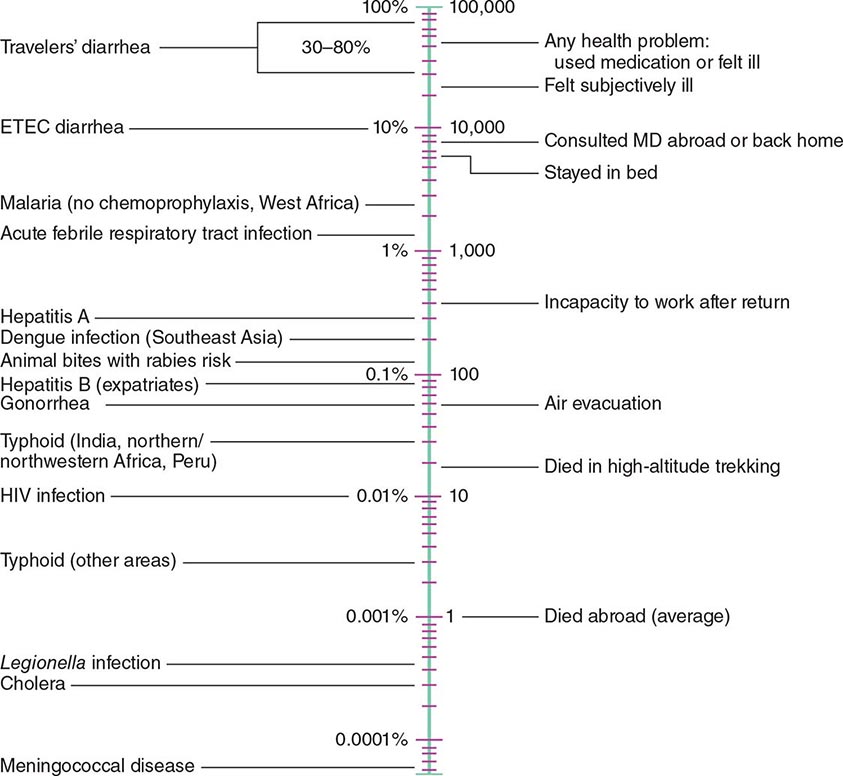
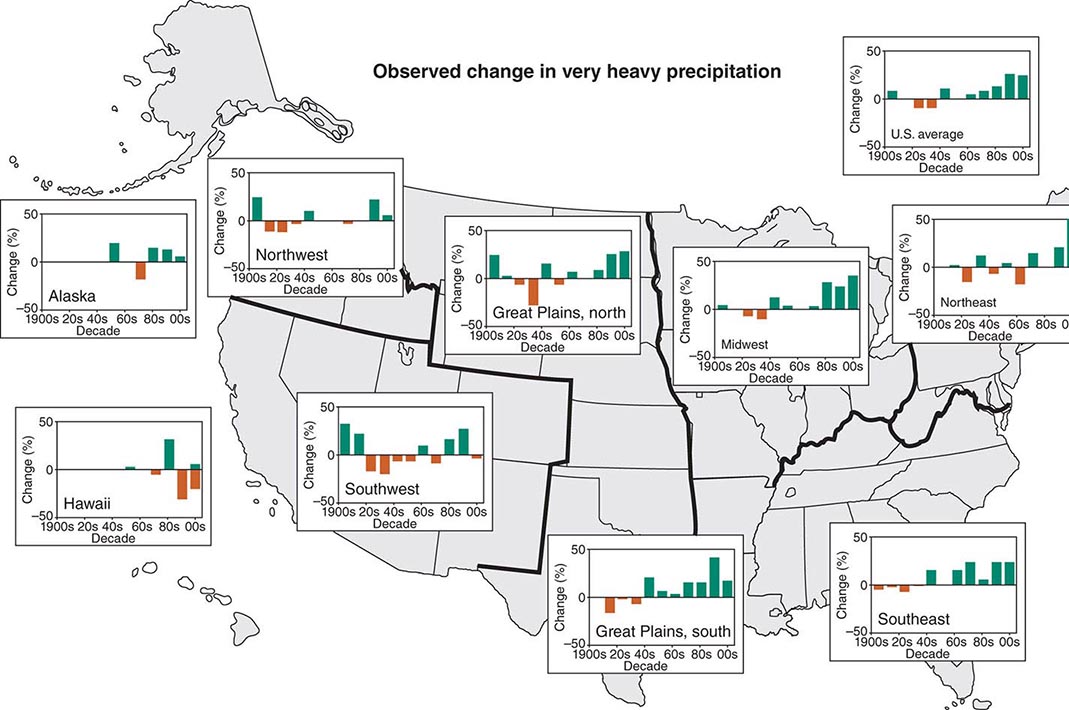
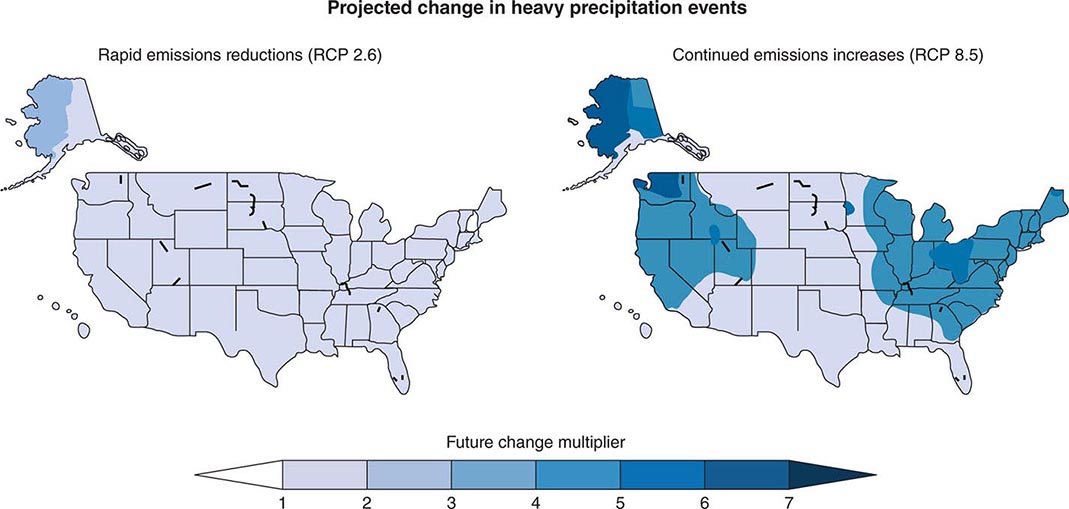
According to the World Tourism Organization, international tourist arrivals grew dramatically from 25 million in 1950 to >1 billion in 2012. Not only are more people traveling; travelers are seeking more exotic and remote destinations. Travel from industrialized to developing regions has been increasing, with Asia and the Pacific, Africa, and the Middle East now emerging destinations. Figure 149-1 summarizes the monthly incidence of health problems during travel in developing countries. Studies continue to show that 50–75% of short-term travelers to the tropics or subtropics report some health impairment. Most of these health problems are minor: only 5% require medical attention, and <1% require hospitalization. Although infectious agents contribute substantially to morbidity among travelers, these pathogens account for only ~1% of deaths in this population. Cardiovascular disease and injuries are the most frequent causes of death among travelers from the United States, accounting for 49% and 22% of deaths, respectively. Age-specific rates of death due to cardiovascular disease are similar among travelers and nontravelers. In contrast, rates of death due to injury (the majority from motor vehicle, drowning, or aircraft accidents) are several times higher among travelers. Motor vehicle accidents account for >40% of travelers’ deaths that are not due to cardiovascular disease or preexisting illness.
FIGURE 149-1 Monthly incidence rates of health problems during stays in developing countries. ETEC, enterotoxigenic Escherichia coli. (From R Steffen et al: Int J Antimicrob Agents 21:89, 2003.)
GENERAL ADVICE
Health maintenance recommendations are based not only on the traveler’s destination but also on assessment of risk, which is determined by such variables as health status, specific itinerary, purpose of travel, season, and lifestyle during travel. Detailed information regarding country-specific risks and recommendations may be obtained from the Centers for Disease Control and Prevention (CDC) publication Health Information for International Travel (available at www.cdc.gov/travel).
Fitness for travel is an issue of growing concern in view of the increased numbers of elderly and chronically ill individuals journeying to exotic destinations (see “Travel and Special Hosts,” below). Since most commercial aircraft are pressurized to 2500 m (8000 ft) above sea level (corresponding to a Pa02 of ~55 mmHg), individuals with serious cardiopulmonary problems or anemia should be evaluated before travel. In addition, those who have recently had surgery, a myocardial infarction, a cerebrovascular accident, or a deep-vein thrombosis may be at high risk for adverse events during flight. A summary of current recommendations regarding fitness to fly has been published by the Aerospace Medical Association Air Transport Medicine Committee (www.asma.org/publications/medical-publications-for-airline-travel). A pretravel health assessment may be advisable for individuals considering particularly adventurous recreational activities, such as mountain climbing and scuba diving.
IMMUNIZATIONS FOR TRAVEL
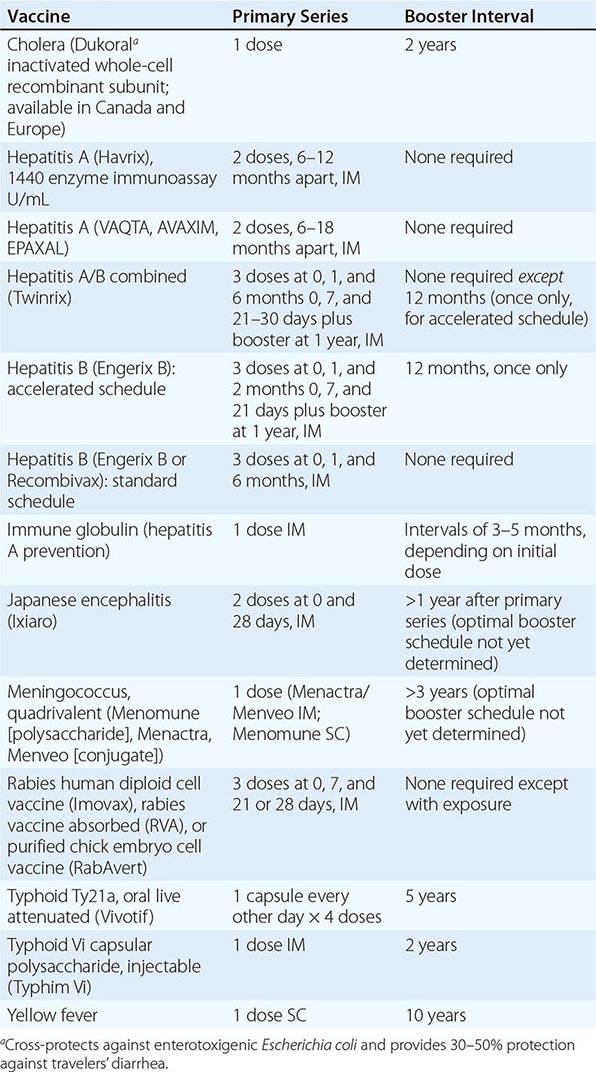
Immunizations for travel fall into three broad categories: routine (childhood/adult boosters that are necessary regardless of travel), required (immunizations that are mandated by international regulations for entry into certain areas or for border crossings), and recommended (immunizations that are desirable because of travel-related risks). Required and recommended vaccines commonly given to travelers are listed in Table 149-1.
|
VACCINES COMMONLY USED FOR TRAVEL IN ADULTS |
Routine Immunizations • DIPHTHERIA, TETANUS, AND POLIO Diphtheria (Chap. 175) continues to be a problem worldwide. Large outbreaks have occurred in countries that do not have rigorous vaccination programs or that have reduced their public vaccination programs. Serologic surveys show that tetanus (Chap. 177) antibodies are lacking in many North Americans, especially in women over the age of 50. The risk of polio (Chap. 228) to the international traveler is extremely low, and wild-type poliovirus has been eradicated from the Western Hemisphere and Europe. However, studies in the United States suggest that 12% of adult travelers are unprotected against at least one poliovirus serogroup. In addition, challenges continue to be faced by polio eradication programs. Foreign travel offers an ideal opportunity to have these immunizations updated. With the recent increase in pertussis among adults, the diphtheria–tetanus–acellular pertussis (Tdap) combination is now recommended for adults as a once-only replacement for the 10-year tetanus–diphtheria (Td) booster.
MEASLES Measles (rubeola) continues to be a major cause of morbidity and death in the developing world (Chap. 229). Several outbreaks of measles in the United States and Canada have been linked to imported cases, especially from Europe, where large outbreaks have occurred recently. The group at highest risk consists of persons born after 1956 and vaccinated before 1980, in many of whom primary vaccination failed. The measles–mumps–rubella (MMR) vaccine is typically used; its coverage of rubella also addresses a growing concern in some areas of Eastern Europe and Asia.
INFLUENZA Influenza (Chap. 224)—possibly the most common vaccine-preventable infection in travelers—occurs year-round in the tropics and during the summer months in the Southern Hemisphere (coinciding with the winter months in the Northern Hemisphere). One prospective study showed that influenza developed in 1% of travelers to Southeast Asia per month of stay. Annual vaccination should be considered for all travelers who do not have a contraindication. Travel-related influenza continues to occur during summer months in Alaska and the Northwest Territories of Canada among cruise-ship passengers and staff. The speed of global spread of the pandemic H1N1 virus once again illustrates why influenza immunization is so important for travelers.
PNEUMOCOCCAL INFECTION Regardless of travel, pneumococcal vaccine (Chap. 171) should be administered routinely to persons over the age of 65 and to persons at high risk of serious infection, including those with chronic heart, lung, or kidney disease; those who have been splenectomized; and those who have sickle cell disease.
Required Immunizations • YELLOW FEVER Documentation of vaccination against yellow fever (Chap. 233) may be required or recommended as a condition for entry into or passage through countries of sub-Saharan Africa and equatorial South America, where the disease is endemic or epidemic, or (by the International Health Regulations) for entry into countries at risk of having the infection introduced. This vaccine is given only by state-authorized yellow fever centers, and its administration must be documented on an official International Certificate of Vaccination. A registry of U.S. clinics that provide the vaccine is available from the CDC (www.cdc.gov/travel). Recent data suggest that fewer than 50% of travelers entering areas endemic for yellow fever are immunized; this lack of coverage is a serious problem, as 13 countries in Central and South America and 30 countries in Africa harbor the illness. Severe adverse events associated with this vaccine have recently increased in incidence. First-time vaccine recipients may present with a syndrome characterized as either neurotropic (1 case per 125,000 doses) or viscerotropic (overall, 1 case per 250,000 doses; among persons 60–69 years of age, 1 case per 100,000 doses; and among persons ≥ 70 years of age, 1 case per 40,000 doses). Immunosuppression and thymic disease increase the risk of these adverse events (www.cdc.gov/vaccines/hcp/vis/vis-statements/yf.pdf).
MENINGOCOCCAL MENINGITIS Protection against meningitis with one of the quadrivalent (preferably conjugate) vaccines is required for entry into Saudi Arabia during the Hajj (Chap. 180).
INFLUENZA Both seasonal and pandemic H1N1 vaccines (the latter, where available) were required for entry into Saudi Arabia during the Hajj in 2013.
Recommended Immunizations • HEPATITIS A AND B Hepatitis A (Chap. 360) is one of the most common vaccine-preventable infections of travelers. The risk is six times greater for travelers who stray from the usual tourist routes. The mortality rate for hepatitis A increases with age, reaching almost 2% among individuals over age 50. Of the four hepatitis A vaccines currently available in North America (two in the United States), all are interchangeable and have an efficacy of >95%. Hepatitis A vaccine is currently given to all children in the United States. Since the most frequently identified risk factor for hepatitis A in the United States is international travel, and since morbidity and mortality increase with age, it seems appropriate that all adults be immune prior to travel.
Long-stay overseas workers appear to be at considerable risk for hepatitis B infection (Chap. 360). The recommendation that all travelers be immunized against hepatitis B before departure is supported by two studies showing that 17% of the assessed travelers who received health care abroad had some type of injection; according to the World Health Organization, nonsterile equipment is used for up to 75% of all injections given in parts of the developing world. A 3-week accelerated schedule of the combined hepatitis A and B vaccine has been approved in the United States. Although no data are available on the specific risk of infection with hepatitis B virus among U.S. travelers, ~240 million people in the world have chronic infection. All children and adolescents in the United States are immunized against this illness. Hepatitis B vaccination should be considered for all travelers.
TYPHOID FEVER Most cases of typhoid fever in North America are due to travel, with ~300 cases seen per year in the United States. The attack rate for typhoid fever (Chap. 190) is 1 case per 30,000 travelers per month of travel to the developing world. However, attack rates in India, Senegal, and North Africa are tenfold higher; rates are especially high among travelers to relatively remote destinations and among immigrants and their families who have returned to their homelands to visit friends or relatives (VFRs). Between 1999 and 2006 in the United States, 66% of imported cases involved the latter group. Unfortunately, data show that the causative organism has become increasingly resistant to fluoroquinolone antibiotics (especially in those cases acquired on the Indian subcontinent). Both of the available vaccines—one oral (live) and the other injectable (polysaccharide)—have efficacy rates of ~70%. In some countries, a combined hepatitis A/typhoid vaccine is available.
MENINGOCOCCAL MENINGITIS Although the risk of meningococcal disease among travelers has not been quantified, it is likely to be higher among travelers who live with poor indigenous populations in overcrowded conditions (Chap. 180). Because of its enhanced ability to prevent nasal carriage (compared with the older polysaccharide vaccine), a quadrivalent conjugate vaccine is the product of choice (regardless of age) for immunization of persons traveling to sub-Saharan Africa during the dry season or to areas of the world where there are epidemics. The vaccine, which protects against serogroups A, C, Y, and W-135, has an efficacy rate of >90%.
JAPANESE ENCEPHALITIS The risk of Japanese encephalitis (Chap. 233), an infection transmitted by mosquitoes in rural Asia and Southeast Asia, can be as high as ~1 case per 5000 travelers per month of stay in an endemic area. Most infections are asymptomatic, with a very small proportion of infected persons becoming ill. However, among those who do become ill, severe neurologic sequelae are common. Most symptomatic infections among U.S. residents have involved military personnel or their families. The vaccine efficacy rate is >90%. The vaccine is recommended for persons staying >1 month in rural endemic areas or for shorter periods if their activities (e.g., camping, bicycling, hiking) in these areas will increase exposure risk.
CHOLERA The risk of cholera (Chap. 193) is extremely low, with ~1 case per 500,000 journeys to endemic areas. Cholera vaccine, not currently available in the United States, was rarely recommended but was considered for aid and health care workers in refugee camps or in disaster-stricken/war-torn areas. A more effective oral cholera vaccine is available in other countries.
RABIES Domestic animals, primarily dogs, are the major transmitters of rabies in developing countries (Chap. 232). Several studies have shown that the risk of rabies posed by a dog bite in an endemic area translates into 1–3.6 cases per 1000 travelers per month of stay. Countries where canine rabies is highly endemic include Mexico, the Philippines, Sri Lanka, India, Thailand, China, and Vietnam. The two vaccines available in the United States provide >90% protection. Rabies vaccine is recommended for long-stay travelers, particularly children (who tend to play with animals and may not report bites), and for persons who may be occupationally exposed to rabies in endemic areas; however, in a large-scale study, almost 50% of potential exposures occurred within the first month of travel. Even after receipt of a preexposure rabies vaccine series, two postexposure doses are required. Travelers who have had the preexposure series do not require rabies immune globulin (which is often unavailable in developing countries) if they are exposed to the disease.
PREVENTION OF MALARIA AND OTHER INSECT-BORNE DISEASES
It is estimated that more than 30,000 American and European travelers develop malaria each year (Chap. 248). The risk to travelers is highest in Oceania and sub-Saharan Africa (estimated at 1:5 and 1:50 per month of stay, respectively, among persons not using chemoprophylaxis); intermediate in malarious areas on the Indian subcontinent and in Southeast Asia (1:250–1:1000 per month); and low in South and Central America (1:2500–1:10,000 per month). Of the 1925 cases of malaria reported in 2011 in the United States (the highest figure in 40 years), 90% of those due to Plasmodium falciparum occurred in travelers returning or emigrating from Africa and Oceania. VFRs are at the highest risk of acquiring malaria and may die of the disease if their immunity has waned after living outside an endemic area for a number of years. According to data from the CDC, VFRs accounted for 59% of severe malaria cases in the United States in 2011. With the worldwide increase in chloroquine- and multidrug-resistant falciparum malaria, decisions about chemoprophylaxis have become more difficult. The case-fatality rate for falciparum malaria in the United States is 4%; however, in only one-third of patients who die is the diagnosis of malaria considered before death.
Several studies indicate that fewer than 50% of travelers adhere to basic recommendations for malaria prevention. Keys to the prevention of malaria include both personal protection measures against mosquito bites (especially between dusk and dawn) and malaria chemoprophylaxis. The former measures entail the use of DEET-containing insect repellents, permethrin-impregnated bed nets and clothing, screened sleeping accommodations, and protective clothing. Thus, in regions where infections such as malaria are transmitted, DEET products (25–50%) are recommended, even for children and infants at birth. Studies suggest that concentrations of DEET above ~50% do not offer a marked increase in protection time against mosquitoes. The CDC also recommends picaridin, oil of lemon eucalyptus (PMD, para-menthane-3,8-diol), and IR3535 (3-[N-butyl-N-acetyl]-aminopropionic acid, ethyl ester). In general, higher concentrations of any active ingredient provide a longer duration of protection. Personal protection measures also help prevent other insect-transmitted illnesses, such as dengue fever (Chap. 233). Over the past decade, the incidence of dengue has markedly increased, particularly in the Caribbean region, Latin America, Southeast Asia, and (more recently) Africa. Chikungunya, another mosquito-borne infection that clinically resembles dengue fever but with arthralgia and arthritis instead of myalgia, has recently crossed to the Western Hemisphere; many thousands of cases are now occurring in the Caribbean. Both dengue and chikungunya viruses are transmitted by an urban-dwelling mosquito that bites primarily at dawn and dusk.
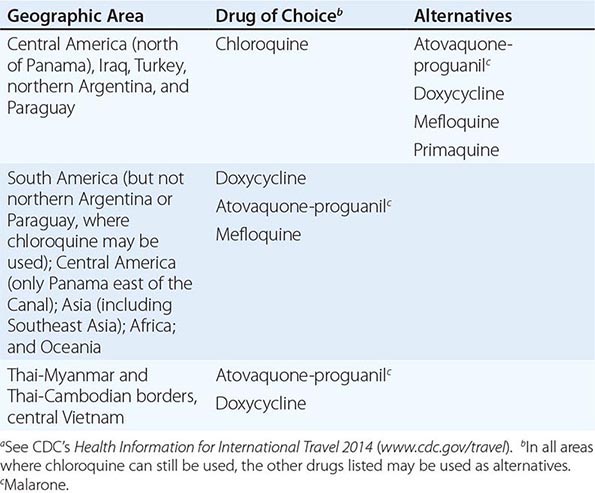
Table 149-2 lists the currently recommended drugs of choice for prophylaxis of malaria, by destination.
|
MALARIA CHEMOSUPPRESSIVE REGIMENS, ACCORDING TO GEOGRAPHIC AREAa |
Note: See also Chap. 248.
PREVENTION OF GASTROINTESTINAL ILLNESS
Diarrhea, the leading cause of illness in travelers (Chap. 160), is usually a short-lived, self-limited condition. However, 40% of affected individuals need to alter their scheduled activities, and another 20% are confined to bed. The most important determinant of risk is the destination. Incidence rates per 2-week stay have been reported to be as low as 8% in industrialized countries and as high as 55% in parts of Africa, Central and South America, and Southeast Asia. Infants and young adults are at particularly high risk for gastrointestinal illness and for complications such as dehydration. Recent reviews suggest that there is little correlation between dietary indiscretions and the occurrence of travelers’ diarrhea. Earlier studies of U.S. students in Mexico showed that eating meals in restaurants and cafeterias or consuming food from street vendors was associated with increased risk. For further discussion, see “Precautions,” below.
Etiology (See also Table 160-3) The most frequently identified pathogens causing travelers’ diarrhea are enterotoxigenic and enteroaggregative Escherichia coli (Chap. 186), although in some parts of the world (notably northern Africa and Southeast Asia) Campylobacter infections (Chap. 192) appear to predominate. Other common causative organisms include Salmonella (Chap. 190), Shigella (Chap. 191), rotavirus (Chap. 227), and norovirus (Chap. 227). The latter virus has caused numerous outbreaks on cruise ships. Except for giardiasis (Chap. 254), parasitic infections are uncommon causes of travelers’ diarrhea in short-term travelers. A growing problem for travelers is the development of antibiotic resistance among many bacterial pathogens. Examples include strains of Campylobacter resistant to quinolones and strains of E. coli, Shigella, and Salmonella resistant to trimethoprim-sulfamethoxazole E. coli. O157 is very rarely a cause of travelers’ diarrhea.
Precautions Some experts think that it is not only what travelers eat but also where they eat that puts them at risk of illness. Food sold by street vendors can carry a high risk, and restaurant hygiene can be a major problem over which the traveler has no control. In addition to discretion in choosing the source of food and water, general precautions include eating foods piping hot; avoiding foods that are raw or poorly cooked; and drinking only boiled or commercially bottled beverages, particularly those that are carbonated. Heating kills diarrhea-causing organisms, whereas freezing does not; therefore, ice cubes made from unpurified water should be avoided. In spite of these recommendations, the literature has repeatedly documented dietary indiscretions by 98% of travelers within the first 72 h after arrival at their destination. The maxim “Boil it, cook it, peel it, or forget it!” is easy to remember but apparently difficult to follow.
Self-Treatment (See also Table 160-5) As travelers’ diarrhea often occurs despite rigorous food and water precautions, travelers should carry medications for self-treatment. An antibiotic is useful in reducing the frequency of bowel movements and the duration of illness in moderate to severe diarrhea. The standard regimen is a 3-day course of a quinolone taken twice daily (or, in the case of some newer formulations, once daily). However, studies have shown that one double dose of a quinolone may be equally effective. For diarrhea acquired in areas such as Thailand, where >90% of Campylobacter infections are quinolone resistant, azithromycin may be a better alternative. Rifaximin, a poorly absorbed rifampin derivative, is highly effective against noninvasive bacterial pathogens such as enterotoxigenic and enteroaggregative E. coli. The current approach to self-treatment of travelers’ diarrhea for the typical short-term traveler is to carry three once-daily doses of an antibiotic and to use as many doses as necessary to resolve the illness. If neither high fever nor blood in the stool accompanies the diarrhea, loperamide should be taken in combination with the antibiotic; studies have shown that this combination is more effective than an antibiotic alone and does not prolong illness.
Prophylaxis Prophylaxis of travelers’ diarrhea with bismuth subsalicylate is widely used but only ~60% effective. For certain individuals (e.g., athletes, persons with a repeated history of travelers’ diarrhea, and persons with chronic diseases), a single daily dose of a quinolone, azithromycin, or rifaximin during travel of <1 month’s duration is 75–90% efficacious in preventing travelers’ diarrhea. Probiotics have been only ~20% effective as prophylaxis. In Europe and Canada, an oral subunit cholera vaccine that cross-protects against enterotoxigenic E. coli (Dukoral) has been shown to provide 30–50% protection against travelers’ diarrhea.
Illness After Return Although extremely common, acute travelers’ diarrhea is usually self-limited or amenable to antibiotic therapy. Persistent bowel problems after the traveler returns home have a less well-defined etiology and may require medical attention from a specialist. Infectious agents (e.g., Giardia lamblia, Cyclospora cayetanensis, Entamoeba histolytica) appear to be responsible for only a small proportion of cases with persistent bowel symptoms. By far the most common causes of persistent diarrhea after travel are postinfectious sequelae such as lactose intolerance and irritable bowel syndrome. A meta-analysis showed that postinfectious irritable bowel syndrome lasting months to years may occur in as many as 4–13% of cases. When no infectious etiology can be identified, a trial of metronidazole therapy for presumed giardiasis, a strict lactose-free diet for 1 week, or a several-week trial of high-dose hydrophilic mucilloid (plus an osmotic laxative such as lactulose or PEG 3350 for persons with alternating diarrhea and constipation) relieves the symptoms of many patients.
PREVENTION OF OTHER TRAVEL-RELATED PROBLEMS
Travelers are at high risk for sexually transmitted diseases (Chap. 163). Surveys have shown that large numbers of travelers engage in casual sex, and there is a reluctance to use condoms consistently. An increasing number of travelers are being diagnosed with illnesses such as schistosomiasis (Chap. 259), dengue (Chap. 233), chikungunya (Chap. 233), and tick-borne rickettsial disease (Chap. 211). Travelers should be cautioned to avoid bathing, swimming, or wading in freshwater lakes, streams, or rivers in parts of northeastern South America, the Caribbean, Africa, and Southeast Asia. Insect repellents are important for prevention not only of malaria but also of other vector-borne diseases. Prevention of travel-associated injury depends mostly on common-sense precautions. Riding on motorcycles (especially without helmets) and in overcrowded public vehicles is not recommended; in developing countries, individuals should never travel by road in rural areas after dark. Of persons who die during travel, fewer than 1% die of infection, whereas 40% die in motor vehicle accidents. Excessive alcohol use has been a significant factor in motor vehicle accidents, drownings, assaults, and injuries. Travelers are cautioned to avoid walking barefoot because of the risk of hookworm and Strongyloides infections (Chap. 257) and snakebites (Chap. 474).
THE TRAVELER’S MEDICAL KIT
A traveler’s medical kit is strongly advisable. The contents may vary widely, depending on the itinerary, duration of stay, style of travel, and local medical facilities. While many medications are available abroad (often over the counter), directions for their use may be nonexistent or in a foreign language, or a product may be outdated or counterfeit. For example, a multicountry study in Southeast Asia showed that a mean of 53% (range, 21–92%) of antimalarial products were counterfeit or contained inadequate amounts of active drug. The sale and marketing of such medications constitute a growing industry. In the medical kit, the short-term traveler should consider carrying an analgesic; an antidiarrheal agent and an antibiotic for self-treatment of travelers’ diarrhea; antihistamines; a laxative; oral rehydration salts; a sunscreen with broad-spectrum protection (UVA and UVB, with the latter at a level of at least 30 SPF); a DEET-containing or equivalent insect repellent for the skin; an insecticide for clothing (permethrin); and, if necessary, an antimalarial drug. To these medications, the long-stay traveler might add a broad-spectrum general-purpose antibiotic (levofloxacin or azithromycin), an antibacterial eye and skin ointment, and a topical antifungal cream. Regardless of the duration of travel, a first-aid kit containing such items as scissors, tweezers, and bandages should be considered. A practical approach to self-treatment of infections in the long-stay traveler who carries a once-daily dose of antibiotics (e.g., levofloxacin) is to use 3 tablets “below the waist” (bowel and bladder infections) and 6 tablets “above the waist” (skin and respiratory infections).
