of HIV-infected patients hospitalized with pneumonia in Thailand harbor cryptococcal infection. Inexpensive point-of-care CRAg tests that are under development could be of great diagnostic benefit in resource-limited regions.
In areas of Africa where there is a high prevalence of HIV infection, routine screening of blood for CRAg in HIV-infected patients with low CD4+ T lymphocyte counts may identify individuals at high risk of cryptococcal disease who are candidates for antifungal therapy. Similarly, CRAg screening has shown that a significant proportion of HIV-infected patients hospitalized with pneumonia in Thailand harbor cryptococcal infection. Inexpensive point-of-care CRAg tests that are under development could be of great diagnostic benefit in resource-limited regions.
PROGNOSIS AND COMPLICATIONS
Even with antifungal therapy, cryptococcosis is associated with high rates of morbidity and death. For the majority of patients with cryptococcosis, the most important prognostic factors are the extent and the duration of the underlying immunologic deficits that predisposed them to develop the disease. Therefore, cryptococcosis is often curable with antifungal therapy in individuals with no apparent immunologic dysfunction, but, in patients with severe immunosuppression (e.g., those with AIDS), the best that can be hoped for is that antifungal therapy will induce remission, which can then be maintained with lifelong suppressive therapy. Before the advent of antiretroviral therapy, the median overall survival period for AIDS patients with cryptococcosis was <1 year. Cryptococcosis in patients with underlying neoplastic disease has a particularly poor prognosis. For CNS cryptococcosis, poor prognostic markers are a CSF assay positive for yeast cells on initial India ink examination (evidence of a heavy fungal burden), high CSF pressure, low CSF glucose levels, low CSF pleocytosis (<2/μL), recovery of yeast cells from extraneural sites, absence of antibody to capsular polysaccharide, a CSF or serum cryptococcal antigen level of ≥1:32, and concomitant glucocorticoid therapy or hematologic malignancy. A response to treatment does not guarantee cure since relapse of cryptococcosis is common even among patients with relatively intact immune systems. Complications of CNS cryptococcosis include cranial nerve deficits, vision loss, and cognitive impairment.
PREVENTION
No vaccine is available for cryptococcosis. In patients at high risk (e.g., those with advanced HIV infection and CD4+ T lymphocyte counts of <200/μL), primary prophylaxis with fluconazole (200 mg/d) is effective in reducing the prevalence of disease. Since antiretroviral therapy raises the CD4+ T lymphocyte count, it constitutes an immunologic form of prophylaxis. However, cryptococcosis in the setting of immune reconstitution has been reported in patients with HIV infection and in recipients of solid organ transplants.
240 |
Candidiasis |
The genus Candida encompasses more than 150 species, only a few of which cause disease in humans. With rare exceptions (although the exceptions are increasing in number), the human pathogens are C. albicans, C. guilliermondii, C. krusei, C. parapsilosis, C. tropicalis, C. kefyr, C. lusitaniae, C. dubliniensis, and C. glabrata. Ubiquitous in nature, they inhabit the gastrointestinal tract (including the mouth and oropharynx), the female genital tract, and the skin. Although cases of candidiasis have been described since antiquity in debilitated patients, the advent of Candida species as common human pathogens dates to the introduction of modern therapeutic approaches that suppress normal host defense mechanisms. Of these relatively recent advances, the most important is the use of antibacterial agents that alter the normal human microbiota and allow nonbacterial species to become more prevalent in the commensal flora. With the introduction of antifungal agents, the causes of Candida infections shifted from an almost complete dominance of C. albicans to the common involvement of C. glabrata and the other species listed above. The non-albicans species now account for approximately half of all cases of candidemia and hematogenously disseminated candidiasis. Recognition of this change is clinically important, since the various species differ in susceptibility to the newer antifungal agents. In developed countries, where medical therapeutics are commonly used, Candida species are now among the most common nosocomial pathogens.
Candida is a small, thin-walled, ovoid yeast that measures 4–6 μm in diameter and reproduces by budding. Organisms of this genus occur in three forms in tissue: blastospores, pseudohyphae, and hyphae. Candida grows readily on simple medium; lysis centrifugation enhances its recovery from blood. Species are identified by biochemical testing (currently with automated devices) or on special agar (e.g., CHROMagar).
EPIDEMIOLOGY
![]() Candida organisms are ubiquitous in nature; worldwide, these fungi are present in humans as commensals, in animals, in foods, and on inanimate objects. In developed countries, where advanced medical therapeutics are commonly used (see “Treatment,” below), Candida species are now among the most common health care–associated pathogens. In the United States, these species are the fourth most common isolates from the blood of hospitalized patients. In countries where advanced medical care is rarely available, mucocutaneous Candida infections, such as thrush, are more common than deep organ infections, which rarely occur; however, the incidence of deep organ candidiasis increases steadily as advances in health care—such as therapy with broad-spectrum antibiotics, more aggressive treatment of cancer, and the use of immunosuppression for sustaining organ transplants—are introduced and implemented. In recent decades, as a result of the HIV epidemic, the incidence of thrush and Candida esophagitis has increased substantially. In aggregate, the global incidence of infections due to Candida species has risen steadily over the past few decades.
Candida organisms are ubiquitous in nature; worldwide, these fungi are present in humans as commensals, in animals, in foods, and on inanimate objects. In developed countries, where advanced medical therapeutics are commonly used (see “Treatment,” below), Candida species are now among the most common health care–associated pathogens. In the United States, these species are the fourth most common isolates from the blood of hospitalized patients. In countries where advanced medical care is rarely available, mucocutaneous Candida infections, such as thrush, are more common than deep organ infections, which rarely occur; however, the incidence of deep organ candidiasis increases steadily as advances in health care—such as therapy with broad-spectrum antibiotics, more aggressive treatment of cancer, and the use of immunosuppression for sustaining organ transplants—are introduced and implemented. In recent decades, as a result of the HIV epidemic, the incidence of thrush and Candida esophagitis has increased substantially. In aggregate, the global incidence of infections due to Candida species has risen steadily over the past few decades.
PATHOGENESIS
In the most serious form of Candida infection, the organisms disseminate hematogenously and form microabscesses and small macroabscesses in major organs. Although the exact mechanism is not known, Candida probably enters the bloodstream from mucosal surfaces after growing to large numbers as a consequence of bacterial suppression by antibacterial drugs; alternatively, in some instances, the organism may enter from the skin. A change from the blastospore stage to the pseudohyphal and hyphal stages is generally considered integral to the organism’s penetration into tissue. However, C. glabrata can cause extensive infection even though it does not transform into pseudohyphae or hyphae. Adherence to both epithelial and endothelial cells, thought to be the first step in invasion and infection, has been studied extensively, and several adhesins have been identified. Biofilm formation also is considered important in pathogenesis. Numerous reviews of cases of hematogenously disseminated candidiasis have identified the predisposing factors or conditions associated with disseminated disease (Table 240-1). Women who receive antibacterial agents may develop vaginal candidiasis.
|
WELL-RECOGNIZED FACTORS AND CONDITIONS PREDISPOSING TO HEMATOGENOUSLY DISSEMINATED CANDIDIASIS |
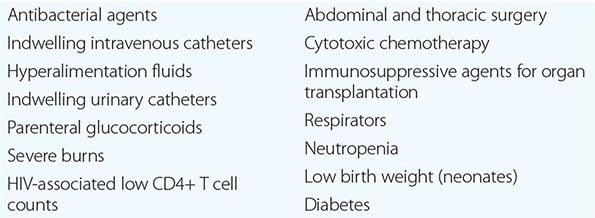
Innate immunity is the most important defense mechanism against hematogenously disseminated candidiasis, and the neutrophil is the most important component of this defense. Macrophages also play an important defensive role. STAT1, Dectin-1, CARD9, and TH1 and TH17 lymphocytes contribute significantly to innate defense (see “Clinical Manifestations,” below). Although many immunocompetent individuals have antibodies to Candida, the role of these antibodies in defense against the organism is not clear. Multiple genetic polymorphisms that predispose to disseminated candidiasis will most likely be identified in future studies.
CLINICAL MANIFESTATIONS
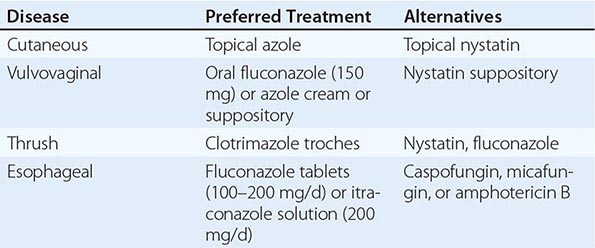
Mucocutaneous Candidiasis Thrush is characterized by white, adherent, painless, discrete or confluent patches in the mouth, on the tongue, or in the esophagus, occasionally with fissuring at the corners of the mouth. This form of disease caused by Candida can also occur at points of contact with dentures. Organisms are identifiable in gram-stained scrapings from lesions. The occurrence of thrush in a young, otherwise healthy-appearing person should prompt an investigation for underlying HIV infection. More commonly, thrush is seen as a nonspecific manifestation of severe debilitating illness. Vulvovaginal candidiasis is accompanied by pruritus, pain, and vaginal discharge which is usually thin but may contain whitish “curds” in severe cases. A subset of patients with recurrent vulvovaginitis have a deficiency in the surface expression of Dectin-1, a major recognition factor for β-glucan on Candida. This deficiency leads to suboptimal functioning of the CARD9 pathway, which ultimately increases the propensity for recurrent vaginal infections.
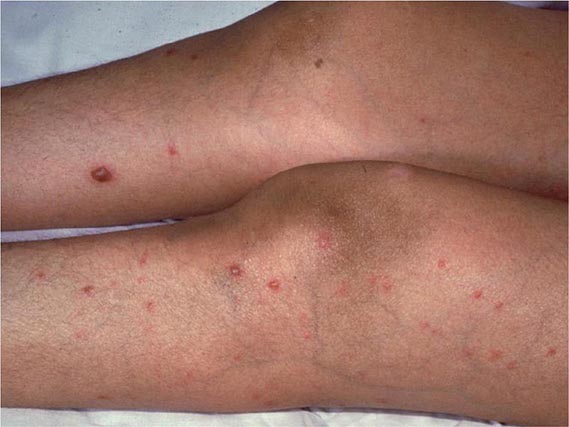
Other Candida skin infections include paronychia, a painful swelling at the nail-skin interface; onychomycosis, a fungal nail infection rarely caused by this genus; intertrigo, an erythematous irritation with redness and pustules in the skin folds; balanitis, an erythematous-pustular infection of the glans penis; erosio interdigitalis blastomycetica, an infection between the digits of the hands or toes; folliculitis, with pustules developing most frequently in the area of the beard; perianal candidiasis, a pruritic, erythematous, pustular infection surrounding the anus; and diaper rash, a common erythematous-pustular perineal infection in infants. Generalized disseminated cutaneous candidiasis, another form of infection that occurs primarily in infants, is characterized by widespread eruptions over the trunk, thorax, and extremities. The diagnostic macronodular lesions of hematogenously disseminated candidiasis (Fig. 240-1) indicate a high probability of dissemination to multiple organs as well as the skin. While the lesions are seen predominantly in immunocompromised patients treated with cytotoxic drugs, they may also develop in patients without neutropenia.
FIGURE 240-1 Macronodular skin lesions associated with hematogenously disseminated candidiasis. Candida organisms are usually but not always visible on histopathologic examination. The fungi grow when a portion of the biopsied specimen is cultured. Therefore, for optimal identification, both histopathology and culture should be performed. (Image courtesy of Dr. Noah Craft and the Victor Newcomer collection at UCLA, archived by Logical Images, Inc.; with permission.)
Chronic mucocutaneous candidiasis is a heterogeneous infection of the hair, nails, skin, and mucous membranes that persists despite intermittent therapy. The onset of disease usually comes in infancy or within the first two decades of life but in rare cases comes in later life. The condition may be mild and limited to a specific area of the skin or nails, or it may take a severely disfiguring form (Candida granuloma) characterized by exophytic outgrowths on the skin. Chronic mucocutaneous candidiasis is usually associated with specific immunologic dysfunction; most frequently reported is a failure of T lymphocytes to proliferate or to excrete cytokines in response to stimulation by Candida antigens in vitro. A subset of the affected patients have mutations in the STAT1 gene resulting in an insufficiency of interferon γ, interleukin 17, and interleukin 22.
Approximately half of patients with chronic mucocutaneous candidiasis have associated endocrine abnormalities that together are designated the autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy (APECED) syndrome. This syndrome is due to mutations in the autoimmune regulator (AIRE) gene and is most prevalent among Finns, Iranian Jews, Sardinians, northern Italians, and Swedes. Conditions that usually follow the onset of the disease include hypoparathyroidism, adrenal insufficiency, autoimmune thyroiditis, Graves’ disease, chronic active hepatitis, alopecia, juvenile-onset pernicious anemia, malabsorption, and primary hypogonadism. In addition, dental enamel dysplasia, vitiligo, pitted nail dystrophy, and calcification of the tympanic membranes may occur. Patients with chronic mucocutaneous candidiasis rarely develop hematogenously disseminated candidiasis, probably because their neutrophil function remains intact.
Deeply Invasive Candidiasis Deeply invasive Candida infections may or may not be due to hematogenous seeding. Deep esophageal infection may result from penetration by organisms from superficial esophageal erosions; joint or deep wound infection from contiguous spread of organisms from the skin; kidney infection from catheter-initiated spread of organisms through the urinary tract; infection of intraabdominal organs and the peritoneum from perforation of the gastrointestinal tract; and gallbladder infection from retrograde migration of organisms from the gastrointestinal tract into the biliary drainage system.
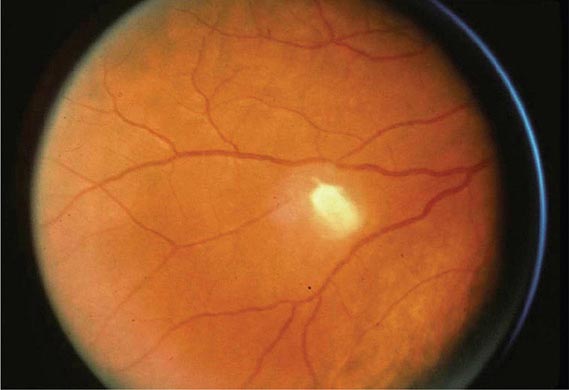
However, far more commonly, deeply invasive candidiasis results from hematogenous seeding of various organs as a complication of candidemia. Once the organism gains access to the intravascular compartment (either from the gastrointestinal tract or, less often, from the skin through the site of an indwelling intravascular catheter), it may spread hematogenously to a variety of deep organs. The brain, chorioretina (Fig. 240-2), heart, and kidneys are most commonly infected and the liver and spleen less commonly so (most often in neutropenic patients). In fact, nearly any organ can become involved, including the endocrine glands, pancreas, heart valves (native or prosthetic), skeletal muscle, joints (native or prosthetic), bone, and meninges. Candida organisms can also spread hematogenously to the skin and cause classic macronodular lesions (Fig. 240-1). Frequently, painful muscular involvement also is evident beneath the area of affected skin. Chorioretinal involvement and skin involvement are highly significant, since both findings are associated with a very high probability of abscess formation in multiple deep organs as a result of generalized hematogenous seeding. Ocular involvement (Fig. 240-2) may require specific treatment (e.g., partial vitrectomy or intraocular injection of antifungal agents) to prevent permanent blindness. An ocular examination is indicated for all patients with candidemia, whether or not they have ocular manifestations.
FIGURE 240-2 Hematogenous Candida endophthalmitis. A classic off-white lesion projecting from the chorioretina into the vitreous causes the surrounding haze. The lesion is composed primarily of inflammatory cells rather than organisms. Lesions of this type may progress to cause extensive vitreal inflammation and eventual loss of the eye. Partial vitrectomy, combined with IV and possibly intravitreal antifungal therapy, may be helpful in controlling the lesions. (Image courtesy of Dr. Gary Holland; with permission.)
DIAGNOSIS
The diagnosis of Candida infection is established by visualization of pseudohyphae or hyphae on wet mount (saline and 10% KOH), tissue Gram’s stain, periodic acid–Schiff stain, or methenamine silver stain in the presence of inflammation. Absence of organisms on hematoxylin-eosin staining does not reliably exclude Candida infection. The most challenging aspect of diagnosis is determining which patients with Candida isolates have hematogenously disseminated candidiasis. For instance, recovery of Candida from sputum, urine, or peritoneal catheters may indicate mere colonization rather than deep-seated infection, and Candida isolation from the blood of patients with indwelling intravascular catheters may reflect inconsequential seeding of the blood from or growth of the organisms on the catheter. Despite extensive research into both antigen and antibody detection systems, there is currently no widely available and validated diagnostic test to distinguish patients with inconsequential seeding of the blood from those whose positive blood cultures represent hematogenous dissemination to multiple organs. Many studies are under way to establish the utility of the β-glucan test; at present, its greatest utility is its negative predictive value (∼90%). Meanwhile, the presence of ocular or macronodular skin lesions is highly suggestive of widespread infection of multiple deep organs. Although extensive research is being conducted on other tests for infection, such as PCR, none of these tests is fully validated or widely available at present.
PROPHYLAXIS
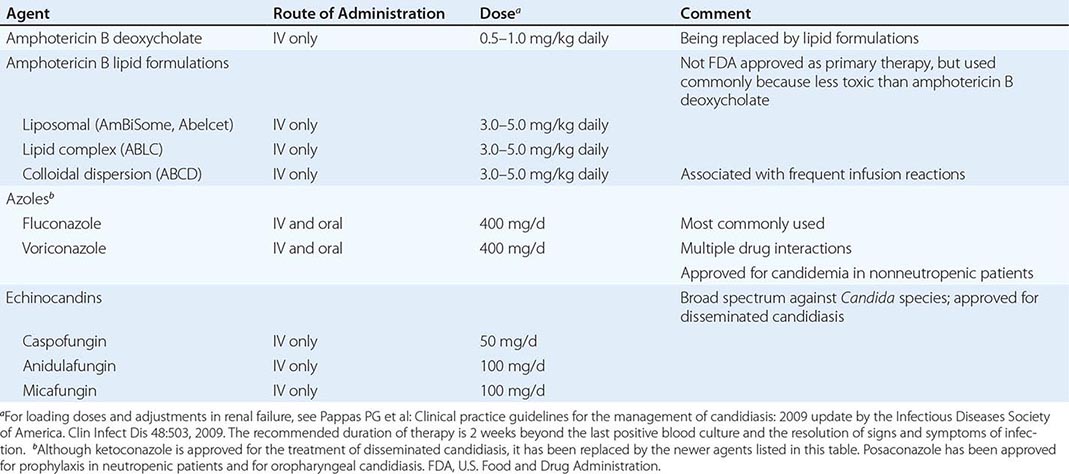
The use of antifungal agents to prevent Candida infections has been controversial, but some general principles have emerged. Most centers administer prophylactic fluconazole (400 mg/d) to recipients of allogeneic stem cell transplants. High-risk liver transplant recipients also are given fluconazole prophylaxis in most centers. The use of prophylaxis for neutropenic patients has varied considerably from center to center; many centers that elect to give prophylaxis to this population use either fluconazole (200–400 mg/d) or a lipid formulation of amphotericin B (AmBiSome, 1–2 mg/d). Caspofungin (50 mg/d) also has been recommended. Some centers have used itraconazole suspension (200 mg/d). Posaconazole (200 mg three times daily) also has been approved by the FDA for prophylaxis in neutropenic patients and is gaining in popularity.
Prophylaxis is sometimes given to surgical patients at very high risk. The widespread use of prophylaxis for nearly all patients in general surgical or medical intensive care units is not—and should not be—a common practice for three reasons: (1) the incidence of disseminated candidiasis is relatively low, (2) the cost-benefit ratio is suboptimal, and (3) increased resistance with widespread prophylaxis is a valid concern.
Prophylaxis for oropharyngeal or esophageal candidiasis in HIV-infected patients is not recommended unless there are frequent recurrences.
241 |
Aspergillosis |
Aspergillosis is the collective term used to describe all disease entities caused by any one of ~50 pathogenic and allergenic species of Aspergillus. Only those species that grow at 37°C can cause invasive infection, although some species without this ability can cause allergic syndromes. A. fumigatus is responsible for most cases of invasive aspergillosis, almost all cases of chronic aspergillosis, and most allergic syndromes. A. flavus is more prevalent in some hospitals and causes a higher proportion of cases of sinus infections, cutaneous infections, and keratitis than A. fumigatus. A. niger can cause invasive infection but more commonly colonizes the respiratory tract and causes external otitis. A. terreus causes only invasive disease, usually with a poor prognosis. A. nidulans occasionally causes invasive infection, primarily in patients with chronic granulomatous disease.
