CHAPTER 33 Kyphoplasty Technique
INTRODUCTION
Pathologic vertebral compression fractures (VCFs) are a leading cause of disability and morbidity in patients with osteoporosis, multiple myeloma, and bone metastases.1–4 The consequences of these fractures include pain and often progressive vertebral collapse with resultant spinal kyphosis. Osteoporotic VCFs have been shown to adversely affect quality of life, physical function, mental health, and survival.4–6 These effects are related to the severity of the spinal deformity and are, in part, independent of pain.4,5 In recent years, researchers have highlighted the reduced quality of life, functional limitations, and impaired pulmonary function associated with spinal kyphotic deformity from osteoporotic VCFs.3,4,7–9 Kyphosis can lead to reduced abdominal space with poor appetite and resultant nutritional problems.4,10 By shifting the patient’s center of gravity forward, kyphotic deformity not only increases the risk of additional fractures,11 but also may lead to poor balance which potentially increases the risk of accidental falls.12,13
The ideal surgical treatment of VCFs should address both the fracture-related pain and the kyphotic deformity. It should be accomplished in a minimally invasive fashion without subjecting the patient to inordinate risks or excessive surgical trauma. Over the past decade, percutaneous vertebroplasty, involving the injection of polymethylmethacrylate (PMMA) into a fractured vertebral body, has been popularized. Substantial alleviation of pain has been reported in a majority of patients treated with vertebroplasty for osteopenic VCF.14–22 Although effective at relieving vertebral fracture pain, vertebroplasty is not designed to address the associated sagittal plane deformity.
Kyphoplasty involves the penetration of the vertebral body with a trochar followed by insertion of an inflatable balloon tamp (IBT). Inflation of the balloon tamp restores the vertebral body back towards its original height, while creating a cavity to be filled with bone void filler. This technique was first performed in 1998. Early results of kyphoplasty suggest significant pain relief as well as the ability to improve the collapsed vertebral body’s height.23–29
The kyphoplasty procedure was designed to address vertebroplasty’s shortcomings such as high rates of cement leakage, although they rarely manifest with symptoms, and inability to correct fracture deformity. As the balloon tamp is inflated in the fractured vertebral body, the vertebral endplates are pushed apart reducing the fracture, and cancellous bone is pushed away from the balloon creating a cavity surrounded by compacted cancellous bone.30–32 The creation of an intravertebral cavity may decrease the potential for cement leakage by allowing for low-pressure, controlled placement of ‘doughy’ cement into the cavity and by creating a dam effect by densely compacting bone around the cavity.
PMMA has been the most common bone void filler used in both vertebroplasty and kyphoplasty. This acrylic cement has a long history of clinical use for the fixation of metal and plastic joint replacements and for the fixation of pathological fractures.33,34 When used to treat vertebral compression fractures, PMMA is usually modified (for example, addition of more barium sulfate, addition of antibiotics, alteration of monomer to powder ratio), in part to attain a viscosity that allows percutaneous insertion into vertebrae while minimizing risk of extravertebral leaks. In April, 2004, the United States FDA approved a formulation of PMMA for use in kyphoplasty procedures.
KYPHOPLASTY PATIENT SELECTION
Surgical timing
To improve reliability and the extent of fracture reduction, one might consider performing kyphoplasty soon after fracture. Some studies suggest improved fracture reduction when kyphoplasty is performed earlier.24,26 However, the appropriate duration of nonoperative treatment prior to consideration of kyphoplasty has not yet been established.
Preoperative evaluation
Preoperative planning for kyphoplasty includes imaging studies to confirm the fracture, estimate the duration of the fracture, and define the fracture anatomy. Lateral radiographs are particularly useful to plan the trajectory for any percutaneous procedure. Magnetic resonance imaging (MRI) can visualize bony edema, which indicates acute fracture, as well as help rule out infection or tumor involvement. Malignant causes of VCF are usually characterized by an ill-defined margin, signal enhancement, and pedicle involvement as well as by paravertebral soft tissue mass.35 Sagittal MRI images with short tau inversion recovery (STIR) sequences highlight the marrow edema changes associated with acute VCFs. STIR sequence MRI has proven useful in determining the acuteness of a VCF.