TRAVEL AND SPECIAL HOSTS
PREGNANCY AND TRAVEL
(See also Chap. 8) A woman’s medical history and itinerary, the quality of medical care at her destinations, and her degree of flexibility determine whether travel is wise during pregnancy. According to the American College of Obstetrics and Gynecology, the safest part of pregnancy in which to travel is between 18 and 24 weeks, when there is the least danger of spontaneous abortion or premature labor. Some obstetricians prefer that women stay within a few hundred miles of home after the 28th week of pregnancy in case problems arise. In general, however, healthy women may be advised that it is acceptable to travel.
Relative contraindications to international travel during pregnancy include a history of miscarriage, premature labor, incompetent cervix, or toxemia. General medical problems such as diabetes, heart failure, severe anemia, or a history of thromboembolic disease also should prompt the pregnant woman to postpone her travels. Finally, regions in which the pregnant woman and her fetus may be at excessive risk (e.g., those at high altitudes, those where live-virus vaccines are required, and those where multidrug-resistant malaria is endemic) are not ideal destinations during any trimester.
Malaria Malaria during pregnancy carries a significant risk of morbidity and death. Levels of parasitemia are highest and failure to clear the parasites after treatment is most frequent among primigravidae. Severe disease, with complications such as cerebral malaria, massive hemolysis, and renal failure, is especially likely in pregnancy. Fetal sequelae include spontaneous abortion, stillbirth, preterm delivery, and congenital infection. Chloroquine and mefloquine are considered to be safe in all trimesters.
Enteric Infections Pregnant travelers must be extremely cautious regarding their food and beverage intake. Dehydration due to travelers’ diarrhea can lead to inadequate placental blood flow. Infections such as toxoplasmosis, hepatitis E, and listeriosis also can cause serious sequelae in pregnancy.
The mainstay of therapy for travelers’ diarrhea is rehydration. Loperamide may be used if necessary. For self-treatment, azithromycin may be the best option. Although quinolones are increasingly being used safely during pregnancy and rifaximin is poorly absorbed from the gastrointestinal tract, these drugs are not approved for this indication.
Because of the serious problems encountered when infants are given local foods and beverages, women are strongly encouraged to breast-feed when traveling with a neonate. A nursing mother with travelers’ diarrhea should not stop breast-feeding but should increase her fluid intake.
Air Travel and High-Altitude Destinations Commercial air travel is not a risk to the healthy pregnant woman or to the fetus. The higher radiation levels reported at altitudes of >10,500 m (>35,000 ft) should pose no problem for the healthy pregnant traveler. Since each airline has a policy regarding pregnancy and flying, it is best to check with the specific carrier when booking reservations. Domestic air travel is usually permitted until the 36th week, whereas international air travel is generally curtailed after the 32nd week.
There are no known risks for pregnant women who travel to high-altitude destinations and stay for short periods. However, there are likewise no data on the safety of pregnant women at altitudes of >4500 m (15,000 ft).
THE HIV-INFECTED TRAVELER
(See also Chap. 226) The HIV-infected traveler is at special risk of serious infections due to a number of pathogens that may be more prevalent at travel destinations than at home. However, the degree of risk depends primarily on the state of the immune system at the time of travel. For persons whose CD4+ T cell counts are normal or >500/μL, data suggest no greater risk during travel than for persons without HIV infection. Individuals with AIDS (CD4+ T cell counts of <200/μL) and others who are symptomatic need special counseling and should visit a travel medicine practitioner before departure, especially when traveling to the developing world.
Several countries routinely deny entry to HIV-positive individuals for prolonged stay, even though these restrictions do not appear to decrease rates of transmission of the virus. In general, HIV testing is required for individuals who wish to stay abroad >3 months or who intend to work or study abroad. Some countries will accept an HIV serologic test done within 6 months of departure, whereas others will not accept a blood test done at any time in the traveler’s home country. Border officials often have the authority to make inquiries of individuals entering a country and to check the medications they are carrying. If antiretroviral drugs are identified, the person may be barred from entering the country. Information on testing requirements for specific countries is available from consular offices but is subject to frequent change.
Immunizations All of the HIV-infected traveler’s routine immunizations should be up to date (Chap. 148). The response to immunization may be impaired at CD4+ T cell counts of <200/μL and in some cases at even higher counts. Thus HIV-infected persons should be vaccinated as early as possible to ensure adequate immune responses. For patients receiving antiretroviral therapy, at least 3 months must elapse before regenerated CD4+ T cells can be considered fully functional; therefore, vaccination of these patients should be delayed. However, when the risk of illness is high or the sequelae of illness are serious, immunization is recommended. In certain circumstances, it may be prudent to check the adequacy of the serum antibody response before departure.
Because of the increased risk of infections due to Streptococcus pneumoniae and other bacterial pathogens that cause pneumonia after influenza, the conjugate pneumococcal vaccine (Prevnar 13) followed by the 23-valent polysaccharide vaccine (Pneumovax) as well as influenza vaccine should be administered. The estimated rates of response to influenza vaccine are >80% among persons with asymptomatic HIV infection and <50% among those with AIDS.
In general, live attenuated vaccines are contraindicated for persons with immune dysfunction. Because measles (rubeola) can be a severe or lethal infection in HIV-positive patients, these patients should receive the measles vaccine (or the combination MMR vaccine) unless the CD4+ T cell count is <200/μL. Between 18% and 58% of symptomatic HIV-infected vaccinees develop adequate measles antibody titers, and 50–100% of asymptomatic HIV-infected persons seroconvert.
It is recommended that the live yellow fever vaccine not be given to HIV-infected travelers. Although the potential adverse effects of a live vaccine in an HIV-infected individual are always a consideration, there appear to have been no reported cases of illness in those who have inadvertently received this vaccine. Nonetheless, if the CD4+ T cell count is <200/μL, an alternative itinerary that poses no risk of exposure to yellow fever is recommended. If the traveler is passing through or traveling to an area where the vaccine is required but the disease risk is low, a physician’s waiver should be issued.
A transient increase in HIV viremia (lasting days to weeks) has been demonstrated in HIV-infected individuals after immunization against influenza, pneumococcal infection, and tetanus (Chap. 226). At this point, however, no evidence indicates that this transient increase is detrimental.
Gastrointestinal Illness Decreased levels of gastric acid, abnormal gastrointestinal mucosal immunity, other complications of HIV infection, and medications taken by HIV-infected patients make travelers’ diarrhea especially problematic in these individuals. Travelers’ diarrhea is likely to occur more frequently, to be more severe, to be accompanied by bacteremia, and to be more difficult to treat. Cryptosporidium, Isospora belli, and Microsporidium infections, although uncommon, are associated with increased morbidity and mortality rates in AIDS patients.
The HIV-infected traveler must be careful to consume only appropriately prepared foods and beverages and may benefit from antibiotic prophylaxis for travelers’ diarrhea. Sulfonamides (as used to prevent pneumocystosis) are ineffective because of widespread resistance.
Other Travel-Related Infections Data are lacking on the severity of many vector-borne diseases in HIV-infected individuals. Malaria is especially severe in asplenic persons and in those with AIDS. The HIV load doubles during malaria, with subsidence in ~8–9 weeks; the significance of this increase in viral load is unknown.
Visceral leishmaniasis (Chap. 251) has been reported in numerous HIV-infected travelers. Diagnosis may be difficult, given that splenomegaly and hyperglobulinemia are often lacking and serologic results are frequently negative. Sandfly bites may be prevented by evening use of insect repellents.
Certain respiratory illnesses, such as histoplasmosis and coccidioidomycosis, cause greater morbidity and mortality among patients with AIDS. Although tuberculosis is common among HIV-infected persons (especially in developing countries), its acquisition by the short-term HIV-infected traveler has not been reported as a major problem. From a prospective study, it is estimated that for travelers not engaged in health care the risk of tuberculosis infection is ~3% per year of travel.
Medications Adverse events due to medications and drug interactions are common and raise complex issues for HIV-infected persons. Rates of cutaneous reaction (e.g., increased cutaneous sensitivity to sulfonamides) are unusually high among patients with AIDS. Since zidovudine is metabolized by hepatic glucuronidation, inhibitors of this process may elevate serum levels of the drug. Concomitant administration of the antimalarial drug mefloquine and the antiretroviral agent ritonavir may result in decreased plasma levels of ritonavir; mefloquine may also interact with many of the other protease inhibitors. In contrast, no significant influence of concomitant mefloquine administration on plasma levels of indinavir or nelfinavir was detected in two HIV-infected travelers. Serum levels of mefloquine may be lowered with the use of efavirenz or nevirapine. There are also potential interactions between atovaquone-proguanil (Malarone) and many of the protease inhibitors as well as between Malarone and the nonnucleoside reverse transcriptase inhibitors (NNRTIs). Because of the increase in antiretroviral agents and the lack of accumulated data on their interactions with antimalarial agents, decisions about malaria chemoprophylaxis continue to be difficult; with a short duration of travel, an interaction may be inconsequential. However, doxycycline appears to have no clinically significant interactions with either the protease inhibitors or the NNRTIs. With regard to malaria treatment, a great hypothetical concern is that the antimalarial drugs lumefantrine (combined with artemisinin in Coartem) and halofantrine may interact with HIV protease inhibitors and NNRTIs since drugs in the latter two categories are known to be potent inhibitors of cytochrome P450. In keeping current with antiretroviral drug interactions, a website from the University of Liverpool (www.hiv-druginteractions.org) is helpful.
CHRONIC ILLNESS, DISABILITY, AND TRAVEL
Chronic health problems need not prevent travel, but special measures can make the journey safer and more comfortable.
Heart Disease Cardiovascular events are the main cause of deaths among travelers and of in-flight emergencies on commercial aircraft. Extra supplies of all medications should be kept in carry-on luggage, along with a copy of a recent electrocardiogram and the name and telephone number of the traveler’s physician at home. Pacemakers are not affected by airport security devices, although electronic telephone checks of pacemaker function cannot be transmitted by international satellites. Travelers with electronic defibrillators should carry a note to that effect and ask for hand screening. A traveler may benefit from supplemental oxygen; since oxygen delivery systems are not standard, supplementary oxygen should be ordered by the traveler’s physician well before flight time. Travelers may benefit from aisle seating and should walk, perform stretching and flexing exercises, consider wearing support hose, and remain hydrated during the flight to prevent venous thrombosis and pulmonary embolism.
Chronic Lung Disease Chronic obstructive pulmonary disease is one of the most common diagnoses in patients who require emergency-department evaluation for symptoms occurring during airline flights. The best predictor of the development of in-flight problems is the sea-level PaO2. A PaO2 of at least 72 mmHg corresponds to an in-flight arterial PaO2 of ~55 mmHg when the cabin is pressurized to 2500 m (8000 ft). If the traveler’s baseline PaO2 is <72 mmHg, the provision of supplemental oxygen should be considered. Contraindications to flight include active bronchospasm, lower respiratory infection, lower-limb deep-vein phlebitis, pulmonary hypertension, and recent thoracic surgery (within the preceding 3 weeks) or pneumothorax. Decreased outdoor activity at the destination should be considered if air pollution is excessive.
Diabetes Mellitus Alterations in glucose control and changes in insulin requirements are common problems among patients with diabetes who travel. Changes in time zone, in the amount and timing of food intake, and in physical activity demand vigilant assessment of metabolic control. Because of the risk of foot ulcers, travelers should wear closed footwear that has been proven to be comfortable. The traveler with diabetes should pack medication (including a bottle of regular insulin for emergencies), insulin syringes and needles, equipment and supplies for glucose monitoring, and snacks in carry-on luggage. Insulin is stable for ~3 months at room temperature but should be kept as cool as possible. The name and telephone number of the home physician and a card and bracelet listing the patient’s medical problems and the type and dose of insulin used should accompany the traveler. In order to facilitate international border crossings, travelers should carry a physician’s letter authorizing the carriage of needles and syringes. In traveling eastward (e.g., from the United States to Europe), the morning insulin dose on arrival may need to be decreased. The blood glucose can then be checked during the day to determine whether additional insulin is required. For flights westward, with lengthening of the day, an additional dose of regular insulin may be required.
Other Special Groups Other groups for whom special travel measures are encouraged include patients undergoing dialysis, those with transplants, and those with other disabilities. Up to 13% of travelers have some disability, but few advocacy groups and tour companies dedicate themselves to this growing population. Medication interactions are a source of serious concern for these travelers, and appropriate medical information should be carried, along with the home physician’s name and telephone number. Some travelers taking glucocorticoids carry stress doses in case they become ill. Immunization of these immunocompromised travelers may result in less than adequate protection. Thus the traveler and the physician must carefully consider which destinations are appropriate.
TRAVEL HEALTH INSURANCE
Today, more elderly or chronically ill individuals travel and more of these individuals journey to remote locations and enjoy adventurous activities. Illness or injury abroad is not uncommon and is best considered before the journey. Persons who develop health problems abroad may incur enormous out-of-pocket expenses. Thus prospective travelers should consider purchasing additional travel health insurance and should check with their health insurance company regarding whether they have coverage for illness or injury overseas. Unfortunately, many insurance companies will not cover pre-existing illness if it is the reason for trip cancellation or illness abroad. Most countries do not accept routine health insurance from other countries unless there is a special traveler supplement. In most circumstances, travelers are asked to pay in cash for services rendered on an emergency basis, whether in a physician’s office, in an emergency or urgent care center, or even in a hospital. There are several types of travel insurance. It is wise to purchase trip cancellation insurance, especially, for example, if the traveler has an underlying chronic illness and may need to cancel a trip due to an exacerbation of disease. Travel health insurance will cover expenses in the event that medical care abroad is needed. Evacuation insurance will cover medical evacuation, usually to a medical center in another location where it is deemed that the care is similar to that available in the traveler’s home country. The cost of medical evacuation can easily exceed $100,000 US. There are a number of travel insurance providers, and it is very important to read the fine print carefully and to determine exactly what each company provides, thereby ensuring an appropriate fit for the individual’s particular circumstances. The U.S. Department of State website lists travel health insurance companies (http://travel.state.gov/travel/tips/emergencies/emergencies_5981.html).
MEDICAL TOURISM
Travel for the purpose of obtaining health care abroad has received a great deal of attention in the medical literature and the media. According to the annual U.S. Department of Commerce In-Flight Survey, there were ~500,000 overseas trips during 2006 in which health treatment was at least one purpose of travel. Lower cost is usually cited as the motivation for this type of tourism, and an entire industry has flourished as a result of this phenomenon. However, the quality of facilities, assistance services, and care is neither uniform nor regulated; thus, in most instances, responsibility for assessing the suitability of an individual program or facility lies solely with the traveler. Persons considering this option must recognize that they are almost always at a disadvantage when being treated in a foreign country, particularly if there are complications. Concerns to be addressed include the quality of the health care facility and its staff; language and cultural differences that may impede accurate interpretation of both verbal and nonverbal communication; religious and ethical differences that may be encountered over issues such as efforts to preserve life and limb or the provision of care for the terminally ill; lack of familiarity with the local medical system; limited access of the care provider to the patient’s medical history; the use of unfamiliar drugs and medicines; the relative difficulty of arranging follow-up care back in the United States; and the possibility that such follow-up care may be fraught with problems should there be complications. If serious issues arise, legal recourse may be difficult or impossible. Patients planning to travel abroad to obtain health care, particularly when surgery is involved, should be immunized for hepatitis B and should consider having baseline hepatitis C and HIV tests preoperatively. Prevalence rates of hepatitis B and C and HIV infection vary considerably around the world and are generally higher in developing regions than in the United States and Western Europe. The latest information available on the safety of the blood supply outside the United States is the World Health Organization’s Global Database on Blood Safety based on data from 2011 (www.who.int/bloodsafety/global_database/en). Persons researching the accreditation status of overseas facilities should note that, although these facilities may be part of a chain, they are surveyed and accredited individually. Accreditation resources include (1) the Joint Commission International (www.jointcommissioninternational.org), (2) the Australian Council for Healthcare Standards International (www.achs.org.au/achs-international/), and (3) the Canadian Council on Health Services (www.cchsa.ca). The American Medical Association also offers guidelines for medical tourism (www.ama-assn.org/ama1/pub/upload/mm/31/medicaltourism.pdf).
PROBLEMS AFTER RETURN
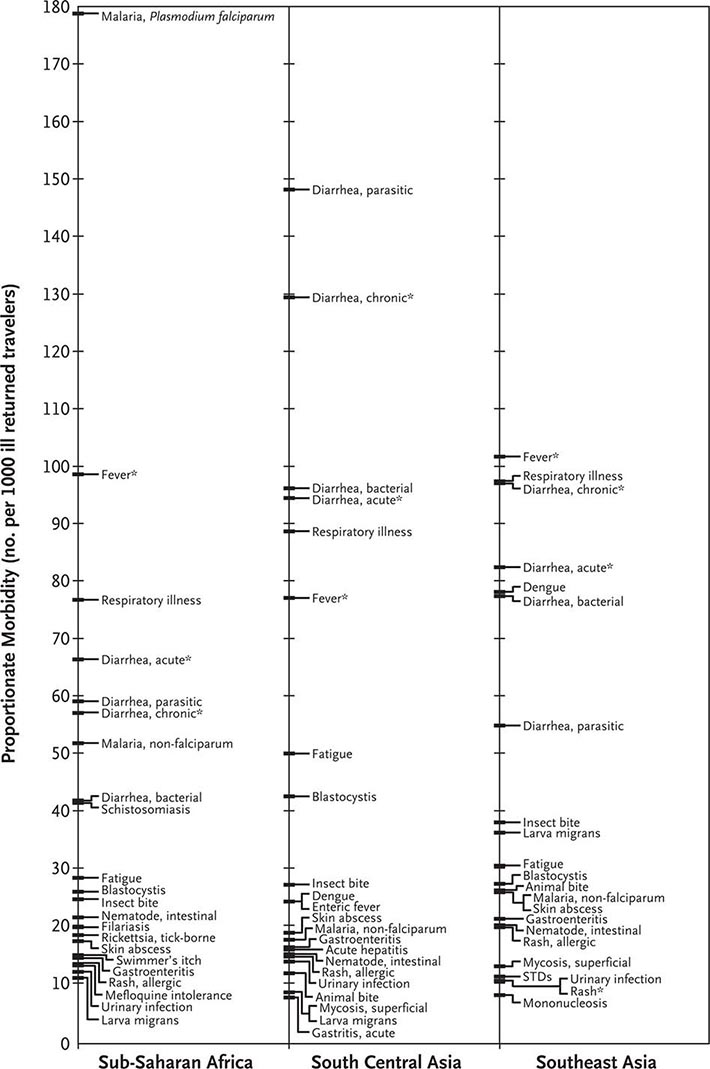
The most common medical problems encountered by travelers after their return home are diarrhea, fever, respiratory illnesses, and skin diseases (Fig. 149-2). Frequently ignored problems are fatigue and emotional stress, especially in long-stay travelers. The approach to diagnosis requires some knowledge of geographic medicine, in particular the epidemiology and clinical presentation of infectious disorders. A geographic history should focus on the traveler’s exact itinerary, including dates of arrival and departure; exposure history (food indiscretions, drinking-water sources, freshwater contact, sexual activity, animal contact, insect bites); location and style of travel (urban vs. rural, first-class hotel accommodation vs. camping); immunization history; and use of antimalarial chemosuppression. Recently, some travelers who have been hospitalized abroad have been shown on return to be colonized with multidrug-resistant bacteria such as Enterobacteriaceae producing extended-spectrum β-lactamases and bacteria producing NDM-1 (New Delhi metallo-β-lactamase 1).
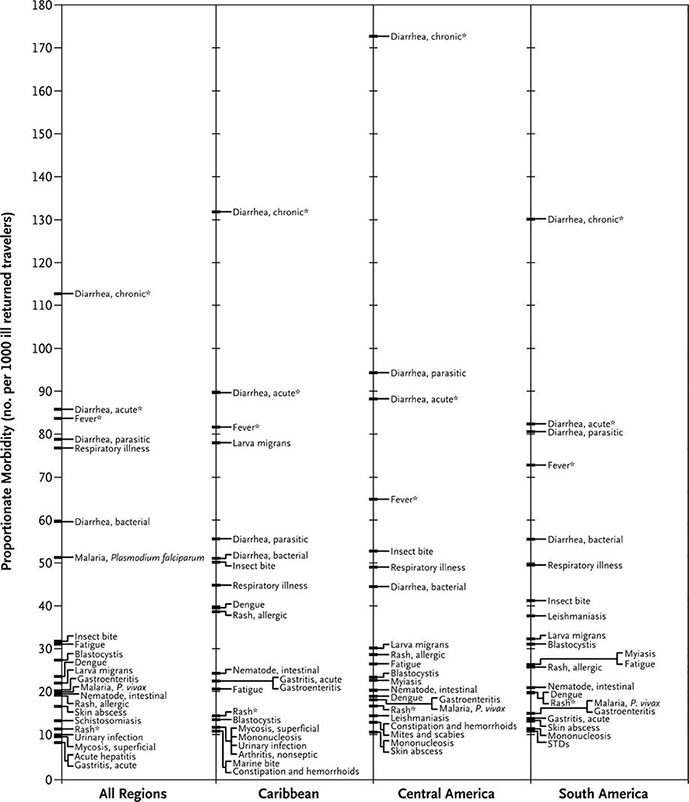
FIGURE 149-2 Proportionate morbidity among ill travelers returning from the developing world, according to region of travel. The proportions (not incidence rates) are shown for each of the top 22 specific diagnoses among all ill returned travelers within each region. STDs, sexually transmitted diseases. Asterisks indicate syndromic diagnoses for which specific etiologies could not be assigned. (Reprinted from DO Freedman et al: N Engl J Med 354:119, 2006.)
DIARRHEA
See “Prevention of Gastrointestinal Illness,” above.
FEVER
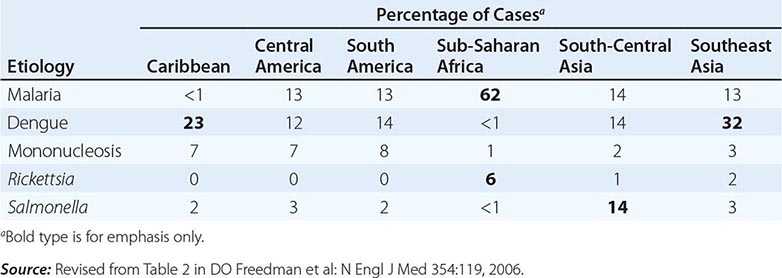
Fever in a traveler who has returned from a malarious area should be considered a medical emergency because death from P. falciparum malaria can follow an illness of only several days’ duration. Although “fever from the tropics” does not always have a tropical cause, malaria should be the first diagnosis considered. The risk of P. falciparum malaria is highest among travelers returning from Africa or Oceania and among those who become symptomatic within the first 2 months after return. Other important causes of fever after travel include viral hepatitis (A and E), typhoid and paratyphoid fever, bacterial enteritis, arboviral infections (e.g., dengue fever), rickettsial infections (including tick typhus, scrub typhus, and Q fever), and—in rare instances—leptospirosis, acute HIV infection, and amebic liver abscess. A cooperative study by GeoSentinel (an emerging infectious disease surveillance group established by the CDC and the International Society of Travel Medicine) showed that, among 3907 febrile returned travelers, malaria was acquired most often in Africa, dengue in Southeast Asia and the Caribbean, typhoid fever in southern Asia, and rickettsial infections (tick typhus) in South Africa (Table 149-3). Outbreaks of dengue, previously considered to be very rare in Africa, have been documented recently in Angola, Kenya, and Tanzania. However, in at least 25% of cases, no etiology of the fever can be found and it resolves spontaneously. Clinicians should keep in mind that no present-day antimalarial agent guarantees protection from malaria and that some immunizations (notably, that against typhoid fever) are only partially protective.
|
ETIOLOGY AND GEOGRAPHIC DISTRIBUTION OF SYSTEMIC FEBRILE ILLNESS IN RETURNED TRAVELERS (N = 3907) |
When no specific diagnosis is forthcoming, the following investigations, where applicable, are suggested: complete blood count, liver function tests, thick/thin blood films or rapid diagnostic testing for malaria (repeated several times if necessary), urinalysis, urine and blood cultures (repeated once), chest x-ray, and collection of an acute-phase serum sample to be held for subsequent examination along with a paired convalescent-phase serum sample.
SKIN DISEASES
Pyodermas, sunburn, insect bites, skin ulcers, and cutaneous larva migrans are the most common skin conditions affecting travelers after their return home. In those with persistent skin ulcers, a diagnosis of cutaneous leishmaniasis, mycobacterial infection, or fungal infection should be considered. Careful, complete inspection of the skin is important in detecting the rickettsial eschar in a febrile patient or the central breathing hole in a “boil” due to myiasis.
EMERGING INFECTIOUS DISEASES
In recent years, travel and commerce have fostered the worldwide spread of HIV infection, led to the reemergence of cholera as a global health threat, and created considerable fear about the possible spread of novel respiratory diseases, including those caused by influenza viruses (H5N1, H1N1, and H7N9). For travelers, there are more common, everyday concerns. One of the largest outbreaks of dengue fever ever documented is now raging in Latin America and Southeast Asia; chikungunya virus has spread rapidly from Africa to southern Asia, southern Europe, and, for the first time in the Western Hemisphere, the Caribbean; schistosomiasis is being described in previously unaffected lakes in Africa; and antibiotic-resistant strains of sexually transmitted and enteric pathogens are emerging at an alarming rate in the developing world. In addition, concerns have been raised about the potential for bioterrorism involving not only standard strains of unusual agents but mutant strains as well.
CONCLUSIONS
The growth of global travel and migration now demand that the clinician become as familiar as possible with travel medicine. Practitioners may choose either to refer their patients to a travel clinic before departure or to acquire knowledge that enables them to provide pretravel counseling and to prescribe appropriate vaccinations and chemoprophylaxis. It is equally important for physicians seeing ill returned travelers to be familiar with common post-travel syndromes and diseases, particularly those that may have been acquired in the developing world, and to identify other physicians who can assist with complex post-travel illnesses. The CDC publishes a biennial text, Health Information for International Travel (accessed through their website at www.cdc.gov/travel) that provides pretravel health recommendations. The International Society of Travel Medicine (www.istm.org) publishes a list of travel clinics, and the American Society of Tropical Medicine and Hygiene (www.astmh.org) publishes a list of clinical tropical medicine specialists.
As Nobel Laureate Dr. Joshua Lederberg pointed out, “The microbe that felled one child in a distant continent yesterday can reach yours today and seed a global pandemic tomorrow.” The vigilant clinician understands that the importance of a thorough travel history cannot be overemphasized.
150e |
Laboratory Diagnosis of Infectious Diseases |
The laboratory diagnosis of infection requires the demonstration—either direct or indirect—of viral, bacterial, fungal, or parasitic agents in tissues, fluids, or excreta of the host. Clinical microbiology laboratories are responsible for processing these specimens and also for determining the antibiotic susceptibility of bacterial and fungal pathogens. Traditionally, detection of pathogenic agents has relied largely on either the microscopic visualization of pathogens in clinical material or the growth of microorganisms in the laboratory. Identification generally is based on phenotypic characteristics such as fermentation profiles for bacteria, cytopathic effects in tissue culture for viral agents, and microscopic morphology for fungi and parasites. These techniques are reliable but are often time-consuming. Increasingly, the use of nucleic acid probes is becoming a standard method for detection, quantitation, and/or identification in the clinical microbiology laboratory, gradually replacing phenotypic characterization and microscopic visualization methods. This chapter discusses general concepts of diagnostic testing, with an emphasis on detection of bacteria. Detection of viral, fungal, and parasitic pathogens is discussed in greater detail in separate chapters (see Chaps. 214e, 235, and 245e, respectively).
DETECTION METHODS
Reappraisal of the methods employed in the clinical microbiology laboratory has led to the development of strategies for detection of pathogenic agents through nonvisual biologic signal detection systems. A biologic signal is generated by detection of a material that can be reproducibly differentiated from other substances present in the sample. Key issues in the use of a biologic signal are distinguishing it from background noise and translating it into meaningful information. Examples of useful materials for detection of biologic signals applicable to clinical microbiology include structural components of bacteria, fungi, and viruses; specific antigens; metabolic end products; unique DNA or RNA base sequences; enzymes; toxins or other proteins; and surface polysaccharides.
A detector is used to sense a signal and discriminate between that signal and background noise. Detection systems range from the trained eyes of a technologist assessing morphologic variations to electronic instruments such as gas-liquid chromatographs or mass spectrometers. The sensitivity with which signals can be detected varies widely. It is essential to use a detection system that discerns small amounts of signal even when biologic background noise is present—i.e., that is both sensitive and specific. Common detection systems include immunofluorescence; chemiluminescence for DNA/RNA probes; flame ionization detection of short- or long-chain fatty acids; and detection of substrate utilization or end-product formation as color changes, of enzyme activity as a change in light absorbance, of turbidity changes as a measure of growth, of cytopathic effects in cell lines, and of particle agglutination as a measure of antigen presence.
Amplification enhances the sensitivity with which weak signals can be detected. The most common microbiologic amplification technique is growth of a single bacterium into a discrete, visible colony on an agar plate or into a suspension containing many identical organisms. The advantage of growth as an amplification method is that it requires only an appropriate growth medium; the disadvantage is the amount of time required. More rapid amplification of biologic signals can be achieved with techniques such as polymerase chain reaction (PCR), ligase chain reaction (LCR), and transcription-mediated amplification (TMA), all of which target the pathogen’s DNA/RNA; enzyme immunoassays (EIAs) for antigens and antibodies; electronic amplification (for gas-liquid chromatography assays); antibody capture methods (for concentration and/or separation); and selective filtration or centrifugation.