EPIDEMIOLOGY AND ECOLOGY
Aspergillus has a worldwide distribution, most commonly growing in decomposing plant materials (i.e., compost) and in bedding. This hyaline (nonpigmented), septate, branching mold produces vast numbers of conidia (spores) on stalks above the surface of mycelial growth. Aspergilli are found in indoor and outdoor air, on surfaces, and in water from surface reservoirs. Daily exposures vary from a few to many millions of conidia; the latter high numbers of conidia are encountered in hay barns and other very dusty environments. The required size of the infecting inoculum is uncertain; however, only intense exposures (e.g., during construction work, handling of moldy bark or hay, or composting) are sufficient to cause disease in healthy immunocompetent individuals. Allergic syndromes may be exacerbated by continuous antigenic exposure arising from sinus or airway colonization or from nail infection. High-efficiency particulate air (HEPA) filtration is often protective against infection; thus HEPA filters should be installed and monitored for efficiency in operating rooms and in areas of the hospital that house high-risk patients.
The incubation period of invasive aspergillosis after exposure is highly variable, extending in documented cases from 2 to 90 days. Thus community acquisition of an infecting strain frequently manifests as invasive infection during hospitalization, although nosocomial acquisition is also common. Outbreaks usually are directly related to a contaminated air source in the hospital.
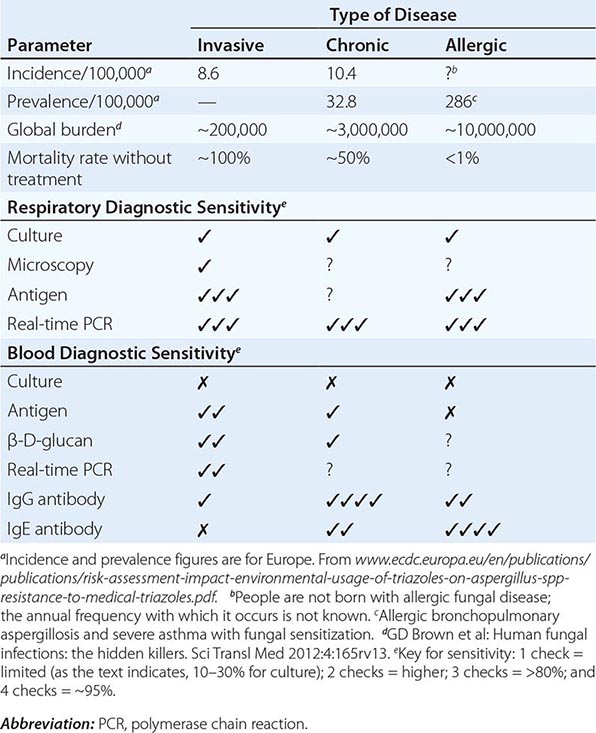
![]() Global aspergillosis incidence and prevalence have been estimated (Table 241-1). However, given the inadequate diagnostic capability in almost all low- and middle-income countries, the accuracy of these estimates is uncertain. The frequency of different manifestations of aspergillosis varies considerably with geographic location; most notably, chronic granulomatous sinusitis is rare outside the Middle East and India, and fungal keratitis is particularly common in Nepal, Myanmar, Bhutan, and India (800 and 113 cases/100,000 population, respectively). The potential effects of chronic pulmonary aspergillosis after pulmonary tuberculosis have only recently been appreciated and include life-threatening hemoptysis, misdiagnosis of smear-negative tuberculosis, and general exacerbation of posttuberculosis morbidity.
Global aspergillosis incidence and prevalence have been estimated (Table 241-1). However, given the inadequate diagnostic capability in almost all low- and middle-income countries, the accuracy of these estimates is uncertain. The frequency of different manifestations of aspergillosis varies considerably with geographic location; most notably, chronic granulomatous sinusitis is rare outside the Middle East and India, and fungal keratitis is particularly common in Nepal, Myanmar, Bhutan, and India (800 and 113 cases/100,000 population, respectively). The potential effects of chronic pulmonary aspergillosis after pulmonary tuberculosis have only recently been appreciated and include life-threatening hemoptysis, misdiagnosis of smear-negative tuberculosis, and general exacerbation of posttuberculosis morbidity.
|
DISEASE FREQUENCY AND DIAGNOSTIC SENSITIVITY FOR DIFFERENT MANIFESTATIONS OF ASPERGILLOSIS |
RISK FACTORS AND PATHOGENESIS
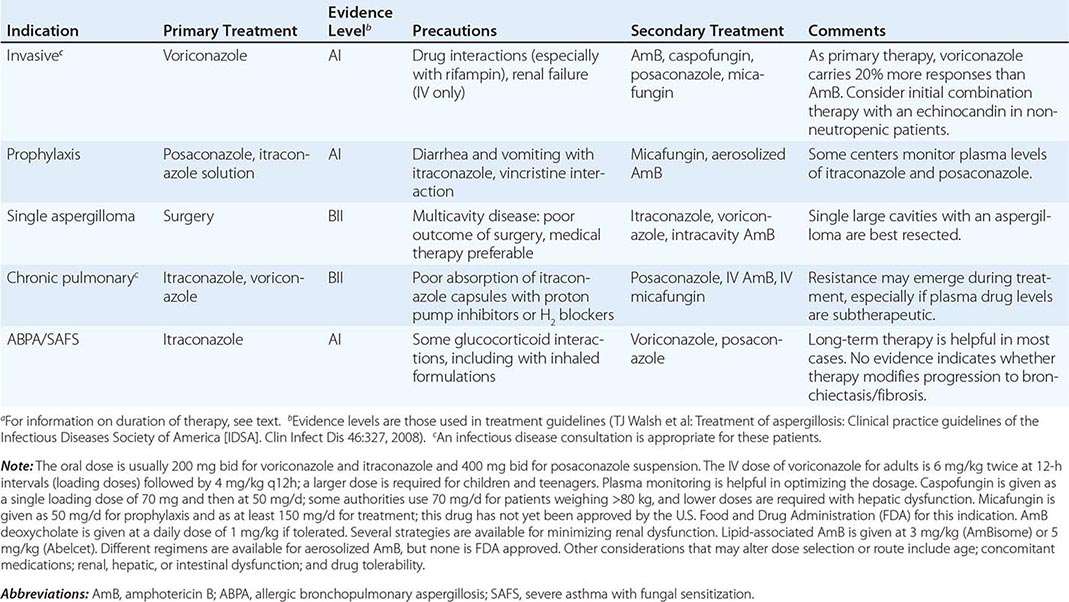
The primary risk factors for invasive aspergillosis are profound neutropenia and glucocorticoid use; risk increases with longer duration of these conditions. Higher doses of glucocorticoids increase the risk of both acquisition of invasive aspergillosis and death from the infection. Neutrophil and/or phagocyte dysfunction also is an important risk factor, as evidenced by aspergillosis in chronic granulomatous disease, advanced HIV infection, and relapsed leukemia. An increasing incidence of invasive aspergillosis in medical intensive care units suggests that, in patients who are not immunocompromised, temporary abrogation of protective responses as a result of glucocorticoid use or a general anti-inflammatory state is a significant risk factor. Many patients have some evidence of prior pulmonary disease—typically, a history of pneumonia or chronic obstructive pulmonary disease. Therapy with infliximab, adalimumab, alemtuzumab, daclizumab, rituximab, and possibly bevacizumab therapy also carries an increased risk of invasive aspergillosis, as do severe liver disease and high levels of stored iron in bone marrow.
Patients with chronic pulmonary aspergillosis have a wide spectrum of underlying pulmonary disease, including tuberculosis, prior pneumothorax, or chronic obstructive pulmonary disease. These patients are immunocompetent except for some cytokine regulation defects, most of which are consistent with an inability to mount an inflammatory immune (TH1-like) response or to control it adequately. Glucocorticoids accelerate disease progression.
![]() Allergic bronchopulmonary aspergillosis (ABPA) is associated with polymorphisms of interleukin (IL) 4Ra, IL-10, and SPA2 genes (and others) and with heterozygosity of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. These associations suggest a strong genetic basis for the development of a TH2-like and “allergic” response to A. fumigatus.
Allergic bronchopulmonary aspergillosis (ABPA) is associated with polymorphisms of interleukin (IL) 4Ra, IL-10, and SPA2 genes (and others) and with heterozygosity of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. These associations suggest a strong genetic basis for the development of a TH2-like and “allergic” response to A. fumigatus.
CD4+CD25+ T (Treg) cells also appear to be pivotal in determining disease phenotype. Remarkably, high-dose glucocorticoid treatment for exacerbations of ABPA almost never leads to invasive aspergillosis.
CLINICAL FEATURES AND APPROACH TO THE PATIENT
|
MAJOR MANIFESTATIONS OF ASPERGILLOSIS |
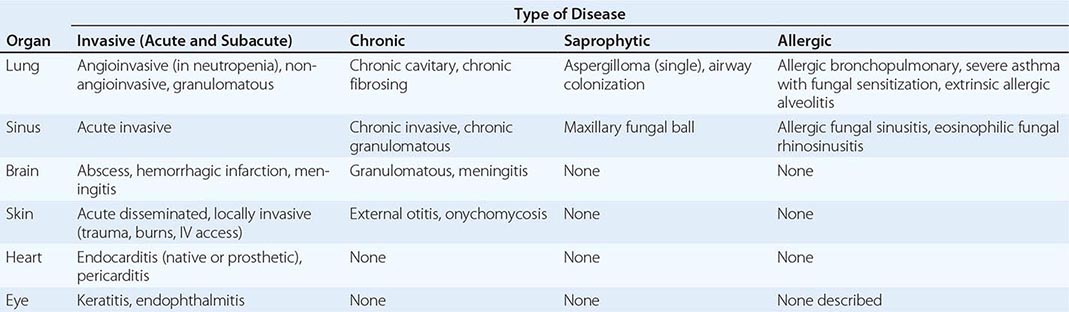
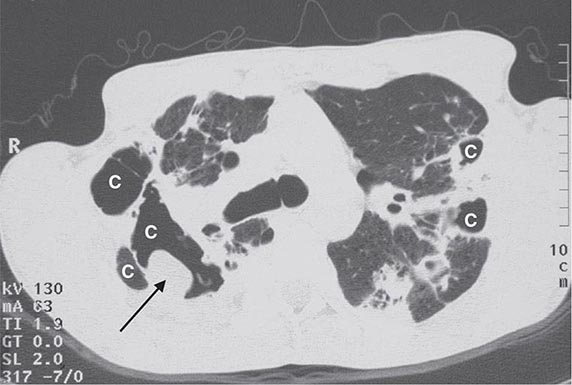
Invasive Pulmonary Aspergillosis Both the frequency of invasive disease and the pace of its progression increase with greater degrees of immunocompromise. Invasive aspergillosis is arbitrarily classified as acute and subacute, with courses of ≤1 month and 1–3 months, respectively. More than 80% of cases of invasive aspergillosis involve the lungs. The most common clinical features are no symptoms at all, fever, cough (sometimes productive), nondescript chest discomfort, trivial hemoptysis, and shortness of breath. Although the fever often responds to glucocorticoids, the disease progresses. The keys to early diagnosis in at-risk patients are a high index of suspicion, screening for circulating antigen (in leukemia), and urgent CT of the thorax. Invasive aspergillosis is one of the most common diagnostic errors revealed at autopsy.
Invasive Sinusitis The sinuses are involved in 5–10% of cases of invasive aspergillosis, especially affecting patients with leukemia and recipients of hematopoietic stem cell transplants. In addition to fever, the most common features are nasal or facial discomfort, blocked nose, and nasal discharge (sometimes bloody). Endoscopic examination of the nose reveals pale, dusky or necrotic-looking tissue in any location. CT or MRI of the sinuses is essential but does not distinguish invasive Aspergillus sinusitis from preexisting allergic or bacterial sinusitis early in the disease process.
Tracheobronchitis Occasionally, only the airways are infected by Aspergillus. The resulting manifestations range from acute or chronic bronchitis to ulcerative or pseudomembranous tracheobronchitis. These entities are particularly common among lung transplant recipients. Obstruction with mucous plugs occurs in normal individuals; in persons with ABPA, cystic fibrosis, and/or bronchiectasis; and in immunocompromised patients.
Disseminated Aspergillosis In the most severely immunocompromised patients, Aspergillus disseminates from the lungs to multiple organs—most often to the brain but also to the skin, thyroid, bone, kidney, liver, gastrointestinal tract, eye (endophthalmitis), and heart valve. Aside from cutaneous lesions, the most common features are gradual clinical deterioration over 1–3 days, with low-grade fever and features of mild sepsis, and nonspecific abnormalities in laboratory tests. In most cases, at least one localization becomes apparent before death. Blood cultures are almost always negative.
Cerebral Aspergillosis Hematogenous dissemination to the brain is a devastating complication of invasive aspergillosis. Single or multiple lesions may develop. In acute disease, hemorrhagic infarction is most typical, and cerebral abscess is common. Rarer manifestations include meningitis, mycotic aneurysm, and cerebral granuloma (mimicking a brain tumor). Local spread from cranial sinuses also occurs. Postoperative infection develops rarely and is exacerbated by glucocorticoids, often given after neurosurgery. The presentation can be either acute or subacute, with mood changes, focal signs, seizures, and decline in mental status. MRI is the most useful immediate investigation; unenhanced CT of the brain is usually nonspecific, and contrast is often contraindicated because of poor renal function.
Endocarditis Most cases of Aspergillus endocarditis are prosthetic valve infections resulting from contamination during surgery. Native valve disease is reported, especially as a feature of disseminated infection and in persons using illicit IV drugs. Culture-negative endocarditis with large vegetations is the most common presentation; embolectomy occasionally reveals the diagnosis.
Cutaneous Aspergillosis Dissemination of Aspergillus occasionally results in cutaneous features, usually an erythematous or purplish nontender area that progresses to a necrotic eschar. Direct invasion of the skin occurs in neutropenic patients at the site of IV catheter insertion and in burn patients; such invasion may also follow trauma. Wounds may become infected with Aspergillus (especially A. flavus) after surgery.
Chronic Pulmonary Aspergillosis The hallmark of chronic cavitary pulmonary aspergillosis (also called semi-invasive aspergillosis, chronic necrotizing aspergillosis, or complex aspergilloma) (Fig. 241-1) is one or more pulmonary cavities expanding over a period of months or years in association with pulmonary symptoms and systemic manifestations such as fatigue and weight loss. (Pulmonary aspergillosis developing over <3 months is better classified as subacute invasive aspergillosis.) Often mistaken initially for tuberculosis, almost all cases occur in patients with prior pulmonary disease (e.g., tuberculosis, atypical mycobacterial infection, sarcoidosis, rheumatoid lung disease, pneumothorax, bullae) or lung surgery. The onset is insidious, and systemic features may be more prominent than pulmonary symptoms. Cavities may have a fluid level or a well-formed fungal ball, but pericavitary infiltrates and multiple cavities—with or without pleural thickening—are typical. An irregular internal cavity surface and thickened cavity walls are indicative of disease activity. IgG antibodies (usually precipitating) to Aspergillus are almost always detectable in blood, and levels fall slowly with successful therapy. Some patients have concurrent infections—even without a fungal ball—with atypical mycobacteria and/or other bacterial pathogens. One or more Aspergillus nodules that resemble early lung carcinoma and may cavitate have been recognized. If untreated, chronic pulmonary aspergillosis typically progresses (sometimes relatively rapidly) to unilateral or upper-lobe fibrosis. This end-stage entity is termed chronic fibrosing pulmonary aspergillosis.
FIGURE 241-1 CT scan image of the chest in a patient with longstanding bilateral chronic cavitary pulmonary aspergillosis. This patient had a history of several bilateral pneumothoraces and had required bilateral pleurodesis in 1990. CT scan then demonstrated multiple bullae, and sputum cultures grew A. fumigatus. The patient had initially weakly and later strongly positive serum Aspergillus antibody tests (precipitins). This scan (2003) shows a mixture of thick- and thin-walled cavities in both lungs (each marked with C), with a probable fungal ball (black arrow) protruding into the large cavity on the patient’s right side (R). There is also considerable pleural thickening bilaterally.
Aspergilloma Aspergilloma (fungal ball) occurs in up to 20% of residual pulmonary cavities ≥2.5 cm in diameter. Signs and symptoms associated with single (simple) aspergillomas are minor, including cough (sometimes productive), hemoptysis, wheezing, and mild fatigue. More significant signs and symptoms are associated with chronic cavitary pulmonary aspergillosis and should be treated as such. The vast majority of fungal balls are caused by A. fumigatus, but A. niger has been implicated, particularly in diabetic patients; aspergillomas due to A. niger can lead to oxalosis with renal dysfunction. The most significant complication of aspergilloma is life-threatening hemoptysis, which may be the presenting manifestation. Some fungal balls resolve spontaneously, but the cavity may still be infected.
Chronic Aspergillus Sinusitis Three entities are subsumed under this broad designation: fungal ball of the sinus, chronic invasive sinusitis, and chronic granulomatous sinusitis. Fungal ball of the sinus is limited to the maxillary sinus (except in rare cases involving the sphenoid sinus) and consists of a chronic saprophytic entity in which the sinus cavity is filled with a fungal ball. Maxillary disease is associated with prior upper-jaw root canal work and chronic (bacterial) sinusitis. About 90% of CT scans show focal hyperattenuation related to concretions; on MRI scans, the T2-weighted signal is decreased, whereas it is increased in bacterial sinusitis. Removal of the fungal ball is curative. No tissue invasion is demonstrable histologically or radiologically.
In contrast, chronic invasive sinusitis is a slowly destructive process that most commonly affects the ethmoid and sphenoid sinuses but can involve any sinus. Patients are usually but not always immunocompromised to some degree (e.g., as a result of diabetes or HIV infection). Imaging of the cranial sinuses shows opacification of one or more sinuses, local bone destruction, and invasion of local structures. The differential diagnosis is wide, including infections caused by numerous other fungi; sphenoid sinusitis is often caused by bacteria. Apart from a history of chronic nasal discharge and blockage, loss of the sense of smell, and persistent headache, the usual presenting features are related to local involvement of critical structures. The orbital apex syndrome (blindness and proptosis) is characteristic. Facial swelling, cavernous sinus thrombosis, carotid artery occlusion, pituitary fossa, and brain and skull base invasion have been described.
Chronic granulomatous sinusitis due to Aspergillus is most commonly seen in the Middle East and India and is often caused by A. flavus. It typically presents late, with facial swelling and unilateral proptosis. The prominent granulomatous reaction histologically distinguishes this disease from chronic invasive sinusitis, in which tissue necrosis with a low-grade mixed-cell infiltrate is typical. IgG antibodies to A. flavus are usually detectable.
Allergic Bronchopulmonary Aspergillosis In almost all cases, ABPA represents a hypersensitivity reaction to A. fumigatus; rare cases are due to other aspergilli and other fungi. ABPA occurs in ~2.5% of patients with asthma who are referred to secondary care and in up to 15% of adults with cystic fibrosis. Episodes of bronchial obstruction with mucous plugs leading to coughing fits, “pneumonia,” consolidation, and breathlessness are typical. Many patients report coughing up thick sputum casts. Eosinophilia commonly develops before systemic glucocorticoids are given. The cardinal diagnostic tests include an elevated serum level of total IgE (usually >1000 IU/mL), a positive skin-prick test in response to A. fumigatus extract, or detection of Aspergillus-specific IgE and IgG antibodies. The presence of hyperattenuated mucus in airways is highly specific. Central bronchiectasis is characteristic, and some patients develop chronic cavitary pulmonary aspergillosis.
Severe Asthma with Fungal Sensitization Many adults with severe asthma do not fulfill the criteria for ABPA and yet are allergic to fungi. Although A. fumigatus is a common allergen, numerous other fungi (e.g., Cladosporium and Alternaria species) are implicated by skin-prick testing and/or specific IgE radioallergosorbent testing. Serum total IgE concentrations are <1000 IU/mL, and bronchiectasis is moderately common.
Allergic Sinusitis Like the lungs, the sinuses manifest allergic responses to Aspergillus and other fungi. The affected patients present with chronic (i.e., perennial) sinusitis that is relatively unresponsive to antibiotics. Many of these patients have nasal polyps, and all have congested nasal mucosae and sinuses full of mucoid material. The histologic hallmarks of allergic fungal sinusitis are local eosinophilia and Charcot-Leyden crystals. Removal of abnormal mucus and polyps, with local and occasionally systemic administration of glucocorticoids, usually leads to resolution. Persistent or recurrent signs and symptoms may require more extensive surgery (ethmoidectomy) and possibly local antifungal therapy. Recurrence is common, often after another bacterial or viral infection.
Superficial Aspergillosis Aspergillus can cause keratitis and otitis externa. The former may be difficult to diagnose early enough to save the patient’s sight. Treatment requires local surgical debridement as well as intensive topical antifungal therapy. Otitis externa usually resolves with debridement and local application of antifungal agents.