SURGICAL ANATOMY
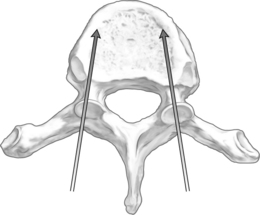
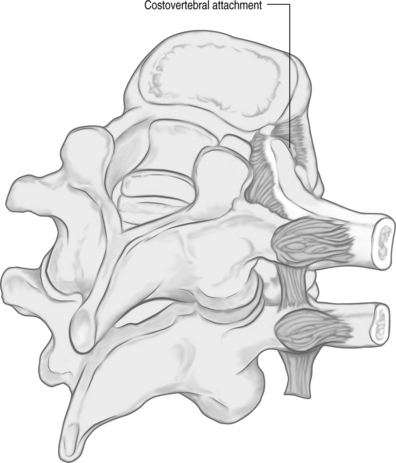
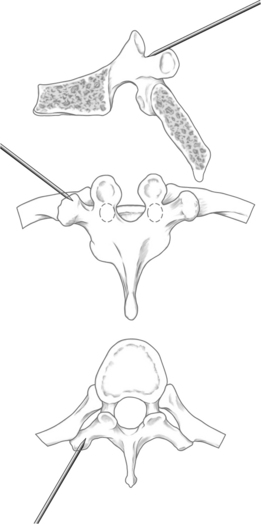
The orientation of the pedicle is important in planning an appropriate trajectory for kyphoplasty. The medial inclination in the transverse plane appears to be greatest in the upper thoracic segments (T1–3), and becomes a straight anterior trajectory in the middle to lower thoracic spinal segments (Fig. 33.1). In the lumbar spine, the medial orientation of the pedicles increases slightly from L1 to L5. In the thoracic spine, the attachment of the ribs to the corresponding vertebral body (i.e. costovertebral joint) protects the lateral side of the pedicle allowing cannulation through an extrapedicular approach. Despite the smaller size of the thoracic pedicle, the costovertebral attachment results in a much larger effective pedicle size (Fig. 33.2)
KYPHOPLASTY SURGICAL TECHNIQUE
Fluoroscopic imaging
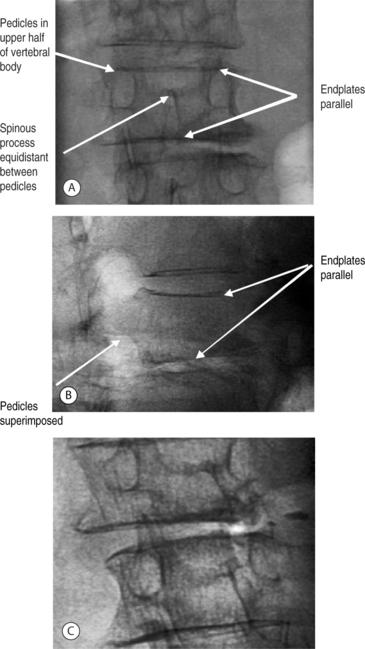
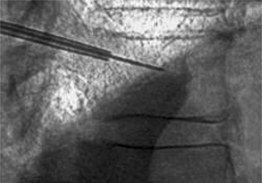
The authors have found simultaneous biplanar fluoroscopy to be advantageous by allowing orthogonal visualization without having to move the C-arm. The ability to visualize the pedicles in both anteroposterior (AP) and lateral views is essential to performing the procedure. Fluoroscopic images confirming the patient’s anatomy must be obtained prior to initiating vertebral cannulation. In the AP view, the superior and inferior endplates are parallel to the fluoroscopic beam and are each visualized as a single cortical shadow, the spinous process is centered in the vertebral body, and the pedicles are symmetric and positioned in the upper half of the vertebral body (Fig. 33.3A). In the lateral view, the endplates are also parallel and the pedicles are superimposed (Fig. 33.3B), Alternatively, the fluoroscope can be rotated 10–20° for a pedicle en-face view, the view down the path of the pedicle (Fig. 33.3C) In this position, the pedicle is visualized directly and can be cannulated by paralleling the pathway of the X-ray beam.