DIRECT DETECTION
Direct detection refers to detection of pathogens without the use of culture. Molecular methods of direct detection are discussed below.
MICROSCOPY AND STAINING
The field of microbiology was defined largely by the development and use of the microscope. The examination of specimens by microscopic methods rapidly provides useful diagnostic information. Staining techniques permit organisms to be seen more clearly.
The simplest method for microscopic evaluation is the wet mount, which is used, for example, to examine cerebrospinal fluid (CSF) for the presence of Cryptococcus neoformans, with India ink as a background against which to visualize large-capsuled yeast cells. Wet mounts with dark-field illumination also are used to detect spirochetes in genital lesions and Borrelia or Leptospira in blood. Skin scrapings and hair samples can be examined with the use of either 10% KOH wet-mount preparations or the calcofluor white method and ultraviolet illumination to detect fungal elements as fluorescing structures. Staining of wet mounts—e.g., with lactophenol cotton blue stain for fungal elements—often is used for morphologic identification.
Bacteria are difficult to see by light microscopy unless they are stained. Although simple one-step stains can be used, differential stains are more common.
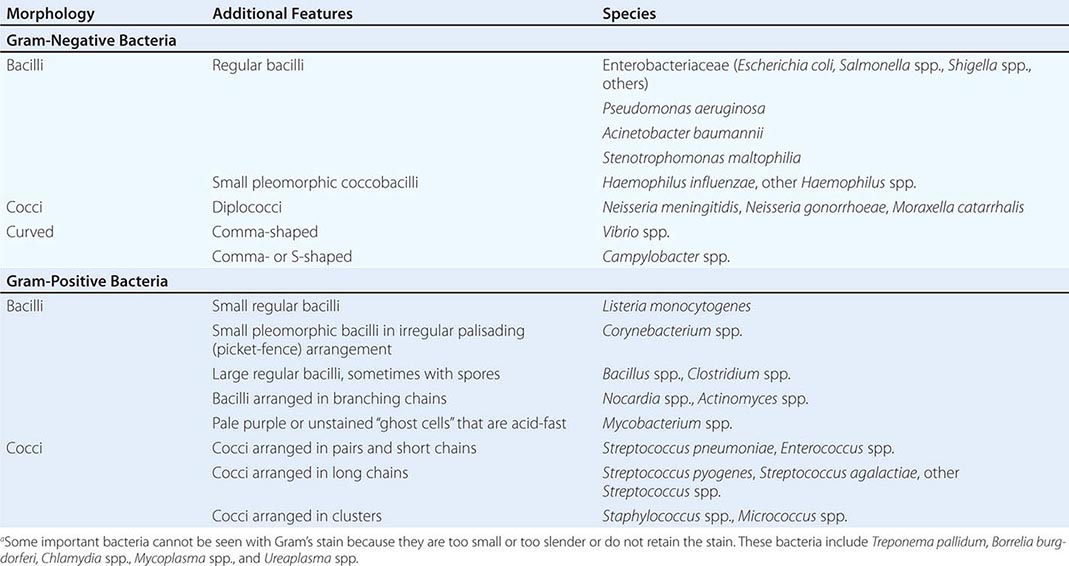
Gram’s Stain Gram’s stain differentiates between organisms with thick peptidoglycan cell walls (gram-positive) and those with thin peptidoglycan cell walls and outer membranes that can be dissolved with alcohol or acetone (gram-negative). Morphology and Gram’s stain characteristics often can be used to categorize stained organisms into groups such as streptococci, staphylococci, and clostridia (Table 150e-1). Gram’s stain is particularly useful for examining sputum for polymorphonuclear leukocytes (PMNs) and bacteria. Sputum specimens from immunocompetent patients with ≥25 PMNs and <10 epithelial cells per low-power field often provide clinically useful information. However, the presence in “sputum” samples of >10 epithelial cells per low-power field and of multiple bacterial types suggests contamination with oral microflora. Despite the difficulty of discriminating between normal microflora and pathogens, Gram’s stain may prove useful for specimens from areas with a large resident microflora if a useful biologic marker (signal) is available. Gram’s staining of vaginal swab specimens can be used to detect epithelial cells covered with gram-positive bacteria in the absence of lactobacilli and the presence of gram-negative rods—a scenario regarded as a sign of bacterial vaginosis. Similarly, examination of stained stool specimens for leukocytes is useful as a screening procedure before testing for Clostridium difficile toxin or other enteric pathogens.
|
INTERPRETATION OF GRAM’S STAINa |
The examination of samples from normally sterile body sites (e.g., CSF or joint, pleural, or peritoneal fluid) with Gram’s stain is useful for determining whether bacteria and/or PMNs are present. The sensitivity is such that >104 bacteria/mL should be detected. Centrifugation often is performed before staining to concentrate specimens thought to contain low numbers of organisms. This simple method is particularly useful for examination of CSF for bacteria and white blood cells or examination of sputum for mycobacteria.
Acid-Fast Stain The acid-fast stain identifies acid-fast bacteria (AFB; mycobacteria) by their retention of carbol fuchsin dye after acid/organic solvent disruption. Modifications of this procedure allow the differentiation of Actinomyces from Nocardia or other weakly (or partially) acid-fast organisms. The acid-fast stain is applied to sputum, other fluids, and tissue samples when Mycobacterium species are suspected. Because few AFB may be present in an entire smear, even when the specimen has been concentrated by centrifugation, identification of the pink/red AFB against the blue background of the counterstain requires a trained eye. An alternative method for detection of AFB is the auramine-rhodamine fluorescent dye stain.
Fluorochrome Stains Fluorochrome stains such as acridine orange are used to identify white blood cells, yeasts, and bacteria in body fluids. Capsular, flagellar, and spore stains are used for identification or demonstration of characteristic structures.
Immunofluorescent Stains The direct immunofluorescent antibody technique uses antibody coupled to a fluorescent compound (e.g., fluorescein) and directed at a specific antigenic target to visualize organisms. When samples are examined under appropriate conditions, the fluorescing compound absorbs ultraviolet light and re-emits light at a higher wavelength that is visible to the human eye. In the indirect immunofluorescent antibody technique, an unlabeled (target) antibody binds a specific antigen. The specimen is then stained with a fluorochrome-labeled antibody directed at the target antibody. Because each unlabeled target antibody attached to the appropriate antigen has multiple sites for attachment of the second antibody, the visual signal is amplified. Immunofluorescence is used to detect viral antigens (e.g., cytomegalovirus, herpes simplex virus, and respiratory viruses) within cultured cells or clinical specimens as well as many difficult-to-grow bacterial agents (e.g., Legionella pneumophila) in clinical specimens.
MACROSCOPIC ANTIGEN DETECTION
Latex agglutination assays and EIAs are rapid and inexpensive methods for identifying organisms, extracellular toxins, and viral agents by means of protein and polysaccharide antigens. Such assays may be performed directly on clinical samples or after growth of organisms on agar plates or in viral cell cultures. Antibodies coupled to a reporter (such as latex particles or an enzyme) are used for detection of antibody–antigen binding reactions.
Direct agglutination of bacterial cells with specific antibody is simple but relatively insensitive; latex agglutination and EIAs are more sensitive. Some cell-associated antigens, such as capsular polysaccharides and lipopolysaccharides, can be detected by agglutination of a suspension of bacterial cells when antibody is added; this method is useful for typing of the somatic antigens of Shigella and Salmonella. EIAs employ antibodies coupled to an enzyme, and an antigen–antibody reaction results in the conversion of a colorless substrate to a colored product. Most of these assays provide information about whether antigen is present but do not quantify the antigen. EIAs are also useful for detecting bacterial toxins—e.g., toxins produced by Shiga toxin–producing Escherichia coli.
Rapid and simple immunoassays for antigens of group A Streptococcus, influenza virus, and respiratory syncytial virus can be used in the clinical setting without a specialized diagnostic laboratory. Such tests usually are reasonably specific but may have only modest sensitivity.
DETECTION OF PATHOGENIC AGENTS BY SEROLOGIC METHODS
Measurement of serum antibody provides an indirect marker for past or current infection with a specific viral agent or other pathogens, including Brucella, Legionella, Rickettsia, and Helicobacter pylori. Serologic methods can be used to determine whether an individual has protective antibody levels or is infected by a specific pathogen. Determination of an antibody level as a measure of current immunity is important in the case of viral agents for which there are vaccines, such as rubella virus and varicella-zoster virus; assays for this purpose normally use one or two dilutions of serum for a qualitative determination of protective antibody levels. Quantitative serologic assays to detect increases in antibody titers most often employ paired serum samples obtained at the onset of illness and 10–14 days later (i.e., acute- and convalescent-phase samples). Since the incubation period before symptoms are noted may be long enough for an antibody response to occur, the demonstration of acute-phase antibody alone is often insufficient to establish the diagnosis of active infection as opposed to past exposure. A fourfold increase in total antibody titer between the acute- and convalescent-phase samples is regarded as evidence for active infection. In addition, IgM may be useful as a measure of an early, acute-phase antibody response. For certain viral agents, such as Epstein-Barr virus, the antibodies produced may be directed at different antigens during different phases of the infection. For this reason, most laboratories test for antibody directed at both viral capsid antigens and antigens associated with recently infected host cells to determine the stage of infection.
DETECTION OF PATHOGENIC AGENTS BY CULTURE
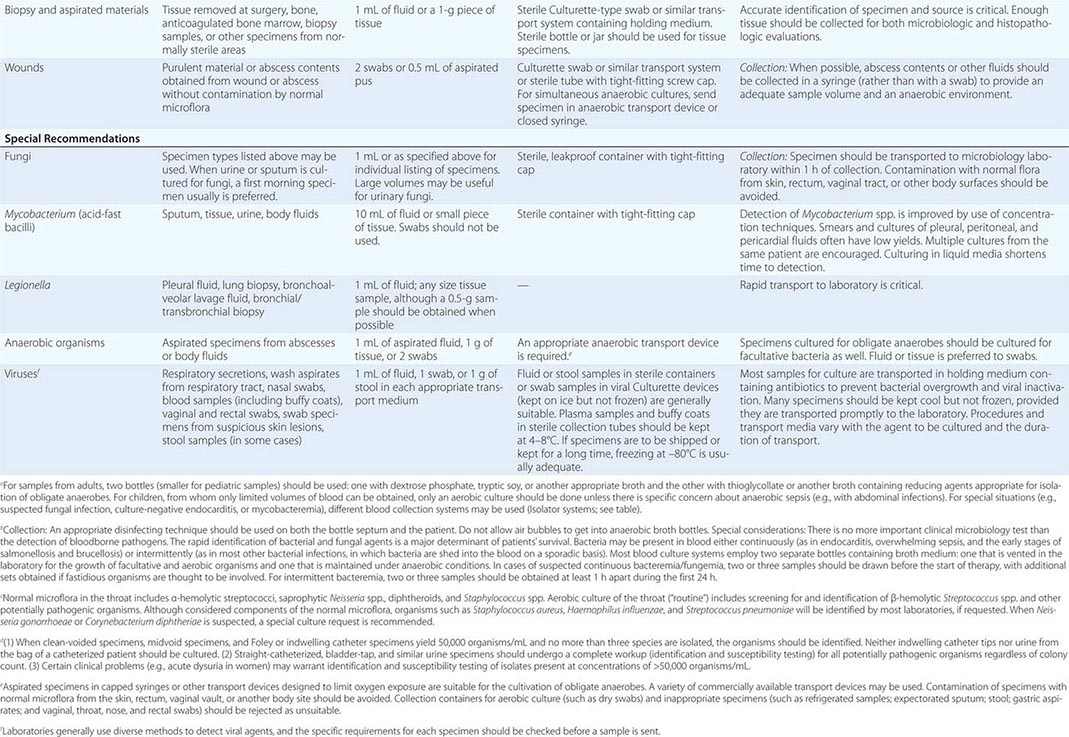
SPECIMEN COLLECTION AND TRANSPORT
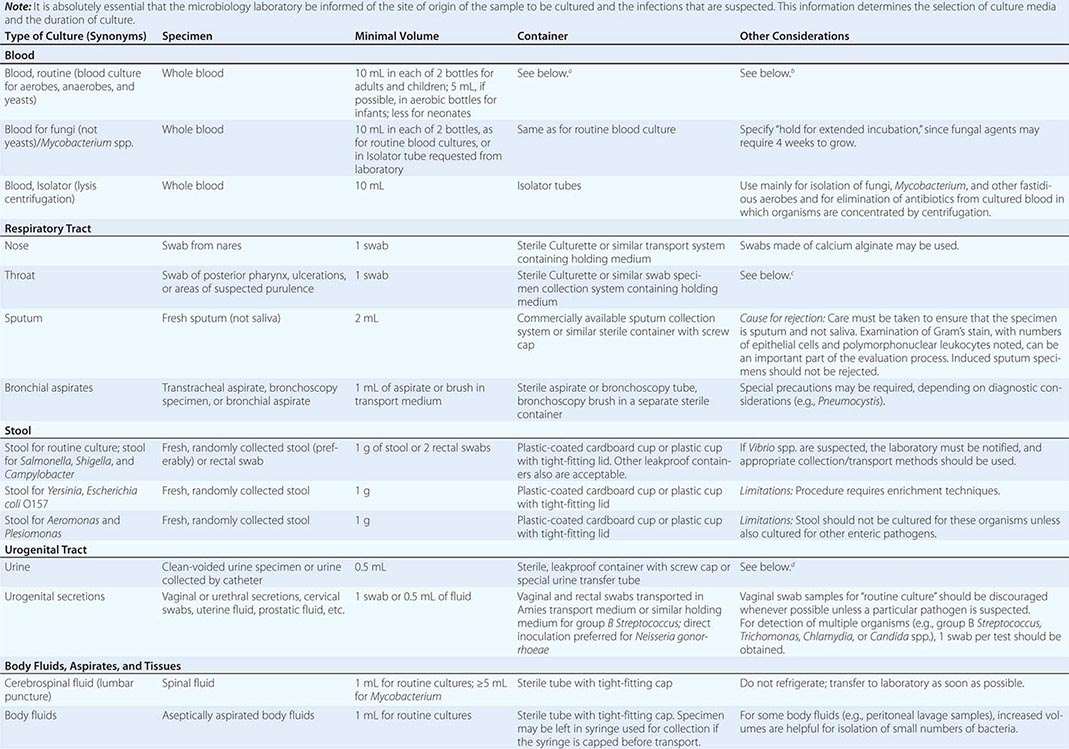
To culture bacterial, fungal, or viral pathogens, an appropriate sample must be placed into the proper medium for growth. The success of efforts to identify a specific pathogen often depends on the collection and transport process coupled to a laboratory-processing algorithm suitable for the specific sample/agent. In some instances, it is better for specimens to be plated at the time of collection rather than first being transported to the laboratory (e.g., urethral swabs being cultured for Neisseria gonorrhoeae). In general, the more rapidly a specimen is plated onto appropriate media, the better the chance is for isolating bacterial pathogens. Deep tissue or fluid (pus) samples are more likely to give useful culture results than are superficial swab specimens. Table 150e-2 lists procedures for collection and transport of common specimens. Because there are many pathogen-specific paradigms for these procedures, it is important to seek advice from the microbiology laboratory when in doubt about a particular situation.
|
INSTRUCTIONS FOR COLLECTION AND TRANSPORT OF SPECIMENS FOR CULTURE |
ISOLATION OF BACTERIAL PATHOGENS
Isolation of pathogens from clinical material relies on the use of artificial media that support bacterial growth. Such media are composed of agar, nutrients, and sometimes substances that inhibit the growth of other bacteria. Broth is employed for growth of organisms from specimens with few bacteria, such as peritoneal dialysis fluid or CSF, or from samples in which anaerobes or other fastidious organisms may be present. Broths that allow the growth of small numbers of organisms may be subcultured onto solid medium once growth is detected. The use of liquid medium for all specimens is not worthwhile.
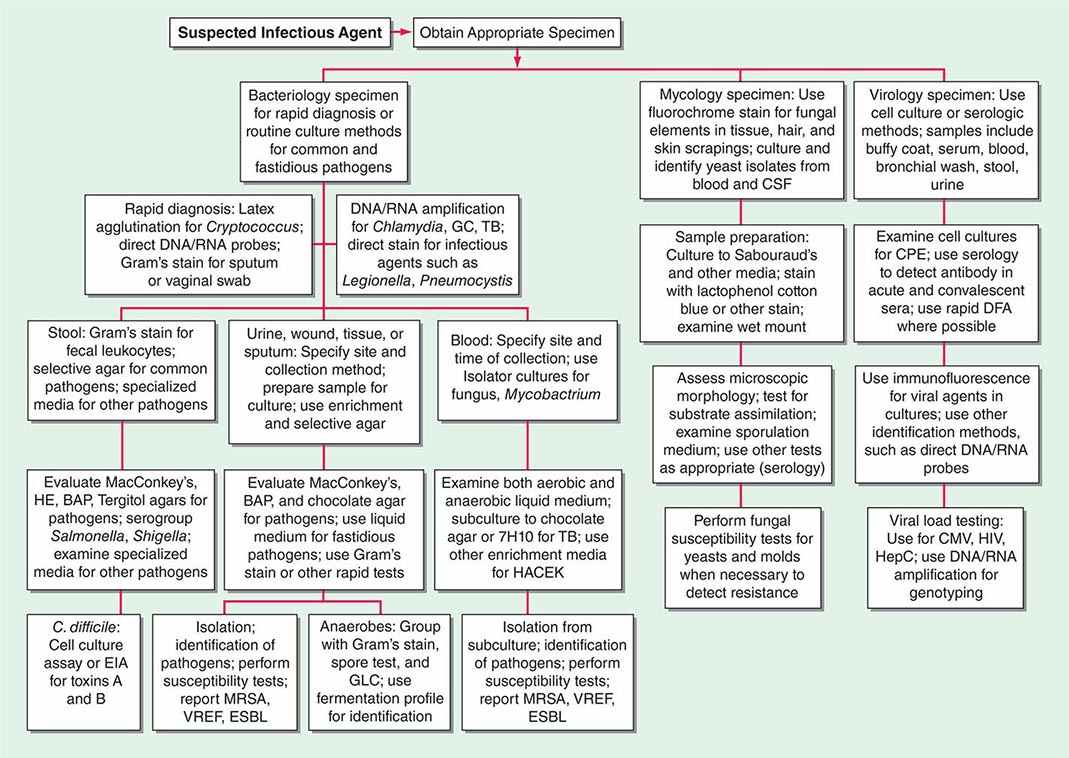
Two basic strategies are used to isolate pathogenic bacteria. The first is to employ enriched media that support the growth of any bacteria that may be present at a site that is normally sterile, such as blood or CSF. The second strategy is to use selective media to isolate specific bacterial species from samples that contain many bacteria under normal conditions (e.g., stool or genital tract secretions). Antimicrobial agents or other substances are incorporated into the agar medium to inhibit growth of all but the bacteria of interest. After incubation, organisms that grow on such media are characterized further to determine whether they are pathogens (Fig. 150e-1).
FIGURE 150e-1 Common specimen-processing algorithms used in clinical microbiology laboratories. BAP, blood agar plate; CMV, cytomegalovirus; CPE, cytopathic effects; CSF, cerebrospinal fluid; DFA, direct fluorescent antibody; EIA, enzyme immunoassay; ESBL, extended-spectrum β-lactamase; GBS, group B Streptococcus; GC, Neisseria gonorrhoeae; GLC, gas-liquid chromatography; HACEK, Haemophilus aphrophilus/parainfluenzae/paraphrophilus, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae; HE, Hektoen enteric medium; HepC, hepatitis C virus; HIV, human immunodeficiency virus; MRSA, methicillin-resistant Staphylococcus aureus; TB, Mycobacterium tuberculosis; VREF, vancomycin-resistant Enterococcus faecium.
BLOOD CULTURE
The detection of microbial pathogens in blood is difficult because the number of organisms present in the sample is often low and the organisms’ integrity and ability to replicate may be damaged by humoral defense mechanisms or antimicrobial agents. Automated blood culture systems detect production of gas (mainly CO2) by bacteria or yeasts growing in broth in a blood culture bottle. Because the bottles are monitored frequently, a positive culture often is detected more rapidly than by manual techniques, and important information, including the results of Gram’s stain and preliminary susceptibility assays, can be obtained sooner. Several factors affect the yield of blood culture from bacteremic patients. Increasing the volume of blood tested increases the chance of a positive culture. For example, a sample-size increase from 10 to 20 mL of blood increases the proportion of positive cultures by ~30%, although this effect is less pronounced in patients with bacterial endocarditis. Obtaining multiple samples for culture (up to three per 24-h period) also increases the chance of detecting a bacterial pathogen. Prolonged culture and blind subculture for detection of most fastidious bacteria (e.g., HACEK organisms) are not needed with automated blood culture systems.
Automated systems also have been applied to the detection of microbial growth from specimens other than blood, such as peritoneal and other normally sterile fluids. Mycobacterium species can be detected in certain automated systems if appropriate liquid media are used for culture. Although automated blood culture systems are more sensitive than lysis-centrifugation methods (e.g., Isolator) for yeasts and most bacteria, lysis-centrifugation culture is recommended for filamentous fungi, Histoplasma capsulatum, and some fastidious bacteria (Legionella and Bartonella).
IDENTIFICATION METHODS
Once bacteria are isolated, characteristics that are readily detectable after growth on agar media (colony size, color, hemolytic reactions, odor, microscopic appearance) may suggest a species, but definitive identification requires additional tests. Identification methods include classic biochemical phenotyping, which is still the most common approach, as well as more sophisticated methods such as mass spectrometry, gas chromatography, and nucleic acid tests (see below).
Biochemical Phenotyping Classic biochemical identification of bacteria entails tests for protein or carbohydrate antigens, the production of specific enzymes, the ability to metabolize specific substrates and carbon sources (such as carbohydrates), or the production of certain metabolites. Rapid versions of some of these tests are available, and many common organisms can be identified on the first day of growth. Other organisms, particularly gram-negative bacteria, require more extensive testing, either manual or automated.
Automated systems allow rapid phenotypic identification of bacterial pathogens. Most of these systems are based on biotyping techniques in which isolates are grown on multiple substrates and the reaction pattern is compared with known patterns for various bacterial species. This procedure is relatively fast. Commercially available systems include miniaturized fermentation, coding to simplify recording of results, and probability calculations for the most likely pathogens. If the biotyping approach is automated and the reading process is coupled to computer-based data analysis, rapidly growing organisms (such as Enterobacteriaceae) can be identified within hours of detection on agar plates.
Several systems use substrates for preformed bacterial enzymes for identification within 2–3 h. These systems do not rely on bacterial growth per se to determine whether a substrate has been used. They employ a heavy inoculum in which specific bacterial enzymes are present in amounts sufficient to convert substrate to product rapidly. In addition, some systems use fluorogenic substrate/end-product detection methods to increase sensitivity through signal amplification.
Gas-Liquid Chromatography Gas-liquid chromatography often is used to detect metabolic end products of bacterial fermentation. One common application is identification of short-chain fatty acids produced by obligate anaerobes during glucose fermentation. Because the types and relative concentrations of volatile acids differ among the various genera and species that make up this group of organisms, such information serves as a metabolic fingerprint for a particular isolate.
Gas-liquid chromatography can be coupled to a sophisticated signal-analysis software system for identification and quantitation of long-chain fatty acids (LCFAs) in the outer membranes and cell walls of bacteria and fungi. For any particular species, the types and relative concentrations of LCFAs are distinctive enough to allow differentiation even from closely related species. An organism may be identified definitively within a few hours after detection of growth on appropriate media.
Matrix-Assisted Laser Desorption/Ionization/Time-of-Flight Mass Spectrometry (MALDI-TOF MS) MALDI-TOF MS is a rapid and accurate method for identifying microorganisms by protein analysis. The organism is mixed with a chemical matrix, dried on a target plate, and then pulsed with a laser. The laser ionizes and vaporizes the microbial proteins, which then travel through a charged vacuum chamber to a detector. The time of flight to the detector is measured for the individual proteins, and the resulting pattern (or fingerprint) is compared with a library of known patterns for various microorganisms to identify the test organism.
The primary clinical advantage of MALDI-TOF is that it takes only minutes to identify an organism, whereas several hours are needed for conventional phenotyping. MALDI-TOF is highly accurate for identification of most bacteria and yeasts grown on solid agar or in blood culture broth. Other potential uses of MALDI-TOF include identification of bacteria and yeasts directly from clinical specimens (e.g., urine), detection of β-lactamase activity, and strain typing of bacteria; however, these applications are still under development.
NUCLEIC ACID TESTS
Techniques for the detection and quantitation of specific DNA and RNA base sequences in clinical specimens have become powerful tools for the diagnosis of bacterial, viral, parasitic, and fungal infections. Nucleic acid tests are used for four purposes. First, they are used to detect, and sometimes to quantify, specific pathogens in clinical specimens. Second, such tests are used for identification of organisms (usually bacteria) that are difficult to identify by conventional methods. Third, nucleic acid tests are used to determine whether two or more isolates of the same pathogen are closely related (i.e., whether they belong to the same “clone” or “strain”). Fourth, these tests are used to predict the sensitivity of organisms to chemotherapeutic agents. Current technology encompasses a wide array of methods for amplification and signal detection, some of which have been approved by the U.S. Food and Drug Administration (FDA) for clinical diagnosis.
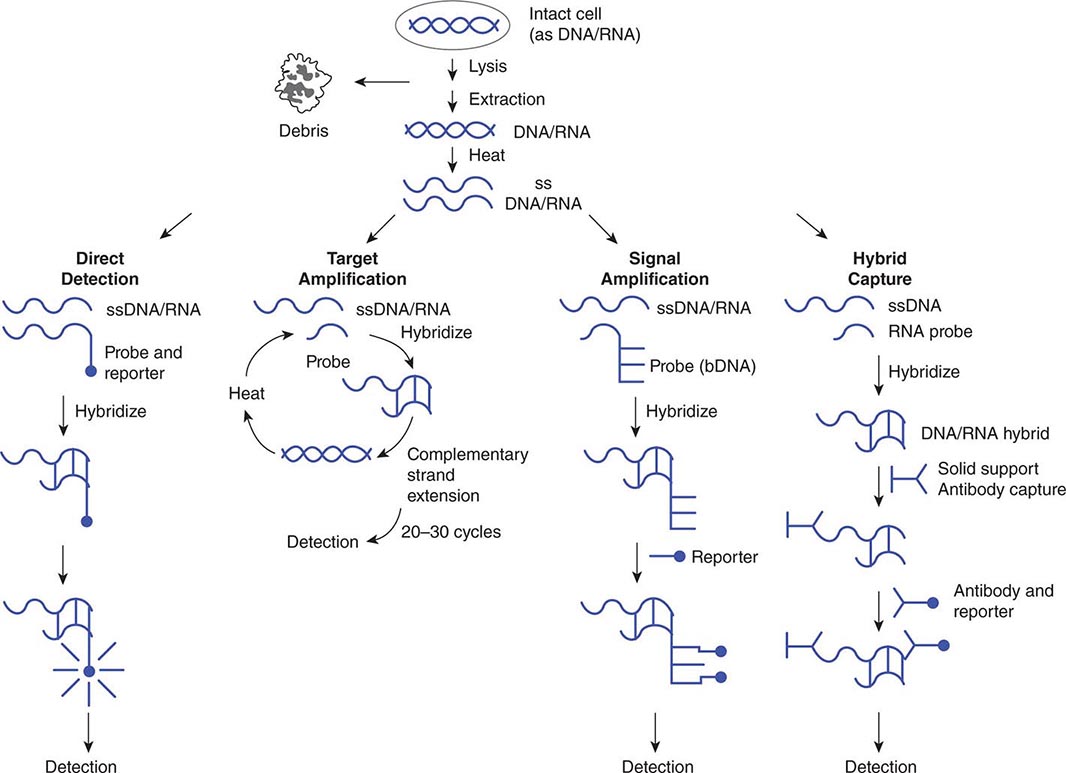
Use of nucleic acid tests generally involves lysis of intact cells or viruses and denaturation of the DNA or RNA to render it single-stranded. Probe(s) or primer(s) complementary to the pathogen-specific target sequence are hybridized to the target sequence in a solution or on a solid support, depending on the system employed. In situ hybridization of a probe to a target also is possible and allows the use of probes with agents present in tissue specimens. Once the probe(s) or primer(s) have been hybridized to the target (biologic signal), a variety of strategies may be employed to detect, amplify, and/or quantify the target–probe complex (Fig. 150e-2).
FIGURE 150e-2 Strategies for amplification and/or detection of a target–probe complex. DNA or RNA extracted from microorganisms is heated to create single-stranded (ss) DNA/RNA containing appropriate target sequences. These target sequences may be hybridized directly (direct detection) with probes attached to reporter molecules; they may be amplified by repetitive cycles of complementary strand extension (polymerase chain reaction) before attachment of a reporter probe; or the original target–probe signal may be amplified via hybridization with an additional probe containing multiple copies of a secondary reporter target sequence (branched-chain DNA, or bDNA). DNA/RNA hybrids also can be “captured” on a solid support (hybrid capture), with antibody to the DNA/RNA hybrids used to concentrate them and a second antibody coupled to a reporter molecule attached to the captured hybrid.
PROBES FOR DIRECT DETECTION OF PATHOGENS IN CLINICAL SPECIMENS
Nucleic acid probes are used for direct detection of pathogens in clinical specimens without amplification of the target strand of DNA or RNA. Such tests detect a relatively short sequence of bases specific for a particular pathogen on single-stranded DNA or RNA by hybridization of a complementary sequence of bases (probe) coupled to a reporter system that serves as the signal for detection. Nucleic acid probes are available commercially for direct detection of various bacterial and parasitic pathogens, including Chlamydia trachomatis, N. gonorrhoeae, and group A Streptococcus. A combined assay to detect and differentiate agents of vaginitis/vaginosis (Gardnerella vaginalis, Trichomonas vaginalis, and Candida species) also has been approved. An assortment of probes is available for confirming the identity of cultured pathogens, including some dimorphic molds, Mycobacterium species, and other bacteria (e.g., Campylobacter species, Streptococcus species, and Staphylococcus aureus). Probes for the direct detection of bacterial pathogens often are aimed at highly conserved 16S ribosomal RNA sequences, of which there are many more copies than there are of any single genomic DNA sequence in a bacterial cell. The sensitivity and specificity of probe assays for direct detection are comparable to those of more traditional assays, including EIA and culture.
