Chapter 42 Knee Pain of Neural Origin
INTRODUCTION
Nerves related to the knee include not only those that go in the skin about the surface of the knee joint but also mixed (motor and sensory) nerves that pass the knee on the way to their targets distal to this joint. In addition to these well-known nerves are those that innervate the knee joint. Each of these nerves is at risk of injury whenever (1) there is blunt force directed at the knee, (2) there is a stretch/traction injury to the musculoskeletal system of the knee joint, (3) there is an inversion sprain to the ankle, and (4) open or arthroscopic knee surgery has been performed. Whereas it is intuitively clear that a joint is innervated, the exact pathways of this innervation are curiously omitted from the classic and even the newer anatomy texts. For the human knee, the innervation pattern was not found to be described until 1994 during the author’s search for an etiology of knee pain unrelated to musculoskeletal dysfunction.25 The approach to knee pain described in this chapter follows closely that developed for wrist pain,8,16,20 which preceded the author’s interest in knee pain and led to a subsequent similar approach to those for shoulder pain1,3 and ankle pain.6,13,29
Nerves are at risk of injury from
Approach: knee pain that exists after musculoskeletal treatment is of neural origin.
PERIPHERAL NERVE ANATOMY RELATED TO THE KNEE
Innervation of the Human Knee Joint
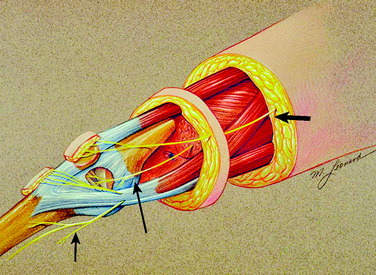
The innervation of the human knee joint is remarkably constant.25 On the medial aspect (Fig. 42-1), the femoral nerve branch that innervates the vastus medialis continues past its motor point and exits deep and distal to the vastus medialis. At this point, it lies deep to the medial retinaculum and becomes related to the medial recurrent geniculate artery and vein. This structure is termed the medial retinacular nerve. These structures, nerve and vessels, continue directly adjacent to the vastus medialis and superficial to the synovium to enter the ligamentous structures of the medial aspect of the knee. These fibers also continue toward the midline of the knee to innervate the undersurface of the patella (see Fig. 42-1).
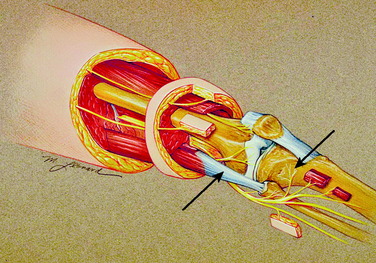
On the lateral aspect of the knee (Fig. 42-2), a branch of the sciatic nerve leaves the popliteal fossa, travels laterally and anteriorly, emerges deep to the biceps tendon, and enters the space beneath the lateral retinaculum. In this location, the nerve is adjacent to the superior lateral geniculate artery and vein. This structure is termed the lateral retinacular nerve. These structures, the nerve and vessels, are immediately distal to the vastus lateralis and superficial to the synovium. The nerve enters the ligamentous structures of the lateral aspect of the knee (which have not yet been further defined histologically) and travels to the midline to innervate the undersurface of the patella.
Anteriorly, another source of innervation is derived from the femoral nerve innervation of the vastus intermedius. This nerve continues on the surface of the periosteum to innervate the tissues around the prepatellar bursa. Finally, posteriorly, branches from the sciatic nerve enter the posterior knee joint capsule to provide innervation (see Fig. 42-2).
Innervation of the Proximal Tibiofibular Joint
Although many patients complain of “knee pain,” they may be pointing or referring to the region distal and lateral to the knee, which is the articulation between the fibular head and the tibia. This joint space is likely to be injured from fractures of the fibula or lateral tibial plateau or from high tibial osteotomy or a Maquet operative procedure. This space is innervated by the common peroneal nerve (see Fig. 42-2). As the common peroneal nerve travels from the popliteal fossa to the fibular head, it gives off a small (~1 mm in diameter) nerve that may enter this space posterior to the fibular head. The common peroneal nerve gives off a second branch to this space immediately as it curves anterior to the fibular head, at the junction with the fibular neck. This branch is also approximately 1 mm in diameter. The next branch of the common peroneal nerve goes to the tibialis anterior, which is a critical motor branch. In the operating room, the only way to identify the branch to the joint is to stimulate it electrically. No motor function will be elicited when the branch to the joint is stimulated. This is a critical step. This small branch often looks like epineurium.