Surgical technique
Starting position
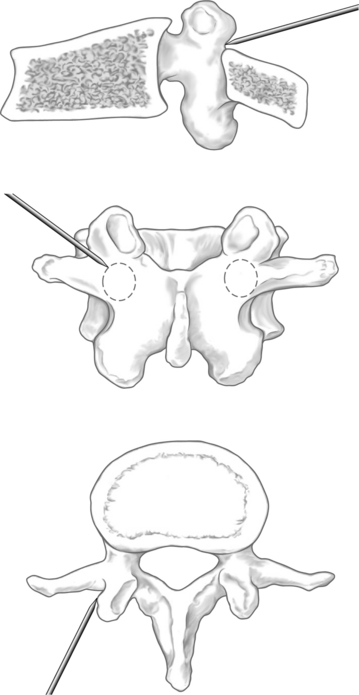
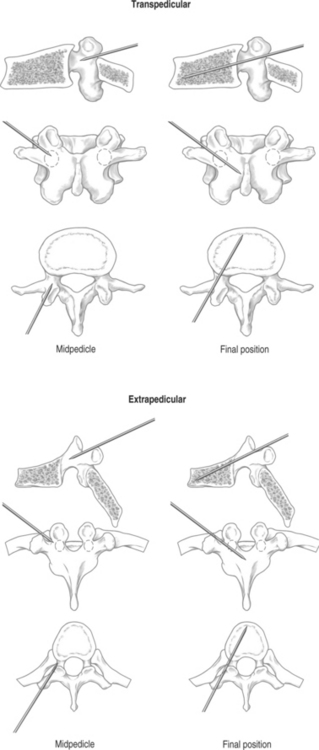
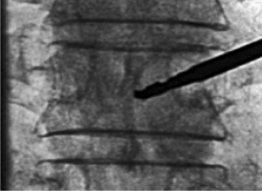
When using the transpedicular approach, the initial instrument should dock on the lateral aspect of the facet joint overlying the lateral aspect of the pedicle on the AP fluoroscopic image (Fig. 33.4). With the extrapedicular approach, the initial instrument is docked at the junction of the transverse process, rib head, and lateral pedicle wall (Fig. 33.5). The tip of the instrument appears outside of the lateral pedicle wall on the AP image. Unless an appropriate starting point is chosen, the balloon tamps cannot be positioned correctly, decreasing the success of the reduction. Once the docking position is selected on the AP view, the position of the starting point must be checked on the lateral view to confirm the appropriate trajectory toward the collapsed vertebral body.
Pedicle cannulation
With both transpedicular and extrapedicular approaches, as the needle is advanced, the tip should remain lateral to the medial pedicle wall on the AP image until it reaches the posterior aspect of the vertebral body on the lateral image (Fig. 33.6). This technique ensures that the instrument remains outside of the spinal canal. Once the vertebral body is entered, the tip of the instrument may be medialized as is appropriate but should not cross the midline. The Jamshidi needle should be advanced 1–2 mm past the posterior vertebral body margin.
Placement of working cannulae
The inner stylet is removed from the introducer needle, and a guide wire is placed into the vertebral body. After the working cannula is advanced over the guide wire into the posterior aspect of the vertebral body under biplanar fluoroscopic guidance, the guide wire is removed (Fig. 33.7). It is important to limit the number of passes through the pedicle as multiple attempts at cannulation create potential paths for cement leakage.