DIAGNOSIS
Several techniques are required to establish the diagnosis of any form of aspergillosis with confidence (Table 241-1). Patients with acute invasive aspergillosis have a relatively heavy load of fungus in the affected organ; thus culture, molecular diagnosis, antigen detection, and histopathology usually confirm the diagnosis. However, the pace of progression leaves only a narrow window for making the diagnosis without losing the patient, and some invasive procedures are not possible because of coagulopathy, respiratory compromise, and other factors. Currently, ~40% of cases of invasive aspergillosis are missed clinically and are diagnosed only at autopsy. Histologic examination of affected tissue reveals either infarction, with invasion of blood vessels by many fungal hyphae, or acute necrosis, with limited inflammation and fewer hyphae. Aspergillus hyphae are hyaline, narrow, and septate, with branching at 45°; no yeast forms are present in infected tissue. Hyphae can be seen in cytology or microscopy preparations, which therefore provide a rapid means of presumptive diagnosis.
Culture is important in confirming the diagnosis, given that multiple other (rarer) fungi can mimic Aspergillus species histologically. Bacterial agar is less sensitive than fungal media for culture. Thus, if physicians do not request fungal culture, the diagnosis may be missed. Culture may be falsely positive (e.g., in patients whose airways are colonized by Aspergillus) or falsely negative. Only 10–30% of patients with invasive aspergillosis have a positive culture at any time. Both antigen detection and real-time polymerase chain reaction (PCR) are faster and much more sensitive than culture of respiratory samples and blood.
The Aspergillus antigen test relies on detection of galactomannan release from Aspergillus organisms during growth. Positive serum antigen results usually precede clinical or radiologic features by several days. The sensitivity of antigen detection is reduced by antifungal prophylaxis and empirical therapy.
Definitive confirmation of the diagnosis requires (1) a positive culture of a sample taken directly from an ordinarily sterile site (e.g., a brain abscess) or (2) positive results of both histologic testing and culture of a sample taken from an affected organ (e.g., sinuses or skin). Most diagnoses of invasive aspergillosis are inferred from fewer data, including the presence of the halo sign on a high-resolution thoracic CT scan, in which a localized ground-glass appearance representing hemorrhagic infarction surrounds a nodule. While a halo sign may be produced by other fungi, Aspergillus species are by far the most common cause. Halo signs are present for ~7 days early in the course of infection in neutropenic patients and are a good prognostic feature, reflecting an early diagnosis. Thick CT sections can give the false appearance of a halo sign, as can other technical factors. Other common radiologic features of invasive pulmonary aspergillosis include nodules and pleural-based infarction or cavitation, with pleural fluid apparent in 10% of patients.
For chronic invasive aspergillosis, Aspergillus antibody testing is invaluable although relatively imprecise. Biopsy of new nodules reveals hyphae surrounded by cells of chronic inflammation and sometimes granulomas. Antibody titers fall with successful therapy. Cultures are infrequently positive but are important in checking for azole resistance. Real-time PCR of sputum is often strongly positive. Some patients with chronic pulmonary aspergillosis also have elevated titers of total serum IgE and Aspergillus-specific IgE.
ABPA and severe asthma with fungal sensitization are diagnosed serologically with elevated total and specific serum IgE levels and with skin-prick tests. Allergic Aspergillus sinusitis is usually diagnosed histologically, although precipitating antibodies in blood also may be useful.
PROPHYLAXIS
In situations in which moderate or high risk is predicted (e.g., after induction therapy for acute myeloid leukemia), the need for antifungal prophylaxis for superficial and systemic candidiasis and for invasive aspergillosis is generally accepted. Fluconazole is commonly used in these situations but has no activity against Aspergillus species. Itraconazole capsules are ineffective, and itraconazole solution offers only modest efficacy. Posaconazole solution is more effective. Some data support the use of IV micafungin. No prophylactic regimen is completely successful.
OUTCOME
Invasive aspergillosis is curable if immune reconstitution occurs, whereas allergic and chronic forms are not. The mortality rate for invasive aspergillosis is ~50% if the infection is treated but is 100% if the diagnosis is missed. Infection with a voriconazole-resistant strain carries a mortality rate of 88%. Cerebral aspergillosis, Aspergillus endocarditis, and bilateral extensive invasive pulmonary aspergillosis have very poor outcomes, as does invasive infection in persons with late-stage AIDS or relapsed uncontrolled leukemia and in recipients of allogeneic hematopoietic stem cell transplants.
The mortality rate for chronic cavitary pulmonary aspergillosis is ~30% 6 months after presentation, falling to ~15% annually thereafter. After 12 months with no antifungal therapy, 70% of patients have deteriorated and 30% are stable (Fig. 241-2). Therapy fails in ~30% of recipients of antifungal therapy and still more often if azole resistance is present.
FIGURE 241-2 Comparison of the impact of itraconazole therapy (400 mg/d) and standard care on chronic cavitary pulmonary aspergillosis at 6 and 12 months. (After R Agarwal et al: Itraconazole in chronic cavitary pulmonary aspergillosis: a randomised controlled trial and systematic review of literature. Mycoses 56:559, 2013.)
Both ABPA and SAFS patients respond to antifungal therapy; ~60% respond to itraconazole and ~80% to voriconazole and posaconazole (if tolerated). If the severity of asthma declines, the inhaled glucocorticoid dose can be reduced and oral glucocorticoids can be stopped.
242 |
Mucormycosis |
Mucormycosis represents a group of life-threatening infections caused by fungi of the order Mucorales of the subphylum Mucoromycotina (formerly known as the class Zygomycetes). Infection caused by the Mucorales is most accurately referred to as mucormycosis, although the term zygomycosis may still be used by some sources. Mucormycosis is highly invasive and relentlessly progressive, resulting in higher rates of morbidity and mortality than many other infections. However, recent studies have suggested that mortality rates from mucormycosis have declined with newer therapies. A high index of suspicion is critical for diagnosis, and early initiation of therapy—often before confirmation of the diagnosis—is necessary to optimize outcomes.
ETIOLOGY
![]() Fungi of the order Mucorales belong to seven medically relevant families (Table 242-1), all of which can cause mucormycosis. Among the Mucorales, Rhizopus oryzae (in the family Mucoraceae) is by far the most common cause of infection in the Western Hemisphere. Less frequently isolated species of the Mucoraceae that cause a similar spectrum of infections include Rhizopus microsporus, Rhizomucor pusillus, Lichtheimia corymbifera (formerly Absidia corymbifera), Apophysomyces elegans, and Mucor species (which, despite its name, only rarely causes mucormycosis). Increasing numbers of cases of mucormycosis due to infection with Cunninghamella species (family Cunninghamellaceae) have also been reported, particularly in highly immunocompromised patients. Rare case reports have demonstrated the ability of fungi in the remaining families of the Mucorales to cause mucormycosis, although other Mucorales can be the major cause of disease in certain geographic areas (e.g., A. elegans in India and Mucor irregularis in China).
Fungi of the order Mucorales belong to seven medically relevant families (Table 242-1), all of which can cause mucormycosis. Among the Mucorales, Rhizopus oryzae (in the family Mucoraceae) is by far the most common cause of infection in the Western Hemisphere. Less frequently isolated species of the Mucoraceae that cause a similar spectrum of infections include Rhizopus microsporus, Rhizomucor pusillus, Lichtheimia corymbifera (formerly Absidia corymbifera), Apophysomyces elegans, and Mucor species (which, despite its name, only rarely causes mucormycosis). Increasing numbers of cases of mucormycosis due to infection with Cunninghamella species (family Cunninghamellaceae) have also been reported, particularly in highly immunocompromised patients. Rare case reports have demonstrated the ability of fungi in the remaining families of the Mucorales to cause mucormycosis, although other Mucorales can be the major cause of disease in certain geographic areas (e.g., A. elegans in India and Mucor irregularis in China).
|
TAXONOMY OF FUNGI CAUSING MUCORMYCOSIS (SUBPHYLUM MUCOROMYCOTINA, ORDER MUCORALES) |
PATHOGENESIS
The Mucorales are ubiquitous environmental fungi to which humans are constantly exposed. These fungi cause infection primarily in patients with diabetes or defects in phagocytic function (e.g., those associated with neutropenia or glucocorticoid treatment). Patients with elevated levels of free iron, which supports fungal growth in serum and tissues, are likewise at increased risk for mucormycosis. In iron-overloaded patients with end-stage renal failure, treatment with deferoxamine predisposes to the development of rapidly fatal disseminated mucormycosis; this agent, an iron chelator for the human host, serves as a fungal siderophore, directly delivering iron to the Mucorales. Furthermore, patients with diabetic ketoacidosis (DKA) are at high risk of developing rhinocerebral mucormycosis. The acidosis causes dissociation of iron from sequestering proteins in serum, resulting in enhanced fungal survival and virulence. Nevertheless, the majority of diabetic patients who present with mucormycosis are not acidotic, and, even absent acidosis, hyperglycemia directly contributes to the risk of mucormycosis by at least three likely mechanisms: (1) hyperglycation of iron-sequestering proteins, disrupting normal iron sequestration; (2) upregulation of a mammalian cell receptor (GRP78) that binds to Mucorales, enabling tissue penetration (due to both a direct effect of hyperglycemia and increasing levels of free iron, which independently enhances GRP78 expression); and (3) induction of poorly characterized defects in phagocytic function.
EPIDEMIOLOGY
Mucormycosis typically occurs in patients with diabetes mellitus, solid organ or hematopoietic stem cell transplantation (HSCT), prolonged neutropenia, or malignancy. The majority of diabetic patients are not acidotic on presentation with mucormycosis. Furthermore, patients often have no previously recognized history of diabetes mellitus when they present with mucormycosis. In these instances, presentation for mucormycosis may result in the first clinical recognition of hyperglycemia, which may have been unmasked by recent glucocorticoid use. Thus a high index of suspicion of mucormycosis must be maintained, even in the absence of a known history of diabetes, if hyperglycemia is present. In patients undergoing HSCT, mucormycosis develops at least as commonly during nonneutropenic as during neutropenic periods, probably because of glucocorticoid treatment of graft-versus-host disease. Mucormycosis can occur as isolated cutaneous or subcutaneous infection in immunologically normal individuals after traumatic implantation of soil or vegetation (e.g., due to natural disasters or motor vehicle accidents) or in nosocomial settings via direct access through IV catheters, SC injections, or maceration of the skin by a moist dressing.
Patients receiving antifungal prophylaxis with either itraconazole or voriconazole may be at increased risk of mucormycosis. These patients typically present with disseminated mucormycosis, the most lethal form of disease. Breakthrough mucormycosis also has been described in patients receiving posaconazole or echinocandin prophylaxis.
CLINICAL MANIFESTATIONS
Mucormycosis can be divided into at least six clinical categories based on clinical presentation and the involvement of a particular anatomic site: rhino-orbital-cerebral, pulmonary, cutaneous, gastrointestinal, disseminated, and miscellaneous. These categories of invasive mucormycosis tend to affect patients with specific defects in host defense. For example, patients with DKA typically develop the rhino-orbital-cerebral form and much more rarely develop pulmonary or disseminated disease. In contrast, pulmonary mucormycosis occurs most commonly in leukemic patients who are receiving chemotherapy and in patients undergoing HSCT.
Rhino-Orbital-Cerebral Disease Rhino-orbital-cerebral mucormycosis continues to be the most common form of the disease. Most cases occur in patients with diabetes, although such cases (probably due to glucocorticoid use) are increasingly being described in the transplantation setting, often along with glucocorticoid-induced diabetes mellitus. The initial symptoms of rhino-orbital-cerebral mucormycosis are nonspecific and include eye or facial pain and facial numbness followed by the onset of conjunctival suffusion and blurry vision. Fever may be absent in up to half of cases. White blood cell counts are typically elevated as long as the patient has functioning bone marrow. If untreated, infection usually spreads from the ethmoid sinus to the orbit, resulting in compromise of extraocular muscle function and proptosis, typically with chemosis. Onset of signs and symptoms in the contralateral eye, with resulting bilateral proptosis, chemosis, vision loss, and ophthalmoplegia, is ominous, suggesting the development of cavernous sinus thrombosis.
Upon visual inspection, infected tissue may appear to be normal during the earliest stages of fungal spread, then progressing through an erythematous phase, with or without edema, before the onset of a violaceous appearance and finally the development of a black necrotic eschar. Infection can sometimes extend from the sinuses into the mouth and produce painful necrotic ulcerations of the hard palate, but this is a late finding that suggests extensive, well-established infection.
Pulmonary Disease Pulmonary mucormycosis is the second most common manifestation. Symptoms include dyspnea, cough, and chest pain; fever is often but not invariably present. Angioinvasion results in necrosis, cavitation, and/or hemoptysis. Lobar consolidation, isolated masses, nodular disease, cavities, or wedge-shaped infarcts may be seen on chest radiography. High-resolution chest CT is the best method for determining the extent of pulmonary mucormycosis and may demonstrate evidence of infection before it is seen on chest x-ray. In the setting of cancer, where mucormycosis may be difficult to differentiate from aspergillosis, the presence of ≥10 pulmonary nodules, pleural effusion, or concomitant sinusitis makes mucormycosis more likely. It is critical to distinguish mucormycosis from aspergillosis as rapidly as possible because treatments for these infections differ. Indeed, voriconazole—the first-line treatment for aspergillosis—exacerbates mucormycosis in mouse and fly models of infection.
Cutaneous Disease Cutaneous mucormycosis may result from external implantation of the fungus or conversely from hematogenous dissemination. External implantation–related infection has been described in the setting of soil exposure from trauma (e.g., in a motor vehicle accident or natural disaster), penetrating injury with plant material (e.g., a thorn), injections of medications (e.g., insulin), catheter insertion, contamination of surgical dressings, and use of tape to secure endotracheal tubes. Cutaneous disease can be highly invasive, penetrating into muscle, fascia, and even bone. In mucormycosis, necrotizing fasciitis carries a mortality rate approaching 80%. Necrotic cutaneous lesions in the setting of hematogenous dissemination also are associated with an extremely high mortality rate. However, with prompt, aggressive surgical debridement, isolated cutaneous mucormycosis has a favorable prognosis and a low mortality rate.
Gastrointestinal Disease In the past, gastrointestinal mucormycosis occurred primarily in premature neonates in association with disseminated disease and necrotizing enterocolitis. However, there has been a marked increase in case reports describing adults with neutropenia or other immunocompromising conditions. In addition, gastrointestinal disease has been reported as a nosocomial process following administration of medications mixed with contaminated wooden applicator sticks. Nonspecific abdominal pain and distention associated with nausea and vomiting are the most common symptoms. Gastrointestinal bleeding is common, and fungating masses may be seen in the stomach at endoscopy. The disease may progress to visceral perforation, with extremely high mortality rates.
Disseminated and Miscellaneous Forms of Disease Hematogenously disseminated mucormycosis may originate from any primary site of infection. The most common site of dissemination is the brain, but metastatic lesions may also be found in any other organ. The mortality rate associated with dissemination to the brain approaches 100%. Even without central nervous system (CNS) involvement, mortality rates for disseminated mucormycosis exceed 90%. Miscellaneous forms of mucormycosis may affect any body site, including bones, mediastinum, trachea, kidneys, and (in association with dialysis) peritoneum.
DIAGNOSIS
A high index of suspicion is required for diagnosis of mucormycosis. Unfortunately, autopsy series have shown that up to half of cases are diagnosed only post-mortem. Because the Mucorales are environmental isolates, definitive diagnosis requires a positive culture from a sterile site (e.g., a needle aspirate, a tissue biopsy specimen, or pleural fluid) or histopathologic evidence of invasive mucormycosis. A probable diagnosis of mucormycosis can be established by culture from a nonsterile site (e.g., sputum or bronchoalveolar lavage) when a patient has appropriate risk factors as well as clinical and radiographic evidence of disease. However, given the urgency of administering therapy early, the patient should be treated while confirmation of the diagnosis is awaited.
Biopsy with histopathologic examination remains the most sensitive and specific modality for definitive diagnosis (Fig. 242-1). Biopsy reveals characteristic wide (≥6- to 30-μm), thick-walled, ribbon-like, aseptate hyphal elements that branch at right angles. Other fungi, including Aspergillus, Fusarium, and Scedosporium species, have septa, are thinner, and branch at acute angles. Because artificial septa may result from folding of tissue during processing (which may also alter the appearance of the angle of branching), the width and the ribbon-like form of the fungus are the most reliable features distinguishing mucormycosis. The Mucorales are visualized most effectively with periodic acid–Schiff or methenamine silver stain or, if the organism burden is high, with hematoxylin and eosin. While histopathology can identify the Mucorales, specific species can be identified only by culture. Polymerase chain reaction (PCR) is being investigated as a diagnostic tool for mucormycosis but is not yet approved by the U.S. Food and Drug Administration (FDA) for this purpose and is not generally available.
FIGURE 242-1 Histopathology sections of Rhizopus oryzae in infected brain. A. Broad, ribbon-like, nonseptate hyphae in the parenchyma (arrows) and a thrombosed blood vessel with extensive intravascular hyphae (arrowhead) (hematoxylin and eosin). B. Extensive, broad, ribbon-like hyphae invading the parenchyma (Gomori methenamine silver).
Unfortunately, cultures are positive in fewer than half of cases of mucormycosis. Nevertheless, the Mucorales are not fastidious organisms and tend to grow quickly (i.e., within 48 h) on culture media. The likely explanation for the low sensitivity of culture is that the Mucorales form long filamentous structures that are killed by tissue homogenization—the standard method for preparing tissue cultures in the clinical microbiology laboratory. Thus the laboratory should be advised when a diagnosis of mucormycosis is suspected, and the tissue should be cut into sections and placed in the center of culture dishes rather than homogenized. There is also substantial variability among isolates in optimal growth temperature, so growth at both room temperature and 37°C is advisable.
Imaging techniques often yield subtle findings that underestimate the extent of disease. For example, the most common finding on CT or MRI of the head or sinuses of a patient with rhino-orbital mucormycosis is sinusitis that is indistinguishable from bacterial sinusitis. It is also common to detect no abnormalities in sinus bones despite clinical evidence of progressive disease. MRI is more sensitive (~80%) for detecting orbital and CNS disease than is CT. High-risk patients should always undergo endoscopy and/or surgical exploration, with biopsy of the areas of suspected infection. If mucormycosis is suspected, initial empirical therapy with a polyene antifungal agent should be initiated while the diagnosis is being confirmed.
DIFFERENTIAL DIAGNOSIS
Other mold infections, including aspergillosis, scedosporiosis, fusariosis, and infections caused by the dematiaceous fungi (brown-pigmented soil organisms), can cause clinical syndromes identical to mucormycosis. Histopathologic examination usually allows distinction of the Mucorales from these other organisms, and a positive culture permits definitive species identification. As stated above, it is important to distinguish the Mucorales from these other fungi, as the preferred antifungal treatments differ (i.e., polyenes for the Mucorales vs. expanded-spectrum triazoles for most septate molds). The entomophthoromycoses caused by Basidiobolus and Conidiobolus also can cause identical clinical syndromes. These fungi may appear similar to the Mucorales on histopathology and can be reliably distinguished from the latter only by culture.
In a patient with sinusitis and proptosis, orbital cellulitis and cavernous sinus thrombosis caused by bacterial pathogens (most commonly Staphylococcus aureus, but also streptococcal and gram-negative species) must be excluded. Klebsiella rhinoscleromatis is a rare cause of an indolent facial rhinoscleroma syndrome that may appear similar to mucormycosis. Finally, the Tolosa-Hunt syndrome causes painful ophthalmoplegia, ptosis, headache, and cavernous sinus inflammation; biopsies and clinical follow-up may be needed to distinguish the Tolosa-Hunt syndrome from mucormycosis by the lack of progression of the former entity.
243 |
Superficial Mycoses and Less Common Systemic Mycoses |
ENDEMIC MYCOSES (DIMORPHIC FUNGI)
Dimorphic fungi exist in discrete environmental niches as molds that produce conidia, which are their infectious form. In tissues and at temperatures of >35°C, the mold converts to the yeast form. Other endemic mycoses—histoplasmosis, coccidioidomycosis, and blastomycosis—are discussed in Chaps. 236, 237, and 238, respectively.
SPOROTRICHOSIS
![]() Etiologic Agent Sporothrix schenckii is a thermally dimorphic fungus that is found worldwide in sphagnum moss, decaying vegetation, and soil.
Etiologic Agent Sporothrix schenckii is a thermally dimorphic fungus that is found worldwide in sphagnum moss, decaying vegetation, and soil.