Common Peroneal Nerve
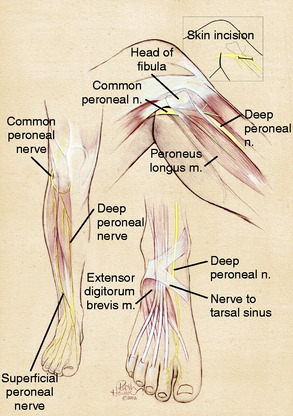
The common peroneal nerve is the lateral branch of the sciatic nerve. It can most often be identified as a distinct branch even to the sciatic notch. Therefore, in the thigh, there is clearly a common peroneal nerve even though it may be referred to as the sciatic nerve. In the distal thigh, the common peroneal nerve courses laterally, approaches the fibular head, and continues anteriorly to cross the fibular neck and enter the leg. As the common peroneal nerve approaches the fibular neck, it clearly divides into the superficial and the deep peroneal nerve, increasing in size as increased epineurium is required to ensheath these two branches (Fig. 42-3). The critical anatomic sites that can cause compression have long been appreciated to occur as the common peroneal nerve crosses the fibular neck, requiring division of the fascia of the peroneus longus fascia.35 With increasing experience, it has become evident that three other anatomic factors must be considered. A fibrous band exists beneath the peroneus longus in 20% of cadavers, but this band is present (and ranges from 5–15 mm in width) in 80% of patients requiring neurolysis.14 The surgeon must identify the common peroneal nerve by elevating it from the fibular neck, determine whether fibrous thickening in the lateral gastrocnemius muscle fascia is present, and finally, be certain that the overall entrance into the anterior and lateral compartments is sufficiently large.
Deep Peroneal Nerve
The deep peroneal nerve arises over the fibular neck as a discrete fascicle. It can be dissected proximally by microtechnique if necessary. Immediately after crossing the fibular neck, it gives off tiny branches to the tibialis anterior and the toe extensor muscles. In so doing, the deep peroneal nerve effectively tethers the common peroneal and its distal branches from having too much excursion. This predisposes the common peroneal nerve and its branches in this region to stretch/traction injuries. The entrapment site for the deep peroneal nerve is at the ankle, the “anterior tarsal tunnel,” which is a rare site for compression unless there has been a direct injury in this location. A more common site for compression is over the dorsum of the foot where the tendon of the extensor hallucis brevis represents the anatomic structure causing the compression. A portion of the tendon of the extensor hallucis brevis is excised in the region of the deep peroneal nerve5 (see Fig. 42-3).
Superficial Peroneal Nerve
The superficial peroneal nerve arises over the fibular neck from the common peroneal nerve. It can be dissected more proximally by microdissection if required. The motor innervation to the foot everters arises within the first 1 to 2 cm distal to the neck of the fibula. The known site of entrapment for this nerve is the region where it transitions from deep to the fascia into the subcutaneous tissues in the distal third of the leg. The typical division into its dorsomedial and dorsolateral branches occurs just proximal to the ankle in approximately 75% of patients (see Fig. 42-3). In the author’s experience, it is now clear that in at least 25% of cadavers and patients coming to surgery, this division can occur in the proximal third of the leg.2,39 This creates the situation in which the superficial peroneal nerve may be found in both the anterior and, its most usually described location, the lateral compartment. Indeed, the superficial peroneal nerve may be present in just the anterior compartment or within the septum between the two compartments.40 The anatomic variability means that any surgical approach to this nerve must open both the anterior and the lateral compartments.