Vertebral body preparation
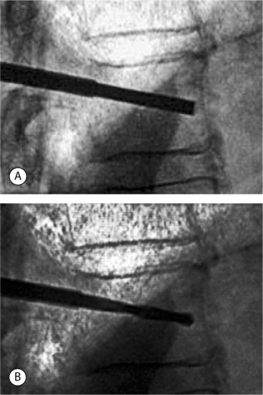
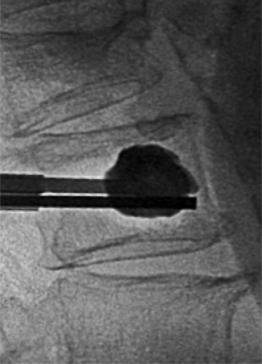
A drill or bone tamp is used to prepare the vertebral body for placement of the IBTs. Under lateral fluoroscopy, the drill or tamp should be advanced to within 2–4 mm of the anterior cortex without perforating the cortex (Fig. 33.8). On the AP view, the tip of the drill should approach the middle of the vertebral body (Fig. 33.9). Once the drill is removed, a dull guide pin can be used to palpate the anterior cortex to confirm that there is no perforation. The vertebral body is now ready for expansion.
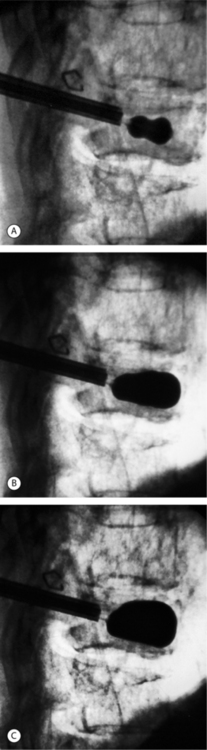
Inflatable balloon tamp inflation
The bilateral IBTs are placed anteriorly in the vertebral body. To create a cavity within the vertebra and to reduce the fracture deformity, the IBTs are inflated in 0.5 cc increments while using visual (radiographic), volume, and pressure controls (digital manometer). Inflation continues until vertebral body height is restored, the IBT contacts a vertebral body cortical wall, the IBT reaches the maximal pressure rating without spontaneous decay, or maximal balloon volume is reached (Fig. 33.10). Four- or six-millimeter-sized balloons are available. We prefer to use 6 mm balloons in larger vertebral bodies (T12–L5) and 4 mm balloons in the midthoracic spine.
Cement application
After the IBTs are withdrawn, a bone filler cannula is used to place partially cured PMMA into the cavity within the fractured vertebral body. To minimize the risk of cement extravasation, the authors allow the cement to become quite viscous prior to placement in the vertebral body. The bone filler device (BFD) filled with cement is positioned anteriorly in the intravertebral cavity created by IBT inflation. As the plunger is advanced, cement is expelled and fills the intravertebral cavity in a retrograde fashion. Cementing should be discontinued if extravertebral extravasation occurs or when cement reaches the posterior 25% of the vertebral body. The cement volume should approximate the volume of the intravertebral cavity. The judicious use of live fluoroscopic imaging is critical during cementing (Fig. 33.11). If cement extravasation occurs, usually through a fractured endplate or vertebral cortex, placing a small amount of viscous cement into the cavity and reinflating the balloon can salvage this situation. This maneuver will line the cavity walls with cement, effectively preventing further extravasation when the remainder of the cavity is filled with cement.
COMPLICATIONS AND PREVENTIVE MEASURES
Errors in patient selection
Poor clinical outcomes may be predicted for kyphoplasty unless careful attention is given to patient screening and work-up. Treating old, healed VCFs is unlikely to affect the patient’s symptoms. The VCF must be confirmed as the likely pain generator if either vertebroplasty or kyphoplasty is being considered. This determination usually requires a combination of clinical findings suggestive of fracture pain and confirmatory imaging studies. VCF pain often increases with weight-bearing activities and eases with recumbency. On history, the presence of abrupt onset of pain that is aggravated by activity or changing positions and that is localized to the area of the radiographically documented fracture suggests the fracture to be responsible for the patient’s symptoms. In contrast to acute fracture pain, the back pain of chronic kyphosis typically worsens as the patient remains erect for periods of time and is not typically exacerbated by changes in position.