Epidemiology and Pathogenesis Sporotrichosis most commonly infects persons who participate in outdoor activities such as landscaping, gardening, and tree farming. Infected animals can transmit S. schenckii to humans. An outbreak of sporotrichosis in Rio de Janeiro that began in 1998 and that has involved >2000 people has been traced to cats, which are highly susceptible to this infection. Sporotrichosis is primarily a localized infection of skin and subcutaneous tissues that follows traumatic inoculation of conidia. Osteoarticular sporotrichosis is uncommon, occurring most often in middle-aged men who abuse alcohol, and pulmonary sporotrichosis occurs almost exclusively in persons with chronic obstructive pulmonary disease who have inhaled the organism from the environment. Dissemination occurs rarely, almost always in markedly immunocompromised patients, especially those with AIDS.
Clinical Manifestations Days or weeks after inoculation, a papule develops at the site and then usually ulcerates but is not very painful. Similar lesions develop sequentially along the lymphatic channels proximal to the original lesion (Fig. 243-1). Some patients develop a fixed cutaneous lesion that can be verrucous or ulcerative and that remains localized without lymphatic extension. The differential diagnosis of lymphocutaneous sporotrichosis includes nocardiosis, tularemia, nontuberculous mycobacterial infection (especially that due to Mycobacterium marinum), and leishmaniasis. Osteoarticular sporotrichosis can present as chronic synovitis or septic arthritis. Pulmonary sporotrichosis must be differentiated from tuberculosis and from other fungal pneumonias. Numerous ulcerated skin lesions, with or without spread to visceral organs (including the central nervous system [CNS]), are characteristic of disseminated sporotrichosis.
FIGURE 243-1 Several nodular lesions that developed after a young boy pricked his index finger with a thorn. A culture yielded S. schenckii. (Courtesy of Dr. Angela Restrepo.)
Diagnosis S. schenckii usually grows readily as a mold when material from a cutaneous lesion is incubated at room temperature. Histopathologic examination of biopsy material shows a mixed granulomatous and pyogenic reaction, and tiny oval or cigar-shaped yeasts are sometimes visualized with special stains. Serologic testing is not useful.
Treatment and Prognosis Guidelines for the management of the various forms of sporotrichosis have been published by the Infectious Diseases Society of America (Table 243-1). Itraconazole is the drug of choice for lymphocutaneous sporotrichosis. Fluconazole is less effective; voriconazole and posaconazole have not been used for sporotrichosis. Saturated solution of potassium iodide (SSKI) also is effective for lymphocutaneous infection and costs much less than itraconazole. However, SSKI is poorly tolerated because of adverse reactions, including metallic taste, salivary gland swelling, rash, and fever. Terbinafine appears to be effective but has been used in few patients. Treatment for lymphocutaneous sporotrichosis is continued for 2–4 weeks after all lesions have resolved, usually for a total of 3–6 months. Pulmonary and osteoarticular forms of sporotrichosis are treated with itraconazole for at least 1 year. Severe pulmonary infection and disseminated sporotrichosis, including that involving the CNS, are treated initially with amphotericin B (AmB), which is followed by itraconazole after improvement has been noted. Lifelong suppressive therapy with itraconazole is required for AIDS patients. The success rate for treatment of lymphocutaneous sporotrichosis is 90–100%, but other forms of the disease respond poorly to antifungal therapy.
|
SUGGESTED TREATMENT FOR ENDEMIC MYCOSES |
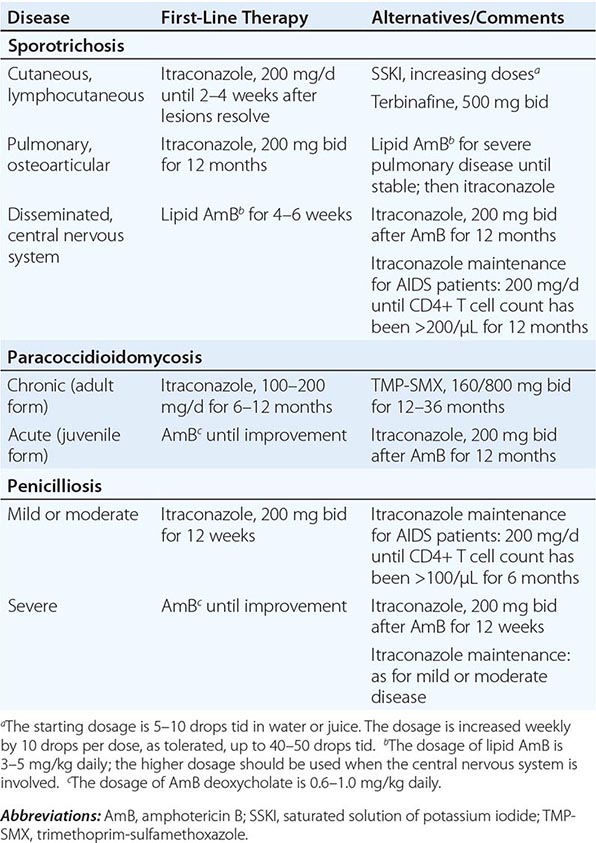
PARACOCCIDIOIDOMYCOSIS
![]() Etiologic Agent, Epidemiology, and Pathogenesis Paracoccidioides brasiliensis is a thermally dimorphic fungus that is endemic in humid areas of Central and South America, especially in Brazil. A striking male-to-female ratio varies from 14:1 to as high as 70:1 (in rural Brazil). Most patients are middle-aged or elderly men from rural areas. Paracoccidioidomycosis develops after the inhalation of aerosolized conidia encountered in the environment. For most patients, disease rarely develops at the time of the initial infection but appears years later, presumably after reactivation of a latent infection.
Etiologic Agent, Epidemiology, and Pathogenesis Paracoccidioides brasiliensis is a thermally dimorphic fungus that is endemic in humid areas of Central and South America, especially in Brazil. A striking male-to-female ratio varies from 14:1 to as high as 70:1 (in rural Brazil). Most patients are middle-aged or elderly men from rural areas. Paracoccidioidomycosis develops after the inhalation of aerosolized conidia encountered in the environment. For most patients, disease rarely develops at the time of the initial infection but appears years later, presumably after reactivation of a latent infection.
Clinical Manifestations Two major syndromes are associated with paracoccidioidomycosis: the acute or juvenile form and the chronic or adult form. The acute form is uncommon, occurs mostly in persons <30 years old, and manifests as disseminated infection of the reticuloendothelial system. Immunocompromised individuals also manifest this type of rapidly progressive disease. The chronic form of paracoccidioidomycosis accounts for ∼90% of cases and predominantly affects older men. The primary manifestation is progressive pulmonary disease, primarily in the lower lobes, with fibrosis. Ulcerative and nodular mucocutaneous lesions in the nares and mouth—another common manifestation of chronic paracoccidioidomycosis—must be differentiated from leishmaniasis (Chap. 251) and squamous cell carcinoma (Chap. 105).
Diagnosis The diagnosis is established by growth of the organism in culture. A presumptive diagnosis can be made by detection of the distinctive thick-walled yeast, with multiple narrow-necked buds attached circumferentially, in purulent material or tissue biopsies.
Treatment and Prognosis Itraconazole is the treatment of choice for paracoccidioidomycosis (Table 243-1). Ketoconazole is also effective but more toxic; voriconazole and posaconazole have been used with success in a few cases. Sulfonamides also are effective and are the least costly agents, but the response is slower and the relapse rate higher. Seriously ill patients should be treated with AmB initially. Patients with paracoccidioidomycosis have an excellent response to therapy, but pulmonary fibrosis is often progressive in those with chronic disease.
PENICILLIOSIS
![]() Etiologic Agent, Epidemiology, and Pathogenesis Penicillium marneffei is a thermally dimorphic fungus that is endemic in the soil in certain areas of Vietnam, Thailand, and several other southeastern Asian countries. The epidemiology of penicilliosis is linked to bamboo rats, which are infected with the fungus but rarely manifest disease. The disease occurs most often among persons living in rural areas in which the rats are found, but there is no evidence for transmission of the infection directly from rats to humans. Infection is rare in immunocompetent hosts, and most cases are reported in persons who have advanced AIDS. Infection results from the inhalation of conidia from the environment. The organism converts to the yeast phase in the lungs and then spreads hematogenously to the reticuloendothelial system.
Etiologic Agent, Epidemiology, and Pathogenesis Penicillium marneffei is a thermally dimorphic fungus that is endemic in the soil in certain areas of Vietnam, Thailand, and several other southeastern Asian countries. The epidemiology of penicilliosis is linked to bamboo rats, which are infected with the fungus but rarely manifest disease. The disease occurs most often among persons living in rural areas in which the rats are found, but there is no evidence for transmission of the infection directly from rats to humans. Infection is rare in immunocompetent hosts, and most cases are reported in persons who have advanced AIDS. Infection results from the inhalation of conidia from the environment. The organism converts to the yeast phase in the lungs and then spreads hematogenously to the reticuloendothelial system.
Clinical Manifestations The clinical manifestations of penicilliosis mimic those of disseminated histoplasmosis and include fever, fatigue, weight loss, dyspnea, diarrhea (in some cases), lymphadenopathy, hepatosplenomegaly, and skin lesions, which appear as papules that often umbilicate and resemble molluscum contagiosum (Chap. 220e).
Diagnosis Penicilliosis is diagnosed by culture of P. marneffei from blood or from biopsy samples of skin, bone marrow, or lymph node. The organism usually grows within 1 week as a mold that produces a distinctive red pigment. Histopathologic examination of tissues and smears of blood or material from skin lesions shows oval or elliptical yeast-like organisms with central septation and can quickly establish a presumptive diagnosis.
Treatment and Prognosis Patients who have severe disease should be treated initially with AmB until their condition improves; therapy can then be changed to itraconazole (Table 243-1). Patients who have mild symptoms can be treated from the start with itraconazole. For patients with AIDS, suppressive therapy with itraconazole is recommended until immune reconstitution (related to successful therapy for HIV infection with antiretroviral drugs) is evident. Disseminated penicilliosis is usually fatal if not treated. With treatment, the mortality rate is ∼10%.
PHAEOHYPHOMYCOSES
In these common soil organisms (also called dematiaceous fungi), melanin causes the hyphae and/or conidia to be darkly pigmented. The term phaeohyphomycosis is used to describe any infection with a pigmented mold. This definition encompasses two specific syndromes—eumycetoma and chromoblastomycosis—as well as all other types of infections caused by these organisms. It is important to note that eumycetomas can be caused by hyaline molds as well as brown-black molds and that only about half of all mycetomas are due to fungi. Actinomycetes cause the remainder (Chap. 199). Most of the involved fungi cause localized subcutaneous infections after direct inoculation, but disseminated infection and serious focal visceral infections also occur, especially in immunocompromised patients.
Etiologic Agents A large number of pigmented molds can cause human infection. All are found in the soil or on plants, and some cause economically important plant diseases. The most common cause of eumycetoma is Madurella species. Fonsecaea and Cladophialophora species are responsible for most cases of chromoblastomycosis. Disseminated infection and focal visceral infections are caused by a variety of dematiaceous fungi; Alternaria, Exophiala, Curvularia, and Wangiella species are among the more common molds reported to cause human infection. Recently, Exserohilum species have caused a large outbreak of severe, sometimes fatal CNS and osteoarticular infections following the injection of methylprednisolone contaminated with this fungus.
Epidemiology and Pathogenesis Eumycetoma and chromoblastomycosis are acquired by inoculation through the skin. These two syndromes are seen almost entirely in tropical and subtropical areas and occur mostly in rural laborers who are frequently exposed to the organisms. Other infections with dematiaceous molds are acquired by inhalation, by traumatic inoculation into the eye or through the skin, or by injection of contaminated medication. Melanin is a virulence factor for all the pigmented molds. Several organisms, specifically Cladophialophora bantiana and Rhinocladiella mackenziei, are neurotropic and likely to cause CNS infection. In an immunocompromised patient or when a pigmented mold is injected directly into a deep structure, these organisms become opportunists, invading blood vessels and mimicking better-known opportunistic infections, such as aspergillosis.
Clinical Manifestations Eumycetoma is a chronic subcutaneous and cutaneous infection that usually occurs on the lower extremities and that is characterized by swelling, development of sinus tracts, and the appearance of grains that are actually colonies of fungi discharged from the sinus tract. As the infection progresses, adjacent fascia and bony structures become involved. The disease is indolent and disfiguring, progressing slowly over years. Complications include fractures of infected bone and bacterial superinfection.
Chromoblastomycosis is an indolent subcutaneous infection characterized by nodular, verrucous, or plaque-like painless lesions that occur predominantly on the lower extremities and grow slowly over months to years. There is hardly ever extension to adjacent structures, as is seen with eumycetoma. Long-term consequences include bacterial superinfection, chronic lymphedema, and (rarely) the development of squamous cell carcinoma.
Dematiaceous molds are the most common cause of allergic fungal sinusitis and a less common cause of invasive fungal sinusitis. Keratitis occurs with traumatic corneal inoculation. Even in many immunocompromised patients, inoculation through the skin generally produces localized cyst-like, nodular lesions at the entry site. However, other immunocompromised patients develop pneumonia, brain abscess, or disseminated infection. Epidural injection of Exserohilum-contaminated steroids has led to meningitis, basilar stroke, epidural abscess or phlegmon, vertebral osteomyelitis, and arachnoiditis.
Diagnosis The specific diagnosis of infection with a pigmented mold is established by growth of the organism in culture. However, in eumycetoma, a tentative clinical diagnosis can be made when a patient presents with a lesion characterized by swelling, sinus tracts, and grains. Histopathologic examination and culture are necessary to confirm that the etiologic agent is a mold and not an actinomycete. In chromoblastomycosis, the diagnosis rests on the histologic demonstration of sclerotic bodies (dark brown, thick-walled, septate fungal forms that resemble large yeasts) in the tissues; culture establishes which pigmented mold is causing the infection. For other infections, growth of the organism is essential to differentiate infection with a hyaline mold (e.g., Aspergillus or Fusarium) from that due to a pigmented mold. No serologic assays for pigmented molds are available. Polymerase chain reaction (PCR) assays are increasingly used in the diagnosis of infection due to pigmented molds but are available only through fungal reference laboratories.
Treatment and Prognosis Treatment of eumycetoma and chromoblastomycosis involves both surgical extirpation of the lesion and use of antifungal agents. Surgical removal of the lesions of both eumycetoma and chromoblastomycosis is most effective if performed before extensive spread has occurred. In chromoblastomycosis, cryosurgery and laser therapy have been used with variable success. The antifungal agents of choice are itraconazole, voriconazole, and posaconazole. The most experience has accrued with itraconazole; less experience has been gained with the newer azoles, which are active in vitro and have been reported to be effective in a few patients. Flucytosine and terbinafine also have been used to treat chromoblastomycosis. Chromoblastomycosis and eumycetoma are chronic indolent infections that are difficult to cure but are not life-threatening.
Disseminated and focal visceral infections are treated with the appropriate antifungal agent; the choice of agent is based on the location and extent of the infection, in vitro testing, and clinical experience with the specific infecting organism. AmB is not effective against many of these organisms but has been used successfully against others. The most experience has accrued with itraconazole in the treatment of localized infections. Voriconazole is increasingly used when infections are disseminated or involve the CNS because this drug reaches adequate concentrations within the CNS and because both IV and well-absorbed oral formulations are available. The role of posaconazole has not been established but will likely expand. Disseminated and focal visceral infections, especially those involving the CNS, are associated with high mortality rates.
OPPORTUNISTIC FUNGAL INFECTIONS
Two genera of hyaline (nonpigmented) molds, Fusarium and Scedosporium, and one yeast-like genus, Trichosporon, have become prominent pathogens among immunocompromised patients. Infections caused by Fusarium and Scedosporium species overlap with invasive aspergillosis in their clinical manifestations, and, when seen in tissues, these organisms appear similar to Aspergillus. In the immunocompetent host, these fungi cause localized infections of skin, skin structures, and subcutaneous tissues, but their role as causes of infection in immunocompromised patients will be emphasized in this section.
FUSARIOSIS
![]() Etiologic Agent, Epidemiology, and Pathogenesis Fusarium species, which are found worldwide in soil and on plants, have emerged as major opportunists in markedly immunocompromised patients. Most human infections follow inhalation of conidia, but ingestion and direct inoculation also can lead to disease. An outbreak of severe Fusarium keratitis among soft contact lens wearers was traced back to a particular brand of contact lens solution and individual contact lens cases that had been contaminated. Disseminated infection is reported most often in patients who have a hematologic malignancy, are neutropenic, have received a stem cell or solid organ transplant, or have a severe burn.
Etiologic Agent, Epidemiology, and Pathogenesis Fusarium species, which are found worldwide in soil and on plants, have emerged as major opportunists in markedly immunocompromised patients. Most human infections follow inhalation of conidia, but ingestion and direct inoculation also can lead to disease. An outbreak of severe Fusarium keratitis among soft contact lens wearers was traced back to a particular brand of contact lens solution and individual contact lens cases that had been contaminated. Disseminated infection is reported most often in patients who have a hematologic malignancy, are neutropenic, have received a stem cell or solid organ transplant, or have a severe burn.
Clinical Manifestations In immunocompetent persons, Fusarium species cause localized infections of various organs. These organisms commonly cause fungal keratitis, which can extend into the anterior chamber of the eye; cause loss of vision; and require corneal transplantation. Onychomycosis due to Fusarium species, while basically an annoyance in immunocompetent patients, is a source of subsequent hematogenous dissemination and should be aggressively sought and treated in neutropenic patients. In profoundly immunocompromised patients, fusariosis is angioinvasive, and clinical manifestations mimic those of aspergillosis. Pulmonary infection is characterized by multiple nodular lesions. Sinus infection is likely to lead to invasion of adjacent structures. Disseminated fusariosis occurs primarily in neutropenic patients with hematologic malignancies and in allogeneic stem cell transplant recipients, especially those with graft-versus-host disease. Disseminated fusariosis differs from disseminated aspergillosis in that skin lesions are extremely common with fusariosis; the lesions are nodular or necrotic, are usually painful, and appear over time in different locations (Fig. 243-2).
FIGURE 243-2 Painful necrotic foot lesion that developed over a week in a woman who had acute leukemia and who had been neutropenic for 2 months. Fusarium species were grown from a punch biopsy. (Courtesy of Dr. Nessrine Ktaich.)
Diagnosis The diagnostic approach usually includes both documentation of the growth of Fusarium species from involved tissue and demonstration of invasion by histopathologic techniques that show septate hyphae in tissues. The organism is difficult to differentiate from Aspergillus species in tissues; thus, identification with culture is imperative. An extremely helpful diagnostic clue is growth in blood cultures, which are positive in as many as 50% of patients with disseminated fusariosis. There are no serologic assays for Fusarium. PCR techniques have proved useful but are available only through fungal reference laboratories.
Treatment and Prognosis Fusarium species are resistant to many antifungal agents. A lipid formulation of AmB (at least 5 mg/kg daily), voriconazole (200–400 mg twice daily), or posaconazole (300 mg daily) is recommended. Many physicians use both a lipid formulation of AmB and either voriconazole or posaconazole because susceptibility information is not available when therapy must be initiated. Serum drug levels should be monitored with either azole to ensure that absorption is adequate and with voriconazole to avoid toxicity. Mortality rates for disseminated fusariosis have been as high as 85%. With the improved antifungal therapy now available, mortality rates have fallen to ∼50%. However, if neutropenia persists, the mortality rate approaches 100%.
SCEDOSPORIOSIS
Etiologic Agent The genus Scedosporium includes several pathogens. The major causes of human infections are Scedosporium apiospermum, which in its sexual state is termed Pseudallescheria boydii, and S. prolificans. The S. apiospermum complex encompasses several species but will be referred to here simply as S. apiospermum.
![]() Epidemiology and Pathogenesis S. apiospermum is found worldwide in temperate climates in tidal flats, swamps, ponds, manure, and soil. This organism is known as a cause of pneumonia, disseminated infection, and brain abscess in near-drowning victims. S. prolificans is also found in soil but is more geographically restricted. Infection occurs predominantly through inhalation of conidia, but direct inoculation through the skin or into the eye also can occur.
Epidemiology and Pathogenesis S. apiospermum is found worldwide in temperate climates in tidal flats, swamps, ponds, manure, and soil. This organism is known as a cause of pneumonia, disseminated infection, and brain abscess in near-drowning victims. S. prolificans is also found in soil but is more geographically restricted. Infection occurs predominantly through inhalation of conidia, but direct inoculation through the skin or into the eye also can occur.
Clinical Manifestations Among immunocompetent persons, Scedosporium species are a prominent cause of eumycetoma. Keratitis as a result of accidental corneal inoculation is a sight-threatening infection. In patients who have hematologic malignancies (especially acute leukemia with neutropenia), recipients of solid organ or stem cell transplants, and patients receiving glucocorticoids, Scedosporium species are angioinvasive, causing pneumonia and widespread dissemination with abscesses. Pulmonary infection mimics aspergillosis; nodules, cavities, and lobar infiltrates are common. Disseminated infection involves the skin, heart, brain, and many other organs. Skin lesions are not as common or as painful as those of fusariosis.