In an alternative probe assay called hybrid capture, an RNA probe anneals to a DNA target, and the resulting DNA/RNA hybrid is captured on a solid support by antibody specific for DNA/RNA hybrids (concentration/amplification) and detected by chemiluminescent-labeled antibody specific for DNA/RNA hybrids. Hybrid capture assays are available for C. trachomatis, N. gonorrhoeae, and human papillomavirus.
Many laboratories have developed their own probes for pathogens; however, unless a method-validation protocol for diagnostic testing has been performed, federal law in the United States restricts the use of such probes to research.
NUCLEIC ACID AMPLIFICATION TEST (NAAT) STRATEGIES
There are several methods for amplification (copying) of small numbers of molecules of nucleic acid to readily detectable levels. These NAATs include PCR, LCR, strand displacement amplification, and self-sustaining sequence replication. In each case, exponential amplification of a pathogen-specific DNA or RNA sequence depends on primers that anneal to the target sequence. The amplified nucleic acid can be detected after the reaction is complete or (in real-time detection) as amplification proceeds. The sensitivity of NAATs is far greater than that of traditional assay methods such as culture. PCR, the first and still the most common NAAT, requires repeated heating of the DNA to separate the two complementary strands of the double helix, hybridization of a primer sequence to the appropriate target sequence, target amplification using PCR for complementary strand extension, and signal detection via a labeled probe. Methods for the monitoring of PCR after each amplification cycle—via either incorporation of fluorescent dyes into the DNA during primer extension or use of fluorescent probes capable of fluorescence resonance energy transfer—have decreased the time required to detect a specific target. An alternative NAAT employs transcription-mediated amplification, in which an RNA target sequence is converted to DNA, which then is exponentially transcribed into an RNA target. The advantage of this method is that only a single heating/annealing step is required for amplification. Identification of otherwise difficult-to-identify bacteria involves an initial amplification of a highly conserved region of 16S rDNA by PCR. Automated sequencing of several hundred bases is then performed, and the sequence information is compared with large databases containing sequence information for thousands of different organisms. Although 16S sequencing is not as rapid as other methods and is still relatively expensive for routine use in a clinical microbiology laboratory, it is becoming the definitive method for identification of unusual or difficult-to-cultivate organisms.
QUANTITATIVE NUCLEIC ACID TEST STRATEGIES
With the advent of newer therapeutic regimens for HIV-associated disease, cytomegalovirus infection, and hepatitis B and C virus infections, the response to therapy has been monitored by determining both genotype and viral load at various times after treatment initiation. Quantitative NAATs are available for HIV (PCR), cytomegalovirus (PCR), hepatitis B virus (PCR), and hepatitis C virus (PCR and TMA). Many laboratories have validated and perform quantitative assays for these and other pathogens (e.g., Epstein-Barr virus), using analyte-specific reagents for NAATs.
Branched-chain DNA (bDNA) testing is an alternative to NAATs for quantitative nucleic acid testing. In such testing, bDNA attaches to a site different from the target-binding sequence of the original probe. Chemiluminescence-labeled oligonucleotides can then bind to multiple repeating sequences on the bDNA. The amplified bDNA signal is detected by chemiluminescence. bDNA assays for viral load of HIV, hepatitis B virus, and hepatitis C virus have been approved by the FDA. The advantage of bDNA assays over PCR is that only a single heating/annealing step is required to hybridize the target-binding probe to the target sequence for amplification.
APPLICATIONS OF NUCLEIC ACID TESTS
The number of FDA-approved NAATS has increased dramatically and includes tests for Mycobacterium tuberculosis, N. gonorrhoeae, C. trachomatis, group B Streptococcus, and methicillin-resistant S. aureus. FDA-approved multiplex NAAT panels for detection of several respiratory or gastrointestinal pathogens also are available. Again, many laboratories have used commercially available reagents and analyte-specific reagents to create laboratory-developed tests for diagnostic use. Nucleic acid tests are useful to detect and identify difficult-to-grow or noncultivable bacterial pathogens such as Legionella, Ehrlichia, Rickettsia, Babesia, Borrelia, and Tropheryma whipplei. In addition, methods for rapid detection of agents of public health concern, such as Franciscella tularensis, Bacillus anthracis, smallpox virus, and Yersinia pestis, have been developed and are available in regional (state) laboratories and at the Centers for Disease Control and Prevention.
Nucleic acid tests are also used to determine how close the relationship is among different isolates of the same species of pathogen. The demonstration that bacteria of a single clone have infected multiple patients in the context of a possible source of transmission (e.g., a health care provider) offers confirmatory evidence for an outbreak. Pulsed-field gel electrophoresis remains the gold standard for bacterial strain analysis. This method involves the use of restriction enzymes that recognize rare sequences of nucleotides to digest bacterial DNA, resulting in large DNA fragments. These fragments are separated by gel electrophoresis with variable polarity of the electrophoretic current and then are visualized. Similar band patterns (i.e., differences in ≤3 bands) suggest that different bacterial isolates are closely related, or clonal. Simpler methods of strain typing include sequencing of single or multiple genes and PCR-based amplification of repetitive DNA sequences in the bacterial chromosome. Whole bacterial genome sequencing has been used in investigation of some outbreaks, but this method is still under development.
Future applications of nucleic acid testing probably will include the replacement of culture for identification of many pathogens with additional multiplex NAAT and solid-state DNA/RNA chip technology, in which thousands of unique nucleic acid sequences can be detected on a single silicon chip.
SUSCEPTIBILITY TESTING OF BACTERIA
A principal responsibility of the clinical microbiology laboratory is to determine which antimicrobial agents inhibit a specific bacterial isolate. Such testing is used for patient care and for monitoring of infection control problems, such as methicillin-resistant S. aureus or vancomycin-resistant Enterococcus faecium. Two approaches are useful. The first is a qualitative assessment of susceptibility, with responses categorized as susceptible, resistant, or intermediate. This approach can involve either the placement of paper disks containing antibiotics on an agar surface inoculated with the bacterial strain to be tested (Kirby-Bauer or disk-diffusion method), with measurement of the zones of growth inhibition after incubation, or the use of broth cultures containing a set concentration of antibiotic (breakpoint method). These methods have been calibrated carefully against quantitative methods and clinical experience with each antibiotic, and zones of inhibition and breakpoints have been determined on a species-by-species basis.
The second approach is to inoculate the test strain of bacteria into a series of cultures with increasing concentrations of antibiotic. The lowest concentration of antibiotic that inhibits visible microbial growth of the bacteria is known as the minimal inhibitory concentration (MIC). If tubes in which no growth is seen are subcultured, the minimal concentration of antibiotic required to kill 99.9% of the starting inoculum also can be determined (minimal bactericidal concentration, or MBC). The MIC value can be given a categorical interpretation of susceptible, resistant, or intermediate and so is more widely used than the MBC. Quantitative susceptibility testing using microbroth dilution in microwell plates or other miniaturized testing platforms has been automated and is used commonly in clinical laboratories. The epsilometer test (E-test), a novel method for determination of the MIC, uses a plastic strip with a known gradient of antibiotic concentrations along its length. When the strip is placed on the surface of an agar plate seeded with the bacterial strain to be tested, antibiotic diffuses into the medium, and bacterial growth is inhibited. For some organisms, such as obligate anaerobes and some β-hemolytic streptococci, routine susceptibility testing generally is not performed because of the difficulty of growing the organisms or the predictable sensitivity of most isolates to specific antibiotics.
SUSCEPTIBILITY TESTING OF FUNGI
With the advent of many new agents for treating yeasts and systemic fungal agents, the need for testing of individual isolates for susceptibility to specific antifungal agents has increased. In the past, few laboratories participated in such testing because of a lack of standard methods like those available for testing bacterial agents. However, several systems have been approved for antifungal susceptibility testing. These methods, which determine the minimal fungicidal concentration (MFC), are similar to the broth microdilution methods used to determine the MIC for bacteria. The E-test method is approved for testing the susceptibility of yeasts to fluconazole, itraconazole, and flucytosine, and disk diffusion can be used to test the susceptibility of Candida species to fluconazole and voriconazole. Methods for determining the MFC of a drug against fungal agents such as Aspergillus species are technically difficult, and most clinical laboratories refer requests for such testing to reference laboratories.
ANTIVIRAL TESTING
See Chaps. 214e and 215e.
151e |
Climate Change and Infectious Disease |
The release of greenhouse gases—principally carbon dioxide—into Earth’s atmosphere since the late nineteenth century has contributed to a climate unfamiliar to our species, Homo sapiens. This new climate has already altered the epidemiology of some infectious diseases. Continued accumulation of greenhouse gases in the atmosphere will further alter the planet’s climate. In some cases climate change may establish conditions favoring the emergence of infectious diseases, while in others it may render areas that are presently suitable for certain diseases unsuitable. This chapter presents the current state of knowledge regarding the known and prospective infectious-disease consequences of climate change.
OVERVIEW
The term climate change refers to long-term alterations in temperature, precipitation, wind, humidity, and other components of weather. Over the past 2.5 million years, the earth has warmed and cooled, cycling between glacial and interglacial periods during which average global temperatures moved up and down by 4–7°C. During the last glacial period, which ended roughly 12,000 years ago, global temperatures were, on average, 5°C cooler than in the mid-twentieth century (Fig. 151e-1).
FIGURE 151e-1 Overview of the earth’s temperature and primary greenhouse gases over the last 600,000 years. Variations of deuterium (δD; black) serve as a proxy for temperature. Atmospheric concentrations of greenhouse gases—CO2 (red), CH4 (blue), and nitrous oxide (N2O; green)—were derived from air trapped within Antarctic ice cores and from recent atmospheric measurements. Shaded areas indicate interglacial periods. Benthic δ18O marine records (dark gray) are a proxy for global ice-volume fluctuations and can be compared to the ice core data. Downward trends in the benthic δ18O curve reflect increasing ice volumes on land. The stars and labels indicate atmospheric concentrations as of the year 2000. CO2 levels surpassed 400 ppm as of 2013 and are rising at a rate of 2–2.5 ppm per year. (From Intergovernmental Panel on Climate Change Fourth Assessment Report. Working Group I, Chapter 6, Figure 6.3. Cambridge University Press, 2007.)
The present climate period, known as the Holocene, is remarkable for its stability: temperatures have largely remained within a range of 2–3°C. This stability has enabled the successful population and cultivation of much of the earth’s landmass by humanity. Current climate change differs from that in the past not only because its primary cause is human activities but also because its pace is faster. The 5°C of warming that occurred at the end of the last ice age took roughly 5000 years, whereas such a temperature increment may occur within the next 150 years unless the release of greenhouse gases is substantially reduced in coming decades. The current rate of warming on Earth is unprecedented in the last 50 million years. Climate science, although still a relatively new discipline, has provided an ever-clearer picture of how the changing chemistry of the atmosphere has influenced, and will continue to influence, the global climate.
GREENHOUSE GASES
Greenhouse gases (Table 151e-1 and Fig. 151e-2) are a group of gases in Earth’s atmosphere that absorb infrared radiation and thus retain heat inside the atmosphere. In the absence of these gases, the earth’s average temperature would be about 33°C colder. Carbon dioxide, released into the atmosphere primarily from fossil fuel combustion and deforestation, has had the greatest effect on climate since the Industrial Revolution. Of note, the Swedish scientist Svante Arrhenius first suggested in the late nineteenth century that the addition of carbon dioxide to the earth’s atmosphere would increase the planet’s surface temperature. Water vapor is the most abundant and a highly potent greenhouse gas but, given its short atmospheric life span and sensitivity to temperature, is not a major factor in recently observed climate change.
|
GREENHOUSE GASES: SOURCES, SINKS, AND FORCINGS |
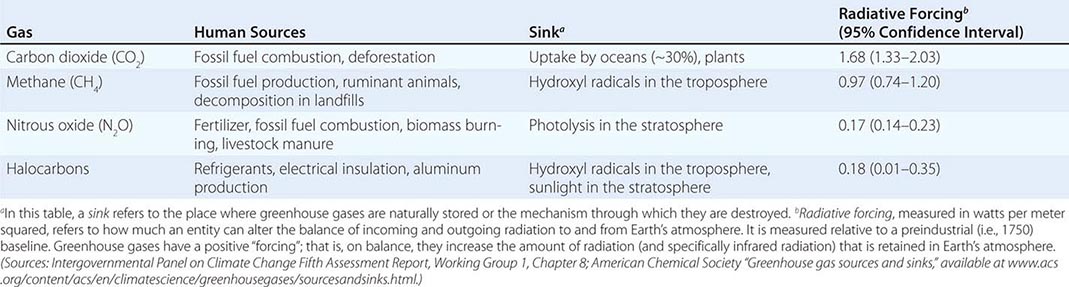
FIGURE 151e-2 Acceleration of radiative forcing (RF) from release of major greenhouse gases, 1850–2011. For definition of radiative forcing, see footnote b to Table 151e-1. (From Intergovernmental Panel on Climate Change Fifth Assessment Report, Working Group 1, Chapter 8, Figure 8.6, p. 677.)
The atmosphere, some of the aerosols suspended in it, and clouds reflect a portion of incoming solar radiation back toward space. The remainder reaches Earth’s surface, where it is absorbed and some is then emitted back at the atmosphere. The earth emits energy absorbed from the sun at longer wavelengths, primarily infrared, that greenhouse gases are able to absorb. The change in wavelength that occurs as solar radiation is absorbed and re-emitted from the earth’s surface is fundamental to the greenhouse effect (Fig. 151e-3).
FIGURE 151e-3 Earth’s energy balance. (Courtesy of NASA CERES project. Data from Trenberth KE et al: Earth’s global energy budget. Bull Am Meteor Soc 90:311, 2009.)
TEMPERATURE
Climate change has become nearly synonymous with global warming, as a clear signal from rising greenhouse gas concentrations has been an increase in the mean global surface temperature of ~0.85°C since 1880. However, this mean warming belies warming that is occurring much faster in certain regions. The Arctic has warmed twice as fast overall, and winters are warming faster than summers. Nighttime minimum temperatures are also rising faster than daytime high temperatures. Each of these nuances bears upon the incidence of infectious diseases in general and vector-borne disease specifically.
A moderate projection based on the best available scientific evidence suggests that average global temperatures will warm an additional 1.4–3.1°C by 2100 as compared to the period 1986–2005. Because of climate change, extreme heat waves have already become more common and are expected to be even more frequent later in this century. Besides contributing directly to morbidity and mortality in human populations, heat waves wilt crops and are expected to contribute substantially to predicted agricultural losses. For example, the 2010 heat wave in Russia, which was unprecedented in its severity, contributed to hundreds of forest fires that generated enough air pollution to kill an estimated 56,000 people and that burned 300,000 acres of crops, including roughly 25% of the nation’s wheat fields. Nutritional deficiencies underlie a substantial portion of the global burden of many infectious diseases.
PRECIPITATION
In addition to changing temperature, the emission of greenhouse gases and the consequent increase in energy in Earth’s atmosphere have influenced the planet’s water cycle. Since 1950, substantial increases in the heaviest precipitation events (i.e., those above the 95th percentile) have been observed in Europe and North America. While trends over that same interval are less clear in other regions because of limited data, regions of Southeast Asia and southern South America have likely experienced increases in heavy precipitation as well. Other areas have seen greater drought, notably southern Australia and the southwestern United States.
A warmer atmosphere holds more water vapor; specifically, there is 6–7.5% more water vapor per degree (Celsius) of warming in the lower atmosphere. For areas that have traditionally had more precipitation on average, warming tends to promote heavier precipitation events. In contrast, in regions prone to drought, warming tends to result in greater periods between rainfalls and in the risk of drought.
HURRICANES
The world’s oceans have absorbed 90% of the excess heat that greenhouse gases have kept in Earth’s atmosphere since the 1960s. Ocean heat provides energy for hurricanes, and warmer years tend to have greater hurricane activity. Atlantic hurricanes are the best studied and have the most data available. An analysis of satellite observations from 1983 to 2005 has shown a trend toward increasing severity—albeit decreasing frequency—of Atlantic hurricanes. Modeling of future tropical cyclones suggests that their intensity may increase 2–11% by 2100 and that the average storm will bring 20% more rainfall.
SEA LEVEL RISE
Between 1901 and 2010, the global sea level rose ~200 mm, or ~1.7 mm per year on average. From 1993 to 2010, the rate of rise nearly doubled—i.e., to 3.2 mm annually. Most of this sea level rise has resulted from the thermal expansion of water. Glacial ice melt is the second greatest factor, and its contribution is accelerating. By 2100, global sea level may rise by ≥500 mm from 1986–2005 levels, with an annual rate of rise of 8–16 mm at the century’s end. A large section of the West Antarctic ice sheet has begun to fall apart, and its melting alone may cause sea level to rise by ≥3 m in coming centuries.
Sea level rise is not uniform. The rate of rise on the eastern seaboard of North America has been roughly double the global rate. Compounding sea level rise is the subsidence of coastal areas due to human settlement. In the absence of levee upgrades, an estimated 170 million people living near coasts worldwide will be at risk of flooding in 2100 because of the combined effects of subsidence, erosion, and sea level rise.
Along with extreme storms and overuse of coastal aquifers, rising seas also contribute to salinization of coastal groundwater. About 1 billion people rely on coastal aquifers for potable water.
EL NIÑO SOUTHERN OSCILLATION
The El Niño Southern Oscillation (ENSO) refers to periodic changes in water temperature in the eastern Pacific Ocean that occur roughly every 5 years. ENSO cycles have dramatic effects on weather around the globe. Warmer-than-average water temperatures in the eastern Pacific define El Niño events (see below), whereas cooler-than-average water temperatures define La Niña periods. Evidence is accruing that climate change may be increasing the frequency and severity of El Niño events.
El Niño events drive alterations in weather worldwide (Fig. 151e-4) and are associated with extreme events and consequently higher rates of morbidity and mortality. Hurricane Mitch, one of the most powerful hurricanes ever observed, with winds reaching 290 km/h, dropped 1–1.8 m (3–6 feet) of rain over 72 h on parts of Honduras and Nicaragua. As a result of this storm, 11,000 people died and 2.7 million were displaced. Outbreaks of cholera, leptospirosis, and dengue occurred in the storm’s aftermath.
FIGURE 151e-4 Characteristic weather anomalies, by season, during El Niño events. (Source: www.cpc.ncep.noaa.gov/products/precip/CWlink/ENSO/ENSO-Global-Impacts/High-Resolution/.)
POPULATION MIGRATION AND CONFLICT
The final common outcome of all climate change effects is population migration. Sea level rise, extreme heat and precipitation, droughts, and salinization of water supplies all conspire to make regions (including some inhabited by humans for millennia) uninhabitable. Among climate change migrants in the near future may be the inhabitants of low-lying South Pacific islands that are vulnerable to sea level rise and residents of the Alaskan archipelago, where melting of permafrost has rendered traditional means of cold food storage difficult.
Climate change may also be contributing to humanitarian crises and conflicts. A severe 2011 drought in East Africa may have incited the Somali famine that resulted in 1 million refugees; mortality rates reached 7.4/10,000 in some refugee camps. Crop losses associated with the 2010 Russian heat wave led Russia to halt grain exports, causing higher grain prices on the world market and food riots in developing nations.
EFFECTS OF CLIMATE CHANGE ON INFECTIOUS DISEASE
The incidence of most, if not all, infectious diseases depends on climate. For any given infection, however, climate change is but one of many factors that determine disease epidemiology, and often it is not the most influential factor. Even in instances in which climate change creates conditions favorable to the spread of infections, diseases may be kept in check through interventions such as vector control or antibiotic treatment.
Detecting climate-change influence on an emerging human disease can be challenging. Research with animal pathogens, which in most instances is less well monitored and intervened upon than that with their human counterparts, has suggested how climate change may influence disease spread. For example, the life cycle of nematode parasites of caribou and musk oxen shortens as temperatures rise. As the Arctic has warmed, higher nematode burdens and consequently higher rates of morbidity and mortality have been observed. Other examples from animals, such as the spread of the protozoan parasite Perkinsus marinus in oysters, demonstrate how warming can enable range expansion of pathogens previously held in check by colder temperatures.
As these and other examples from studies of animals make clear, the influence of climate change on infectious diseases can be pronounced. The following sections deal with the infectious diseases for which research has explored the influence of climate change.
VECTOR-BORNE DISEASE
Because insects are cold-blooded, ambient temperature dictates their geographic distribution. With increases in temperatures (in particular, nighttime minimum temperatures), insects are freed to move poleward and up mountainsides. At the same time, as new areas become climatically suitable, current mosquito habitats may become unsuitable as a result of heat extremes.
In addition, insects tend to be sensitive to water availability. Mosquitoes that transmit malaria, dengue, and other infections may breed in pools of water created by heavy downpours. As has been observed in the Amazon, breeding pools can also appear during periods of drought when rivers recede and leave behind stagnant pools of water for Anopheles mosquitoes. These circumstances have raised interest in the potentially favorable impact of water-cycle intensification on the spread of mosquito-borne disease.
Malaria • TEMPERATURE Higher temperatures promote higher mosquito-biting rates, shorter parasite reproductive cycles, and the potential for the survival of mosquito vectors of Plasmodium infection in locations previously too cold to sustain them. Recent modeling experiments identify highland areas of East Africa and South America as perhaps most vulnerable to increased malarial incidence as a result of rising temperatures. In addition, a recent analysis of interannual malaria in Ecuador and Colombia has documented a greater incidence of malaria at higher altitudes in warmer years. Highland populations may be more vulnerable to malaria epidemics because they lack immunity.
Although rising temperature has the potential to expand the viable range of disease, malaria incidence is not associated with temperature in a strictly linear fashion. While mosquitoes and parasites may adapt to a warming climate, the present optimal temperature for malaria transmission is ~25°C, with a range of transmission temperatures between 16°C and 34°C. Rising temperatures also can have differential effects on parasite development during external incubation and on the mosquitoes’ gonotrophic cycle. Asynchrony between these two temperature-sensitive processes has been shown to decrease the vectorial capacity of mosquitoes.1
PRECIPITATION The abundance of Anopheles mosquitoes is strongly correlated with the availability of surface water pools for mosquito breeding, and biting rates have been linked to soil moisture (a surrogate for breeding pools). Research in the East African highlands has documented that increased variance in rainfall over time has strengthened the association between precipitation and disease incidence. These disease-promoting effects of precipitation may be countered by the potential for extreme rainfall to flush mosquito larvae from breeding sites.
PROJECTIONS Climate models have begun to deliver output on regional scales, permitting projections of climate-suitable regions to assist national and local health authorities. Climate models speak to the temperature and precipitation ranges necessary for malaria transmission but do not—and cannot—account for the capacity of malaria control programs to halt the spread of disease. The global reduction in malaria distribution over the past century makes it clear that, even with climate change, malaria occurs in far fewer places today because of public health interventions.
Despite intensive efforts, malaria remains the single greatest vector-borne disease cause of morbidity and death in the world. Particularly in regions that are most affected by malaria and where the public health infrastructure is inadequate to contain it, climate modeling may provide a useful tool in determining where the disease may spread. Recent modeling studies in sub-Saharan Africa have suggested that, although East African nations may encompass regions that will become more climatically suitable for malaria over this century, West African nations may not. By 2100, temperatures in West Africa may largely exceed those optimal for malaria transmission, and the climate may become drier; in contrast, higher temperatures and changes in precipitation may allow malaria to move up the mountainsides of East African countries. Climate change may create conditions favorable to malaria in subtropical and temperate regions of the Americas, Europe, and Asia as well.
Dengue Like malaria epidemics, dengue fever epidemics depend on temperature (Fig. 151e-5). Higher temperatures increase the rate of larval development and accelerate the emergence of adult Aedes mosquitoes. The daily temperature range may also influence dengue virus transmission, with a smaller range corresponding to a higher transmission potential. Temperatures <15°C or >36°C also substantially reduce mosquito feeding. In a Rhesus model of dengue, viral replication can occur in as little as 7 days with temperatures of >32–35°C; at 30°C, replication takes ≥12 days; and replication does not reliably occur at 26°C. Research on dengue in New Caledonia has shown peak transmission at ~32°C, reflecting combined effects of a shorter extrinsic incubation period, a higher feeding frequency, and more rapid development of mosquitoes. Along with temperature, peak relative humidity is a strong predictor of dengue outbreaks.
FIGURE 151e-5 Effects of temperature on variables associated with dengue transmission. Shown are the number of days required for development of immature Aedes aegypti mosquitoes to adults, the length of the dengue virus type 2 extrinsic incubation period (EIP), the percentage of A. aegypti mosquitoes that complete a blood meal within 30 min after a blood source is made available, and the percentage of hatched A. aegypti larvae surviving to adulthood. (Reproduced from CW Morin et al: Climate and dengue transmission: evidence and implications. Environ Health Perspect 121:1264, 2013.)
The association between dengue epidemics and precipitation is less consistent in the peer-reviewed literature, possibly because of the mosquito vector’s greater reliance on domestic breeding sites than on natural pools of water. For instance, in some studies, increased access to a piped water supply has been linked to dengue epidemics, presumably because of associated increased domestic water storage. Nonetheless, several studies have established rainfall as a predictor of the seasonal timing of dengue epidemics.
The current global distribution of dengue largely overlaps the geographic spread of Aedes mosquitoes (Fig. 151e-6). The presence of Aedes without dengue endemicity in large regions of North and South America and Africa illustrates the relevance of variables other than climate to disease incidence. Nevertheless, coupled climatic–epidemiologic modeling suggests dramatic shifts in the relative vectorial capacity for dengue by the end of this century should little or no mitigation of greenhouse gas emissions occur (Fig. 151e-7).
FIGURE 151e-6 Distribution of Aedes aegypti mosquitoes (turquoise) and dengue fever epidemics (red). (Map produced by the Agricultural Research Service of the U.S. Department of Agriculture.)
FIGURE 151e-7 Trend of annually averaged global dengue epidemic potential (rVc). Differences in rVc are based on 30-year averages of temperature and daily temperature range. A. Differences between 1980–2009 and 1901–1930. B. Differences between 2070–2099 and 1980–2009. The mean value of rVc was averaged from five global climate models under RCP8.5, a scenario of high greenhouse-gas emission. The color bar describes the values of the rVc. (From J Liu-Helmersson et al: Vectorial capacity of Aedes aegypti: effects of temperature and implications for global dengue epidemic potential. PLoS ONE 9:e89783, 2014 [doi:10.1371/journal.pone.0089783].)
Other Arbovirus Infections Climate change may favor increased geographic spread of other arboviral diseases, including Chikungunya virus disease, West Nile virus disease, and eastern equine encephalitis. Chikungunya virus disease emerged in Italy in 2007, having previously been mostly a disease of African nations. Climate models predict that, should competent vectors be present, conditions will be suitable for Chikungunya virus to gain a foothold in Western Europe, especially France, in the first half of the twenty-first century. In North America, areas favorable to West Nile virus outbreaks are expected to shift northward in this century. Current hotspots in North America are the California Central Valley, southwestern Arizona, southern Texas, and Louisiana, which have both compatible climates and avian reservoirs for the disease. By mid-century, the upper Midwest and New England will be more climatically suited to West Nile virus; by the end of the century, the area of risk may shift even further north to southern Canada. Whether the disease will ultimately move northward will depend on reservoir availability and mosquito control programs, among other factors.
Lyme Disease In the past few decades, Ixodes scapularis, the primary tick vector for Lyme disease as well as for anaplasmosis and babesiosis in New England, has become established in Canada because of warming temperatures. With climate change, the range of the tick is expected to expand further (Fig. 151e-8).
FIGURE 151e-8 Present and projected probability of establishment of Ixodes scapularis. (From U.S. National Climate Assessment 2014, adapted from JS Brownstein et al: Effect of climate change on Lyme disease risk in North America. Ecohealth 2:38, 2005.)
Lyme disease, caused by the spirochete Borrelia burgdorferi, is the most commonly reported vector-borne disease in North America, with ~30,000 cases per year. The model used in Fig. 151e-8 showed 95% accuracy in predicting current I. scapularis distribution and suggests substantial expansion of tick habitat and consequently of populations at risk for the diseases this tick transmits, particularly in Quebec, Iowa, and Arkansas, by 2080. Of note, some areas on the Gulf Coast may become less suitable for ticks by the end of the century.
WATERBORNE DISEASE
Outbreaks of waterborne disease are associated with heavy rainfall events. A review of 548 waterborne disease outbreaks in the United States found that 51% were preceded by precipitation levels above the 90th percentile. Since 1900, most regions of the United States except the Southwest and Hawaii have experienced an increase in heavy downpours (Fig. 151e-9), with the greatest intensification of the water cycle in New England and Alaska. Climate models suggest that by 2100 daily heavy-precipitation events, which are defined as a cumulative daily amount that now occurs once every 20 years, will increase nationwide (Fig. 151e-10). This scenario may be from two to as much as five times more likely, depending on the extent of greenhouse gas emission reductions achieved early in the twenty-first century.
FIGURE 151e-9 Percentage changes in the annual amount of precipitation falling in very heavy events, defined as the heaviest 1% of all daily events from 1901 to 2012 for each region. Changes are relative to a 1901–1960 average for all regions except values for Alaska and Hawaii, which are relative to the 1951–1980 average. (From U.S. National Climate Assessment 2014, NOAA National Climate Data Center/Cooperative Institute for Climate and Satellites, North Carolina.)
FIGURE 151e-10 Increased frequency of extreme daily precipitation events (defined as a daily amount that now occurs once in 20 years) by the latter part of the twenty-first century (2081–2100) compared to the frequency in the latter part of the twentieth century (1981–2000). A representative concentration pathway (RCP) describes a plausible climate future based on a net radiative forcing (e.g., 2.6 or 8.5) in 2100. (From U.S. National Climate Assessment 2014, NOAA National Climate Data Center/Cooperative Institute for Climate and Satellites, North Carolina.)
Most disease outbreaks after heavy precipitation occur through contamination of drinking-water supplies. While outbreaks related to surface-water contamination generally occur within a month of the precipitation event, disease outbreaks from groundwater contamination tend to occur ≥2 months later. According to a review of published reports of waterborne disease outbreaks, Vibrio and Leptospira species are the pathogens most commonly involved in the wake of heavy precipitation.