Proximal Tibial Nerve
Although the tibial nerve (like the common peroneal nerve) arises at the sciatic notch as the medial component of the sciatic nerve, and is often clearly a distinct nerve in the thigh, the only well-described site for tibial nerve compression was at the medial ankle in the tarsal tunnel.31 It is now evident that the tibial nerve can be compressed in a site more proximal than the tarsal tunnel, and this site (just distal to the knee) is best described as the proximal tibial nerve to distinguish it from the tarsal tunnel region. Tibial nerve compression in the popliteal fossa has been described related to the presence of compartment syndrome or space-occupying masses, such as a popliteal artery or a Baker cyst.26,34 Although anatomy texts clearly depict a fibrous arch or soleal sling from which the soleus muscle arises, only recently has there been an anatomic study describing the relationship of this sling to the proximal tibial nerve.41 This sling lies at a mean distance of 9.3 cm (range, 7–13 cm) from the middle of the popliteal fossa and causes a visible narrowing of the tibial nerve over a length of 1.5 cm in 55% of the cadavers, with a severe constriction being found in 2% of the specimens. With injury to the knee, especially one associated with postoperative bleeding into the popliteal fossa, tibial nerve compression at this proximal site must be considered as a source of symptoms of numbness in the plantar aspect of the foot, with proximal referral to the knee region.
Lateral Femoral Cutaneous Nerve
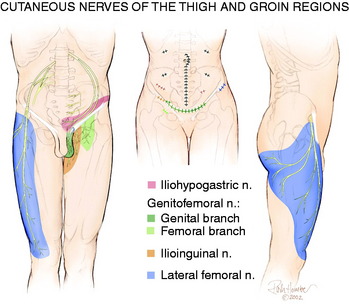
The lateral femoral cutaneous nerve has a great deal of anatomic variability related both to its location at the anterior superior iliac crest2 and to its innervated skin territory. If the location is within the inguinal ligament instead of inferior to it, the lateral femoral cutaneous nerve is at risk for compression or stretch/traction injury during falls, while wearing tight belts, during a seatbelt injury, or during manipulation of the knee with the patient on the operating room table. From Figure 42-4, it can be appreciated that compression of the lateral femoral cutaneous nerve can produce symptoms that include numbness or pain about the lateral aspect of the knee.37 This nerve, or the femoral nerve itself, can be injured during regional anesthesia given for knee surgery.22
Saphenous Nerve
The saphenous nerve is a branch of the femoral nerve that arises from the femoral nerve in the proximal thigh. Its cutaneous branches to the skin below the knee are well described and lie in a position to be injured directly from either a medial ankle arthroscopy portal or the midline incisions used for many surgical approaches to the knee. These branches can be directly injured by blunt trauma as well. Less well recognized is the nerve to the skin overlying the patellar itself. This is a branch of the femoral nerve, which may include sensory contribution from the obturator nerve joining within Hunter’s canal. The medial cutaneous nerve of the thigh (see Fig. 42-1) approaches from the medial aspect of the knee compared with the vertical approach taken by the anterior femoral cutaneous nerves.1 Even less well appreciated is that the saphenous nerve can be compressed in the distal thigh within Hunter’s canal. This is called adductor canal syndrome, which is rare in the absence of direct trauma to this region. Entrapment of the saphenous nerve in this location can present as medial knee pain.36
CLINICAL EVALUATION OF KNEE PAIN OF NEURAL ORIGIN
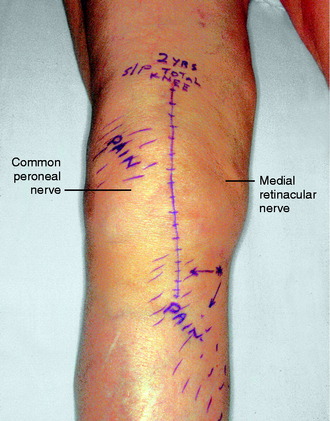
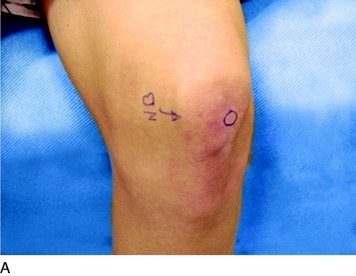
The physical examination is directed to identifying one or more sources of neural origin of the pain. First, the clinician should try to distinguish whether any skin territories are dysesthetic or painful when touched lightly. If so, these areas should be outlined. The most common distribution is that of the infrapatellar branch of the saphenous nerve; the second most common is the skin over the patella itself. These areas might be numb, indicating loss of sensation (Fig. 42-5). Once the pattern is identified, examine proximally along the course of the given nerve, observing for a trigger spot, which is either a true end-bulb neuroma or an in-continuity nerve lesion. The hypothesis is made that this nerve or these nerves are the source of the cutaneous pain; a diagnostic nerve block will be required to confirm this hypothesis.