Diagnosis Diagnosis depends on the growth of Scedosporium species from involved tissue and the demonstration of invasion by histopathologic techniques that show septate hyphae in tissues. Culture evidence is essential because Scedosporium species are difficult to differentiate from Aspergillus in tissues. Demonstration of tissue invasion is essential because these ubiquitous environmental molds can be mere contaminants or colonizers. S. prolificans can grow in blood cultures, but S. apiospermum usually does not. There are no serologic assays for Scedosporium. PCR techniques have proved useful but are available only through fungal reference laboratories.
Treatment and Prognosis Scedosporium species are resistant to AmB, echinocandins, and some azoles. Voriconazole is the agent of choice for S. apiospermum, and posaconazole also has been used for this infection. S. prolificans is resistant in vitro to almost every available antifungal agent; the addition of agents such as terbinafine to a voriconazole regimen has been attempted because in vitro data suggest possible synergy against some strains of S. prolificans. Mortality rates for invasive S. apiospermum infection are ∼50%, but those for invasive S. prolificans infection remain as high as 85–100%.
TRICHOSPORONOSIS
Etiologic Agent The genus Trichosporon contains many species, some of which cause localized infection of hair and nails. The major pathogen responsible for invasive infection is Trichosporon asahii. Trichosporon species grow as yeast-like colonies in vitro; in vivo, however, hyphae, pseudohyphae, and arthroconidia can also be seen.
Epidemiology and Pathogenesis These yeasts are commonly found in soil, sewage, and water and in rare instances can colonize human skin and the human gastrointestinal tract. Most infections follow fungal inhalation or entry via central venous catheters. Systemic infection occurs almost exclusively in immunocompromised hosts, including those who have hematologic malignancies, are neutropenic, have received a solid organ transplant, or are receiving glucocorticoids.
Clinical Manifestations Disseminated trichosporonosis resembles invasive candidiasis, and fungemia is often the initial manifestation of infection. Pneumonia, skin lesions, and sepsis syndrome are common. The skin lesions begin as papules or nodules surrounded by erythema and progress to central necrosis. A chronic form of infection mimics hepatosplenic candidiasis (chronic disseminated candidiasis).
Diagnosis The diagnosis of systemic Trichosporon infection is established by growth of the organism from involved tissues or from blood. Histopathologic examination of a skin lesion showing a mixture of yeast forms, arthroconidia, and hyphae can lead to an early presumptive diagnosis of trichosporonosis. The serum cryptococcal antigen latex agglutination test may be positive in patients with disseminated trichosporonosis because T. asahii and Cryptococcus neoformans share polysaccharide antigens.
Treatment and Prognosis Rates of response to AmB have been disappointing, and many Trichosporon isolates are resistant in vitro. Voriconazole appears to be the antifungal agent of choice and is used at a dosage of 200–400 mg twice daily. The mortality rates for disseminated Trichosporon infection have been as high as 70% but are decreasing with the use of newer azoles, such as voriconazole; however, patients who remain neutropenic are likely to succumb to this infection.
SUPERFICIAL CUTANEOUS INFECTIONS
Fungal infections of the skin and skin structures are caused by molds and yeasts that do not invade deeper tissues but rather cause disease merely by inhabiting the superficial layers of skin, hair follicles, and nails. These agents are the most common cause of fungal infections of humans but only rarely cause serious infections.
YEAST INFECTIONS
Etiologic Agents The lipophilic yeast Malassezia is dimorphic in that it lives on the skin in the yeast phase but transforms to the mold phase as it causes disease. Most species require exogenous lipids for growth.
Epidemiology and Pathogenesis Malassezia species are part of the indigenous human flora found in the stratum corneum of the back, chest, scalp, and face—areas rich in sebaceous glands. Disease is more common in humid areas. The organisms do not invade below the stratum corneum and generally elicit little if any inflammatory response.
Clinical Manifestations Malassezia species cause tinea versicolor (also called pityriasis versicolor), folliculitis, and seborrheic dermatitis. Tinea versicolor presents as flat round scaly patches of hypo- or hyperpigmented skin on the neck, chest, or upper arms. The lesions are usually asymptomatic but can be pruritic. They can be mistaken for vitiligo, but the latter is not scaly. Folliculitis occurs over the back and chest and mimics bacterial folliculitis. Seborrheic dermatitis manifests as erythematous pruritic scaly lesions in the eyebrows, moustache, nasolabial folds, and scalp. The scalp lesions are termed cradle cap in babies and dandruff in adults. Seborrheic dermatitis can be severe in patients with advanced AIDS. Fungemia and disseminated infection occur rarely with Malassezia species—almost always in premature neonates receiving parenteral lipid preparations through a central venous catheter.
Diagnosis Malassezia infections are diagnosed clinically in most cases. If scrapings are collected on a microscope slide on which a drop of potassium hydroxide has been placed, a mixture of budding yeasts and short septate hyphae is seen. In order to culture M. furfur from those patients in whom disseminated infection is suspected, sterile olive oil must be added to the medium.
Treatment and Prognosis Topical creams and lotions, including selenium sulfide shampoo, ketoconazole shampoo or cream, terbinafine cream, and ciclopirox cream, are effective in treating Malassezia infections and are usually given for 2 weeks. Mild topical steroid creams are sometimes used to treat seborrheic dermatitis. For extensive disease, itraconazole (200 mg/d) or fluconazole (200 mg/d) can be used for 5–7 days. The rare cases of fungemia caused by Malassezia species are treated with AmB or fluconazole, prompt removal of the catheter, and discontinuance of parenteral lipid infusions. Malassezia skin infections are benign and self-limited, although recurrences are the rule. The outcome of systemic infection depends on the host’s underlying conditions, but most infected infants do well.
DERMATOPHYTE (MOLD) INFECTIONS
Etiologic Agents The molds that cause skin infections in humans include the genera Trichophyton, Microsporum, and Epidermophyton. These organisms, which are not components of the normal skin flora, can live within the keratinized structures of the skin—hence the term dermatophytes.
![]() Epidemiology and Pathogenesis Dermatophytes occur worldwide, and infections with these organisms are extremely common. Some organisms cause disease only in humans and can be transmitted by person-to-person contact and by fomites, such as hairbrushes or wet floors, that have been contaminated by infected individuals. Several species cause infections in cats and dogs and can readily be transmitted from these animals to humans. Finally, some dermatophytes are spread from contact with soil. The characteristic ring shape of cutaneous lesions is the result of the organisms’ outward growth in a centrifugal pattern in the stratum corneum. Fungal invasion of the nail usually occurs through the lateral or superficial nail plates and then spreads throughout the nail; when hair shafts are invaded, the organisms can be found either within the shaft or surrounding it. Symptoms are caused by the inflammatory reaction elicited by fungal antigens and not by tissue invasion. Dermatophyte infections occur more commonly in male than in female patients, and progesterone has been shown to inhibit dermatophyte growth.
Epidemiology and Pathogenesis Dermatophytes occur worldwide, and infections with these organisms are extremely common. Some organisms cause disease only in humans and can be transmitted by person-to-person contact and by fomites, such as hairbrushes or wet floors, that have been contaminated by infected individuals. Several species cause infections in cats and dogs and can readily be transmitted from these animals to humans. Finally, some dermatophytes are spread from contact with soil. The characteristic ring shape of cutaneous lesions is the result of the organisms’ outward growth in a centrifugal pattern in the stratum corneum. Fungal invasion of the nail usually occurs through the lateral or superficial nail plates and then spreads throughout the nail; when hair shafts are invaded, the organisms can be found either within the shaft or surrounding it. Symptoms are caused by the inflammatory reaction elicited by fungal antigens and not by tissue invasion. Dermatophyte infections occur more commonly in male than in female patients, and progesterone has been shown to inhibit dermatophyte growth.
Clinical Manifestations Dermatophyte infection of the skin is often called ringworm. This term is confusing because worms are not involved. Tinea, the Latin word for worm, describes the serpentine nature of the skin lesions and is a less confusing designation that is used in conjunction with the name of the body part affected—e.g., tinea capitis (head), tinea pedis (feet), tinea corporis (body), tinea cruris (crotch), and tinea unguium (nails, although infection at this site is more often termed onychomycosis).
Tinea capitis occurs most commonly in children 3–7 years old. Children with tinea capitis usually present with well-demarcated scaly patches in which hair shafts are broken off right above the skin; alopecia can result. Tinea corporis is manifested by well-demarcated, annular, pruritic, scaly lesions that undergo central clearing. Usually one or several small lesions are present. In some cases, tinea corporis can involve much of the trunk or manifest as folliculitis with pustule formation. The rash should be differentiated from contact dermatitis, eczema, and psoriasis. Tinea cruris is seen almost exclusively in men. The perineal rash is erythematous and pustular, has a discrete scaly border, is without satellite lesions, and is usually pruritic. The rash must be differentiated from intertriginous candidiasis, erythrasma, and psoriasis.
Tinea pedis also is more common among men than among women. It usually starts in the web spaces of the toes; peeling, maceration, and pruritus are followed by development of a scaly pruritic rash along the lateral and plantar surfaces of the feet. Hyperkeratosis of the soles of the feet often ensues. Tinea pedis has been implicated in lower-extremity cellulitis, as streptococci and staphylococci can gain entrance to the tissues through fissures between the toes. Onychomycosis affects toenails more often than fingernails and is most common among persons who have tinea pedis. The nail becomes thickened and discolored and may crumble; onycholysis almost always occurs. Onychomycosis is more common in older adults and in persons with vascular disease, diabetes mellitus, and trauma to the nails. Fungal infection must be differentiated from psoriasis, which can mimic onychomycosis but usually has associated skin lesions.
Diagnosis Many dermatophyte infections are diagnosed by their clinical appearance. If the diagnosis is in doubt, as is often the case in children with tinea capitis, scrapings should be taken from the edge of a lesion with a scalpel blade, transferred to a slide to which a drop of potassium hydroxide is added, and examined under a microscope for the presence of hyphae. Cultures are indicated if an outbreak is suspected or the patient does not respond to therapy. Culture of the nail is especially useful as an aid to decisions about both diagnosis and treatment.
Treatment and Prognosis Dermatophyte infections usually respond to topical therapy. Lotions or sprays are easier than creams to apply to large or hairy areas. Particularly for tinea cruris, the affected area should be kept as dry as possible. When patients have extensive skin lesions, oral itraconazole or terbinafine can hasten resolution (Table 243-2). Terbinafine interacts with fewer drugs than itraconazole and is generally the first-line agent. Onychomycosis does not respond to topical therapy, although ciclopirox nail lacquer applied daily for a year is occasionally beneficial. Itraconazole and terbinafine both accumulate in the nail plate and can be used to treat onychomycosis (Table 243-2). These agents are more effective and better tolerated than griseofulvin and ketoconazole. The major decision to be made with regard to therapy is whether the extent of nail involvement justifies the use of systemic antifungal agents that have adverse effects, may interact with other drugs, and are costly. Treating for cosmetic reasons alone is discouraged. Relapses of tinea cruris and tinea pedis are common and should be treated early with topical creams to avoid development of more extensive disease. Relapses of onychomycosis follow treatment in 25–30% of cases.
|
SUGGESTED ORAL TREATMENT FOR EXTENSIVE TINEA INFECTIONS AND ONYCHOMYCOSIS |
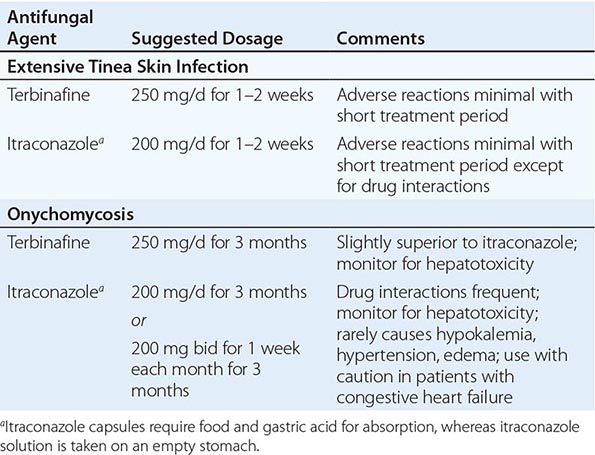
244 |
Pneumocystis Infections |
DEFINITION AND DESCRIPTION
Pneumocystis is an opportunistic pathogen that is an important cause of pneumonia in immunocompromised hosts, particularly those with HIV infection (Chap. 226), and in individuals with organ transplants, those with hematologic malignancies, and those receiving immunosuppressive therapy. The organism was discovered in rodents in 1906 and was initially believed to be a protozoan. Because Pneumocystis cannot be cultured, our understanding of its biology has been limited, but molecular techniques have demonstrated that the organism is actually a fungus. Formerly known as Pneumocystis carinii, the species infecting humans has been renamed Pneumocystis jirovecii.
EPIDEMIOLOGY
P. jirovecii pneumonia (PCP) came to medical attention when cases were reported in malnourished orphans in Europe during World War II. The disease was later recognized in other immunosuppressed populations but was rare in the era before HIV/AIDS and before intensive immunosuppressive therapy for organ transplantation and autoimmune disorders. In 1981, PCP was first reported in men who had sex with men and in IV drug users who had no obvious cause of immunosuppression. These cases were subsequently recognized as the first cases of what came to be known as the acquired immunodeficiency syndrome (AIDS) (Chap. 226). The incidence of PCP increased dramatically as the AIDS epidemic grew: without chemoprophylaxis or antiretroviral therapy (ART), 80–90% of patients with HIV/AIDS in North America and Western Europe ultimately develop one or more episodes of PCP. While its incidence declined with the introduction of anti-Pneumocystis prophylaxis and combination ART, PCP has continued to be a leading cause of AIDS-associated morbidity in the United States and Western Europe, particularly in individuals who do not know they are infected with HIV until they are profoundly immunosuppressed and in HIV-infected patients who are not receiving ART or PCP prophylaxis.
PCP also develops in HIV-uninfected patients who are immunocompromised secondary to hematologic or malignant neoplasms, stem cell or solid organ transplantation, and immunosuppressive medications. The incidence of PCP depends on the degree of immunosuppression. PCP is increasingly reported among individuals receiving tumor necrosis factor α inhibitors and antilymphocyte monoclonal antibodies for rheumatologic or other diseases. While clinical PCP in immunocompetent hosts has not been clearly documented, studies have shown that Pneumocystis organisms can colonize the airways of children and adults who are not overtly immunocompromised. The relevance of these organisms to acute or chronic syndromes, such as chronic obstructive pulmonary disease (COPD), in immunocompetent patients is being investigated.
![]() In some developing countries, the incidence of PCP among HIV-infected individuals has been found to be lower than that in industrialized countries. This lower incidence may be due to competing mortality from infectious diseases such as tuberculosis and bacterial pneumonia, which typically occur before patients become immunosuppressed enough to develop PCP. Geographic variations in Pneumocystis exposure and underdiagnosis also may explain the apparent lower frequency of PCP in some countries.
In some developing countries, the incidence of PCP among HIV-infected individuals has been found to be lower than that in industrialized countries. This lower incidence may be due to competing mortality from infectious diseases such as tuberculosis and bacterial pneumonia, which typically occur before patients become immunosuppressed enough to develop PCP. Geographic variations in Pneumocystis exposure and underdiagnosis also may explain the apparent lower frequency of PCP in some countries.
PATHOGENESIS AND PATHOLOGY
Life Cycle and Transmission The life cycle of Pneumocystis involves both sexual and asexual reproduction, and the organism exists as a trophic form, a cyst, and a precyst at various points. Serologic and molecular studies have demonstrated that most humans are exposed to Pneumocystis early in life. It was historically thought that Pneumocystis developed from reactivation of latent infection, but de novo infections from environmental sources and person-to-person transmission occur as well. Outbreaks of PCP suggest that nosocomial transmission can take place, and studies with rodents show that immunocompetent animals can serve as reservoirs for transmission of P. carinii (the infecting species in rodents) to immunocompetent and immunosuppressed animals. However, Pneumocystis organisms are species specific, and thus humans are infected only by other humans who transmit P. jirovecii; humans cannot be infected with animal species of Pneumocystis such as P. murina (mice) or P. oryctolagi (rabbits). The utility of respiratory isolation in preventing transmission from patients with PCP to other immunosuppressed individuals has been debated; no clear evidence exists, although it seems prudent to isolate patients with active PCP from other immunosuppressed patients.
Role of Immunity Defects in cellular and/or humoral immunity predispose to development of PCP. CD4+ T cells are critical in host defense against Pneumocystis. For HIV-infected patients, the incidence is inversely related to the CD4+ T cell count: at least 80% of cases occur at counts of <200 cells/μL, and most of these cases develop at counts of <100 cells/μL. CD4+ T cell counts are less specific and thus less useful in predicting the risk of PCP in HIV-uninfected, immunosuppressed patients.
Lung Pathology Pneumocystis has a unique tropism for the lung. It is presumably inhaled into the alveolar space. Clinically apparent pneumonia occurs only if an individual is immunocompromised. Pneumocystis proliferates in the lung, provoking a mononuclear cell response. The alveoli become filled with proteinaceous material, and alveolar damage results in increased alveolar-capillary injury and surfactant abnormalities. Stained lung sections typically show foamy, vacuolated alveolar exudates composed largely of viable and nonviable organisms (Fig. 244-1A). Interstitial edema and fibrosis may develop, and organisms can be seen in the alveolar space with silver or other stains. Moreover, the organisms can be seen when tissue is subjected to colorimetric or immunofluorescent staining (Fig. 244-1B–D).
FIGURE 244-1 Direct microscopy of Pneumocystis pneumonia. A. Transbronchial lung biopsy stained with hematoxylin and eosin shows eosinophilic alveolar filling. B. Methenamine silver–stained bronchoalveolar lavage (BAL) fluid. C. Giemsa-stained BAL fluid. D. Immunofluorescent stain of BAL fluid.
CLINICAL FEATURES
Clinical Presentation PCP presents as acute or subacute pneumonia that may initially be characterized by a vague sense of dyspnea alone but that subsequently manifests as fever and nonproductive cough with progressive shortness of breath ultimately resulting in respiratory failure and death. Extrapulmonary manifestations of PCP are rare but can include involvement of almost any organ, most notably lymph nodes, spleen, and liver.
Physical Examination The physical examination findings in PCP are nonspecific. Patients have decreased oxygen saturation—at rest or with exertion—that, without treatment, progresses to severe hypoxemia. Patients may initially have a normal chest examination and no adventitious sounds but later, without treatment, develop diffuse rales and signs of consolidation. Oral thrush in a patient with HIV infection indicates an increased risk for PCP.
Laboratory Findings The results of routine laboratory tests are nonspecific in PCP. Serum levels of lactate dehydrogenase (LDH) are often elevated due to pulmonary damage; however, a normal LDH level does not rule out PCP, nor is an elevated LDH value specific for PCP. The peripheral white blood cell count may be elevated, but the increase is usually modest. Hepatic and renal function are typically normal.
Radiographic Findings Although the initial chest radiograph may be normal when patients have mild symptoms, the classic radiographic appearance of PCP consists of diffuse bilateral interstitial infiltrates that are perihilar and symmetric (Fig. 244-2A)—yet another finding that is not specific for PCP. The interstitial infiltrates can progress to alveolar filling (Fig. 244-2B). High-resolution chest CT shows diffuse ground-glass opacities in virtually all patients with PCP (Fig. 244-2C). A normal chest CT essentially rules out the diagnosis of PCP. Cysts and pneumothoraces are common chest radiographic findings (Fig. 244-2D). A wide variety of atypical radiographic findings have been described, including asymmetric patterns, upper lobe infiltrates, mediastinal adenopathy, nodules, cavities, and effusions.
FIGURE 244-2 Radiographs in Pneumocystis pneumonia. A. Posterior-anterior chest radiograph showing symmetric interstitial infiltrates. B. Posterior-anterior chest radiograph showing symmetric alveolar infiltrates (courtesy of Alison Morris). C. CT image demonstrating symmetric interstitial infiltrates and ground-glass opacities. D. CT image showing symmetric interstitial infiltrates, ground-glass opacities, and pneumatoceles.
DIAGNOSIS
The optimal sample for diagnostic examination depends on how ill the patient is and what resources are available. Before the 1990s, diagnoses of PCP were usually established by open lung biopsy; later, transbronchial lung biopsy was employed. Hematoxylin and eosin staining of pulmonary tissue demonstrates a foamy alveolar infiltrate and a mononuclear interstitial infiltrate (Fig. 244-2A). This appearance is pathognomonic for PCP even though the organisms cannot be specifically identified with this stain. The diagnosis is typically established in lung tissue or pulmonary secretions by highly specific staining of the cyst—e.g., with methenamine silver (Fig. 244-2B), toluidine blue O, or Giemsa (Fig. 244-2C)—or by staining with a specific immunofluorescent antibody (Fig. 244-2D).