Combined Sewer Systems Roughly 40 million people in the United States and millions more around the world rely on combined sewer systems in which storm water and sanitary wastewater are conveyed in the same pipe to treatment facilities. These systems were designed on the basis of the nineteenth-century climate, in which heavy downpours were less frequent than they are today. The frequency of combined sewer overflows resulting in untreated sewage discharge, usually into freshwater bodies, has been increasing in cities worldwide. Overflows are associated with discharges of heavy metals and other chemical pollutants as well as a variety of pathogens. Outbreaks of hepatitis A, Escherichia coli O157:H7 infection, and cryptosporidial disease have been associated with sewer overflows in the United States.
Rising Temperatures and Vibrio Species Warmer temperatures favor proliferation of Vibrio species and disease outbreaks, as has been demonstrated in countries surrounding the Baltic Sea, Chile, Israel, northwestern Spain, and the U.S. Pacific Northwest. Around the Baltic Sea, outbreaks of Vibrio infection may be particularly likely because of faster warming near the poles and the sea’s relatively low salt content. In 2004, a Vibrio parahaemolyticus outbreak occurred from consumption of Alaskan oysters. This pathogen was unknown in Alaskan oysters prior to this event and extended the known geographic range of the disease 1000 km northward.
ENSO-RELATED OUTBREAKS
In the past, El Niño events were used as a model to investigate the potential for extreme weather–related infectious disease epidemics occurring in association with climate change. Recent evidence has indicated that climate change itself may be strengthening El Niño events. These events tend to promote epidemic infections in certain regions (Fig. 151e-11).
FIGURE 151e-11 Characteristic patterns of disease outbreaks associated with El Niño events, determined on the basis of 2006–2007 conditions. (From A Anyamba et al: Developing global climate anomalies suggest potential disease risks for 2006–2007. Int J Health Geogr 5:60, 2006.)
Associations of El Niño with outbreaks of Rift Valley fever in eastern and southern Africa have been known since the 1950s. El Niño favors wet conditions suitable for the insect vectors of the disease in these regions. Given the strong association between El Niño conditions and disease incidence, models have successfully predicted Rift Valley fever epidemics in humans and animals. In the 2006–2007 El Niño season, for example, outbreaks of Rift Valley fever were accurately predicted 2–6 weeks prior to epidemics in Somalia, Kenya, and Tanzania.
El Niño has had inconsistent associations with malaria incidence in African countries. Some of the strongest associations between El Niño and malaria have been identified in South Africa and Swaziland, where available data on incidence are relatively robust; however, even in these instances, the observed increased risk did not reach statistical significance. A stronger link to El Niño has been found in several studies done in South America. Research on malaria incidence in Colombia between 1960 and 2006 found that a 1°C temperature rise contributed to a 20% increase in incidence.
El Niño years are often associated with an increased incidence of dengue. Research on dengue outbreaks in Thailand from 1996 to 2005 revealed that 15–22% of the variance in monthly dengue disease incidence was attributable to El Niño. In South America, data on dengue outbreaks between 1995 and 2010 showed an increased incidence during the El Niño events of 1997–1998 and 2006–2007.
CLIMATE CHANGE, POPULATION DISPLACEMENT, AND INFECTIOUS DISEASE EPIDEMICS
For many reasons, including freshwater shortages, flooding, food shortages, and climate change–driven conflicts, climate change has and will continue to put pressure on human populations to move. Human migrations have long been associated with epidemic disease in the migrating populations themselves and in the communities in which they settle. The specific pathogens and patterns of disease that may appear after population migration relate to endemic diseases present in the migrant populations.
Large-scale migrations are common after extreme precipitation events. Hurricane Katrina, for instance, displaced about 1 million people from the U.S. Gulf Coast. Among Katrina refugees, outbreaks of respiratory, diarrheal, and skin diseases were most common. While attribution of a single weather event to increased greenhouse gas emissions is difficult, research can provide information on the likelihood of such events. It is expected, for example, that warming by 1°C increases the odds of a storm as strong as or stronger than Katrina by two- to sevenfold.
In the developing world, infectious disease outbreaks associated with population displacement due to extreme weather events may be especially hard to detect and respond to. Mitigation of disease risk requires overlaying of climate-related migration risk with foci of disease epidemics.
A BROADER VIEW OF CLIMATE CHANGE AND HEALTH
Climate change has far-reaching implications for the distribution and spread of infectious diseases worldwide. However, the greatest disease burdens related to climate change may not be due to infections. Because climate change disrupts the foundations of health, such as access to safe drinking water and food, it has the potential to undermine progress against major existing health problems such as malnutrition. In addition, resource scarcity and climate instability are increasingly associated with conflicts. Scholars have argued that events related to climate change were a factor in the revolutions of the Arab Spring and the Syrian civil war.
The public health response to climate change entails both mitigation and adaptation measures. Mitigation represents primary prevention and involves the reduction of greenhouse gas emissions into the atmosphere. Although no clear safety threshold of greenhouse gas emissions has been agreed upon, national governments from the major industrialized countries have agreed to set a warming target of <2°C above preindustrial levels by 2050; the attainment of this goal will require reducing greenhouse gas emissions by 40–70% below 2010 levels. To date, no international agreement exists to facilitate this reduction. Adaptation represents secondary prevention and is aimed at reducing the harms associated with sea level rise, heat waves, floods, droughts, wildfires, and other greenhouse gas–driven events. The efficacy of adaptation is constrained by the challenges inherent in predicting the precise location, duration, and severity of extreme weather events and flooding related to sea level rise, among other considerations.
1rVc is the vectorial capacity relative to the vector-to-human population ratio and is defined by the equation rVc = a2bhbme-μmn/μm where a is the vector biting rate; bh is the probability of vector-to-human transmission per bite; bm is the probability of human-to-vector infection per bite; n is the duration of the extrinsic incubation period; and μm is the vector mortality rate.
152e |
Infections in Veterans Returning from Foreign Wars |
Wars are an unfortunate but apparently inescapable consequence of human socialization. In the past quarter-century alone, there have been dozens of major armed conflicts worldwide. Several of these wars have been multinational in scope and have involved the deployment of large numbers of ground forces from their home countries to distinct areas of conflict in developing portions of the world, such as southwest and central Asia (e.g., Iraq, Afghanistan) and Africa.
Troops who are deployed as combatants or in other military capacities on foreign soil are at risk of acquiring infectious diseases endemic to that region on the basis of intimate human and environmental exposures and their immunologic naiveté with regard to local endemic or enzootic pathogens. This risk is magnified by the crowded social conditions engendered by mass troop deployments, infrastructure destruction, and population displacement; it is further amplified by vulnerabilities in public health, such as the lapses in hygiene and sanitation that invariably accompany armed conflicts. The clinical spectrum of infectious illness acquired in this setting includes acute infections in the combat theater, acute infections with delayed symptoms, and chronic or relapsing infections. The latter two scenarios have the potential to cause illness in veterans returning from foreign wars.
The impact of acute infectious diseases of war, which were once a major cause of noncombat mortality, has significantly lessened in modern conflicts, largely because of the use of preventive vaccines and the early institution of antimicrobial therapy. Nonetheless, these acute diseases remain an important cause of morbidity in deployed military personnel (Table 152e-1). Many acute infectious diseases, such as influenza, meningococcal meningitis, hepatitis A, and adenoviral respiratory disease, can largely be prevented by routine vaccination of troops. Others, such as bacterial gastroenteritis and viral respiratory tract infections, continue to represent common causes of minor morbidity among deployed forces. The incidence of several other acute infections, such as malaria and dengue, can be favorably impacted—although not completely abrogated—by the use of chemoprophylaxis, personal protective measures, and vector control. Uncommonly, infections that have short incubation periods and are acquired just days before leaving a combat theater may become clinically manifest only upon the return of troops to their countries of origin. Such was the case in a cluster of African tick typhus that occurred during short-term U.S. troop deployments to Somalia and Botswana in the early 1990s. However, because most acute infections with brief clinical incubation periods are self-limited or responsive to treatment, they are not typically seen in returning war veterans and will not be addressed further in this chapter.
|
ACUTE INFECTIOUS DISEASES OF WAR THAT HAVE BRIEF CLINICAL INCUBATION PERIODS AND THEREFORE ARE LIKELY TO CAUSE SYMPTOMATIC ILLNESS IN MILITARY PERSONNEL DURING DEPLOYMENT |
A number of infectious diseases acquired in a theater of military operations may become apparent in veterans only after they have returned to their home countries. Although their incidences have not been precisely defined, these complications of military service—manifesting clinically with either acute or chronic signs and symptoms—may cause significant morbidity in affected veterans and in some settings may endanger public health through secondary transmission or contamination of the blood supply. Of a sample of nearly 53,000 U.S. veterans of the Persian Gulf War (1990–1991), 7% were diagnosed with an infectious disease in the aftermath of the war; no excess risk of mortality was observed over a 6-year follow-up comparison with nondeployed veterans.
This chapter focuses on infectious diseases that have occurred or have been a source of concern in returning veterans of foreign wars over the past quarter-century. During this period, several pathogens have been associated with disease in this population, as discussed below. Some pathogens have been associated with only rare case reports in war veterans, and some, given their epidemiology, may pose a risk in future conflicts. In general, it is practical to classify infections with delayed signs and symptoms related to prolonged incubation periods or significant clinical latency in terms of their potential to manifest clinically as acute illnesses or chronic/relapsing diseases. Table 152e-2 provides details regarding the epidemiology, clinical characteristics, diagnosis, therapy, and prevention of infectious diseases of concern in returning war veterans. Figure 152e-1 illustrates a differential diagnostic approach—based on prominent signs or symptoms—to suspected infections in this population.
|
INFECTIOUS DISEASES ASSOCIATED WITH DELAYED CLINICAL MANIFESTATIONS THAT MAY PRESENT WITH ACUTE, CHRONIC, OR RELAPSING COURSES IN VETERANS RETURNING FROM RECENT FOREIGN WARS |
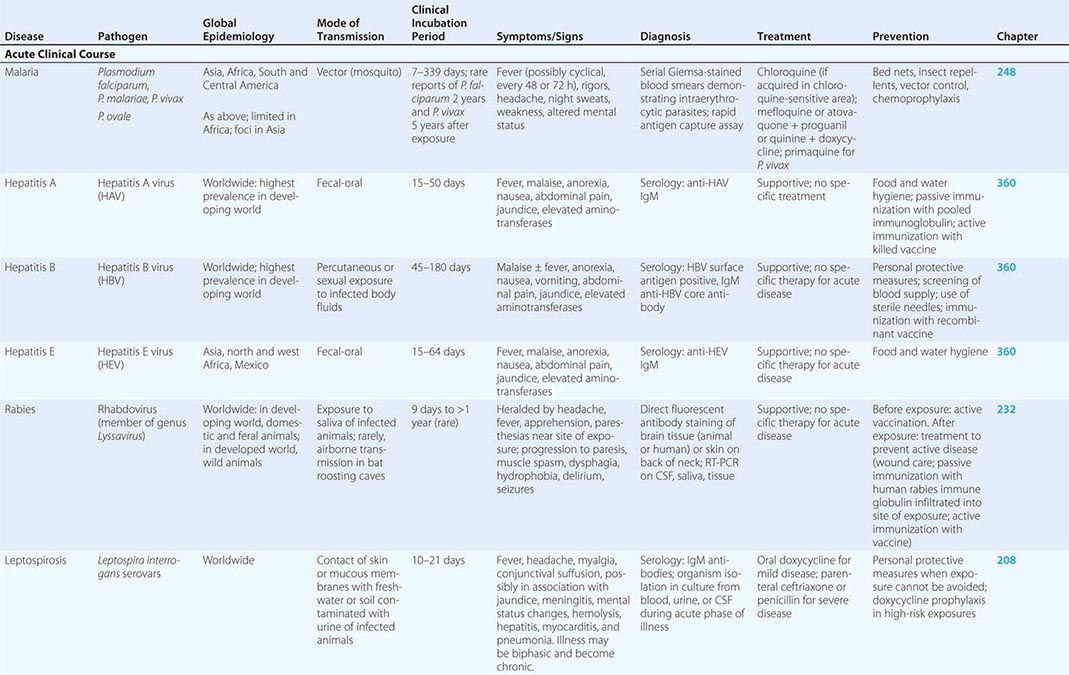
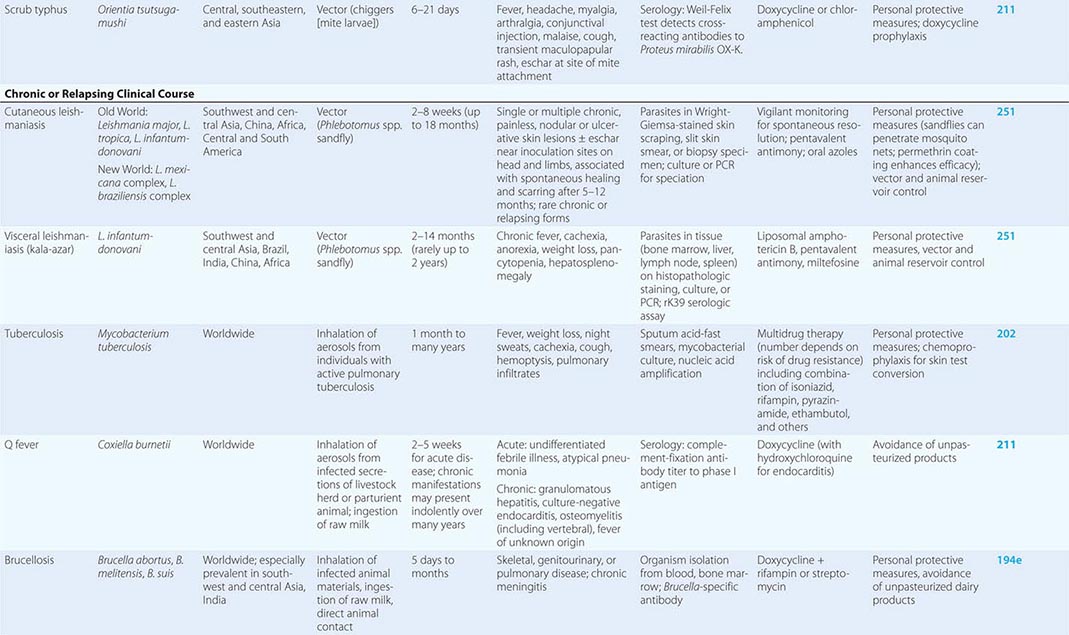
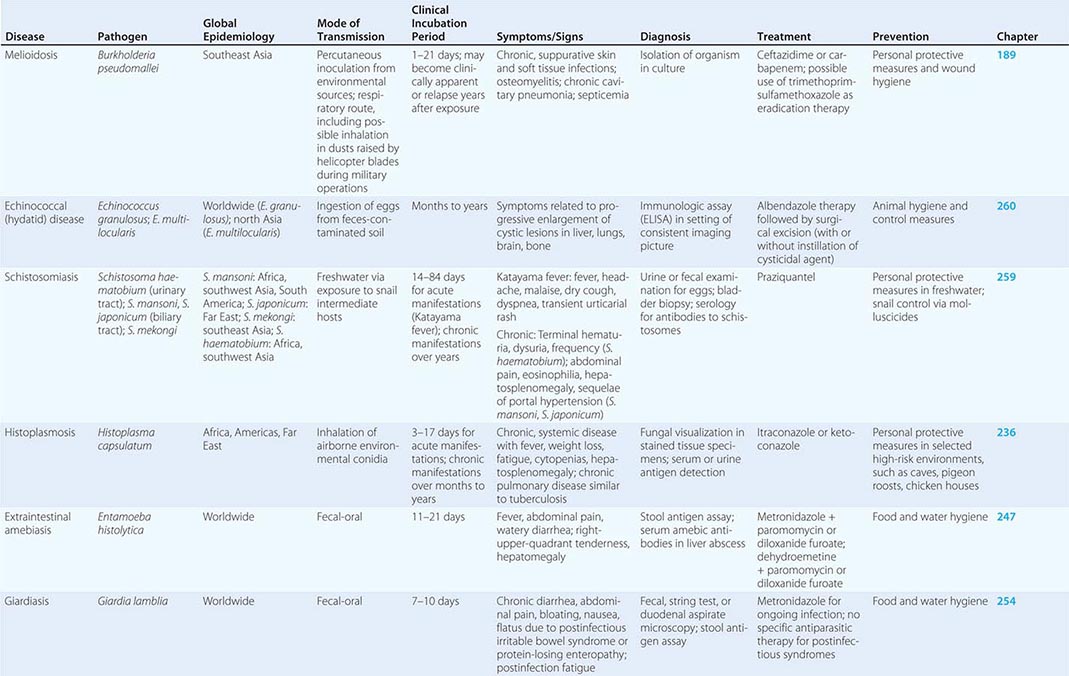
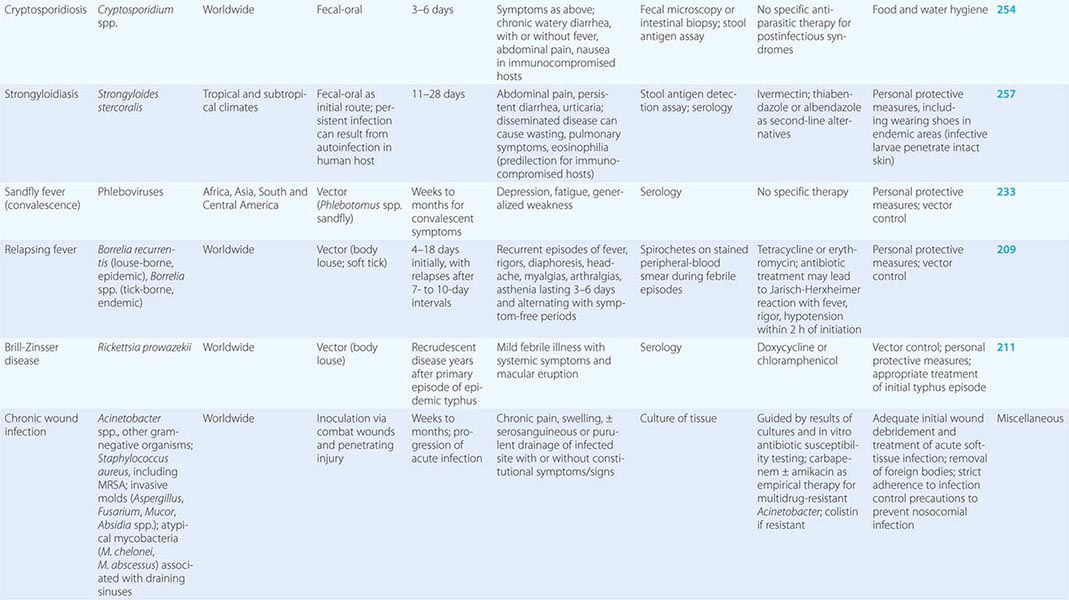
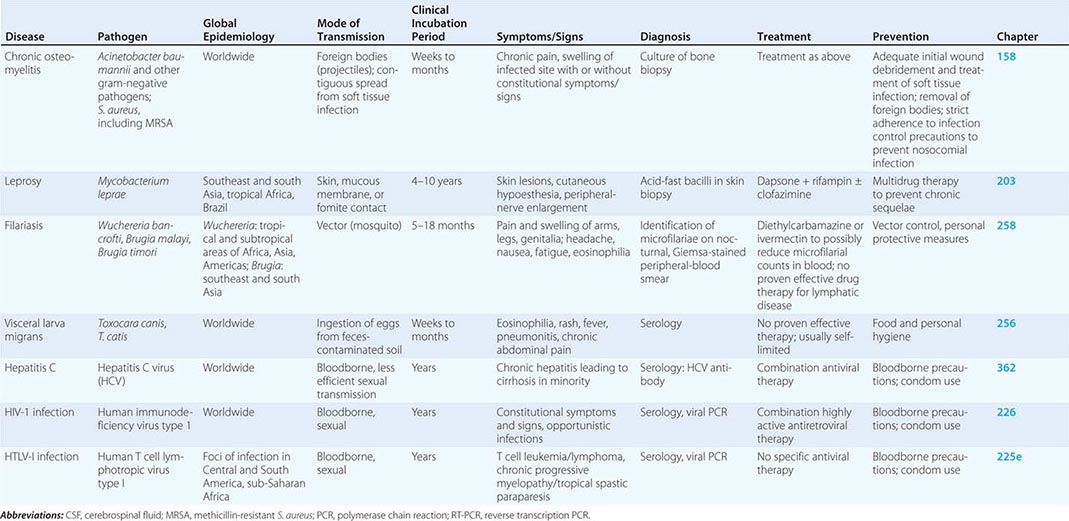
FIGURE 152e-1 Syndromic approach to the differential diagnosis of suspected infection in a veteran who has returned from a foreign war in southwest Asia, central Asia, or Africa at least 2 weeks prior to clinical presentation. HBV, hepatitis B virus.
ACUTE INFECTIOUS DISEASES WITH DELAYED CLINICAL PRESENTATIONS
Malaria (See Chap. 248) Malaria, which is due to infection with Plasmodium species of protozoa, has historically caused significant battlefield morbidity and lost duty time among armed forces; these phenomena have been affirmed by recent U.S. military experiences in Africa and central Asia. Because of its worldwide prevalence and its pathophysiology, malaria remains an important cause of infection both during military operations and in returning war veterans.
The risk of malaria is exacerbated by several factors inherent to war: inadequate shelter promoting increased troop exposure to vectors; abeyance of governmental programs for vector control; and ecologic changes leading to an increased vector presence in the contested environment. Because of the complex life cycle of the parasite and the predilection of P. vivax and P. ovale to remain latent in their liver stages of development for prolonged periods, malaria in foreign-stationed troops may become clinically apparent only after their return home. In the aftermath of the Vietnam War, more than 13,000 cases of malaria—the vast majority due to P. vivax—were imported into the United States. Of the 7683 Soviet troops diagnosed with P. vivax malaria acquired during the U.S.S.R.’s war in Afghanistan in the 1980s, 76% developed clinical manifestations >1 month after their return to the U.S.S.R., with some cases developing as long as 3 years later.
Imported malaria with prolonged clinical latency periods remains a problem among veterans returning from wars in endemic areas. In a cluster of 112 cases of imported disease in U.S. Marines returning to the United States from deployment to Somalia in 1993, falciparum malaria was diagnosed as late as 12 weeks after return; some cases due to P. vivax were diagnosed after an additional 2 months. In an outbreak of imported P. vivax malaria among U.S. Army Rangers deployed to Afghanistan in 2002, the median time to diagnosis was nearly 8 months after return.
Although malaria is largely preventable through the combined use of vector control, personal protective measures (e.g., bed nets, insect repellent, long sleeves, permethrin-treated clothing), and chemoprophylaxis, nonadherence to these measures and/or to chemotherapy (including terminal primaquine prophylaxis to eradicate the liver stage of P. vivax) appears to be responsible for the majority of cases in recent U.S. military experiences. However, there is also evidence to support chemoprophylaxis failures in a small subgroup of cases of P. falciparum and P. vivax malaria acquired during U.S. deployments to Somalia. Thus, imported malaria in returning war veterans remains possible despite appropriate adherence to chemoprophylaxis.
Viral Hepatitis (See Chap. 360) The incidence of viral hepatitis, once a major scourge of military campaigns and their aftermath, has declined considerably over the past half-century of military engagements. Although more than 115,000 cases of viral hepatitis, most due to hepatitis A virus, were reported among Soviet troops serving in Afghanistan in the 1980s, only rare reports of hepatitis A and B were noted during the massive, short-term deployment of U.S. troops to the Persian Gulf in the early 1990s. Hepatitis A and E, endemic in many parts of the developing world, present clinically as acute infections transmitted by the fecal-oral route and can be largely controlled with interventions practiced widely among military forces from industrialized countries: appropriate food and water hygiene and pre-deployment vaccination against hepatitis A. Hepatitis B contamination of serum-stabilized yellow fever vaccine caused a large outbreak of the disease among U.S. forces during World War II; such events are currently unlikely, given the use of modern virus-inactivation techniques in vaccine manufacturing. Despite their potential—as a consequence of their long clinical incubation and latency periods—to cause disease in returning veterans, hepatitis B and hepatitis C have been acquired relatively rarely in theater because of risk factor mitigation: routine drug testing of modern armies and screening of the blood supply to exclude viral contamination.
Rabies (See Chap. 232) Deployed soldiers are often in close contact with feral dogs and other potentially rabid animals in both urban and remote combat environments. In the period 2001–2010, 643 animal bites were documented during medical encounters among U.S. forces in the combat theaters of southwest and central Asia; the majority of these potential exposures were dog bites. Of bitten personnel, 18% received rabies postexposure prophylaxis. Recently, a U.S. soldier developed rapidly progressive signs and symptoms of rabies 8 months after a dog bite in Afghanistan and died within 17 days of illness onset. This case, which represents the first rabies death in nearly 40 years in a member of the U.S. military who acquired the infection overseas, reinforces both the lethality of the disease and the need to practice vigilant exposure precautions among deployed military personnel and to administer postexposure prophylaxis to troops who are bitten by animals in high-risk areas.
CHRONIC OR RELAPSING INFECTIOUS DISEASES ACQUIRED IN THE THEATER OF WAR
Leishmaniasis (See Chap. 251) Various forms of leishmaniasis may be acquired during military deployment and may present with myriad chronic clinical manifestations in war veterans. Because the protozoal pathogens (Leishmania species) are endemic throughout much of southwest and central Asia, their associated diseases have occurred in veterans returning from several recent conflicts there. The widespread distribution of various species of Leishmania elsewhere throughout the developing world suggests that leishmaniasis may complicate future conflicts as well.
Leishmaniasis may present clinically as cutaneous, mucocutaneous, or visceral disease; all forms are transmitted to humans by the bite of infected phlebotomine sandflies via zoonotic (small mammal) reservoirs. In rare circumstances, infection may be transmitted through blood transfusion. Transmission to humans is enhanced by factors that bring them into close proximity to animal reservoirs, such as life in dense, mobile populations; disruption of ecologic niches; and infrastructural breakdown. All these factors are common sequelae of war.
At least 1300 cases of cutaneous leishmaniasis caused by L. major or L. tropica have been diagnosed in American soldiers deployed to Iraq and Afghanistan over the past decade; however, the actual burden of infection may be higher due to underreporting, as lesions spontaneously resolve in many cases. The infection manifests clinically as one or more chronic, painless skin ulcers or nodules that may persist for 6–12 months. Rarely, lesions of cutaneous leishmaniasis may disseminate locally or diffusely.
Visceral disease (kala-azar) is typically caused by L. donovani and may be life-threatening. There have been at least five confirmed reports of U.S. veterans returning from recent deployments with classic visceral leishmaniasis associated with chronic fever, weight loss, pancytopenia, hypergammaglobulinemia, and organomegaly. As systemic leishmanial infection is known to manifest clinically years after exposure and may recrudesce if host immunity wanes due to unrelated causes, it remains possible that additional cases may yet surface.
Chronic Diarrhea Although acute bacterial gastroenteritis remains a major noncombat cause of morbidity and duty days lost during troop deployments, chronic illness is unusual. However, selected bacterial and parasitic enteric pathogens may cause chronic infections or illnesses in returning veterans. Although such infections have been uncommonly noted in recent deployments, they pose potential threats in future wars because of their worldwide prevalence.
Giardiasis (Chap. 254), amebiasis (Chap. 247), and cryptosporidiosis (Chap. 254), which usually cause self-limited protozoal gastroenteritis in immunocompetent hosts, may result in persistent symptoms in immunocompromised populations or when complicated by secondary illness. Giardia infection has been associated with chronic diarrhea due to postinfectious irritable bowel syndrome and with chronic signs and symptoms of systemic illness in association with postinfection fatigue or protein-losing enteropathy. Cryptosporidiosis also may cause chronic diarrhea or malabsorptive syndromes in immunocompromised individuals. Amebic infection of the colon may be associated with several serious complications, including perforation, fistulae, and obstruction; extraintestinal spread of amebiasis may result in hepatic invasion leading to abscess formation.
Systemic Illness Due to Enteric Pathogens Certain helminthic infections are endemic in many parts of the developing world and may pose continuing risks to infected military personnel after their return. Larvae of the intestinal nematode Strongyloides stercoralis (Chap. 257) either may be passed in the feces and develop into the infective stage in the external environment or may persist in the human small intestine and initiate new infective cycles in a process known as autoinfection. Autoinfection with Strongyloides may result in chronic clinical manifestations such as pruritus, rash, abdominal pain, weight loss, diarrhea, and eosinophilia. In immunocompromised hosts, chronic strongyloidiasis may cause a life-threatening hyperinfection syndrome, likely triggered by high parasite burdens and resulting in a multiorgan, systemic illness consistent with severe inflammatory response syndrome. In some cases, Strongyloides hyperinfection syndrome may be complicated by gram-negative sepsis or meningitis related to bacterial seeding from parasitic involvement of the lungs or gastrointestinal tract. Although not described in association with recent wars, chronic strongyloidiasis was an uncommon phenomenon affecting a small number of World War II and Vietnam War veterans; one study estimated that there were up to 400 affected individuals still living in Great Britain. The pathogen may pose a risk to troops deployed in the future to tropical and subtropical regions of the world where the parasite is endemic.
Chronic schistosomiasis (Chap. 259) results from intravascular infection by trematode parasites whose larval forms penetrate the skin of humans through contact with freshwater inhabited by the snail intermediate host. The pathogens are widely distributed throughout large portions of the developing world. A chronic inflammatory response in the portal circulation to the eggs of S. mansoni and S. japonicum leads to fibrosing disease in the liver and eventually to cirrhosis. Similar pathophysiologic events occur in the vascular plexus of the human genitourinary tract in response to chronic S. haematobium infection, leading to fibrosing changes in the human bladder and ureters—a precursor to bladder cancer. Rarely, individuals with chronic schistosomiasis develop a syndrome of persistent or relapsing bacteremia with Salmonella typhi, which is the etiologic agent of typhoid fever and is not otherwise a typical infectious cause of chronic disease in veterans.
Other Chronic Infections/Syndromes Awareness of the potential threat of troop exposure to agents of biological warfare (Chap. 261e) has been heightened over the past two decades by revelations regarding Iraq’s state-sponsored chemical weapons program in the 1990s, the known broad availability of such technology, and escalations of global and geopolitical conflicts as well as of international acts of terrorism. Most of the high-risk pathogens posing a threat of bioterrorism cause acute clinical manifestations; however, selected agents, such as those causing Q fever and brucellosis, may result in chronic diseases, whether exposure is natural or intentional. Isolated cases of naturally acquired Q fever and brucellosis have been reported in recent U.S. veterans of the wars in Iraq and Afghanistan. To date, there has been no confirmed evidence of infections related to exposure to biological weapons in returning war veterans.