The existence of multiple fractures may complicate the diagnosis, so that advanced imaging studies such as MRI or computed tomography (CT) with bone scans are usually required to identify recent fractures. Sagittal T1-weighted MR sequences can distinguish acute or nonhealed fractures from healed fractures. Edema associated with acute VCFs produces low signal intensity, whereas more chronic fractures tend to produce signals that are similar to those of nonfractured vertebrae. As mentioned in the Preoperative Evaluation section, sagittal STIR (heavily T2-weighted) MRI sequences are the most sensitive way to distinguish marrow fat from marrow edema. In STIR-MR images, edema in acute fractures produces high-intensity signal.36–38 On bone scan analyses, recently fractured vertebrae show an increased uptake of 99mTc compared to nonfractured vertebrae. CT plus bone scans may be used when MR images cannot be obtained.
Cement complications
The majority of complications reported for vertebral augmentation procedures relate to extravertebral cement extravasation. Cement may leak out of the vertebral body directly through deficiencies in the vertebral body cortex or via the venous system. If PMMA extravasates outside of the vertebral body, complications related to mechanical or thermal injury of adjacent anatomic structures may occur. The risk of local cement leakage is likely affected by cement injection pressure and cement viscosity as well as the ability of the bone, particularly the vertebral body cortex, to resist cement leakage. In addition to the risks of local cement leakage, systemic exposure to cement has been associated with cardiovascular collapse.39,40 It has been hypothesized that pressurization of PMMA into cancellous bone predisposes to embolization of cement, methylmethacrylate monomer, and bone marrow contents to the lungs with resulting adverse cardiopulmonary sequelae.39–42 This theorized result is certainly a cause for concern during vertebral augmentation procedures when high-pressure PMMA injection into vertebral bodies is performed.
Extravertebral cement extravasation commonly occurs during vertebroplasty with reported leak rates of up to 65%;14 however, clinical sequelae of the leakage have been infrequently reported. In contrast, the reported rate of cement extravasation with kyphoplasty is typically less than 10%.23,26–29,43 With kyphoplasty, the creation of an intravertebral cavity surrounded by compacted bone allows for the placement of higher-viscosity cement under lower pressure compared to the injection conditions needed for vertebroplasty.
Failure of reduction
The deleterious effects of spinal kyphosis on physical function, mental, respiratory, and gastrointestinal health are well established.3–5,9,44–46 Kyphoplasty attempts to reduce the fracture and associated deformity in a reliable and predictable fashion. Some degree of fracture reduction has been achieved in more than 60% of treated fractures.27,28 Factors that seem to limit reduction achieved with kyphoplasty include partial healing of bone, suboptimal placement of the IBT, and collapse of vertebral endplates after IBT removal and before cement placement. In cases where healed bone limits IBT expansion and fracture reduction, high IBT pressures at low balloon volumes and distorted IBT inflation shapes will be observed.
SUMMARY
Kyphoplasty is a technically demanding procedure offering a much needed treatment option for patients with symptomatic VCFs that do not respond to medical therapy or that are associated with progressive kyphosis. Rapid pain reduction, improved quality of life, and often fracture reduction have been observed in several consecutive case series.23,25–28,43,47 Further study is required to determine the optimal time for surgical intervention, to refine the patient selection criteria, and to delineate specific long-term effects of correcting vertebral deformity.
1 Barrick MC, Mitchell SA. Multiple myeloma. AJN. 2001;101(Suppl)(4):6-12.
2 Schachar NS. An update on the nonoperative treatment of patients with metastatic bone disease. Clin Orthop. 2001;382:75-81.