The demonstration of organisms in bronchoalveolar lavage fluid is almost 100% sensitive and specific for PCP in patients with either HIV infection or immunosuppression of other etiologies. The organisms are identified with the specific stains indicated above for lung biopsy. While expectorated sputum or throat swabs have very low sensitivity, an induced sputum sample obtained and interpreted by an experienced provider can be highly sensitive and specific. The reported sensitivity of induced sputum for PCP is widely variable (55–90%), however, and is dependent on both the characteristics of the patient and the experience of the center conducting the test.
Recently, many laboratories have offered polymerase chain reaction (PCR) testing of respiratory specimens for Pneumocystis. However, these PCR tests are so sensitive that it is difficult to distinguish patients with colonization (i.e., those whose acute lung disease is due to some other process but who have low levels of Pneumocystis DNA in the lungs) from those with acute pneumonia due to Pneumocystis. Such PCR tests on appropriate samples may be more useful for ruling out a diagnosis of PCP if they are negative than for definitively attributing the disease to Pneumocystis.
There has been considerable interest in serologic tests such as assays for (1→3)-β-D-glucan, levels of which are frequently elevated in patients with PCP. However, no serologic assays developed to date offer substantial sensitivity or specificity.
COURSE AND PROGNOSIS
Untreated, PCP is invariably fatal. Patients with HIV infection often have an indolent course that presents as mild exercise intolerance or chest tightness without fever or cough and a normal or nearly normal posterior-anterior chest radiograph, with progression over days, weeks, or even a few months to fever, cough, diffuse alveolar infiltrates, and profound hypoxemia. Some patients with HIV infection and most patients with other types of immunosuppression have more acute disease that progresses over a few days to respiratory failure. Rare patients also develop distributive shock. A few unusual patients present with extrapulmonary manifestations in the skin or soft tissue, retina, brain, liver, kidney, or spleen that are nonspecific in presentation and can be diagnosed only by histology.
Factors that influence mortality risk include the patient’s age and degree of immunosuppression as well as the presence of preexisting lung disease, a low serum albumin level, the need for mechanical ventilation, and the development of pneumothorax. With advances in supportive critical care, the prognosis for patients with PCP who require intubation and respiratory support has improved and now depends to a large extent on comorbidities and the prognosis of the underlying disease. Since patients typically do not respond to therapy for 4–8 days, supportive care for a minimum of 10 days is a reasonable consideration if such support is compatible with the patient’s wishes and the prognosis of comorbidities. Patients whose condition continues to deteriorate after 3 or 4 days or has not improved after 7–10 days should be reevaluated to determine whether other infectious processes are present (either having been missed on initial evaluation or having developed during treatment) or whether noninfectious processes (e.g., congestive heart failure, pulmonary emboli, pulmonary hypertension, drug toxicity, or a neoplastic process) are causing pulmonary dysfunction.
PREVENTION
The most effective method for preventing PCP is to eliminate the cause of immunosuppression by withdrawing immunosuppressive therapy or treating the underlying cause, e.g., HIV infection. Patients who are susceptible to PCP benefit from chemoprophylaxis during the period of susceptibility. For patients with HIV infection, CD4+ T cell counts are a reliable marker of susceptibility, and counts below 200 cells/μL are an indication to start prophylaxis (Table 244-2). For patients with HIV infection who are not receiving ART, oral candidiasis or prior PCP also is an indication for chemoprophylaxis, regardless of CD4+ T cell count. For such patients not receiving ART, any prior episode of an AIDS-defining illness or pneumonia should encourage the use of chemoprophylaxis. However, patients who are not adherent to ART are not likely to take PCP chemoprophylaxis.
|
PROPHYLAXIS OF PNEUMOCYSTOSIS |
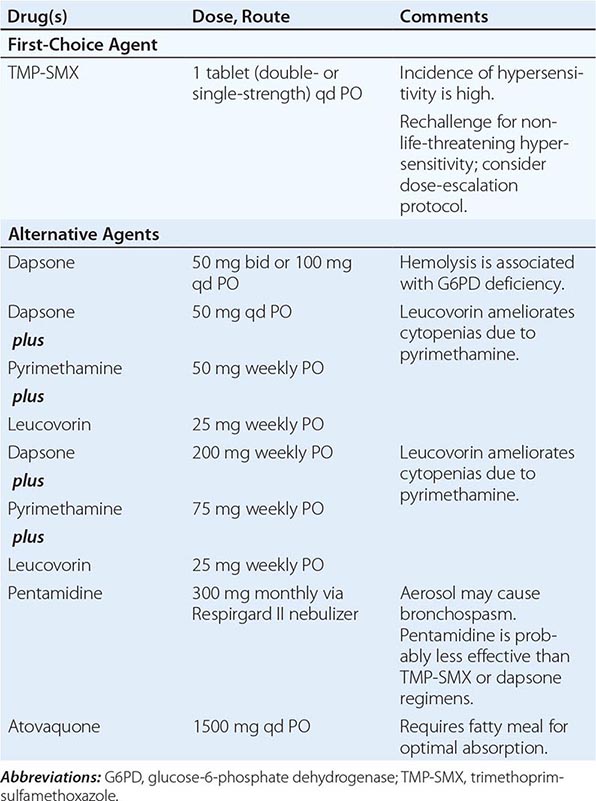
For patients without HIV infection, there is no laboratory parameter, including the CD4+ T cell count, that predicts susceptibility to PCP with adequate positive and negative accuracy. The period of susceptibility is usually estimated on the basis of experience with the underlying disease and immunosuppressive regimen. Patients receiving a prolonged course of high-dose glucocorticoids appear to be particularly susceptible to PCP. The glucocorticoid exposure threshold that warrants chemoprophylaxis is controversial, but such preventive therapy should be strongly considered for any patient receiving more than the equivalent of 20 mg of prednisone daily for 30 days.
TMP-SMX is the most effective prophylactic drug: few patients experience a PCP breakthrough when they are reliably taking a recommended TMP-SMX chemoprophylactic regimen. Several TMP-SMX regimens have been used successfully. One double-strength tablet daily is the regimen with which there is the most experience, but either one single-strength tablet daily or one double-strength tablet two or three times weekly also has been recommended for various populations of patients.
For patients who cannot tolerate TMP-SMX (usually because of hypersensitivity or bone marrow suppression), alternative drugs include daily dapsone, weekly dapsone-pyrimethamine, and monthly aerosol pentamidine. Patients who develop hypersensitivity to TMP-SMX can sometimes tolerate the drug if a gradual dose-escalation protocol is used. Dapsone cross-reacts with sulfonamides in a substantial fraction of patients and therefore is rarely useful in patients with a history of life-threatening reactions to TMP-SMX. Aerosolized pentamidine is highly effective, but it is not as effective as TMP-SMX and may not provide protection in areas of the lung that are not well ventilated. Atovaquone is also effective and well tolerated; however, this drug is available only as an oral preparation, and gastrointestinal absorption is unpredictable in patients with abnormal gastrointestinal motility or function.
SECTION 17 |
PROTOZOAL AND HELMINTHIC INFECTIONS: GENERAL CONSIDERATIONS |
245e |
Laboratory Diagnosis of Parasitic Infections |
The cornerstone for the diagnosis of parasitic infections is a thorough history of the patient’s illness. Epidemiologic aspects of the illness are especially important because the risks of acquiring many parasites are closely related to occupation, recreation, or travel to areas of high endemicity. Without a basic knowledge of the epidemiology and life cycles of the major parasites, it is difficult to approach the diagnosis of parasitic infections systematically. Accordingly, the medical classification of important human parasites in this chapter emphasizes their geographic distribution, their transmission, and the anatomic location and stages of their life cycle in humans. The text and tables are intended to serve as a guide to the correct diagnostic procedures for the major parasitic infections; in addition, the reader is referred to other chapters that contain more comprehensive information about each infection (Chaps. 247–260). Tables 245e-1, 245e-2, and 245e-3 summarize the geographic distributions, the anatomic locations, and the methods employed for the diagnosis of flatworm, roundworm, and protozoal infections, respectively.
|
FLATWORM INFECTIONS |
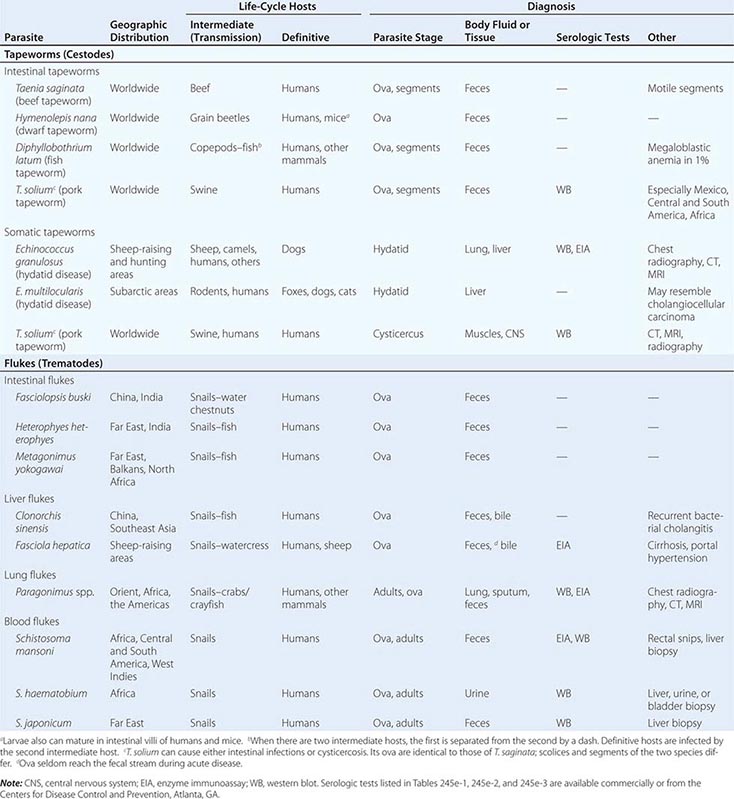
|
ROUNDWORM INFECTIONS |
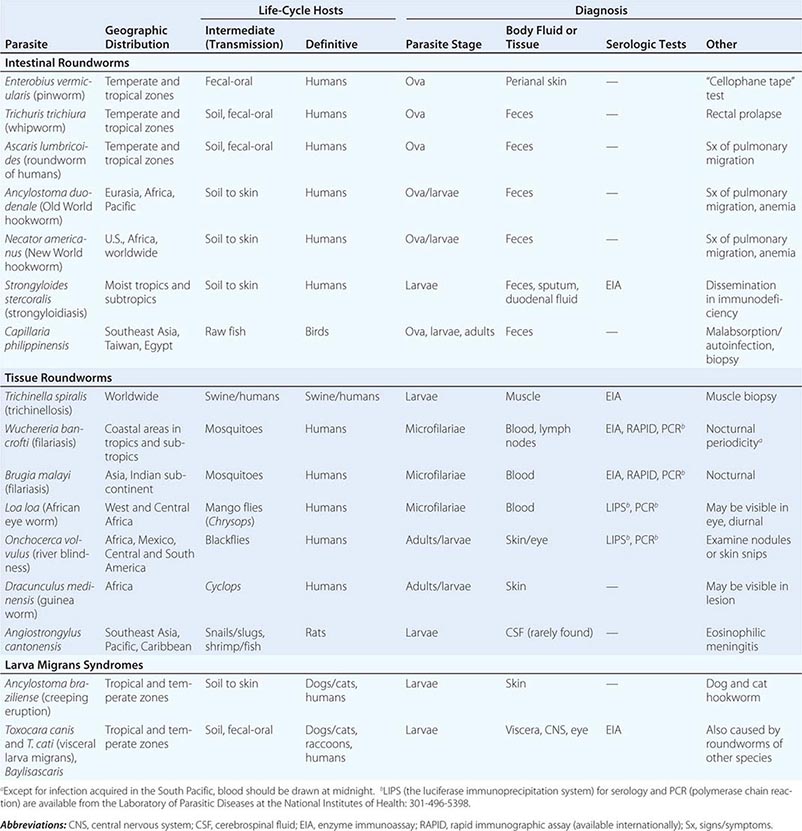
|
PROTOZOAL INFECTIONS |
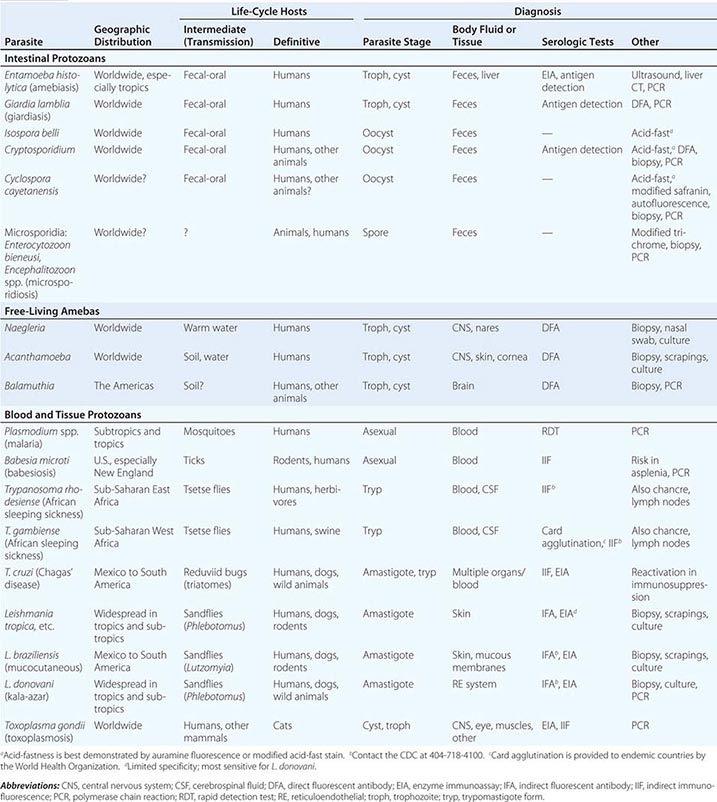
In addition to selecting the correct diagnostic procedures, physicians must counsel their patients to ensure that specimens are collected properly and arrive at the laboratory promptly. For example, the diagnosis of bancroftian filariasis is unlikely to be confirmed by the laboratory unless blood is drawn near midnight, when the nocturnal microfilariae are active. Laboratory personnel and surgical pathologists should be notified in advance when a parasitic infection is suspected. Continuing interaction with the laboratory staff and the surgical pathologists increases the likelihood that parasites in body fluids or biopsy specimens will be examined carefully by the most capable individuals.
INTESTINAL PARASITES
Most helminths and protozoa exit the body in the fecal stream. Many laboratories now use a stool collection kit with instructions for patients to transfer portions of the sample directly into bacterial carrier medium and fixative, which increases yield. If kits are not available, the patient should be instructed to collect feces in a clean waxed or cardboard container and to record the time of collection on the container. Refrigeration will preserve trophozoites for a few hours and protozoal cysts and helminthic ova for several days. Contamination with water (which could contain free-living protozoa) or with urine (which can damage trophozoites) should be avoided. Fecal samples should be collected before ingestion of barium or other contrast agents for radiologic procedures and before treatment with antidiarrheal agents and antacids; these substances change the consistency of the feces and interfere with microscopic detection of parasites. Because of the cyclic shedding of most parasites in the feces, a minimum of three samples collected on alternate days should be examined. Examination of a single sample can be up to 50% less sensitive.
Analysis of fecal samples entails both macroscopic and microscopic examination. Watery or loose stools are more likely to contain protozoal trophozoites, but protozoal cysts and all stages of helminths may be found in formed feces. If adult worms or tapeworm segments are observed, they should be transported promptly to the laboratory or washed and preserved in fixative for later examination. The only tapeworm with motile segments is Taenia saginata, the beef tapeworm, which patients sometimes bring to the physician. Because the ova of T. saginata are morphologically indistinguishable from those of Taenia solium (the cause of cysticercosis), motility is an important distinguishing characteristic.
Microscopic examination of feces is not complete until direct wet mounts have been evaluated and concentration techniques as well as permanent stains have been applied. Before accepting a report of negativity for ova and parasites as final, the physician should insist that the laboratory undertake each of these procedures. Some intestinal parasites are more readily detected in material other than feces. For example, examination of duodenal contents is sometimes necessary to detect Giardia lamblia, Cryptosporidium, and Strongyloides larvae. Use of the “cellophane tape” technique to detect pinworm ova on the perianal skin sometimes also reveals ova of T. saginata deposited perianally when the motile segments disintegrate (Table 245e-4).
|
ALTERNATIVE PROCEDURES FOR LABORATORY DIAGNOSIS OF PARASITES FOUND IN FECESa |
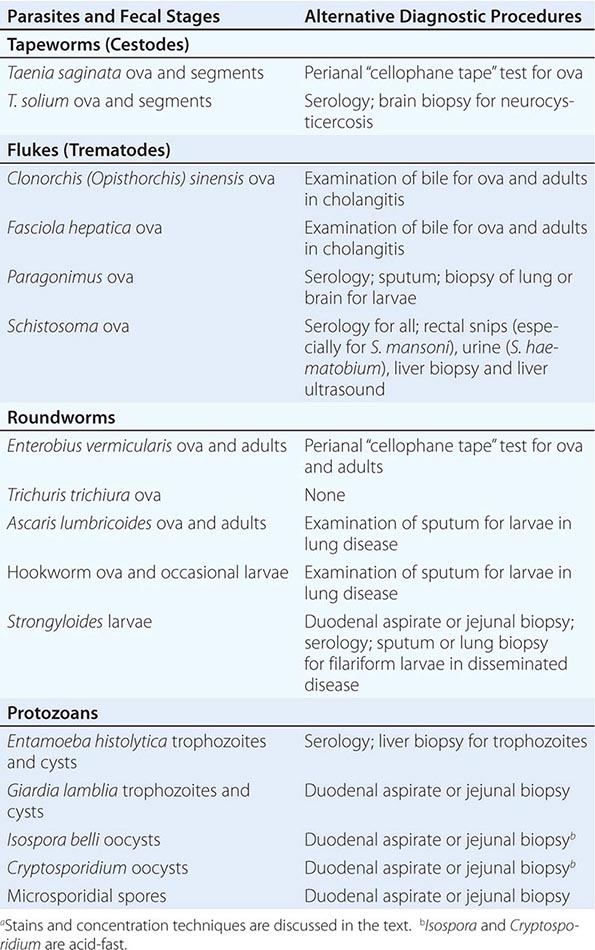
Two routine solutions are used to make wet mounts for identification of the various life stages of helminths and protozoa: physiologic saline for trophozoites, cysts, ova, and larvae and dilute iodine solution for protozoal cysts and ova. Iodine solution must never be used to examine specimens for trophozoites because it kills the parasites and thus eliminates their characteristic motility.
The two most common concentration procedures for detecting small numbers of cysts and ova are formalin-ether sedimentation and zinc sulfate flotation. The formalin-ether technique is preferable because all parasites sediment but not all float. Slides permanently stained for trophozoites should be prepared before concentration. Additional slides stained for cysts and ova may be made from the concentrate.
In many instances, especially in the differentiation of Entamoeba histolytica from other amebas, identification of parasites from wet mounts or concentrates must be considered tentative. Permanently stained smears allow study of the cellular detail necessary for definitive identification. The iron-hematoxylin stain is excellent for critical work, but trichrome staining, which can be completed in 1 h, is a satisfactory alternative that also reveals parasites in specimens preserved in polyvinyl alcohol fixative. Modified acid-fast staining and fluorescent auramine microscopy are useful adjuncts for detection and identification of several intestinal protozoa, including Cryptosporidium and Cyclospora, while direct fluorescent antibody testing is in common use for Cryptosporidium and Giardia. Microsporidia, which cause chronic diarrhea in HIV-infected patients, may be missed unless a special modified trichrome stain or fluorescent screening with calcofluor white is requested (Table 245e-3).