HIV-1 infection (Chap. 226), ubiquitous throughout the world, continues to pose a potential bloodborne and sexually transmitted risk to soldiers engaged in armed conflict in high-prevalence areas. Several reports describe war veterans returning to their countries of origin harboring HIV-1; in some of these cases, novel viral genotypes have been imported into the population. Tuberculosis (Chap. 202) also is endemic throughout much of the developing world and is prevalent in several areas of recent multinational conflicts. Although there is no evidence that active, chronic tuberculosis has affected veterans of recent wars, the rate of tuberculin skin test conversion, which indicates new infections, was noted to be 2.5% among U.S. military personnel deployed to southwest Asia in the early 2000s.
Several chronic infections that pose a risk to war veterans tend to recur or become clinically active in immunocompromised individuals and may be particularly aggressive in this population. Latent infections such as leishmaniasis, tuberculosis, histoplasmosis, brucellosis, Q fever, and strongyloidiasis in otherwise healthy veterans returning from war may become clinically expressed only much later in the setting of chronic glucocorticoid use, monoclonal antibody therapy, organ transplantation, cytotoxic chemotherapy, advanced HIV-1 disease, hematologic malignancy, or other immunosuppressive conditions. Thus, physicians should remain vigilant regarding the potential development of symptomatic disease due to such latent microbes in immunologically compromised veterans who may have initially acquired an infection years previously while serving in the military.
A number of syndromes of possible infectious etiology, some of which may present with chronic clinical manifestations, have been noted in veterans returning from recent wars. After the Gulf War in 1990–1991, numerous veterans from several countries experienced constellations of various common, nonspecific symptoms, including fatigue, musculoskeletal pain, sleep disturbances, and difficulty concentrating. Despite exhaustive investigations and several hypotheses regarding potential etiologies of this chronic multisystemic illness, including infectious agents, no unifying or single cause has been identified. In a randomized, placebo-controlled trial, a prolonged course of doxycycline failed to alleviate such symptoms at 1 year. In 2003, a small outbreak of acute idiopathic eosinophilic pneumonia was reported among U.S. troops serving in southwest Asia. Although a thorough investigation failed to elucidate an infectious etiology, it is noteworthy that symptoms developed up to 11 months after arrival in the theater of combat; this time frame suggests that such cases may become clinically manifest after return to the home front.
Chronic Wound Infections and Osteomyelitis War wounds, an important cause of morbidity in all armed conflicts, are at high risk of infection due to contamination with environmental bacteria and the presence of retained foreign bodies. In recent conflicts, improved survival rates due to enhanced and expedited care of combat casualties have had the unintended consequence of increasing the potential for infectious complications, a situation exacerbated by repeated and at times prolonged exposure to health care environments and their associated pathogens. Many wounds sustained in recent wars have resulted from penetrating soft-tissue trauma and open fractures of the extremities—injuries attributable to improvised explosive devices used as antipersonnel weapons and to body armor that leaves the limbs unprotected. Cultures of samples taken at the time of injury at a combat support hospital in Iraq revealed that most contaminated wounds harbored gram-positive commensal skin bacteria; other investigators, however, have noted an early predominance of gram-negative bacteria, including multidrug-resistant (MDR) pathogens.
Approximately 3% of nearly 17,000 combat injuries sustained between 2003 and 2009 in U.S. military operations in Iraq and Afghanistan involved soft tissue infections. Although it is not clear how many of these infections became chronic or progressed to involve deeper tissue structures, a significant number were managed in tertiary care facilities, many on the home front. The bacteriology of infected combat wounds comprises predominantly gram-negative bacilli and MDR organisms. Broad-spectrum antimicrobial prophylaxis administered at the time of injury appears to be a risk factor for subsequent infection; nosocomial acquisition of health care–associated MDR pathogens likely contributes as well. Invasive fungal infections have recently emerged as a significant cause of morbidity and death in the context of combat wounds.
During the past decade of wars in Iraq and Afghanistan, MDR strains of Acinetobacter baumannii (Chap. 187) have emerged as important pathogens in both wound and bloodstream infections among returning veterans treated at U.S. health care facilities. The majority of isolates display in vitro susceptibility to amikacin and variable susceptibility to carbapenems but are largely resistant to other commonly used antimicrobial agents. Antimicrobial treatment should be guided by in vitro susceptibility data; patients who are critically ill, are immunocompromised, or have significant medical co-morbidities may benefit from combination therapy. Colistin (polymyxin E) has been shown to be clinically effective against Acinetobacter infections caused by isolates resistant to both aminoglycosides and carbapenems. Mortality rates have been low among immunocompetent hosts receiving appropriate antimicrobial treatment and undergoing debridement; however, Acinetobacter infections in immunocompromised individuals are associated with higher mortality risk. Strict adherence to hand-washing and other infection control procedures is important to limit the nosocomial spread of MDR organisms.
Chronic osteomyelitis related to either extension of a contiguous soft tissue infection or an infected prosthesis also represents a burgeoning problem for wounded veterans of recent wars. Limited microbiologic data have shown a predominance of gram-negative etiologic agents—most often Acinetobacter and Pseudomonas aeruginosa—in the initial episodes of osteomyelitis but a shift to staphylococcal isolates in the majority of recurrent cases—a change that may perhaps be related to nosocomial acquisition. Relapses have been noted to occur 1 month to 1 year after treatment of the initial infection.
Veterans with traumatic brain injury, who have accounted for 22% of American casualties in recent wars in Iraq and Afghanistan, are at risk for infectious complications due to several factors: the presence of foreign bodies or prosthetic material related to their traumatic wounds; acquisition of a wide range of nosocomial infections during repeated interactions with the health care system; and injury-induced cognitive changes that may increase impulsivity and risk-taking behaviors. In line with the last-mentioned factor, this subgroup of veterans may be at heightened risk for substance abuse and other practices that expose them to various bloodborne and sexually transmitted infections. Moreover, they may be at risk for post-neurosurgical complications, such as pyogenic meningitis due to multidrug-resistant Acinetobacter species.
SECTION 2 |
CLINICAL SYNDROMES: COMMUNITY-ACQUIRED INFECTI ONS |
153 |
Pneumonia |
DEFINITION
Pneumonia is an infection of the pulmonary parenchyma. Despite being the cause of significant morbidity and mortality, pneumonia is often misdiagnosed, mistreated, and underestimated. In the past, pneumonia was typically classified as community-acquired (CAP), hospital-acquired (HAP), or ventilator-associated (VAP). Over the past two decades, however, some persons presenting with onset of pneumonia as outpatients have been found to be infected with the multidrug-resistant (MDR) pathogens previously associated with HAP. Factors responsible for this phenomenon include the development and widespread use of potent oral antibiotics, earlier transfer of patients out of acute-care hospitals to their homes or various lower-acuity facilities, increased use of outpatient IV antibiotic therapy, general aging of the population, and more extensive immunomodulatory therapies. The potential involvement of these MDR pathogens has led to a designation for a new category of pneumonia—health care–associated pneumonia (HCAP)—that is distinct from CAP. Conditions associated with HCAP and the likely pathogens are listed in Table 153-1.
| TABLE 153-1 |
CLINICAL CONDITIONS ASSOCIATED WITH AND LIKELY PATHOGENS IN HEALTH CARE–ASSOCIATED PNEUMONIA |
Although the new classification system has been helpful in designing empirical antibiotic strategies, it is not without its disadvantages. Not all MDR pathogens are associated with all risk factors (Table 153-1). Moreover, HCAP is a distillation of multiple risk factors, and each patient must be considered individually. For example, the risk of infection with MDR pathogens for a nursing home resident who has dementia but can independently dress, ambulate, and eat is quite different from the risk for a patient who is in a chronic vegetative state with a tracheostomy and a percutaneous feeding tube in place. In addition, risk factors for MDR infection do not preclude the development of pneumonia caused by the usual CAP pathogens.
This chapter deals with pneumonia in patients who are not considered to be immunocompromised. Pneumonia in severely immunocompromised patients, some of whom overlap with the groups of patients considered in this chapter, warrants separate discussion (see Chaps. 104, 169, and 226).
PATHOPHYSIOLOGY
Pneumonia results from the proliferation of microbial pathogens at the alveolar level and the host’s response to those pathogens. Microorganisms gain access to the lower respiratory tract in several ways. The most common is by aspiration from the oropharynx. Small-volume aspiration occurs frequently during sleep (especially in the elderly) and in patients with decreased levels of consciousness. Many pathogens are inhaled as contaminated droplets. Rarely, pneumonia occurs via hematogenous spread (e.g., from tricuspid endocarditis) or by contiguous extension from an infected pleural or mediastinal space.
Mechanical factors are critically important in host defense. The hairs and turbinates of the nares capture larger inhaled particles before they reach the lower respiratory tract. The branching architecture of the tracheobronchial tree traps microbes on the airway lining, where mucociliary clearance and local antibacterial factors either clear or kill the potential pathogen. The gag reflex and the cough mechanism offer critical protection from aspiration. In addition, the normal flora adhering to mucosal cells of the oropharynx, whose components are remarkably constant, prevents pathogenic bacteria from binding and thereby decreases the risk of pneumonia caused by these more virulent bacteria.
When these barriers are overcome or when microorganisms are small enough to be inhaled to the alveolar level, resident alveolar macrophages are extremely efficient at clearing and killing pathogens. Macrophages are assisted by proteins that are produced by the alveolar epithelial cells (e.g., surfactant proteins A and D) and that have intrinsic opsonizing properties or antibacterial or antiviral activity. Once engulfed by the macrophage, the pathogens—even if they are not killed—are eliminated via either the mucociliary elevator or the lymphatics and no longer represent an infectious challenge. Only when the capacity of the alveolar macrophages to ingest or kill the microorganisms is exceeded does clinical pneumonia become manifest. In that situation, the alveolar macrophages initiate the inflammatory response to bolster lower respiratory tract defenses. The host inflammatory response, rather than proliferation of microorganisms, triggers the clinical syndrome of pneumonia. The release of inflammatory mediators, such as interleukin 1 and tumor necrosis factor, results in fever. Chemokines, such as interleukin 8 and granulocyte colony-stimulating factor, stimulate the release of neutrophils and their attraction to the lung, producing both peripheral leukocytosis and increased purulent secretions. Inflammatory mediators released by macrophages and the newly recruited neutrophils create an alveolar capillary leak equivalent to that seen in the acute respiratory distress syndrome, although in pneumonia this leak is localized (at least initially). Even erythrocytes can cross the alveolar-capillary membrane, with consequent hemoptysis. The capillary leak results in a radiographic infiltrate and rales detectable on auscultation, and hypoxemia results from alveolar filling. Moreover, some bacterial pathogens appear to interfere with the hypoxemic vasoconstriction that would normally occur with fluid-filled alveoli, and this interference can result in severe hypoxemia. Increased respiratory drive in the systemic inflammatory response syndrome (Chap. 325) leads to respiratory alkalosis. Decreased compliance due to capillary leak, hypoxemia, increased respiratory drive, increased secretions, and occasionally infection-related bronchospasm all lead to dyspnea. If severe enough, the changes in lung mechanics secondary to reductions in lung volume and compliance and the intrapulmonary shunting of blood may cause respiratory failure and the patient’s death.
PATHOLOGY
Classic pneumonia evolves through a series of pathologic changes. The initial phase is one of edema, with the presence of a proteinaceous exudate—and often of bacteria—in the alveoli. This phase is rarely evident in clinical or autopsy specimens because of the rapid transition to the red hepatization phase. The presence of erythrocytes in the cellular intraalveolar exudate gives this second stage its name, but neutrophil influx is more important with regard to host defense. Bacteria are occasionally seen in pathologic specimens collected during this phase. In the third phase, gray hepatization, no new erythrocytes are extravasating, and those already present have been lysed and degraded. The neutrophil is the predominant cell, fibrin deposition is abundant, and bacteria have disappeared. This phase corresponds with successful containment of the infection and improvement in gas exchange. In the final phase, resolution, the macrophage reappears as the dominant cell type in the alveolar space, and the debris of neutrophils, bacteria, and fibrin has been cleared, as has the inflammatory response.
This pattern has been described best for lobar pneumococcal pneumonia and may not apply to pneumonia of all etiologies, especially viral or Pneumocystis pneumonia. In VAP, respiratory bronchiolitis may precede the development of a radiologically apparent infiltrate. Because of the microaspiration mechanism, a bronchopneumonia pattern is most common in nosocomial pneumonias, whereas a lobar pattern is more common in bacterial CAP. Despite the radiographic appearance, viral and Pneumocystis pneumonias represent alveolar rather than interstitial processes.
COMMUNITY-ACQUIRED PNEUMONIA
ETIOLOGY
The extensive list of potential etiologic agents in CAP includes bacteria, fungi, viruses, and protozoa. Newly identified pathogens include metapneumoviruses, the coronaviruses responsible for severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome, and community-acquired strains of methicillin-resistant Staphylococcus aureus (MRSA). Most cases of CAP, however, are caused by relatively few pathogens (Table 153-2). Although Streptococcus pneumoniae is most common, other organisms also must be considered in light of the patient’s risk factors and severity of illness. Separation of potential agents into either “typical” bacterial pathogens or “atypical” organisms may be helpful. The former category includes S. pneumoniae, Haemophilus influenzae, and (in selected patients) S. aureus and gram-negative bacilli such as Klebsiella pneumoniae and Pseudomonas aeruginosa. The “atypical” organisms include Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella species (in inpatients) as well as respiratory viruses such as influenza viruses, adenoviruses, human metapneumovirus, and respiratory syncytial viruses. Viruses may be responsible for a large proportion of CAP cases that require hospital admission, even in adults. Atypical organisms cannot be cultured on standard media or seen on Gram’s stain. The frequency and importance of atypical pathogens have significant implications for therapy. These organisms are intrinsically resistant to all β-lactam agents and must be treated with a macrolide, a fluoroquinolone, or a tetracycline. In the ∼10–15% of CAP cases that are polymicrobial, the etiology usually includes a combination of typical and atypical pathogens.
|
MICROBIAL CAUSES OF COMMUNITY-ACQUIRED PNEUMONIA, BY SITE OF CARE |
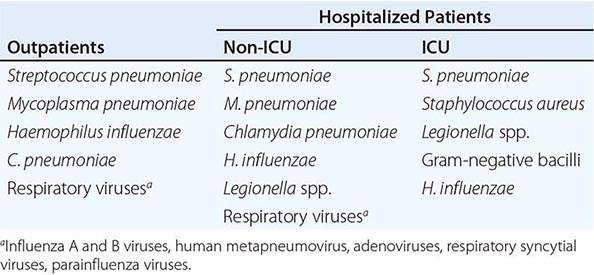
Note: Pathogens are listed in descending order of frequency. ICU, intensive care unit.
Anaerobes play a significant role only when an episode of aspiration has occurred days to weeks before presentation of pneumonia. The combination of an unprotected airway (e.g., in patients with alcohol or drug overdose or a seizure disorder) and significant gingivitis constitutes the major risk factor. Anaerobic pneumonias are often complicated by abscess formation and by significant empyemas or parapneumonic effusions.
S. aureus pneumonia is well known to complicate influenza infection. However, MRSA has been reported as the primary etiologic agent of CAP. While this entity is still relatively uncommon, clinicians must be aware of its potentially serious consequences, such as necrotizing pneumonia. Two important developments have led to this problem: the spread of MRSA from the hospital setting to the community and the emergence of genetically distinct strains of MRSA in the community. The former circumstance is more likely to result in HCAP, whereas the novel community-acquired MRSA (CA-MRSA) strains may infect healthy individuals with no association with health care.
Unfortunately, despite a careful history and physical examination as well as routine radiographic studies, the causative pathogen in a case of CAP is difficult to predict with any degree of certainty; in more than one-half of cases, a specific etiology is never determined. Nevertheless, epidemiologic and risk factors may suggest the involvement of certain pathogens (Table 153-3).
|
EPIDEMIOLOGIC FACTORS SUGGESTING POSSIBLE CAUSES OF COMMUNITY-ACQUIRED PNEUMONIA |
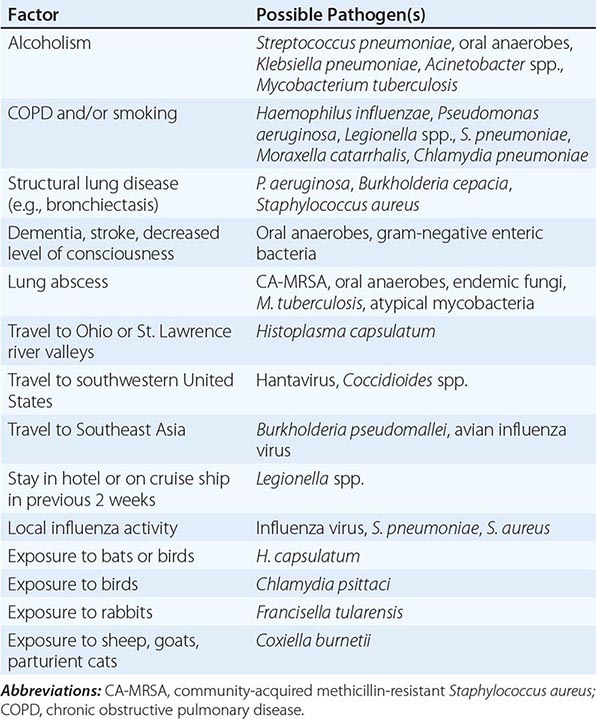
EPIDEMIOLOGY
More than 5 million CAP cases occur annually in the United States; usually, 80% of the affected patients are treated as outpatients and 20% as inpatients. The mortality rate among outpatients is usually ≤1%, whereas among hospitalized patients the rate can range from ∼12% to 40%, depending on whether treatment is provided in or outside of the intensive care unit (ICU). CAP results in more than 1.2 million hospitalizations and more than 55,000 deaths annually. The overall yearly cost associated with CAP is estimated at $12 billion. The incidence rates are highest at the extremes of age. The overall annual rate in the United States is 12 cases/1000 persons, but the figure increases to 12–18/1000 among children <4 years of age and to 20/1000 among persons >60 years of age.
The risk factors for CAP in general and for pneumococcal pneumonia in particular have implications for treatment regimens. Risk factors for CAP include alcoholism, asthma, immunosuppression, institutionalization, and an age of ≥70 years. In the elderly, factors such as decreased cough and gag reflexes as well as reduced antibody and Toll-like receptor responses increase the likelihood of pneumonia. Risk factors for pneumococcal pneumonia include dementia, seizure disorders, heart failure, cerebrovascular disease, alcoholism, tobacco smoking, chronic obstructive pulmonary disease, and HIV infection. CA-MRSA pneumonia is more likely in patients with skin colonization or infection with CA-MRSA. Enterobacteriaceae tend to infect patients who have recently been hospitalized and/or received antibiotic therapy or who have comorbidities such as alcoholism, heart failure, or renal failure. P. aeruginosa is a particular problem in patients with severe structural lung disease, such as bronchiectasis, cystic fibrosis, or severe chronic obstructive pulmonary disease. Risk factors for Legionella infection include diabetes, hematologic malignancy, cancer, severe renal disease, HIV infection, smoking, male gender, and a recent hotel stay or ship cruise. (Many of these risk factors would now reclassify as HCAP some cases that were previously designated CAP.)
CLINICAL MANIFESTATIONS
CAP can vary from indolent to fulminant in presentation and from mild to fatal in severity. Manifestations of progression and severity include both constitutional findings and those limited to the lung and associated structures. In light of the pathobiology of the disease, many of the findings are to be expected.
The patient is frequently febrile with tachycardia or may have a history of chills and/or sweats. Cough may be either nonproductive or productive of mucoid, purulent, or blood-tinged sputum. Gross hemoptysis is suggestive of CA-MRSA pneumonia. Depending on severity, the patient may be able to speak in full sentences or may be very short of breath. If the pleura is involved, the patient may experience pleuritic chest pain. Up to 20% of patients may have gastrointestinal symptoms such as nausea, vomiting, and/or diarrhea. Other symptoms may include fatigue, headache, myalgias, and arthralgias.
Findings on physical examination vary with the degree of pulmonary consolidation and the presence or absence of a significant pleural effusion. An increased respiratory rate and use of accessory muscles of respiration are common. Palpation may reveal increased or decreased tactile fremitus, and the percussion note can vary from dull to flat, reflecting underlying consolidated lung and pleural fluid, respectively. Crackles, bronchial breath sounds, and possibly a pleural friction rub may be heard on auscultation. The clinical presentation may not be so obvious in the elderly, who may initially display new-onset or worsening confusion and few other manifestations. Severely ill patients may have septic shock and evidence of organ failure.
DIAGNOSIS
When confronted with possible CAP, the physician must ask two questions: Is this pneumonia, and, if so, what is the likely etiology? The former question is typically answered by clinical and radiographic methods, whereas the latter requires the aid of laboratory techniques.
Clinical Diagnosis The differential diagnosis includes both infectious and noninfectious entities such as acute bronchitis, acute exacerbations of chronic bronchitis, heart failure, pulmonary embolism, hypersensitivity pneumonitis, and radiation pneumonitis. The importance of a careful history cannot be overemphasized. For example, known cardiac disease may suggest worsening pulmonary edema, while underlying carcinoma may suggest lung injury secondary to irradiation.
Unfortunately, the sensitivity and specificity of the findings on physical examination are less than ideal, averaging 58% and 67%, respectively. Therefore, chest radiography is often necessary to differentiate CAP from other conditions. Radiographic findings may include risk factors for increased severity (e.g., cavitation or multilobar involvement). Occasionally, radiographic results suggest an etiologic diagnosis. For example, pneumatoceles suggest infection with S. aureus, and an upper-lobe cavitating lesion suggests tuberculosis. CT may be of value in a patient with suspected postobstructive pneumonia caused by a tumor or foreign body or suspected cavitary disease. For outpatients, the clinical and radiologic assessments are usually all that is done before treatment for CAP is started since most laboratory results are not available soon enough to influence initial management significantly. In certain cases, the availability of rapid point-of-care outpatient diagnostic tests can be very important; for example, rapid diagnosis of influenza virus infection can prompt specific anti-influenza drug treatment and secondary prevention.
Etiologic Diagnosis The etiology of pneumonia usually cannot be determined solely on the basis of clinical presentation. Except for CAP patients admitted to the ICU, no data exist to show that treatment directed at a specific pathogen is statistically superior to empirical therapy. The benefit of establishing a microbial etiology can therefore be questioned, particularly in light of the cost of diagnostic testing. However, a number of reasons can be advanced for attempting an etiologic diagnosis. Identification of an unexpected pathogen allows narrowing of the initial empirical regimen, thereby decreasing antibiotic selection pressure and lessening the risk of resistance. Pathogens with important public safety implications, such as Mycobacterium tuberculosis and influenza virus, may be found in some cases. Finally, without culture and susceptibility data, trends in resistance cannot be followed accurately, and appropriate empirical therapeutic regimens are harder to devise.
GRAM’S STAIN AND CULTURE OF SPUTUM The main purpose of the sputum Gram’s stain is to ensure that a sample is suitable for culture. However, Gram’s staining may also identify certain pathogens (e.g., S. pneumoniae, S. aureus, and gram-negative bacteria) by their characteristic appearance. To be adequate for culture, a sputum sample must have >25 neutrophils and <10 squamous epithelial cells per low-power field. The sensitivity and specificity of the sputum Gram’s stain and culture are highly variable. Even in cases of proven bacteremic pneumococcal pneumonia, the yield of positive cultures from sputum samples is ≤50%.
Some patients, particularly elderly individuals, may not be able to produce an appropriate expectorated sputum sample. Others may already have started a course of antibiotics that can interfere with culture results at the time a sample is obtained. Inability to produce sputum can be a consequence of dehydration, and the correction of this condition may result in increased sputum production and a more obvious infiltrate on chest radiography. For patients admitted to the ICU and intubated, a deep-suction aspirate or bronchoalveolar lavage sample (obtained either via bronchoscopy or non-bronchoscopically) has a high yield on culture when sent to the microbiology laboratory as soon as possible. Since the etiologies in severe CAP are somewhat different from those in milder disease (Table 153-2), the greatest benefit of staining and culturing respiratory secretions is to alert the physician of unsuspected and/or resistant pathogens and to permit appropriate modification of therapy. Other stains and cultures (e.g., specific stains for M. tuberculosis or fungi) may be useful as well.
BLOOD CULTURES The yield from blood cultures, even when samples are collected before antibiotic therapy, is disappointingly low. Only 5–14% of cultures of blood from patients hospitalized with CAP are positive, and the most frequently isolated pathogen is S. pneumoniae. Since recommended empirical regimens all provide pneumococcal coverage, a blood culture positive for this pathogen has little, if any, effect on clinical outcome. However, susceptibility data may allow narrowing of antibiotic therapy in appropriate cases. Because of the low yield and the lack of significant impact on outcome, blood cultures are no longer considered de rigueur for all hospitalized CAP patients. Certain high-risk patients—including those with neutropenia secondary to pneumonia, asplenia, complement deficiencies, chronic liver disease, or severe CAP—should have blood cultured.
URINARY ANTIGEN TESTS Two commercially available tests detect pneumococcal and Legionella antigen in urine. The test for Legionella pneumophila detects only serogroup 1, but this serogroup accounts for most community-acquired cases of Legionnaires’ disease in the United States. The sensitivity and specificity of the Legionella urine antigen test are as high as 90% and 99%, respectively. The pneumococcal urine antigen test also is quite sensitive and specific (80% and >90%, respectively). Although false-positive results can be obtained with samples from pneumococcus-colonized children, the test is generally reliable. Both tests can detect antigen even after the initiation of appropriate antibiotic therapy.
POLYMERASE CHAIN REACTION Polymerase chain reaction (PCR) tests, which amplify a microorganism’s DNA or RNA, are available for a number of pathogens. PCR of nasopharyngeal swabs has become the standard for diagnosis of respiratory viral infection. In addition, PCR can detect the nucleic acid of Legionella species, M. pneumoniae, C. pneumoniae, and mycobacteria. In patients with pneumococcal pneumonia, an increased bacterial load in whole blood documented by PCR is associated with an increased risk of septic shock, the need for mechanical ventilation, and death. Clinical availability of such a test could conceivably help identify patients suitable for ICU admission.
SEROLOGY A fourfold rise in specific IgM antibody titer between acute- and convalescent-phase serum samples is generally considered diagnostic of infection with the pathogen in question. In the past, serologic tests were used to help identify atypical pathogens as well as selected unusual organisms such as Coxiella burnetii. Recently, however, they have fallen out of favor because of the time required to obtain a final result for the convalescent-phase sample.
BIOMARKERS A number of substances can serve as markers of severe inflammation. The two currently in use are C-reactive protein (CRP) and procalcitonin (PCT). Levels of these acute-phase reactants increase in the presence of an inflammatory response, particularly to bacterial pathogens. CRP may be of use in the identification of worsening disease or treatment failure, and PCT may play a role in determining the need for antibacterial therapy. These tests should not be used on their own but, when interpreted in conjunction with other findings from the history, physical examination, radiology, and laboratory tests, may help with antibiotic stewardship and appropriate management of seriously ill patients with CAP.
|
TREATMENT |
COMMUNITY-ACQUIRED PNEUMONIA |
SITE OF CARE
The cost of inpatient management exceeds that of outpatient treatment by a factor of 20, and hospitalization accounts for most CAP-related expenditures. Thus the decision to admit a patient with CAP to the hospital has considerable implications. Certain patients clearly can be managed at home, and others clearly require treatment in the hospital, but the choice is sometimes difficult. Tools that objectively assess the risk of adverse outcomes, including severe illness and death, can minimize unnecessary hospital admissions. There are currently two sets of criteria: the Pneumonia Severity Index (PSI), a prognostic model used to identify patients at low risk of dying; and the CURB-65 criteria, a severity-of-illness score.
To determine the PSI, points are given for 20 variables, including age, coexisting illness, and abnormal physical and laboratory findings. On the basis of the resulting score, patients are assigned to one of five classes with the following mortality rates: class 1, 0.1%; class 2, 0.6%; class 3, 2.8%; class 4, 8.2%; and class 5, 29.2%. Determination of the PSI is often impractical in a busy emergency-department setting because of the number of variables that must be assessed. However, clinical trials demonstrate that routine use of the PSI results in lower admission rates for class 1 and class 2 patients. Patients in class 3 could ideally be admitted to an observation unit until a further decision can be made.
The CURB-65 criteria include five variables: confusion (C); urea >7 mmol/L (U); respiratory rate ≥30/min (R); blood pressure, systolic ≤90 mmHg or diastolic ≤60 mmHg (B); and age ≥65 years. Patients with a score of 0, among whom the 30-day mortality rate is 1.5%, can be treated outside the hospital. With a score of 2, the 30-day mortality rate is 9.2%, and patients should be admitted to the hospital. Among patients with scores of ≥3, mortality rates are 22% overall; these patients may require ICU admission.
It is not clear which assessment tool is superior. Whichever system is used, these objective criteria must always be tempered by careful consideration of factors relevant to individual patients, including the ability to comply reliably with an oral antibiotic regimen and the resources available to the patient outside the hospital.
Neither PSI nor CURB-65 is accurate in determining the need for ICU admission. Septic shock or respiratory failure in the emergency department is an obvious indication for ICU care. However, mortality rates are higher among less ill patients who are admitted to the floor and then deteriorate than among equally ill patients monitored in the ICU. A variety of scores have been proposed to identify patients most likely to have early deterioration (Table 153-4). Most factors in these scores are similar to the minor severity criteria proposed by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) in their guidelines for the management of CAP.
|
RISK FACTORS FOR EARLY DETERIORATION IN CAP |
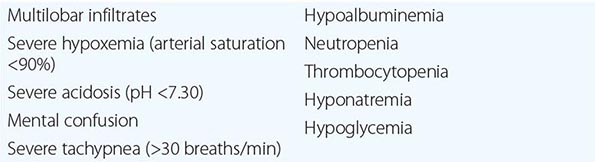
ANTIBIOTIC RESISTANCE
Antimicrobial resistance is a significant problem that threatens to diminish our therapeutic armamentarium. Misuse of antibiotics results in increased antibiotic selection pressure that can affect resistance locally or even globally by clonal dissemination. For CAP, the main resistance issues currently involve S. pneumoniae and CA-MRSA.