3 Lyles KW, Gold DT, Shipp KM, et al. Association of osteoporotic vertebral compression fractures with impaired functional status. Am J Med. 1993;94(6):595-601.
4 Silverman SL. The clinical consequences of vertebral compression fracture. Bone. 1992;13(Suppl 2):S27-S31.
5 Gold DT. The clinical impact of vertebral fractures: quality of life in women with osteoporosis. Bone. 1996;18(3 Suppl):185S-189S.
6 Gold DT, Lyles KW. Fractures: effects on quality of life. In: Rosen CJ, Glowacki J, Bilezikian JP, editors. The aging skeleton. San Diego, CA: Academic Press; 1999:632.
7 Leidig G, Minne HW, Sauer P, et al. A study of complaints and their relation to vertebral destruction in patients with osteoporosis. Bone Miner. 1990;8(3):217-229.
8 Pluijm SM, Tromp AM, Smit JH, et al. Consequences of vertebral deformities in older men and women. J Bone Miner Res. 2000;15(8):1564-1572.
9 Schlaich C, Minne HW, Bruckner T, et al. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporos Int. 1998;8(3):261-267.
10 Ross PD, Davis JW, Epstein RS, et al. Pain and disability associated with new vertebral fractures and other spinal conditions. J Clin Epidemiol. 1994;47(3):231-239.
11 Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285(3):320-323.
12 White AA3rd, Panjabi MM, Thomas CL. The clinical biomechanics of kyphotic deformities. Clin Orthop. 1977;128:8-17.
13 Keller TS, Harrison DE, Colloca CJ, et al. Prediction of osteoporotic spinal deformity. Spine. 2003;28(5):455-462.
14 Cortet B, Cotten A, Boutry N, et al. Percutaneous vertebroplasty in the treatment of osteoporotic vertebral compression fractures: an open prospective study. J Rheumatol. 1999;26(10):2222-2228.
15 Cortet B, Cotten A, Boutry N, et al. Percutaneous vertebroplasty in patients with osteolytic metastases or multiple myeloma. Rev Rhum Engl Ed. 1997;64(3):177-183.
16 Cyteval C, Sarrabere MP, Roux JO, et al. Acute osteoporotic vertebral collapse: open study on percutaneous injection of acrylic surgical cement in 20 patients. AJR Am J Roentgenol. 1999;173(6):1685-1690.
17 Deramond H, Depriester C, Galibert P, et al. Percutaneous vertebroplasty with polymethylmethacrylate. Technique, indications, and results. Radiol Clin North Am. 1998;36(3):533-546.
18 Evans AJ, Jensen ME, Kip KE, et al. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty: retrospective report of 245 cases. Radiology. 2003;226(2):366-372.
19 Gangi A, Kastler BA, Dietemann JL. Percutaneous vertebroplasty guided by a combination of CT and fluoroscopy. AJNR Am J Neuroradiol. 1994;15(1):83-86.
20 Grados F, Depriester C, Cayrolle G, et al. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology. 2000;39(12):1410-1414.
21 Hodler J, Peck D, Gilula LA. Midterm outcome after vertebroplasty: predictive value of technical and patient-related factors. Radiology. 2003;227(3):662-668.
22 Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol. 1997;18(10):1897-1904.
23 Rhyne A3rd, Banit D, Laxer E, et al. Kyphoplasty: report of eighty-two thoracolumbar osteoporotic vertebral fractures. J Orthop Trauma. 2004;18(5):294-299.
24 Wong WH, Reiley MA, Garfin SR. Vertebroplasty/kyphoplasty. J Women’s Imaging. 2000;2(3):117-124.
25 Theodorou DJ, Theodorou SJ, Duncan TD, et al. Percutaneous balloon kyphoplasty for the correction of spinal deformity in painful vertebral body compression fractures. Clin Imaging. 2002;26(1):1-5.