BLOOD AND TISSUE PARASITES
Invasion of tissue by protozoa and helminths renders the choice of diagnostic techniques more difficult. For example, physicians must understand that aspiration of an amebic liver abscess rarely reveals E. histolytica because the trophozoites are located primarily in the abscess wall. They must remember that the urine sediment offers the best opportunity to detect Schistosoma haematobium in the young Ethiopian immigrant or the American traveler who returns from Africa with hematuria. Tables 245e-1, 245e-2, and 245e-3, which offer a quick guide to the geographic distribution and anatomic locations of the major tissue parasites, should help the physician to select the appropriate body fluid or biopsy site for microscopic examination. Tables 245e-5 and 245e-6 provide additional information about the identification of parasites in samples from specific anatomic locations. The laboratory procedures for detection of parasites in other body fluids are similar to those used in the examination of feces. The physician should insist on wet mounts, concentration techniques, and permanent stains for all body fluids. The trichrome or iron-hematoxylin stain is satisfactory for all tissue helminths in body fluids other than blood, but microfilarial worms and blood protozoa are more easily visualized with Giemsa or Wright’s staining.
|
IDENTIFICATION OF PARASITES IN BLOOD AND OTHER BODY FLUIDS |
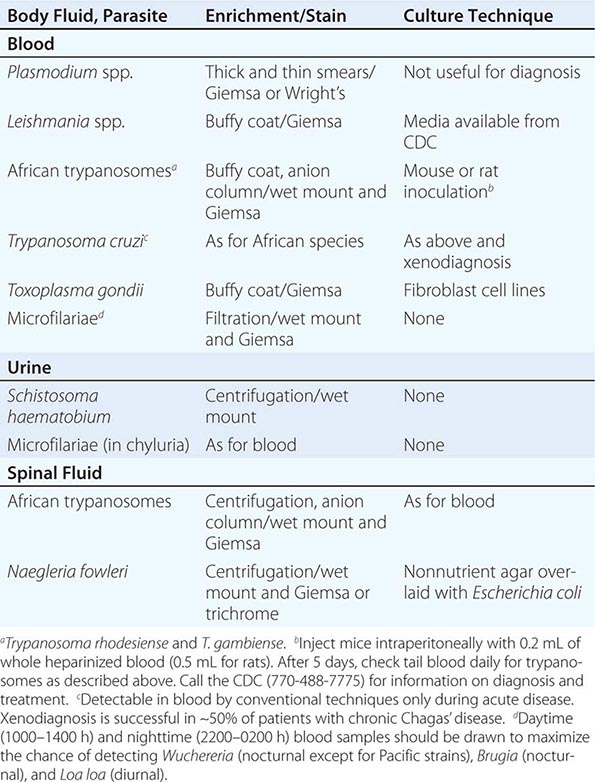
|
MINOR PROCEDURES FOR DIAGNOSIS OF PARASITIC INFECTIONS |

The parasites most commonly detected in Giemsa-stained blood smears are the plasmodia, microfilariae, and African trypanosomes (Table 245e-5). Most patients with Chagas’ disease present in the chronic phase, when Trypanosoma cruzi is no longer microscopically detectable in blood smears. Wet mounts are sometimes more sensitive than stained smears for the detection of microfilariae and African trypanosomes because these active parasites cause noticeable movement of the erythrocytes in the microscopic field. Filtration of blood through a polycarbonate filter (pore size, 3–5 μm) facilitates the detection of microfilariae. The intracellular amastigote forms of Leishmania species and T. cruzi can sometimes be visualized in stained smears of peripheral blood, but aspirates of the bone marrow, liver, and spleen are the best sources for microscopic detection and culture of Leishmania in kala-azar and of T. cruzi in chronic Chagas’ disease. The diagnosis of malaria and the critical distinction among the various Plasmodium species are made by microscopic examination of stained thick and thin blood films (Chap. 248). Because the lab infrastructure and technical expertise may not be available in many rural areas with high levels of malaria, rapid detection tests (RDTs) are increasingly being used to fill this gap. These are immunochromatographic capture assays with monoclonal antibodies to species-specific antigens (histidine-rich protein 2 [PfHRP2] or aldolase of Plasmodium falciparum) or conserved Plasmodium antigens (lactate dehydrogenase). The World Health Organization sponsored a major testing program evaluating the different RDTs. Lower performance rates have been reported in a variety of field sites, especially in areas where deletions of the pfhrp2 gene have been detected. Subpatent infection and identification of Plasmodium species can also be confirmed by polymerase chain reaction (PCR), but for this purpose PCR is primarily a research tool. P. knowlesi, a simian parasite, has been identified as the cause of an increasing number of infections in Malaysian Borneo and other areas of Southeast Asia; PCR or another molecular method is required to differentiate P. knowlesi from P. malariae.
Although most tissue parasites stain with the traditional hematoxylin and eosin, appropriate special stains should also be applied to surgical biopsy specimens. The surgical pathologist who is accustomed to applying silver stains for Pneumocystis to induced sputum and transbronchial biopsies may need to be reminded to examine wet mounts and iron-hematoxylin–stained preparations of pulmonary specimens for helminthic ova and E. histolytica. The clinician should also be able to advise the surgeon and pathologist about optimal techniques for the identification of parasites in specimens obtained by certain specialized minor procedures (Table 245e-6). For example, the excision of skin snips for the diagnosis of onchocerciasis, the collection of rectal snips for the diagnosis of schistosomiasis, and punch biopsy of skin lesions for the identification and culture of cutaneous and mucocutaneous species of Leishmania are simple procedures, but the diagnosis can be missed if the specimens are improperly obtained or processed.
NONSPECIFIC TESTS
Eosinophilia (>500/μL) commonly accompanies infections with most of the tissue helminths; absolute numbers of eosinophils may be high in trichinellosis and the migratory phases of filariasis (Table 245e-7). Intestinal helminths provoke eosinophilia only during pulmonary migration of the larval stages. Eosinophilia is not a manifestation of protozoal infections. Parasitic causes of eosinophilia in cerebrospinal fluid include nematodes (e.g., Angiostrongylus, Gnathostoma, Toxocara, and Baylisascaris species) as well as flatworms (e.g., T. solium and Schistosoma species).
|
PARASITES FREQUENTLY ASSOCIATED WITH EOSINOPHILIAa |
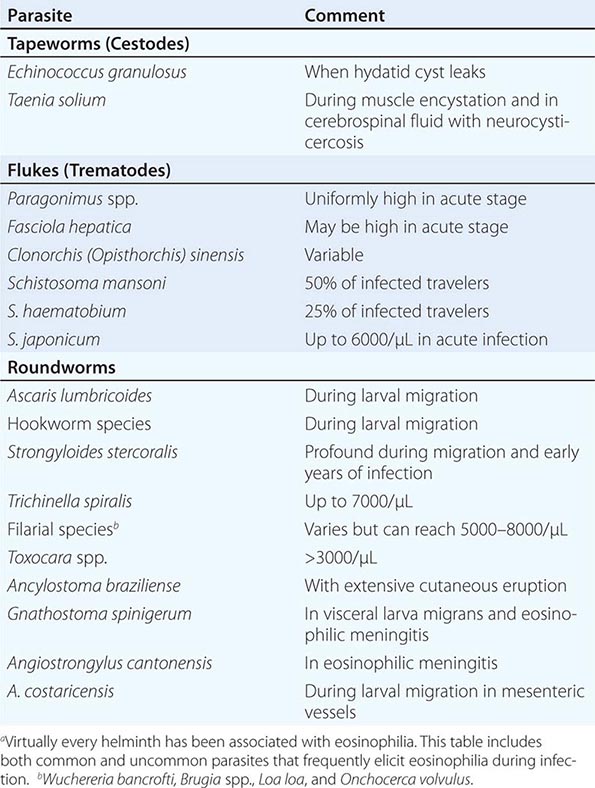
Like the hypochromic microcytic anemia of heavy hookworm infections, other nonspecific laboratory abnormalities may suggest parasitic infection in patients with appropriate geographic and/or environmental exposures. Biochemical evidence of cirrhosis or an abnormal urine sediment in an African immigrant certainly raises the possibility of schistosomiasis, and anemia and thrombocytopenia in a febrile traveler or immigrant are among the hallmarks of malaria. CT and MRI also contribute to the diagnosis of infections with many tissue parasites and have become invaluable adjuncts in the diagnosis of neurocysticercosis and cerebral toxoplasmosis.
ANTIBODY AND ANTIGEN DETECTION
Useful antibody assays for many of the important tissue parasites are available; most of those listed in Table 245e-8 can be obtained from the Centers for Disease Control and Prevention (CDC) in Atlanta. The results of serologic tests not listed in the tables should be interpreted with caution.
|
SEROLOGIC AND MOLECULAR TESTS FOR PARASITIC INFECTIONSa |
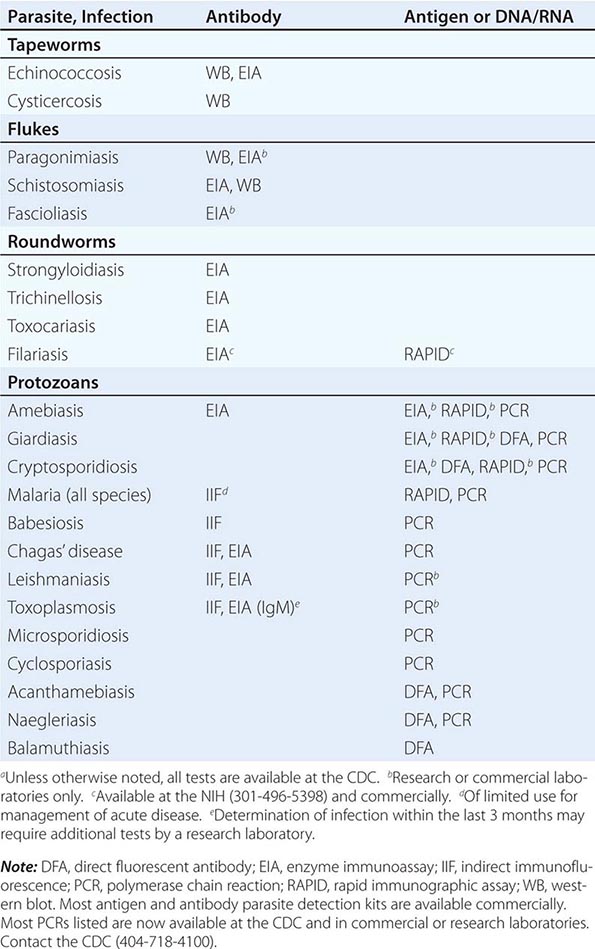
The value of antibody assays is limited by several factors. For example, the preparation of thick and thin blood smears remains the procedure of choice for the diagnosis of malaria in individual patients because diagnostic titers to plasmodia develop slowly and do not differentiate species—a critical step in patient management. Filarial antigens cross-react with those from other nematodes; as in assays for antibody to most parasites, the presence of antibody in the filarial assay fails to distinguish between past and current infection. Despite these specific limitations, the restricted geographic distribution of many tropical parasites increases the diagnostic usefulness of both the presence and the absence of antibody in travelers from industrialized countries. In contrast, a large proportion of the world’s population has been exposed to Toxoplasma gondii, and the presence of IgG antibody to T. gondii does not constitute proof of active disease.
Fewer antibody assays are available for the diagnosis of infection with intestinal parasites. E. histolytica is the major exception. Sensitive, specific serologic tests are invaluable in the diagnosis of amebiasis. Commercial kits for the detection of antigen by enzyme-linked immunosorbent assay or of whole organisms by fluorescent antibody assay are now available for several protozoan parasites (Table 245e-8).
MOLECULAR TECHNIQUES
DNA hybridization with probes that are repeated many times in the genome of a specific parasite and amplification of a specific DNA fragment by PCR have now been established as useful techniques for the diagnosis of several protozoan infections (Table 245e-8). Although PCR is very sensitive, it is an adjunct to conventional techniques for parasite detection and should be requested only when microscopic and immunodiagnostic procedures fail to establish the probable diagnosis. For example, only multiple negative blood smears or the failure to identify the infecting species justifies PCR for the diagnosis or proper management of malaria. In addition to PCR of anticoagulated blood, the CDC (www.cdc.gov/dpdx/) and several commercial laboratories now perform PCRs for detection of certain specific parasites in stool samples, biopsy specimens, and bronchoalveolar lavage fluid (Table 245e-8). Although PCRs are now used primarily for the detection of protozoans, active research efforts are likely to establish their feasibility for the detection of several helminths.
246e |
Agents Used to Treat Parasitic Infections |
Parasitic infections afflict more than half of the world’s population and impose a substantial health burden, particularly in underdeveloped nations, where they are most prevalent. The reach of some parasitic diseases, including malaria, has expanded over the past few decades as a result of factors such as deforestation, population shifts, global warming, and other climatic events. Despite major efforts at vaccine development and vector control, chemotherapy remains the single most effective means of controlling parasitic infections. Efforts to combat the spread of some diseases are hindered by the development and spread of drug resistance, the limited introduction of new antiparasitic agents, and the proliferation of counterfeit medications. However, there are good reasons to be optimistic. Ambitious global initiatives aimed at controlling or eliminating threats such as AIDS, tuberculosis, and malaria have demonstrated some early successes. Recognition of the substantial burden imposed by the “neglected” tropical diseases has generated multinational partnerships to develop and deploy effective antiparasitic agents. Vaccines against several tropical diseases are being developed, and clinical trials for vaccines against parasites continue.
This chapter deals exclusively with the agents used to treat infections due to parasites. Specific treatment recommendations for the parasitic diseases of humans are listed in subsequent chapters. Many of the agents discussed herein are approved by the U.S. Food and Drug Administration (FDA) but are considered investigational for the treatment of certain infections. Drugs marked in the text with an asterisk (*) are available through the Centers for Disease Control and Prevention (CDC) Drug Service (telephone: 404-639-3670 or 404-639-2888; www.cdc.gov/ncpdcid/dsr/). Drugs marked with a dagger (†) are available only through their manufacturers; contact information for these manufacturers may be available from the CDC.
Table 246e-1 presents a brief overview of each agent (including some drugs that are covered in other chapters), along with major toxicities, spectrum of activity, and safety for use during pregnancy and lactation.
|
OVERVIEW OF AGENTS USED FOR THE TREATMENT OF PARASITIC INFECTIONS |
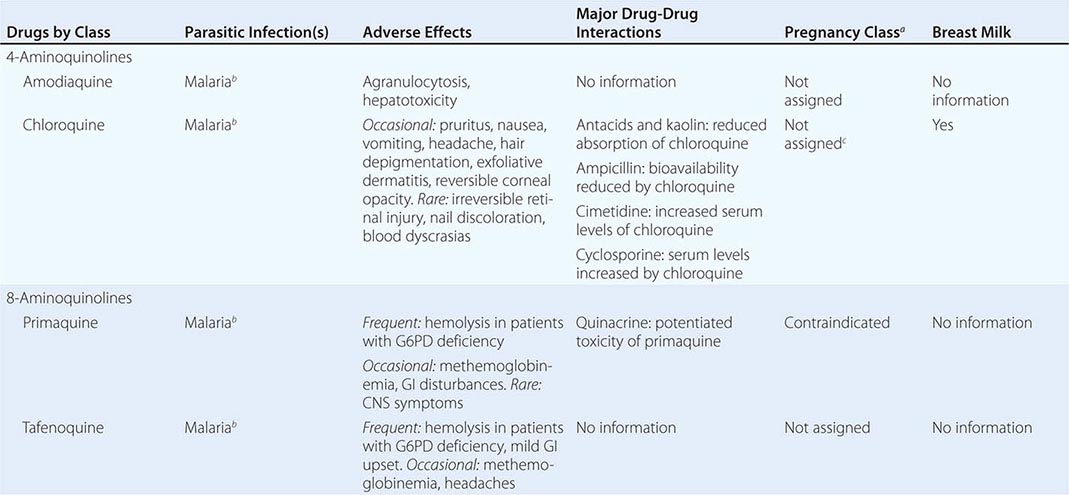
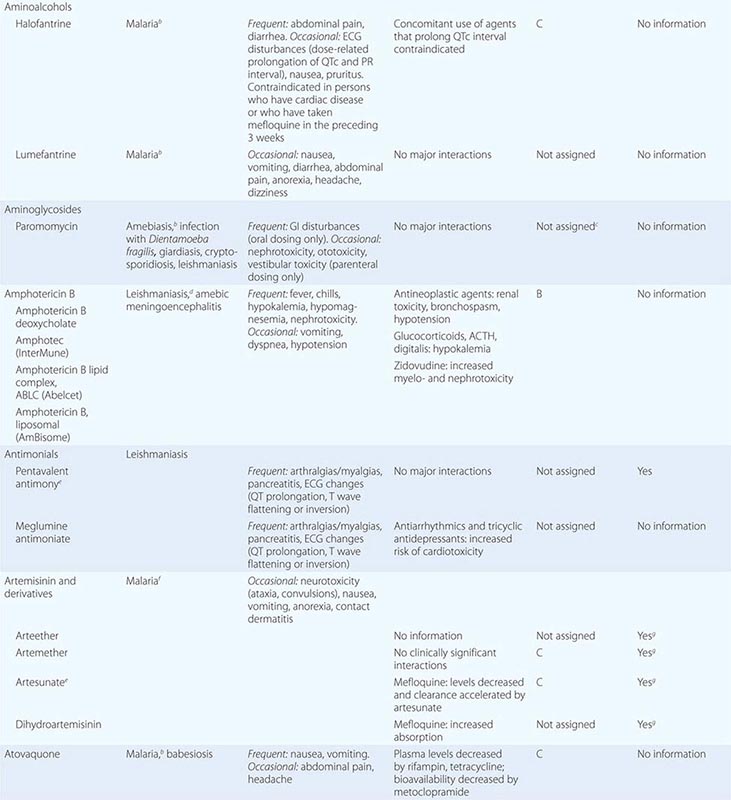
Albendazole Like all benzimidazoles, albendazole acts by selectively binding to free β-tubulin in nematodes, inhibiting the polymerization of tubulin and the microtubule-dependent uptake of glucose. Irreversible damage occurs in gastrointestinal (GI) cells of the nematodes, resulting in starvation, death, and expulsion by the host. This fundamental disruption of cellular metabolism offers treatment for a wide range of parasitic diseases.
Albendazole is poorly absorbed from the GI tract. Administration with a fatty meal increases its absorption by two- to sixfold. Poor absorption may be advantageous for the treatment of intestinal helminths, but successful treatment of tissue helminth infections (e.g., hydatid disease and neurocysticercosis) requires that a sufficient amount of active drug reach the site of infection. The metabolite albendazole sulfoxide is responsible for the drug’s therapeutic effect outside the gut lumen. Albendazole sulfoxide crosses the blood-brain barrier, reaching a level significantly higher than that achieved in plasma. The high concentrations of albendazole sulfoxide attained in cerebrospinal fluid (CSF) may explain the efficacy of albendazole in the treatment of neurocysticercosis.
Albendazole is extensively metabolized in the liver, but there are few data regarding the drug’s use in patients with hepatic disease. Single-dose albendazole therapy in humans is largely without side effects (overall frequency, ≤1%). More prolonged courses (e.g., as administered for cystic and alveolar echinococcal disease) have been associated with liver function abnormalities and bone marrow toxicity. Thus, when prolonged use is anticipated, the drug should be administered in treatment cycles of 28 days interrupted by 14-day intervals off therapy. Prolonged therapy with full-dose albendazole (800 mg/d) should be approached cautiously in patients also receiving drugs with known effects on the cytochrome P450 system.
Amodiaquine Amodiaquine has been widely used in the treatment of malaria for >40 years. Like chloroquine (the other major 4-aminoquinoline), amodiaquine is now of limited use because of the spread of resistance. Amodiaquine interferes with hemozoin formation through complexation with heme. It is rapidly absorbed and acts as a prodrug after oral administration; the principal plasma metabolite monodesethylamodiaquine is the predominant antimalarial agent. Amodiaquine and its metabolites are all excreted in urine, but there are no recommendations concerning dosage adjustment in patients with impaired renal function. Agranulocytosis and hepatotoxicity can develop with repeated use; therefore, this drug should not be used for prophylaxis. Despite widespread resistance, amodiaquine has been shown to be effective in some areas when combined with other antimalarial drugs (e.g., artesunate, sulfadoxine-pyrimethamine), particularly in children. Although licensed, amodiaquine is not yet available in the United States.
Amphotericin B See Table 246e-1 and Chap. 235.
Antimonials* Despite associated adverse reactions and the need for prolonged parenteral treatment, the pentavalent antimonial compounds (designated Sbv) have remained the first-line therapy for all forms of leishmaniasis throughout the world, primarily because they are affordable and effective and have survived the test of time. Pentavalent antimonials are active only after bioreduction to the trivalent Sb(III) form, which inhibits trypanothione reductase, a critical enzyme involved in the oxidative stress management of Leishmania species. The fact that Leishmania species use trypanothione rather than glutathione (which is used by mammalian cells) may explain the parasite-specific activity of antimonials. The drugs are taken up by the reticuloendothelial system, and their activity against Leishmania species may be enhanced by this localization. Sodium stibogluconate is the only pentavalent antimonial available in the United States; meglumine antimoniate is used principally in francophone countries.
Resistance is a major problem in some areas. Although low-level unresponsiveness to Sbv was identified in India in the 1970s, incremental increases in both the recommended daily dosage (to 20 mg/kg) and the duration of treatment (to 28 days) satisfactorily compensated for the growing resistance until around 1990. There has since been steady erosion in the capacity of Sbv to induce long-term cure in patients with kala-azar who live in eastern India. Co-infection with HIV impairs the treatment response.