S. pneumoniae In general, pneumococcal resistance is acquired (1) by direct DNA incorporation and remodeling resulting from contact with closely related oral commensal bacteria, (2) by the process of natural transformation, or (3) by mutation of certain genes.
The minimal inhibitory concentration (MIC) cutoffs for penicillin in pneumonia are ≤2 μg/mL for susceptibility, >2–4 μg/mL for intermediate, and ≥8 μg/mL for resistant. A change in susceptibility thresholds resulted in a dramatic decrease in the proportion of pneumococcal isolates considered nonsusceptible. For meningitis, MIC thresholds remain at the former higher levels. Fortunately, resistance to penicillin appeared to plateau even before the change in MIC thresholds. Pneumococcal resistance to β-lactam drugs is due solely to low-affinity penicillin-binding proteins. Risk factors for penicillin-resistant pneumococcal infection include recent antimicrobial therapy, an age of <2 years or >65 years, attendance at day-care centers, recent hospitalization, and HIV infection.
![]() In contrast to penicillin resistance, resistance to macrolides is increasing through several mechanisms. Target-site modification caused by ribosomal methylation in 23S rRNA encoded by the ermB gene results in high-level resistance (MICs, ≥64 μg/mL) to macrolides, lincosamides, and streptogramin B–type antibiotics. The efflux mechanism encoded by the mef gene (M phenotype) is usually associated with low-level resistance (MICs, 1–32 μg/mL). These two mechanisms account for ∼45% and ∼65%, respectively, of resistant pneumococcal isolates in the United States. High-level resistance to macrolides is more common in Europe, whereas lower-level resistance predominates in North America.
In contrast to penicillin resistance, resistance to macrolides is increasing through several mechanisms. Target-site modification caused by ribosomal methylation in 23S rRNA encoded by the ermB gene results in high-level resistance (MICs, ≥64 μg/mL) to macrolides, lincosamides, and streptogramin B–type antibiotics. The efflux mechanism encoded by the mef gene (M phenotype) is usually associated with low-level resistance (MICs, 1–32 μg/mL). These two mechanisms account for ∼45% and ∼65%, respectively, of resistant pneumococcal isolates in the United States. High-level resistance to macrolides is more common in Europe, whereas lower-level resistance predominates in North America.
Pneumococcal resistance to fluoroquinolones (e.g., ciprofloxacin and levofloxacin) has been reported. Changes can occur in one or both target sites (topoisomerases II and IV) from mutations in the gyrA and parC genes, respectively. In addition, an efflux pump may play a role in pneumococcal resistance to fluoroquinolones.
Isolates resistant to drugs from three or more antimicrobial classes with different mechanisms of action are considered MDR strains. The propensity for an association of pneumococcal resistance to penicillin with reduced susceptibility to other drugs, such as macrolides, tetracyclines, and trimethoprim-sulfamethoxazole, also is of concern. In the United States, 58.9% of penicillin-resistant pneumococcal isolates from blood are also resistant to macrolides.
The most important risk factor for antibiotic-resistant pneumococcal infection is use of a specific antibiotic within the previous 3 months. Therefore, a patient’s history of prior antibiotic treatment is a critical factor in avoiding the use of an inappropriate antibiotic.
CA-MRSA CAP due to MRSA may be caused by the classic hospital-acquired strains or by the more recently identified, genotypically and phenotypically distinct community-acquired strains. Most infections with the former strains have been acquired either directly or indirectly by contact with the health care environment and would now be classified as HCAP. However, in some hospitals, the CA-MRSA strains are displacing the classic hospital-acquired strains—a trend suggesting that the newer strains may be more robust and blurring this distinction.
![]() Methicillin resistance in S. aureus is determined by the mecA gene, which encodes for resistance to all β-lactam drugs. At least five staphylococcal chromosomal cassette mec (SCCmec) types have been described. The typical hospital-acquired strain usually has type II or III, whereas CA-MRSA has a type IV SCCmec element. CA-MRSA isolates tend to be less resistant than the older hospital-acquired strains and are often susceptible to trimethoprim-sulfamethoxazole, clindamycin, and tetracycline in addition to vancomycin and linezolid. However, the most important distinction is that CA-MRSA strains also carry genes for superantigens, such as enterotoxins B and C and Panton-Valentine leukocidin, a membrane-tropic toxin that can create cytolytic pores in polymorphonuclear neutrophils, monocytes, and macrophages.
Methicillin resistance in S. aureus is determined by the mecA gene, which encodes for resistance to all β-lactam drugs. At least five staphylococcal chromosomal cassette mec (SCCmec) types have been described. The typical hospital-acquired strain usually has type II or III, whereas CA-MRSA has a type IV SCCmec element. CA-MRSA isolates tend to be less resistant than the older hospital-acquired strains and are often susceptible to trimethoprim-sulfamethoxazole, clindamycin, and tetracycline in addition to vancomycin and linezolid. However, the most important distinction is that CA-MRSA strains also carry genes for superantigens, such as enterotoxins B and C and Panton-Valentine leukocidin, a membrane-tropic toxin that can create cytolytic pores in polymorphonuclear neutrophils, monocytes, and macrophages.
Gram-Negative Bacilli A detailed discussion of resistance among gram-negative bacilli is beyond the scope of this chapter (Chap. 186). Fluoroquinolone resistance among isolates of Escherichia coli from the community appears to be increasing. Enterobacter species are typically resistant to cephalosporins; the drugs of choice for use against these bacteria are usually fluoroquinolones or carbapenems. Similarly, when infections due to bacteria producing extended-spectrum β-lactamases are documented or suspected, a fluoroquinolone or a carbapenem should be used; these MDR strains are more likely to be involved in HCAP.
INITIAL ANTIBIOTIC MANAGEMENT
Since the etiology of CAP is rarely known at the outset of treatment, initial therapy is usually empirical, designed to cover the most likely pathogens (Table 153-5). In all cases, antibiotic treatment should be initiated as expeditiously as possible. The CAP treatment guidelines in the United States (summarized in Table 153-5) represent joint statements from the IDSA and the ATS; the Canadian guidelines come from the Canadian Infectious Disease Society and the Canadian Thoracic Society. In all these guidelines, coverage is always provided for the pneumococcus and the atypical pathogens. In contrast, guidelines from some European countries do not always include atypical coverage based on local epidemiologic data. The U.S./Canadian approach is supported by retrospective data derived from administrative databases including thousands of patients. Atypical pathogen coverage provided by the addition of a macrolide to a cephalosporin or by the use of a fluoroquinolone alone has been consistently associated with a significant reduction in mortality rates compared with those for β-lactam coverage alone.
|
EMPIRICAL ANTIBIOTIC TREATMENT OF COMMUNITY-ACQUIRED PNEUMONIA |
aDoxycycline (100 mg PO bid) is an alternative to the macrolide. bMICs of >16 μg/mL in 25% of isolates. cA respiratory fluoroquinolone should be used for penicillin-allergic patients. dDoxycycline (100 mg IV q12h) is an alternative to the macrolide. eFor penicillin-allergic patients, use a respiratory fluoroquinolone and aztreonam (2 g IV q8h). fFor penicillin-allergic patients, substitute aztreonam.
Abbreviations: CA-MRSA, community-acquired methicillin-resistant Staphylococcus aureus; ICU, intensive care unit.
Therapy with a macrolide or a fluoroquinolone within the previous 3 months is associated with an increased likelihood of infection with a resistant strain of S. pneumoniae. For this reason, a fluoroquinolone-based regimen should be used for patients recently given a macrolide, and vice versa (Table 153-5).
Once the etiologic agent(s) and susceptibilities are known, therapy may be altered to target the specific pathogen(s). However, this decision is not always straightforward. If blood cultures yield S. pneumoniae sensitive to penicillin after 2 days of treatment with a macrolide plus a β-lactam or with a fluoroquinolone alone, should therapy be switched to penicillin alone? The concern here is that a β-lactam alone would not be effective in the potential 15% of cases with atypical co-infection. No standard approach exists. In all cases, the individual patient and the various risk factors must be considered.
Management of bacteremic pneumococcal pneumonia also is controversial. Data from nonrandomized studies suggest that combination therapy (especially macrolide/β-lactam) is associated with a lower mortality rate than monotherapy, particularly in severely ill patients. The exact reason is unknown, but possible explanations include an additive or synergistic antibacterial effect, antimicrobial tolerance, atypical co-infection, or the immunomodulatory effects of the macrolides.
For CAP patients admitted to the ICU, the risk of infection with P. aeruginosa or CA-MRSA is increased. Empirical coverage should be considered when a patient has risk factors or a Gram’s stain suggestive of these pathogens (Table 153–5). If CA-MRSA is suspected, either linezolid or vancomycin can be added to the initial empirical regimen; however, there is increasing concern about vancomycin’s loss of potency against MRSA, poor penetration into epithelial lining fluid, and lack of effect on toxin production relative to linezolid.
Although hospitalized patients have traditionally received initial therapy by the IV route, some drugs—particularly the fluoroquinolones—are very well absorbed and can be given orally from the outset to select patients. For patients initially treated IV, a switch to oral treatment is appropriate as long as the patient can ingest and absorb the drugs, is hemodynamically stable, and is showing clinical improvement.
The duration of treatment for CAP has generated considerable interest. Patients were previously treated for 10–14 days, but studies with fluoroquinolones and telithromycin suggest that a 5-day course is sufficient for otherwise uncomplicated CAP. Even a single dose of ceftriaxone has been associated with a significant cure rate. A longer course may be required for patients with bacteremia, metastatic infection, or infection with a virulent pathogen such as P. aeruginosa or CA-MRSA.
GENERAL CONSIDERATIONS
In addition to appropriate antimicrobial therapy, certain general considerations apply in dealing with CAP, HCAP, or HAP/VAP. Adequate hydration, oxygen therapy for hypoxemia, and assisted ventilation when necessary are critical to successful treatment. Patients with severe CAP who remain hypotensive despite fluid resuscitation may have adrenal insufficiency and may respond to glucocorticoid treatment. The value of adjunctive therapy, such as glucocorticoids, statins, and angiotensin-converting enzyme inhibitors, remains unproven in the management of CAP.
Failure to Improve Patients slow to respond to therapy should be reevaluated at about day 3 (sooner if their condition is worsening rather than simply not improving), and several possible scenarios should be considered. A number of noninfectious conditions mimic pneumonia, including pulmonary edema, pulmonary embolism, lung carcinoma, radiation and hypersensitivity pneumonitis, and connective tissue disease involving the lungs. If the patient truly has CAP and empirical treatment is aimed at the correct pathogen, lack of response may be explained in a number of ways. The pathogen may be resistant to the drug selected, or a sequestered focus (e.g., lung abscess or empyema) may be blocking access of the antibiotic(s) to the pathogen. The patient may be getting either the wrong drug or the correct drug at the wrong dose or frequency of administration. Another possibility is that CAP is the correct diagnosis but an unsuspected pathogen (e.g., CA-MRSA, M. tuberculosis, or a fungus) is the cause. Nosocomial superinfections—both pulmonary and extrapulmonary—are other possible explanations for a patient’s failure to improve or deterioration. In all cases of delayed response or deteriorating condition, the patient must be carefully reassessed and appropriate studies initiated, possibly including such diverse procedures as CT or bronchoscopy.
Complications As in other severe infections, common complications of severe CAP include respiratory failure, shock and multiorgan failure, coagulopathy, and exacerbation of comorbid illnesses. Three particularly noteworthy conditions are metastatic infection, lung abscess, and complicated pleural effusion. Metastatic infection (e.g., brain abscess or endocarditis) is very unusual and will require a high degree of suspicion and a detailed workup for proper treatment. Lung abscess may occur in association with aspiration or with infection caused by a single CAP pathogen, such as CA-MRSA, P. aeruginosa, or (rarely) S. pneumoniae. Aspiration pneumonia is typically a polymicrobial infection involving both aerobes and anaerobes. A significant pleural effusion should be tapped for both diagnostic and therapeutic purposes. If the fluid has a pH of <7, a glucose level of <2. 2 mmol/L, and a lactate dehydrogenase concentration of >1000 U/L or if bacteria are seen or cultured, then it should be completely drained; a chest tube is often required and video-assisted thoracoscopy may be needed for late treatment or difficult cases.
Follow-Up Fever and leukocytosis usually resolve within 2–4 days in otherwise healthy patients with CAP, but physical findings may persist longer. Chest radiographic abnormalities are slowest to resolve (4–12 weeks), with the speed of clearance depending on the patient’s age and underlying lung disease. Patients may be discharged from the hospital once their clinical conditions, including comorbidities, are stable. The site of residence after discharge (nursing home, home with family, home alone) is an important discharge timing consideration, particularly for elderly patients. For a hospitalized patient, a follow-up radiograph ∼4–6 weeks later is recommended. If relapse or recurrence is documented, particularly in the same lung segment, the possibility of an underlying neoplasm must be considered.
PROGNOSIS
The prognosis of CAP depends on the patient’s age, comorbidities, and site of treatment (inpatient or outpatient). Young patients without comorbidity do well and usually recover fully after ~2 weeks. Older patients and those with comorbid conditions can take several weeks longer to recover fully. The overall mortality rate for the outpatient group is <1%. For patients requiring hospitalization, the overall mortality rate is estimated at 10%, with ~50% of deaths directly attributable to pneumonia.
PREVENTION
The main preventive measure is vaccination (Chap. 148). Recommendations of the Advisory Committee on Immunization Practices should be followed for influenza and pneumococcal vaccines.
A pneumococcal polysaccharide vaccine (PPV23) and a protein conjugate pneumococcal vaccine (PCV13) are available in the United States. The former product contains capsular material from 23 pneumococcal serotypes; in the latter, capsular polysaccharide from 13 of the most frequent pneumococcal pathogens affecting children is linked to an immunogenic protein. PCV13 produces T cell–dependent antigens that result in long-term immunologic memory. Administration of this vaccine to children has led to an overall decrease in the prevalence of antimicrobial-resistant pneumococci and in the incidence of invasive pneumococcal disease among both children and adults. However, vaccination can be followed by the replacement of vaccine serotypes with nonvaccine serotypes, as was seen with serotypes 19A and 35B after introduction of the original 7-valent conjugate vaccine. PCV13 now is also recommended for the elderly and for younger immunocompromised patients. Because of an increased risk of pneumococcal infection, even among patients without obstructive lung disease, smokers should be strongly encouraged to stop smoking.
Two forms of influenza vaccine are available: intramuscular inactivated vaccine and intranasal live-attenuated cold-adapted vaccine. The latter is contraindicated in immunocompromised patients. In the event of an influenza outbreak, unprotected patients at risk from complications should be vaccinated immediately and given chemoprophylaxis with either oseltamivir or zanamivir for 2 weeks—i.e., until vaccine-induced antibody levels are sufficiently high.
HEALTH CARE–ASSOCIATED PNEUMONIA
HCAP represents a transition between classic CAP and typical HAP. The definition of HCAP is still in some flux because of a lack of consistent large-scale studies. Several early studies were limited to patients with culture-positive pneumonia. In these studies, the incidence of MDR pathogens in HCAP was as high as or higher than in HAP/VAP. MRSA in particular was more common in HCAP than in traditional HAP/VAP. Conversely, prospective studies in nontertiary-care centers have found a low incidence of MDR pathogens in HCAP.
The patients at greatest risk for HCAP are not well defined. Patients from nursing homes are not always at elevated risk for infection with MDR pathogens. Careful evaluation of nursing home residents with pneumonia suggests that their risk of MDR infection is low if they have not recently received antibiotics and are independent in most activities of daily living. Recent hospitalization (i.e., in the preceding 90 days) is also a major risk factor for infection with MDR pathogens. Conversely, nursing home patients are at increased risk of infection with influenza virus and other atypical pneumonia pathogens. Undue concern about MDR pathogens occasionally results in failure to cover atypical pathogens when treating nursing home patients. In addition, patients receiving home infusion therapy or undergoing chronic dialysis are probably at particular risk for MRSA pneumonia but may not be at greater risk for infection with Pseudomonas or Acinetobacter than are other patients who develop CAP.
In general, the management of HCAP due to MDR pathogens is similar to that of MDR HAP/VAP. This topic will therefore be covered in subsequent sections on HAP and VAP. The prognosis of HCAP is intermediate between that of CAP and VAP and is closer to that of HAP.
VENTILATOR-ASSOCIATED PNEUMONIA
Most hospital-acquired pneumonia research has focused on VAP. However, the information and principles based on this research can be applied to non-ICU HAP and HCAP as well. The greatest difference between VAP and HCAP/HAP studies is the dependence on expectorated sputum for a microbiologic diagnosis of VAP (as for that of CAP), which is further complicated by frequent colonization by pathogens in patients with HAP or HCAP. Therefore, most of the literature has focused on HCAP or HAP resulting in intubation, where, once again, access to the lower respiratory tract facilitates an etiologic diagnosis.
Etiology Potential etiologic agents of VAP include both MDR and non-MDR bacterial pathogens (Table 153-6). The non-MDR group is nearly identical to the pathogens found in severe CAP (Table 153-2); it is not surprising that such pathogens predominate if VAP develops in the first 5–7 days of the hospital stay. However, if patients have other risk factors for HCAP, MDR pathogens are a consideration, even early in the hospital course. The relative frequency of individual MDR pathogens can vary significantly from hospital to hospital and even between different critical care units within the same institution. Most hospitals have problems with P. aeruginosa and MRSA, but other MDR pathogens are often institution-specific. Less commonly, fungal and viral pathogens cause VAP, usually affecting severely immunocompromised patients. Rarely, community-associated viruses cause mini-epidemics, usually when introduced by ill health care workers.
|
MICROBIOLOGIC CAUSES OF VENTILATOR-ASSOCIATED PNEUMONIA |
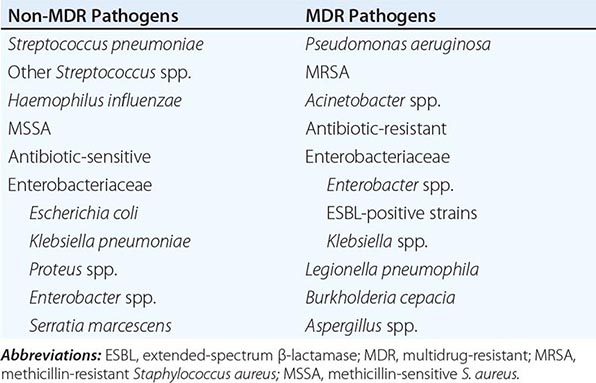
Epidemiology Pneumonia is a common complication among patients requiring mechanical ventilation. Prevalence estimates vary between 6 and 52 cases per 100 patients, depending on the population studied. On any given day in the ICU, an average of 10% of patients will have pneumonia—VAP in the overwhelming majority of cases. The frequency of diagnosis is not static but changes with the duration of mechanical ventilation, with the highest hazard ratio in the first 5 days and a plateau in additional cases (1% per day) after ~2 weeks. However, the cumulative rate among patients who remain ventilated for as long as 30 days is as high as 70%. These rates often do not reflect the recurrence of VAP in the same patient. Once a ventilated patient is transferred to a chronic-care facility or to home, the incidence of pneumonia drops significantly, especially in the absence of other risk factors for pneumonia. However, in chronic ventilator units, purulent tracheobronchitis becomes a significant issue, often interfering with efforts to wean patients off mechanical ventilation (Chap. 323).
Three factors are critical in the pathogenesis of VAP: colonization of the oropharynx with pathogenic microorganisms, aspiration of these organisms from the oropharynx into the lower respiratory tract, and compromise of the normal host defense mechanisms. Most risk factors and their corresponding prevention strategies pertain to one of these three factors (Table 153-7).
|
PATHOGENIC MECHANISMS AND CORRESPONDING PREVENTION STRATEGIES FOR VENTILATOR-ASSOCIATED PNEUMONIA |
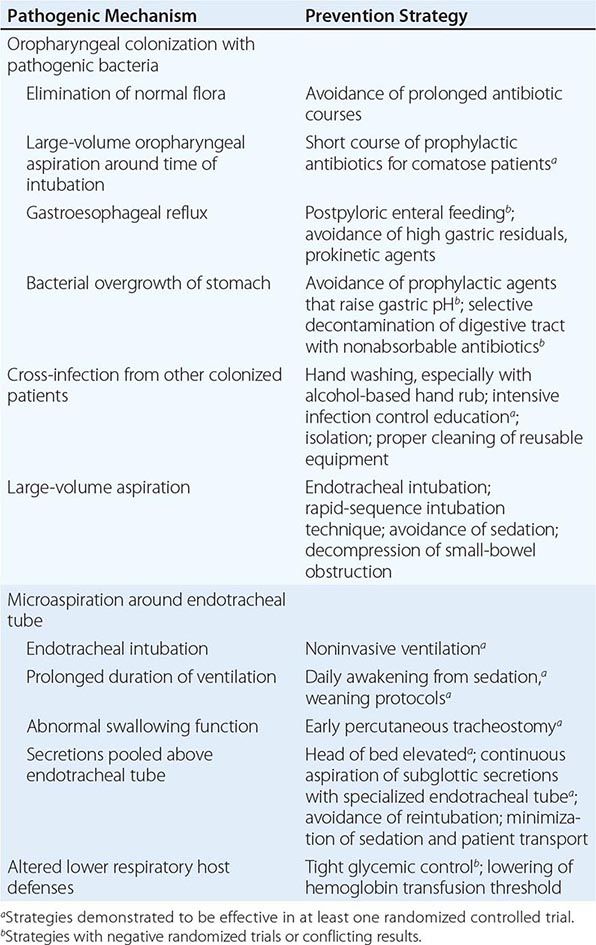
The most obvious risk factor is the endotracheal tube, which bypasses the normal mechanical factors preventing aspiration. While the presence of an endotracheal tube may prevent large-volume aspiration, microaspiration is actually exacerbated by secretions pooling above the cuff. The endotracheal tube and the concomitant need for suctioning can damage the tracheal mucosa, thereby facilitating tracheal colonization. In addition, pathogenic bacteria can form a glycocalyx biofilm on the tube’s surface that protects them from both antibiotics and host defenses. The bacteria can also be dislodged during suctioning and can reinoculate the trachea, or tiny fragments of glycocalyx can embolize to distal airways, carrying bacteria with them.
In a high percentage of critically ill patients, the normal oropharyngeal flora is replaced by pathogenic microorganisms. The most important risk factors are antibiotic selection pressure, cross-infection from other infected/colonized patients or contaminated equipment, and malnutrition. Of these factors, antibiotic exposure poses the greatest risk by far. Pathogens such as P. aeruginosa almost never cause infection in patients without prior exposure to antibiotics. The recent emphasis on hand hygiene has lowered the cross-infection rate.
How the lower respiratory tract defenses become overwhelmed remains poorly understood. Almost all intubated patients experience microaspiration and are at least transiently colonized with pathogenic bacteria. However, only around one-third of colonized patients develop VAP. Colony counts increase to high levels, sometimes days before the development of clinical pneumonia; these increases suggest that the final step in VAP development, independent of aspiration and oropharyngeal colonization, is the overwhelming of host defenses. Severely ill patients with sepsis and trauma appear to enter a state of immunoparalysis several days after admission to the ICU—a time that corresponds to the greatest risk of developing VAP. The mechanism of this immunosuppression is not clear, although several factors have been suggested. Hyperglycemia affects neutrophil function, and trials suggest that keeping the blood sugar level close to normal with exogenous insulin may have beneficial effects, including a decreased risk of infection. More frequent transfusions also adversely affect the immune response.
Clinical Manifestations The clinical manifestations are generally the same in VAP as in all other forms of pneumonia: fever, leukocytosis, increase in respiratory secretions, and pulmonary consolidation on physical examination, along with a new or changing radiographic infiltrate. The frequency of abnormal chest radiographs before the onset of pneumonia in intubated patients and the limitations of portable radiographic technique make interpretation of radiographs more difficult than in patients who are not intubated. Other clinical features may include tachypnea, tachycardia, worsening oxygenation, and increased minute ventilation.
Diagnosis No single set of criteria is reliably diagnostic of pneumonia in a ventilated patient. The inability to identify such patients compromises efforts to prevent and treat VAP and even calls into question estimates of the impact of VAP on mortality rates.
Application of clinical criteria consistently results in overdiagnosis of VAP, largely because of three common findings in at-risk patients: (1) tracheal colonization with pathogenic bacteria in patients with endotracheal tubes, (2) multiple alternative causes of radiographic infiltrates in mechanically ventilated patients, and (3) the high frequency of other sources of fever in critically ill patients. The differential diagnosis of VAP includes a number of entities such as atypical pulmonary edema, pulmonary contusion, alveolar hemorrhage, hypersensitivity pneumonitis, acute respiratory distress syndrome, and pulmonary embolism. Clinical findings in ventilated patients with fever and/or leukocytosis may have alternative causes, including antibiotic-associated diarrhea, sinusitis, urinary tract infection, pancreatitis, and drug fever. Conditions mimicking pneumonia are often documented in patients in whom VAP has been ruled out by accurate diagnostic techniques. Most of these alternative diagnoses do not require antibiotic treatment; require antibiotics different from those used to treat VAP; or require some additional intervention, such as surgical drainage or catheter removal, for optimal management.
This diagnostic dilemma has led to debate and controversy. The major question is whether a quantitative-culture approach as a means of eliminating false-positive clinical diagnoses is superior to the clinical approach enhanced by principles learned from quantitative-culture studies. The most recent IDSA/ATS guidelines for HAP/VAP suggest that either approach is clinically valid.
QUANTITATIVE-CULTURE APPROACH The essence of the quantitative-culture approach is to discriminate between colonization and true infection by determining the bacterial burden. The more distal in the respiratory tree the diagnostic sampling, the more specific the results and therefore the lower the threshold of growth necessary to diagnose pneumonia and exclude colonization. For example, a quantitative endotracheal aspirate yields proximate samples, and the diagnostic threshold is 106 cfu/mL. The protected specimen brush method, in contrast, obtains distal samples and has a threshold of 103 cfu/mL. Conversely, sensitivity declines as more distal secretions are obtained, especially when they are collected blindly (i.e., by a technique other than bronchoscopy). Additional tests that may increase the diagnostic yield include Gram’s staining, differential cell counts, staining for intracellular organisms, and detection of local protein levels elevated in response to infection.
The Achilles heel of the quantitative approach is the effect of antibiotic therapy. With sensitive microorganisms, a single antibiotic dose can reduce colony counts below the diagnostic threshold. Recent changes in antibiotic therapy are the most significant. After 3 days, the operating characteristics of the tests are almost the same as if no antibiotic therapy has been given. Conversely, colony counts above the diagnostic threshold during antibiotic therapy suggest that the current antibiotics are ineffective. Even the normal host response may be sufficient to reduce quantitative-culture counts below the diagnostic threshold if sampling is delayed. In short, expertise in quantitative-culture techniques is critical, with a specimen obtained as soon as pneumonia is suspected and before antibiotic therapy is initiated or changed.
In a study comparing the quantitative with the clinical approach, use of bronchoscopic quantitative cultures resulted in significantly less antibiotic use at 14 days after study entry and in lower rates of mortality and severity-adjusted mortality at 28 days. In addition, more alternative sites of infection were found in patients randomized to the quantitative-culture strategy. A critical aspect of this study was that antibiotic treatment was initiated only in patients whose gram-stained respiratory sample was positive or who displayed signs of hemodynamic instability. Fewer than one-half as many patients were treated for pneumonia in the bronchoscopy group, and only one-third as many microorganisms were cultured.
CLINICAL APPROACH The lack of specificity of a clinical diagnosis of VAP has led to efforts to improve the diagnostic criteria. The Clinical Pulmonary Infection Score (CPIS) was developed by weighting of the various clinical criteria usually used for the diagnosis of VAP (Table 153-8). Use of the CPIS allows the selection of low-risk patients who may need only short-course antibiotic therapy or no treatment at all. Moreover, studies have demonstrated that the absence of bacteria in gram-stained endotracheal aspirates makes pneumonia an unlikely cause of fever or pulmonary infiltrates. These findings, coupled with a heightened awareness of the alternative diagnoses possible in patients with suspected VAP, can prevent inappropriate overtreatment for pneumonia. Furthermore, data show that the absence of an MDR pathogen in tracheal aspirate cultures eliminates the need for MDR coverage when empirical antibiotic therapy is narrowed. Since the most likely explanations for the mortality benefit of bronchoscopic quantitative cultures are decreased antibiotic selection pressure (which reduces the risk of subsequent infection with MDR pathogens) and identification of alternative sources of infection, a clinical diagnostic approach that incorporates such principles may result in similar outcomes.
|
CLINICAL PULMONARY INFECTION SCORE (CPIS) |
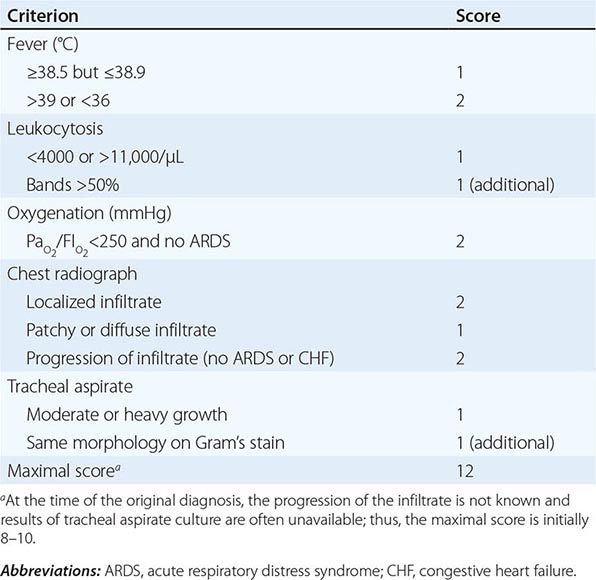
Other large randomized studies that did not demonstrate a similar beneficial impact of quantitative culture on outcomes did not tightly link antibiotic treatment to the results of quantitative culture and other tests. Given the conflicting results only partially explained by methodologic issues, the IDSA/ATS guidelines therefore suggest that the choice depends on availability and local expertise.
|
TREATMENT |
VENTILATOR-ASSOCIATED PNEUMONIA |
Many studies have demonstrated higher mortality rates with initially inappropriate empirical antibiotic therapy. The key to appropriate antibiotic management of VAP is an appreciation of the resistance patterns of the most likely pathogens in a given patient.