26 Phillips FM, Ho E, Campbell-Hupp M, et al. Early radiographic and clinical results of balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures. Spine. 2003;28(19):2260-2265.
27 Lieberman IH, Dudeney S, Reinhardt MK, et al. Initial outcome and efficacy of ‘kyphoplasty’ in the treatment of painful osteoporotic vertebral compression fractures. Spine. 2001;26(14):1631-1638.
28 Ledlie JT, Renfro M. Balloon kyphoplasty: one-year outcomes in vertebral body height restoration, chronic pain, and activity levels. J Neurosurg. 2003;98(1 Suppl):36-42.
29 Dudeney S, Lieberman IH, Reinhardt MK, et al. Kyphoplasty in the treatment of osteolytic vertebral compression fractures as a result of multiple myeloma. J Clin Oncol. 2002;20(9):2382-2387.
30 Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26(14):1511-1515.
31 Lane JM, Johnson CE, Khan SN, et al. Minimally invasive options for the treatment of osteoporotic vertebral compression fractures. Orthop Clin North Am. 2002;33(2):431-438. viii
32 Phillips FM, Wetzel FT, Lieberman I, et al. An in vivo comparison of the potential for extravertebral cement leak after vertebroplasty and kyphoplasty. Spine. 2002;27(19):2173-2178. discussion 2178–2179
33 Jang JS, Lee SH, Rhee CH. Polymethylmethacrylate-augmented screw fixation for stabilization in metastatic spinal tumors. Technical note. J Neurosurg. 2002;96(1 Suppl):131-134.
34 Bauer TW, Schils J. The pathology of total joint arthroplasty. I. Mechanisms of implant fixation. Skeletal Radiol. 1999;28(8):423-432.
35 Shih TT, Huang KM, Li YW. Solitary vertebral collapse: distinction between benign and malignant causes using MR patterns. J Magn Reson Imaging. 1999;9(5):635-642.
36 Do HM. Magnetic resonance imaging in the evaluation of patients for percutaneous vertebroplasty. Top Magn Reson Imaging. 2000;11(4):235-244.
37 Mathis JM, Barr JD, Belkoff SM, et al. Percutaneous vertebroplasty: a developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol. 2001;22(2):373-381.
38 Phillips FM, Pfeifer BA, Lieberman IH, et al. Minimally invasive treatments of osteoporotic vertebral compression fractures: vertebroplasty and kyphoplasty. Instr Course Lect. 2003;52:559-567.
39 Pinto PW. Cardiovascular collapse associated with the use of methylmethacrylate. AANA J. 1993;61(6):613-616.
40 Rudigier JF, Ritter G. Pathogenesis of circulatory reactions triggered by nervous reflexes during the implantation of bone cements. Res Exp Med (Berl). 1983;183(2):77-94.
41 Markel DC, Femino JE, Farkas P, et al. Analysis of lower extremity embolic material after total knee arthroplasty in a canine model. J Arthroplasty. 1999;14(2):227-232.
42 Orsini EC, Byrick RJ, Mullen JB, et al. Cardiopulmonary function and pulmonary microemboli during arthroplasty using cemented or non-cemented components. The role of intramedullary pressure. J Bone Joint Surg Am. 1987;69(6):822-832.
43 Coumans JV, Reinhardt MK, Lieberman IH. Kyphoplasty for vertebral compression fractures: 1-year clinical outcomes from a prospective study. J Neurosurg. 2003;99(1 Suppl):44-50.
44 Leech JA, Dulberg C, Kellie S, et al. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis. 1990;141(1):68-71.
45 Kado DM, Browner WS, Palermo L, et al. Vertebral fractures and mortality in older women: a prospective study. Arch Intern Med. 1999;159(11):1215-1220.
46 Cooper C, Atkinson EJ, Jacobsen SJ, et al. Population-based study of survival after osteoporotic fractures. Am J Epidemiol. 1993;137(9):1001-1005.
47 Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(1 Suppl):21-30.