Sodium stibogluconate is available in aqueous solution and is administered parenterally. Antimony appears to have two elimination phases. When the drug is administered IV, the mean half-life of the first phase is <2 h; the mean half-life of the terminal elimination phase is nearly 36 h. This slower phase may be due to conversion of pentavalent antimony to a trivalent form that is the likely cause of the side effects often seen with prolonged therapy.
Artemisinin Derivatives* Artesunate, artemether, arteether, and the parent compound artemisinin are sesquiterpene lactones derived from the wormwood plant Artemisia annua. These agents are at least tenfold more potent in vivo than other antimalarial drugs and presently show no cross-resistance with known antimalarial drugs; thus, they have become first-line agents for the treatment of severe falciparum malaria. The artemisinin compounds are rapidly effective against the asexual blood forms of Plasmodium species but are not active against intrahepatic forms. Artemisinin and its derivatives are highly lipid soluble and readily cross both host and parasite cell membranes. One factor that explains the drugs’ highly selective toxicity against malaria is that parasitized erythrocytes concentrate artemisinin and its derivatives to concentrations 100-fold higher than those in uninfected erythrocytes. The antimalarial effect of these agents results primarily from dihydroartemisinin, a compound to which artemether and artesunate are both converted. In the presence of heme or molecular iron, the endoperoxide moiety of dihydroartemisinin decomposes, generating free radicals and other metabolites that damage parasite proteins. The compounds are available for oral, rectal, IV, or IM administration, depending on the derivative. In the United States, IV artesunate is available for the treatment of severe, quinidine-unresponsive malaria through the CDC malaria hotline (770-488-7788, M–F, 0800–1630 EST; 770-488-7100 after hours). Artemisinin and its derivatives are cleared rapidly from the circulation. Their short half-lives limit their value for prophylaxis and monotherapy. These agents should be used only in combination with another, longer-acting agent (e.g., artesunate-mefloquine, dihydroartemisinin-piperaquine). A combined formulation of artemether and lumefantrine is available for the treatment of acute uncomplicated falciparum malaria acquired in areas where Plasmodium falciparum is resistant to chloroquine and antifolates.
Atovaquone Atovaquone is a hydroxynaphthoquinone that exerts broad-spectrum antiprotozoal activity via selective inhibition of parasite mitochondrial electron transport. This agent exhibits potent activity against toxoplasmosis and babesiosis when used with pyrimethamine and azithromycin, respectively. Atovaquone possesses a novel mode of action against Plasmodium species, inhibiting the electron transport system at the level of the cytochrome bc1 complex. The drug is active against both the erythrocytic and the exoerythrocytic stages of Plasmodium species; however, because it does not eradicate hypnozoites from the liver, patients with Plasmodium vivax or Plasmodium ovale infections must be given radical prophylaxis.
Malarone® is a fixed-dose combination of atovaquone and proguanil used for malaria prophylaxis as well as for the treatment of acute, uncomplicated P. falciparum malaria. Malarone has been shown to be effective in regions with multidrug-resistant P. falciparum. Resistance to atovaquone has yet to be reported.
The bioavailability of atovaquone varies considerably. Absorption after a single oral dose is slow, increases two- to threefold with a fatty meal, and is dose-limited above 750 mg. The elimination half-life is increased in patients with moderate hepatic impairment. Because of the potential for drug accumulation, the use of atovaquone is generally contraindicated in persons with a creatinine clearance rate <30 mL/min. No dosage adjustments are needed in patients with mild to moderate renal impairment.
Azithromycin See Table 246e-1 and Chap. 170.
Azoles See Table 246e-1 and Chap. 235.
Benznidazole* This oral nitroimidazole derivative is used to treat Chagas’ disease, with cure rates of 80–90% recorded in acute infections. Benznidazole is believed to exert its trypanocidal effects by generating oxygen radicals to which the parasites are more sensitive than mammalian cells because of a relative deficiency in antioxidant enzymes. Benznidazole also appears to alter the balance between pro- and anti-inflammatory mediators by downregulating the synthesis of nitrite, interleukin (IL) 6, and IL-10 in macrophages. Benznidazole is highly lipophilic and readily absorbed. The drug is extensively metabolized; only 5% of the dose is excreted unchanged in the urine. Benznidazole is well tolerated; adverse effects are rare and usually manifest as GI upset or pruritic rash.
Chloroquine This 4-aminoquinoline has marked, rapid schizonticidal and gametocidal activity against blood forms of P. ovale and Plasmodium malariae and against susceptible strains of P. vivax and P. falciparum. It is not active against intrahepatic forms (P. vivax and P. ovale). Parasitized erythrocytes accumulate chloroquine in significantly greater concentrations than do normal erythrocytes. Chloroquine, a weak base, concentrates in the food vacuoles of intraerythrocytic parasites because of a relative pH gradient between the extracellular space and the acidic food vacuole. Once it enters the acidic food vacuole, chloroquine is rapidly converted to a membrane-impermeable protonated form and is trapped. Continued accumulation of chloroquine in the parasite’s acidic food vacuoles results in drug levels that are 600-fold higher at this site than in plasma. The high accumulation of chloroquine results in an increase in pH within the food vacuole to a level above that required for the acid proteases’ optimal activity, inhibiting parasite heme polymerase; as a result, the parasite is effectively killed with its own metabolic waste. Compared with susceptible strains, chloroquine-resistant plasmodia transport chloroquine out of intraparasitic compartments more rapidly and maintain lower chloroquine concentrations in their acid vesicles. Hydroxychloroquine, a congener of chloroquine, is equivalent to chloroquine in its antimalarial efficacy but is preferred to chloroquine for the treatment of autoimmune disorders because it produces less ocular toxicity when used in high doses.
Chloroquine is well absorbed. However, because it exhibits extensive tissue binding, a loading dose is required to yield effective plasma concentrations. A therapeutic drug level in plasma is reached 2–3 h after oral administration (the preferred route). Chloroquine can be administered IV, but excessively rapid parenteral administration can result in seizures and death from cardiovascular collapse. The mean half-life of chloroquine is 4 days, but the rate of excretion decreases as plasma levels decline, making once-weekly administration possible for prophylaxis in areas with sensitive strains. About one-half of the parent drug is excreted in urine, but the dose should not be reduced for persons with acute malaria and renal insufficiency.
Ciprofloxacin See Table 246e-1 and Chap. 170.
Clindamycin See Table 246e-1 and Chap. 170.
Dapsone See Table 246e-1 and Chap. 205e.
Dehydroemetine Emetine is an alkaloid derived from ipecac; dehydroemetine is synthetically derived from emetine and is considered less toxic. Both agents are active against Entamoeba histolytica and appear to work by blocking peptide elongation and thus inhibiting protein synthesis. Emetine is rapidly absorbed after parenteral administration, rapidly distributed throughout the body, and slowly excreted in the urine in unchanged form. Both agents are contraindicated in patients with renal disease.
Diethylcarbamazine* A derivative of the antihelminthic agent piperazine with a long history of successful use, diethylcarbamazine (DEC) remains the treatment of choice for lymphatic filariasis and loiasis and has also been used for visceral larva migrans. Although piperazine itself has no antifilarial activity, the piperazine ring of DEC is essential for the drug’s activity. DEC’s mechanism of action remains to be fully defined. Proposed mechanisms include immobilization due to inhibition of parasite cholinergic muscle receptors, disruption of microtubule formation, and alteration of helminthic surface membranes resulting in enhanced killing by the host’s immune system. DEC enhances adherence properties of eosinophils. The development of resistance under drug pressure (i.e., a progressive decrease in efficacy when the drug is used widely in human populations) has not been observed, although DEC has variable effects when administered to persons with filariasis. Monthly administration provides effective prophylaxis against both bancroftian filariasis and loiasis.
DEC is well absorbed after oral administration, with peak plasma concentrations reached within 1–2 h. No parenteral form is available. The drug is eliminated largely by renal excretion, with <5% found in feces. If more than one dose is to be administered to an individual with renal dysfunction, the dose should be reduced commensurate with the reduction in creatinine clearance rate. Alkalinization of the urine prevents renal excretion and increases the half-life of DEC. Use in patients with onchocerciasis can precipitate a Mazzotti reaction, with pruritus, fever, and arthralgias. Like other piperazines, DEC is active against Ascaris species. Patients co-infected with this nematode may expel live worms after treatment.
Diloxanide Furoate Diloxanide furoate, a substituted acetanilide, is a luminally active agent used to eradicate the cysts of E. histolytica. After ingestion, diloxanide furoate is hydrolyzed by enzymes in the lumen or mucosa of the intestine, releasing furoic acid and the ester diloxanide; the latter acts directly as an amebicide.
Diloxanide furoate is given alone to asymptomatic cyst passers. For patients with active amebic infections, diloxanide is generally administered in combination with a 5-nitroimidazole such as metronidazole or tinidazole. Diloxanide furoate is rapidly absorbed after oral administration. When coadministered with a 5-nitroimidazole, only diloxanide appears in the systemic circulation; levels peak within 1 h and disappear within 6 h. About 90% of an oral dose is excreted in the urine within 48 h, chiefly as the glucuronide metabolite. Diloxanide furoate is contraindicated in pregnant and breast-feeding women and in children <2 years of age.
Eflornithine* Eflornithine (difluoromethylornithine, or DFMO) is a fluorinated analogue of the amino acid ornithine. Although originally designed as an antineoplastic agent, eflornithine has proven effective against some trypanosomatids. At one point, the production of this effective agent ceased despite the increasing incidence of human African trypanosomiasis; however, production resumed after eflornithine was discovered to be an effective cosmetic depilatory agent.
Eflornithine has specific activity against all stages of infection with Trypanosoma brucei gambiense; however, it is inactive against Trypanosoma brucei rhodesiense. The drug acts as an irreversible suicide inhibitor of ornithine decarboxylase, the first enzyme in the biosynthesis of the polyamines putrescine and spermidine. Polyamines are essential for the synthesis of trypanothione, an enzyme required for the maintenance of intracellular thiols in the correct redox state and for the removal of reactive oxygen metabolites. However, polyamines are also essential for cell division in eukaryotes, and ornithine decarboxylase is similar in trypanosomes and mammals. The selective antiparasitic activity of eflornithine is partly explained by the structure of the trypanosomal enzyme, which lacks a 36-amino-acid C-terminal sequence found on mammalian ornithine decarboxylase. This difference results in a lower turnover of ornithine decarboxylase and a more rapid decrease of polyamines in trypanosomes than in the mammalian host. the diminished effectiveness of eflornithine against T. b. rhodesiense appears to be due to the parasite’s ability to replace the inhibited enzyme more rapidly than T. b. gambiense.
Eflornithine is less toxic but more costly than conventional therapy. It can be administered IV or PO. The dose should be reduced in renal failure. Eflornithine readily crosses the blood-brain barrier; CSF levels are highest in persons with the most severe central nervous system (CNS) involvement.
Fumagillin† Fumagillin is a water-insoluble antibiotic that is derived from the fungus Aspergillus fumigatus and is active against microsporidia. This drug is effective when used topically to treat ocular infections due to Encephalitozoon species. When given systemically, fumagillin was effective but caused thrombocytopenia in all recipients in the second week of treatment; this side effect was readily reversed when administration of the drug was stopped. The mechanisms by which fumagillin inhibits microsporidial replication are poorly understood, although the drug may inhibit methionine aminopeptidase 2 by irreversibly blocking the active site.
Furazolidone This nitrofuran derivative is an effective alternative agent for the treatment of giardiasis and also exhibits activity against Isospora belli. Because it is the only agent active against Giardia that is available in liquid form, it is most often used to treat young children. Furazolidone undergoes reductive activation in Giardia lamblia trophozoites—an event that, unlike the reductive activation of metronidazole, involves an NADH oxidase. The killing effect correlates with the toxicity of reduced products, which damage important cellular components, including DNA. Although furazolidone had been thought to be largely unabsorbed when administered orally, the occurrence of systemic adverse reactions indicates that this is not the case. More than 65% of the drug dose can be recovered from the urine as colored metabolites. Omeprazole reduces the oral bioavailability of furazolidone.
Furazolidone is a monoamine oxidase (MAO) inhibitor; thus, caution should be used in its concomitant administration with other drugs (especially indirectly acting sympathomimetic amines) and in the consumption of food and drink containing tyramine during treatment. However, hypertensive crises have not been reported in patients receiving furazolidone, and it has been suggested that—because furazolidone inhibits MAOs gradually over several days—the risks are small if treatment is limited to a 5-day course. Because hemolytic anemia can occur in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency and glutathione instability, furazolidone treatment is contraindicated in mothers who are breast-feeding and in neonates.
Halofantrine This 9-phenanthrenemethanol is one of three classes of arylaminoalcohols first identified as potential antimalarial agents by the World War II Malaria Chemotherapy Program. Its activity is believed to be similar to that of chloroquine, although it is an oral alternative for the treatment of malaria due to chloroquine-resistant P. falciparum.
Although the mechanism of action is poorly understood, halofantrine is thought to share mechanism(s) with the 4-aminoquinolines, forming a complex with ferriprotoporphyrin IX and interfering with the degradation of hemoglobin.
Halofantrine exhibits erratic bioavailability, but its absorption is significantly enhanced when it is taken with a fatty meal. The elimination half-life of halofantrine is 1–2 days; it is excreted mainly in feces. Halofantrine is metabolized into N-debutyl-halofantrine by the cytochrome P450 enzyme CYP3A4. Grapefruit juice should be avoided during treatment because it increases both halofantrine’s bioavailability and halofantrine-induced QT interval prolongation by inhibiting CYP3A4 at the enterocyte level.
Iodoquinol Iodoquinol (diiodohydroxyquin), a hydroxyquinoline, is an effective luminal agent for the treatment of amebiasis, balantidiasis, and infection with Dientamoeba fragilis. Its mechanism of action is unknown. It is poorly absorbed. Because the drug contains 64% organically bound iodine, it should be used with caution in patients with thyroid disease. Iodine dermatitis occurs occasionally during iodoquinol treatment. Protein-bound serum iodine levels may be increased during treatment and can interfere with certain tests of thyroid function. These effects may persist for as long as 6 months after discontinuation of therapy. Iodoquinol is contraindicated in patients with liver disease. Most serious are the reactions related to prolonged high-dose therapy (optic neuritis, peripheral neuropathy), which should not occur if the recommended dosage regimens are followed.
Ivermectin Ivermectin (22,23-dihydroavermectin) is a derivative of the macrocyclic lactone avermectin produced by the soil-dwelling actinomycete Streptomyces avermitilis. Ivermectin is active at low doses against a wide range of helminths and ectoparasites. It is the drug of choice for the treatment of onchocerciasis, strongyloidiasis, cutaneous larva migrans, and scabies. Ivermectin is highly active against microfilariae of the lymphatic filariases but has no macrofilaricidal activity. When ivermectin is used in combination with other agents such as DEC or albendazole for treatment of lymphatic filariasis, synergistic activity is seen. Although active against the intestinal helminths Ascaris lumbricoides and Enterobius vermicularis, ivermectin is only variably effective in trichuriasis and is ineffective against hookworms. Widespread use of ivermectin for treatment of intestinal nematode infections in sheep and goats has led to the emergence of drug resistance in veterinary practice; this development may portend problems in human medical use.
Data suggest that ivermectin acts by opening the neuromuscular membrane-associated, glutamate-dependent chloride channels. The influx of chloride ions results in hyperpolarization and muscle paralysis—particularly of the nematode pharynx, with consequent blockage of the oral ingestion of nutrients. As these chloride channels are present only in invertebrates, paralysis is seen only in the parasite.
Ivermectin is available for administration to humans only as an oral formulation. The drug is highly protein bound; it is almost completely excreted in feces. Both food and beer increase the bioavailability of ivermectin significantly. Ivermectin is distributed widely throughout the body; animal studies indicate that it accumulates at the highest concentration in adipose tissue and liver, with little accumulation in the brain. Few data exist to guide therapy in hosts with conditions that may influence drug pharmacokinetics.
Ivermectin is generally administered as a single dose of 150–200 μg/kg. In the absence of parasitic infection, the adverse effects of ivermectin in therapeutic doses are minimal. Adverse effects in patients with filarial infections include fever, myalgia, malaise, lightheadedness, and (occasionally) postural hypotension. The severity of such side effects is related to the intensity of parasite infection, with more symptoms in individuals with a heavy parasite burden. In onchocerciasis, skin edema, pruritus, and mild eye irritation may also occur. The adverse effects are generally self-limiting and only occasionally require symptom-based treatment with antipyretics or antihistamines. More severe complications of ivermectin therapy for onchocerciasis include encephalopathy in patients heavily infected with Loa loa.
Lumefantrine Lumefantrine (benflumetol), a fluorene arylaminoalcohol derivative synthesized in the 1970s by the Chinese Academy of Military Medical Sciences (Beijing), has marked blood schizonticidal activity against a wide range of plasmodia. This agent conforms structurally and in mode of action to other arylaminoalcohols (quinine, mefloquine, and halofantrine). Lumefantrine exerts its antimalarial effect as a consequence of its interaction with heme, a degradation product of hemoglobin metabolism. Although its antimalarial activity is slower than that of the artemisinin-based drugs, the recrudescence rate with the recommended lumefantrine regimen is lower. The pharmacokinetic properties of lumefantrine are reminiscent of those of halofantrine, with variable oral bioavailability, considerable augmentation of oral bioavailability by concomitant fat intake, and a terminal elimination half-life of ~4–5 days in patients with malaria.
Artemether and lumefantrine have synergistic activity, and the combined formulation of artemether and lumefantrine is effective for the treatment of falciparum malaria in areas where P. falciparum is resistant to chloroquine and antifolates.
Mebendazole This benzimidazole is a broad-spectrum antiparasitic agent widely used to treat intestinal helminthiases. Its mechanism of action is similar to that of albendazole; however, it is a more potent inhibitor of parasite malic dehydrogenase and exhibits a more specific and selective effect against intestinal nematodes than the other benzimidazoles.
Mebendazole is available only in oral form but is poorly absorbed from the GI tract; only 5–10% of a standard dose is measurable in plasma. The proportion absorbed from the GI tract is extensively metabolized in the liver. Metabolites appear in the urine and bile; impaired liver or biliary function results in higher plasma mebendazole levels in treated patients. No dose reduction is warranted in patients with renal function impairment. Because mebendazole is poorly absorbed, its incidence of side effects is low. Transient abdominal pain and diarrhea sometimes occur, usually in persons with massive parasite burdens.
Mefloquine Mefloquine is the preferred drug for prophylaxis of chloroquine-resistant malaria; high doses can be used for treatment. Despite the development of drug-resistant strains of P. falciparum in parts of Africa and Southeast Asia, mefloquine is an effective drug throughout most of the world. Cross-resistance of mefloquine with halofantrine and with quinine has been documented in limited areas. Like quinine and chloroquine, this quinoline is active only against the asexual erythrocytic stages of malarial parasites. Unlike quinine, however, mefloquine has a relatively poor affinity for DNA and, as a result, does not inhibit the synthesis of parasitic nucleic acids and proteins. Although both mefloquine and chloroquine inhibit hemozoin formation and heme degradation, mefloquine differs in that it forms a complex with heme that may be toxic to the parasite.
Mefloquine HCl is poorly water soluble and intensely irritating when given parenterally; thus it is available only in tablet form. Its absorption is adversely affected by vomiting and diarrhea but is significantly enhanced when the drug is administered with or after food. About 98% of the drug binds to protein. Mefloquine is excreted mainly in the bile and feces; therefore, no dose adjustment is needed in persons with renal insufficiency. The drug and its main metabolite are not appreciably removed by hemodialysis. No special chemoprophylactic dosage adjustments are indicated for the achievement of plasma concentrations in dialysis patients that are similar to those in healthy persons. Pharmacokinetic differences have been detected among various ethnic populations. In practice, however, these distinctions are of minor importance compared with host immune status and parasite sensitivity. In patients with impaired liver function, the elimination of mefloquine may be prolonged, leading to higher plasma levels.
Mefloquine should be used with caution by individuals participating in activities requiring alertness and fine-motor coordination. A recent FDA review found that dizziness, vertigo, or tinnitus can persist or become permanent as a result of treatment with the drug; thus, a boxed warning was mandated. If the drug is to be administered for a prolonged period, periodic evaluations are recommended, including liver function tests and ophthalmic examinations. Sleep abnormalities (insomnia, abnormal dreams) have occasionally been reported. Psychosis and seizures occur rarely; mefloquine should not be prescribed to patients with neuropsychiatric conditions, including depression, generalized anxiety disorder, psychosis, schizophrenia, and seizure disorder. If acute anxiety, depression, restlessness, or confusion develops during prophylaxis, these psychiatric symptoms may be considered prodromal to a more serious event, and the drug should be discontinued.