ANTIBIOTIC RESISTANCE
If not for the higher risk of infection with MDR pathogens (Table 153-1), VAP could be treated with the same antibiotics used for severe CAP. However, antibiotic selection pressure leads to the frequent involvement of MDR pathogens by selecting either for drug-resistant isolates of common pathogens (MRSA and Enterobacteriaceae producing extended-spectrum β-lactamases or carbapenemases) or for intrinsically resistant pathogens (P. aeruginosa and Acinetobacter species). Frequent use of β-lactam drugs, especially cephalosporins, appears to be the major risk factor for infection with MRSA and extended-spectrum β-lactamase–positive strains.
P. aeruginosa has demonstrated the ability to develop resistance to all routinely used antibiotics. Unfortunately, even if initially sensitive, P. aeruginosa isolates also have a propensity to develop resistance during treatment. Either de-repression of resistance genes or selection of resistant clones within the large bacterial inoculum associated with most pneumonias may be the cause. Acinetobacter species, Stenotrophomonas maltophilia, and Burkholderia cepacia are intrinsically resistant to many of the empirical antibiotic regimens employed (see later in this chapter). VAP caused by these pathogens emerges during treatment of other infections, and resistance is always evident at initial diagnosis.
EMPIRICAL THERAPY
Recommended options for empirical therapy are listed in Table 153-9. Treatment should be started once diagnostic specimens have been obtained. The major factor in the selection of agents is the presence of risk factors for MDR pathogens. Choices among the various options listed depend on local patterns of resistance and—a very important factor—the patient’s prior antibiotic exposure.
|
EMPIRICAL ANTIBIOTIC TREATMENT OF HEALTH CARE–ASSOCIATED PNEUMONIA |
Abbreviation: MDR, multidrug-resistant.
The majority of patients without risk factors for MDR infection can be treated with a single agent. The major difference from CAP is the markedly lower incidence of atypical pathogens in VAP; the exception is Legionella, which can be a nosocomial pathogen, especially with breakdowns in the treatment of potable water in the hospital. The standard recommendation for patients with risk factors for MDR infection is for three antibiotics: two directed at P. aeruginosa and one at MRSA. The choice of a β-lactam agent provides the greatest variability in coverage, yet the use of the broadest-spectrum agent—a carbapenem, even in an antibiotic combination—still represents inappropriate initial therapy in 10–15% of cases.
SPECIFIC TREATMENT
Once an etiologic diagnosis is made, broad-spectrum empirical therapy can be modified to specifically address the known pathogen. For patients with MDR risk factors, antibiotic regimens can be reduced to a single agent in more than one-half of cases and to a two-drug combination in more than one-quarter of cases. Only a minority of cases require a complete course with three drugs. A negative tracheal-aspirate culture or growth below the threshold for quantitative cultures, especially if the sample was obtained before any antibiotic change, strongly suggests that antibiotics should be discontinued. Identification of other confirmed or suspected sites of infection may require ongoing antibiotic therapy, but the spectrum of pathogens (and the corresponding antibiotic choices) may be different from those for VAP. If the CPIS decreases over the first 3 days, antibiotics should be stopped after 8 days. An 8-day course of therapy is just as effective as a 2-week course and is associated with less frequent emergence of antibiotic-resistant strains.
The major controversy regarding specific therapy for VAP concerns the need for ongoing combination treatment of Pseudomonas infection. No randomized controlled trials have demonstrated a benefit of combination therapy with a β-lactam and an aminoglycoside, nor have subgroup analyses in other trials found a survival benefit with such a regimen. The unacceptably high rates of clinical failure and death for VAP caused by P. aeruginosa despite combination therapy (see “Failure to Improve,” later) indicate that better regimens are needed—including, perhaps, aerosolized antibiotics. VAP caused by MRSA is associated with a 40% clinical failure rate when treated with standard-dose vancomycin. One proposed solution is the use of high-dose individualized treatment, although the risk of renal toxicity increases with this strategy. In addition, the MIC of vancomycin has been increasing, and a high percentage of clinical failures occur when the MIC is in the upper range of sensitivity (i.e., 1. 5–2 μg/mL). Linezolid appears to be 15% more efficacious than even adjusted-dose vancomycin and is clearly preferred in patients with renal insufficiency and those infected with high-MIC isolates of MRSA.
FAILURE TO IMPROVE
Treatment failure is not uncommon in VAP, especially that caused by MDR pathogens. In addition to the 40% failure rate for MRSA infection treated with vancomycin, VAP due to Pseudomonas has a 50% failure rate, no matter what the regimen. Causes of clinical failure vary with the pathogen(s) and the antibiotic(s). Inappropriate therapy can usually be minimized by use of the recommended triple-drug regimen (Table 153-9). However, the emergence of β-lactam resistance during therapy is an important problem, especially in infection with Pseudomonas and Enterobacter species. Recurrent VAP caused by the same pathogen is possible because the biofilm on endotracheal tubes allows reintroduction of the microorganism. However, studies of VAP caused by Pseudomonas show that approximately half of recurrent cases are caused by a new strain. Inadequate local levels of vancomycin are the likely cause of treatment failure in VAP due to MRSA.
Treatment failure is very difficult to diagnose. Pneumonia due to a new superinfection, the presence of extrapulmonary infection, and drug toxicity must be considered in the differential diagnosis of treatment failure. Serial CPIS calculations appear to track the clinical response accurately, while repeat quantitative cultures may clarify the microbiologic response. A persistently elevated or rising CPIS by day 3 of therapy is likely to indicate treatment failure. The most sensitive component of the CPIS is improvement in oxygenation.
COMPLICATIONS
Apart from death, the major complication of VAP is prolongation of mechanical ventilation, with corresponding increases in length of stay in the ICU and in the hospital. In most studies, an additional week of mechanical ventilation resulting from VAP is common. The additional expense of this complication often warrants costly and aggressive efforts at prevention.
In rare cases, some types of necrotizing pneumonia (e.g., that due to P. aeruginosa) result in significant pulmonary hemorrhage. More commonly, necrotizing infections result in the long-term complications of bronchiectasis and parenchymal scarring leading to recurrent pneumonias. The long-term complications of pneumonia are underappreciated. Pneumonia results in a catabolic state in a patient already nutritionally at risk. The muscle loss and general debilitation from an episode of VAP often require prolonged rehabilitation and, in the elderly, commonly result in an inability to return to independent function and the need for nursing home placement.
FOLLOW-UP
Clinical improvement, if it occurs, is usually evident within 48–72 h of the initiation of antimicrobial treatment. Because findings on chest radiography often worsen initially during treatment, they are less helpful than clinical criteria as an indicator of clinical response in severe pneumonia. Seriously ill patients with pneumonia often undergo follow-up chest radiography daily, at least until they are being weaned off mechanical ventilation.
Prognosis VAP is associated with significant mortality. Crude mortality rates of 50–70% have been reported, but the real issue is attributable mortality. Many patients with VAP have underlying diseases that would result in death even if VAP did not occur. Attributable mortality exceeded 25% in one matched-cohort study, while more recent studies have suggested much lower rates. Patients who develop VAP are at least twice as likely to die as those who do not. Some of the variability in VAP mortality rates is clearly related to the type of patient and ICU studied. VAP in trauma patients is not associated with attributable mortality, possibly because many of the patients were otherwise healthy before being injured. However, the causative pathogen also plays a major role. Generally, MDR pathogens are associated with significantly greater attributable mortality than non-MDR pathogens. Pneumonia caused by some pathogens (e.g., S. maltophilia) is simply a marker for a patient whose immune system is so compromised that death is almost inevitable.
Prevention (Table 153-7) Because of the significance of the endotracheal tube as a risk factor for VAP, the most important preventive intervention is to avoid endotracheal intubation or minimize its duration. Successful use of noninvasive ventilation via a nasal or full-face mask avoids many of the problems associated with endotracheal tubes. Strategies that minimize the duration of ventilation through daily holding of sedation and formal weaning protocols also have been highly effective in preventing VAP.
Unfortunately, a tradeoff in risks is sometimes required. Aggressive attempts to extubate early may result in reintubation(s) and increase aspiration, posing a risk of VAP. Heavy continuous sedation increases the risk, but self-extubation because of insufficient sedation also is a risk. The tradeoffs also apply to antibiotic therapy. Short-course antibiotic prophylaxis can decrease the risk of VAP in comatose patients requiring intubation, and data suggest that antibiotics decrease VAP rates in general. However, the major benefit appears to be a decrease in the incidence of early-onset VAP, which is usually caused by the less pathogenic non-MDR microorganisms. Conversely, prolonged courses of antibiotics consistently increase the risk of VAP caused by the more lethal MDR pathogens. Despite its virulence and associated mortality, VAP caused by Pseudomonas is rare among patients who have not recently received antibiotics.
Minimizing the amount of microaspiration around the endotracheal tube cuff also is a strategy for avoidance of VAP. Simply elevating the head of the bed (at least 30° above horizontal but preferably 45°) decreases VAP rates. Specially modified endotracheal tubes that allow removal of the secretions pooled above the cuff also may prevent VAP. The risk-to-benefit ratio of transporting the patient outside the ICU for diagnostic tests or procedures should be carefully considered, since VAP rates are increased among transported patients.
Emphasis on the avoidance of agents that raise gastric pH and on oropharyngeal decontamination has been diminished by the equivocal and conflicting results of recent clinical trials. The role in the pathogenesis of VAP that is played by the overgrowth of bacterial components of the bowel flora in the stomach also has been downplayed. MRSA and the nonfermenters P. aeruginosa and Acinetobacter species are not normally part of the bowel flora but reside primarily in the nose and on the skin, respectively. Therefore, emphasis on controlling overgrowth of the bowel flora may be relevant only in certain populations, such as liver transplant recipients and patients who have undergone other major intraabdominal procedures or who have bowel obstruction.
In outbreaks of VAP due to specific pathogens, the possibility of a breakdown in infection control measures (particularly contamination of reusable equipment) should be investigated. Even high rates of pathogens that are already common in a particular ICU may be a result of cross-infection. Education and reminders of the need for consistent hand washing and other infection-control practices can minimize this risk.
HOSPITAL-ACQUIRED PNEUMONIA
While significantly less well studied than VAP, HAP in nonintubated patients—both inside and outside the ICU—is similar to VAP. The main differences are the higher frequency of non-MDR pathogens and the better underlying host immunity in nonintubated patients. The lower frequency of MDR pathogens allows monotherapy in a larger proportion of cases of HAP than of VAP.
The only pathogens that may be more common in the non-VAP population are anaerobes. The greater risk of macroaspiration by nonintubated patients and the lower oxygen tensions in the lower respiratory tract of these patients increase the likelihood of a role for anaerobes. While more common in patients with HAP, anaerobes are usually only contributors to polymicrobial pneumonias except in patients with large-volume aspiration or in the setting of bowel obstruction/ileus. As in the management of CAP, specific therapy targeting anaerobes probably is not indicated (unless gross aspiration is a concern) since many of the recommended antibiotics are active against anaerobes.
Diagnosis is even more difficult for HAP in the nonintubated patient than for VAP. Lower respiratory tract samples appropriate for culture are considerably more difficult to obtain from nonintubated patients. Many of the underlying diseases that predispose a patient to HAP are also associated with an inability to cough adequately. Since blood cultures are infrequently positive (<15% of cases), the majority of patients with HAP do not have culture data on which antibiotic modifications can be based. Therefore, de-escalation of therapy is less likely in patients with risk factors for MDR pathogens. Despite these difficulties, the better host defenses in non-ICU patients result in lower mortality rates than are documented for VAP. In addition, the risk of antibiotic failure is lower in HAP.
154 |
Lung Abscess |
Lung abscess represents necrosis and cavitation of the lung following microbial infection. Lung abscesses can be single or multiple but usually are marked by a single dominant cavity >2 cm in diameter.
ETIOLOGY
The low prevalence of lung abscesses makes them difficult to study in randomized controlled trials. Although the incidence of lung abscesses has decreased in the postantibiotic era, they are still a source of significant morbidity and mortality.
Lung abscesses are usually characterized as either primary (~80% of cases) or secondary. Primary lung abscesses usually arise from aspiration, are often caused principally by anaerobic bacteria, and occur in the absence of an underlying pulmonary or systemic condition. Secondary lung abscesses arise in the setting of an underlying condition, such as a postobstructive process (e.g., a bronchial foreign body or tumor) or a systemic process (e.g., HIV infection or another immunocompromising condition). Lung abscesses can also be characterized as acute (<4–6 weeks in duration) or chronic (~40% of cases).
EPIDEMIOLOGY
The majority of the existing epidemiologic information involves primary lung abscesses. In general, middle-aged men are more commonly affected than middle-aged women. The major risk factor for primary lung abscesses is aspiration. Patients at particular risk for aspiration, such as those with altered mental status, alcoholism, drug overdose, seizures, bulbar dysfunction, prior cerebrovascular or cardiovascular events, or neuromuscular disease, are most commonly affected. In addition, patients with esophageal dysmotility or esophageal lesions (strictures or tumors) and those with gastric distention and/or gastroesophageal reflux, especially those who spend substantial time in the recumbent position, are at risk for aspiration.
It is widely thought that colonization of the gingival crevices by anaerobic bacteria or microaerophilic streptococci (especially in patients with gingivitis and periodontal disease), combined with a risk of aspiration, is important in the development of lung abscesses. In fact, many physicians consider it extremely rare for lung abscesses to develop in the absence of teeth as a nidus for bacterial colonization.
The importance of these risk factors in the development of lung abscesses is highlighted by a significant reduction in abscess incidence in the late 1940s that coincided with a change in oral surgical technique: beginning at that time, these operations were no longer performed with the patient in the seated position without a cuffed endotracheal tube, and the frequency of perioperative aspiration events was thus decreased. In addition, the introduction of penicillin around the same time significantly reduced the incidence of and mortality rate from lung abscess.
PATHOGENESIS
Primary Lung Abscesses The development of primary lung abscesses is thought to originate when chiefly anaerobic bacteria (as well as microaerophilic streptococci) in the gingival crevices are aspirated into the lung parenchyma in a susceptible host (Table 154-1). Thus, patients who develop primary lung abscesses usually carry an overwhelming burden of aspirated material or are unable to clear the bacterial load. Pneumonitis develops initially (exacerbated in part by tissue damage caused by gastric acid); then, over a period of 7–14 days, the anaerobic bacteria produce parenchymal necrosis and cavitation whose extent depends on the host–pathogen interaction (Fig. 154-1). Anaerobes are thought to produce more extensive tissue necrosis in polymicrobial infections in which virulence factors of the various bacteria can act synergistically to cause more significant tissue destruction.
|
EXAMPLES OF MICROBIAL PATHOGENS THAT CAN CAUSE LUNG ABSCESSES |
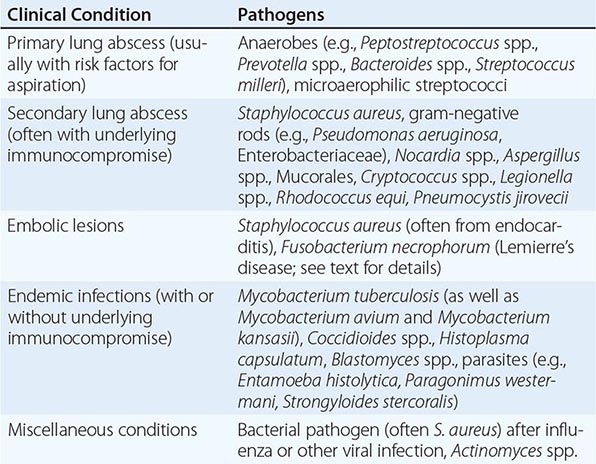
FIGURE 154-1 Representative chest CT scans demonstrating development of lung abscesses. This patient was immunocompromised due to underlying lymphoma and developed severe Pseudomonas aeruginosa pneumonia, as represented by a left lung infiltrate with concern for central regions of necrosis (panel A, black arrow). Two weeks later, areas of cavitation with air fluid levels were visible in this region and were consistent with the development of lung abscesses (panel B, white arrow). (Images provided by Dr. Ritu Gill, Division of Chest Radiology, Brigham and Women’s Hospital, Boston.)
Secondary Lung Abscesses The pathogenesis of secondary abscesses depends on the predisposing factor. For example, in cases of bronchial obstruction from malignancy or a foreign body, the obstructing lesion prevents clearance of oropharyngeal secretions, leading to abscess development. With underlying systemic conditions (e.g., immunosuppression after bone marrow or solid organ transplantation), impaired host defense mechanisms lead to increased susceptibility to development of lung abscesses caused by a broad range of pathogens, including opportunistic organisms (Table 154-1).
Lung abscesses also arise from septic emboli, either in tricuspid valve endocarditis (often involving Staphylococcus aureus) or in Lemierre’s syndrome, in which an infection begins in the pharynx (classically involving Fusobacterium necrophorum) and then spreads to the neck and the carotid sheath (which contains the jugular vein) to cause septic thrombophlebitis.
PATHOLOGY AND MICROBIOLOGY
Primary Lung Abscesses In primary lung abscesses, the dependent segments (posterior upper lobes and superior lower lobes) are the most common locations, given the predisposition of aspirated materials to be deposited in these areas. Generally, the right lung is affected more commonly than the left because the right mainstem bronchus is less angulated. In secondary abscesses, the location of the abscess may vary with the underlying cause.
The microbiology of primary lung abscesses is often polymicrobial, primarily including anaerobic organisms as well as microaerophilic streptococci (Table 154-1). The retrieval and culture of anaerobes can be complicated by the contamination of samples with microbes from the oral cavity, the need for expeditious transport of the cultures to the laboratory, the need for early plating with special culture techniques, the prolonged time required for culture growth, and the need for collection of specimens prior to administration of antibiotics. When attention is paid to these factors, rates of recovery of specific isolates have been reported to be as high as 78%.
Because it is not clear that knowing the identity of the causative anaerobic isolate alters the response to treatment of a primary lung abscess, practice has shifted away from the use of specialized techniques to obtain material for culture, such as transtracheal aspiration and bronchoalveolar lavage with protected brush specimens that allow recovery of culture material while avoiding contamination from the oral cavity. When no pathogen is isolated from a primary lung abscess (which is the case as often as 40% of the time), the abscess is termed a nonspecific lung abscess, and the presence of anaerobes is often presumed. A putrid lung abscess refers to foul-smelling breath, sputum, or empyema and is essentially diagnostic of an anaerobic lung abscess.
Secondary Lung Abscesses In contrast, the microbiology of secondary lung abscesses can encompass quite a broad bacterial spectrum, with infection by Pseudomonas aeruginosa and other gram-negative rods most common. In addition, a broad array of pathogens can be identified in patients from certain endemic areas and in specific clinical scenarios (e.g., a significant incidence of fungal infections among immunosuppressed patients following bone marrow or solid organ transplantation). Because immunocompromised hosts and patients without the classic presentation of a primary lung abscess can be infected with a wide array of unusual organisms (Table 154-1), it is of special importance to obtain culture material in order to target therapy.
CLINICAL MANIFESTATIONS
Clinical manifestations may initially be similar to those of pneumonia, with fevers, cough, sputum production, and chest pain; a more chronic and indolent presentation that includes night sweats, fatigue, and anemia is often observed with anaerobic lung abscesses. A subset of patients with putrid lung abscesses may report discolored phlegm and foul-tasting or foul-smelling sputum. Patients with lung abscesses due to non-anaerobic organisms, such as S. aureus, may present with a more fulminant course characterized by high fevers and rapid progression.
Findings on physical examination may include fevers, poor dentition, and/or gingival disease as well as amphoric and/or cavernous breath sounds on lung auscultation. Additional findings may include digital clubbing and the absence of a gag reflex.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of lung abscesses includes other noninfectious processes that result in cavitary lung lesions, including lung infarction, malignancy, sequestration, vasculitides (e.g., granulomatosis with polyangiitis), lung cysts or bullae containing fluid, and septic emboli (e.g., from tricuspid valve endocarditis).
DIAGNOSIS
The presence of a lung abscess is determined by chest imaging. Although a chest radiograph usually detects a thick-walled cavity with an air-fluid level, computed tomography (CT) permits better definition and may provide earlier evidence of cavitation. CT may also yield additional information regarding a possible underlying cause of lung abscess, such as malignancy, and may help distinguish a peripheral lung abscess from a pleural infection. This distinction has important implications for treatment, because a pleural space infection, such as an empyema, may require urgent drainage.
As described earlier (see “Pathology and Microbiology,” above), more invasive diagnostics (such as transtracheal aspiration) were traditionally undertaken for primary lung abscesses, whereas empirical therapy that includes drugs targeting anaerobic organisms currently is used more often. While sputum can be collected noninvasively for Gram’s stain and culture, which may yield a pathogen, it is likely that the infection will be polymicrobial, and culture results may not reflect the presence of anaerobic organisms. Many physicians consider putrid-smelling sputum to be virtually diagnostic of an anaerobic infection.
When a secondary lung abscess is present or empirical therapy fails to elicit a response, sputum and blood cultures are advised in addition to serologic studies for opportunistic pathogens (e.g., viruses and fungi causing infections in immunocompromised hosts). Additional diagnostics, such as bronchoscopy with bronchoalveolar lavage or protected brush specimen collection and CT-guided percutaneous needle aspiration, can be undertaken. Risks posed by these more invasive diagnostics include spillage of abscess contents into the other lung (with bronchoscopy) and pneumothorax and bronchopleural fistula development (with CT-guided needle aspiration). However, early diagnostics in secondary abscesses, especially in immunocompromised hosts, are particularly important, because the patients involved may be especially fragile and at risk for infection with a broad array of pathogens and, therefore, less likely than other patients to respond to empirical therapy.
|
TREATMENT |
LUNG ABSCESS |
The availability of antibiotics in the 1940s and 1950s established therapy with this drug class as the primary approach to the treatment of lung abscess. Previously, surgery had been relied upon much more frequently. For many decades, penicillin was the antibiotic of choice for primary lung abscesses in light of its anaerobic coverage; however, because oral anaerobes can produce β-lactamases, clindamycin has proved superior to penicillin in clinical trials. For primary lung abscesses, the recommended regimens are (1) clindamycin (600 mg IV three times daily; then, with the disappearance of fever and clinical improvement, 300 mg PO four times daily) or (2) an IV-administered β-lactam/β-lactamase combination, followed—once the patient’s condition is stable—by orally administered amoxicillin-clavulanate. This therapy should be continued until imaging demonstrates that the lung abscess has cleared or regressed to a small scar. Treatment duration may range from 3–4 weeks to as long as 14 weeks. One small study suggested that moxifloxacin (400 mg/d PO) is as effective and well tolerated as ampicillin-sulbactam. Notably, metronidazole is not effective as a single agent: it covers anaerobic organisms but not the microaerophilic streptococci that are often components of the mixed flora of primary lung abscesses.
In secondary lung abscesses, antibiotic coverage should be directed at the identified pathogen, and a prolonged course (until resolution of the abscess is documented) is often required. Treatment regimens and courses vary widely, depending on the immune state of the host and the identified pathogen. Other interventions may be necessary as well, such as relief of an obstructing lesion or treatment directed at the underlying condition predisposing the patient to lung abscess. Similarly, if the condition of patients with presumed primary lung abscess fails to improve, additional studies to rule out an underlying predisposing cause for a secondary lung abscess are indicated.
Although it can take as long as 7 days for patients receiving appropriate therapy to defervesce, as many as 10–20% of patients may not respond at all, with continued fevers and progression of the abscess cavity on imaging. An abscess >6–8 cm in diameter is less likely to respond to antibiotic therapy without additional interventions. Options for patients who do not respond to antibiotics and whose additional diagnostic studies fail to identify an additional pathogen that can be treated include surgical resection and percutaneous drainage of the abscess, especially in poor surgical candidates. Possible complications of percutaneous drainage include bacterial contamination of the pleural space as well as pneumothorax and hemothorax.
COMPLICATIONS
Larger cavity size on presentation may correlate with the development of persistent cystic changes (pneumatoceles) or bronchiectasis. Additional possible complications include recurrence of abscesses despite appropriate therapy, extension to the pleural space with development of empyema, life-threatening hemoptysis, and massive aspiration of lung abscess contents.
PROGNOSIS AND PREVENTION
Reported mortality rates for primary abscesses have been as low as 2%, while rates for secondary abscesses are generally higher—as high as 75% in some case series. Other poor prognostic factors include an age >60, the presence of aerobic bacteria, sepsis at presentation, symptom duration of >8 weeks, and abscess size >6 cm.
Mitigation of underlying risk factors may be the best approach to prevention of lung abscesses, with attention directed toward airway protection, oral hygiene, and minimized sedation with elevation of the head of the bed for patients at risk for aspiration. Prophylaxis against certain pathogens in at-risk patients (e.g., recipients of bone marrow or solid organ transplants or patients whose immune systems are significantly compromised by HIV infection) may be undertaken.
155 |
Infective Endocarditis |
The prototypic lesion of infective endocarditis, the vegetation (Fig. 155-1), is a mass of platelets, fibrin, microcolonies of microorganisms, and scant inflammatory cells. Infection most commonly involves heart valves but may also occur on the low-pressure side of a ventricular septal defect, on mural endocardium damaged by aberrant jets of blood or foreign bodies, or on intracardiac devices themselves. The analogous process involving arteriovenous shunts, arterio-arterial shunts (patent ductus arteriosus), or a coarctation of the aorta is called infective endarteritis.
FIGURE 155-1 Vegetations (arrows) due to viridans streptococcal endocarditis involving the mitral valve.
Endocarditis can be classified according to the temporal evolution of disease, the site of infection, the cause of infection, or the predisposing risk factor (e.g., injection drug use). While each classification criterion provides therapeutic and prognostic insight, none is sufficient alone. Acute endocarditis is a hectically febrile illness that rapidly damages cardiac structures, seeds extracardiac sites, and, if untreated, progresses to death within weeks. Subacute endocarditis follows an indolent course; causes structural cardiac damage only slowly, if at all; rarely metastasizes; and is gradually progressive unless complicated by a major embolic event or a ruptured mycotic aneurysm.
In developed countries, the incidence of endocarditis ranges from 4 to 7 cases per 100,000 population per year and has remained relatively stable during recent decades. While congenital heart diseases remain a constant predisposition, predisposing conditions in developed countries have shifted from chronic rheumatic heart disease (still a common predisposition in developing countries) to illicit IV drug use, degenerative valve disease, and intracardiac devices. The incidence of endocarditis is notably increased among the elderly. In developed countries, 25–35% of cases of native valve endocarditis (NVE) are associated with health care, and 16–30% of all cases of endocarditis involve prosthetic valves. The risk of prosthesis infection is greatest during the first 6–12 months after valve replacement; gradually declines to a low, stable rate thereafter; and is similar for mechanical and bioprosthetic devices. The incidence of endocarditis involving cardiovascular implantable electronic devices (CIED), primarily permanent pacemakers and implantable cardioverter-defibrillators, ranges from 0.5 to 1.14 cases per 1000 device recipients and is higher among patients with an implantable cardioverter-defibrillator than among those with a permanent pacemaker.
ETIOLOGY
Although many species of bacteria and fungi cause sporadic episodes of endocarditis, a few bacterial species cause the majority of cases (Table 155-1). The oral cavity, skin, and upper respiratory tract are the respective primary portals for viridans streptococci, staphylococci, and HACEK organisms (Haemophilus species, Aggregatibacter aphrophilus, A. actinomycetemcomitans, Cardiobacterium species, Eikenella species, and Kingella species). Streptococcus gallolyticus subspecies gallolyticus (formerly S. bovis biotype 1) originates from the gastrointestinal tract, where it is associated with polyps and colonic tumors, and enterococci enter the bloodstream from the genitourinary tract. Health care–associated NVE, most commonly caused by Staphylococcus aureus, coagulase-negative staphylococci (CoNS), and enterococci, may have either a nosocomial onset (55%) or a community onset (45%); community-onset cases develop in patients who have had extensive contact with the health care system over the preceding 90 days. Endocarditis complicates 6–25% of episodes of catheter-associated S. aureus bacteremia; the higher rates are detected in high-risk patients studied by transesophageal echocardiography (TEE) (see “Echocardiography,” later).
|
ORGANISMS CAUSING MAJOR CLINICAL FORMS OF ENDOCARDITIS |
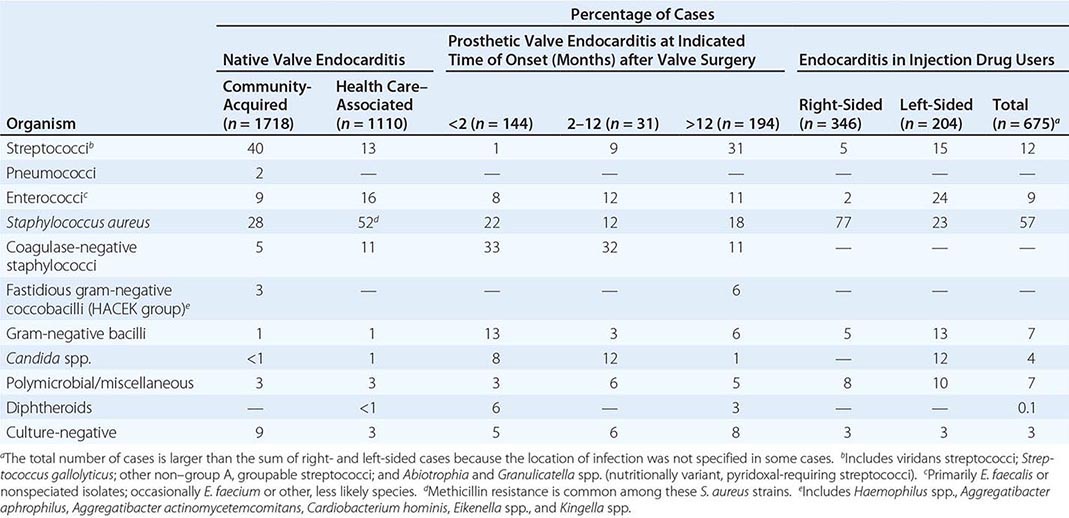
Note: Data are compiled from multiple studies.