Critical Points CLINICAL EVALUATION OF KNEE PAIN OF NEURAL ORIGIN
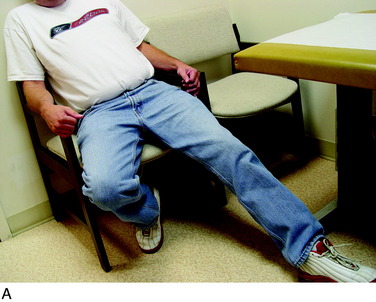
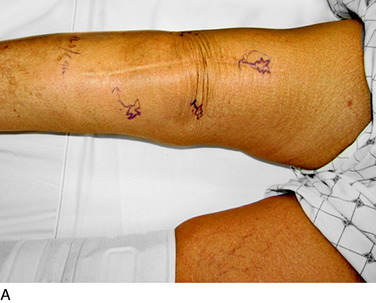
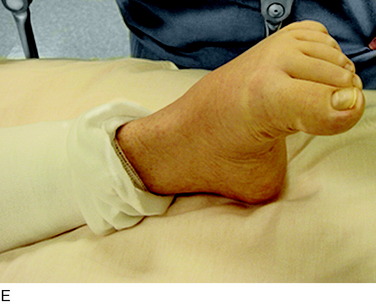
The physical examination is then directed to the joint afferents. Palpation is done deeply to the spot located just distal to the vastus medialis muscle, through the medial retinaculum to elicit pain from the medial retinacular nerve. Palpation is next done deeply to the area located just distal to the vastus lateralis muscle, through the lateral retinaculum, to elicit pain from the lateral retinacular nerve (Fig. 42-5). The hypothesis is made that the knee joint pain is due to an injury to one or both of the joint afferents; a diagnostic nerve block will be required to confirm this hypothesis. The medial cutaneous nerve to the thigh and the medial retinacular nerve can be blocked at the same area. Ten minutes after the nerve blocks, the patient is instructed to walk in the hallway, climb and descend a few steps, and even to kneel on a padded chair (Fig. 42-6). A reduction of 5 points on a visual analog scale; for example, from 10 to 5, where 10 represents the worst pain, is confirmation that sufficient relief of pain has occurred to permit surgery for partial knee denervation and resection of cutaneous neuromas.
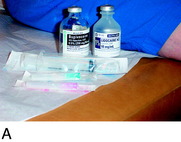
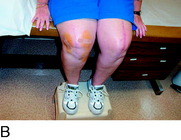


FIGURE 42-6 Demonstration of the use of local anesthesia to block the suspected joint and cutaneous afferents in the patient with knee pain. A mixture of 0.5% bupivacaine (Marcaine) and 1% lidocaine (Xylocaine), each without epinephrine (upper left), is used to conduct the local block at the two sites indicated in Figure 42-5 to block the joint afferents most likely to be the source of joint pain. Additional blocks would also be done of cutaneous afferents. After the block, the patient should be able to climb stairs and kneel without the previous pain (as shown). There should be a reduction of at least 5 on the 10-point Likert scale in order for this block to be considered successful enough to recommend a partial knee denervation. If there is significant residual pain after the block, additional nerves causing this pain should be identified.
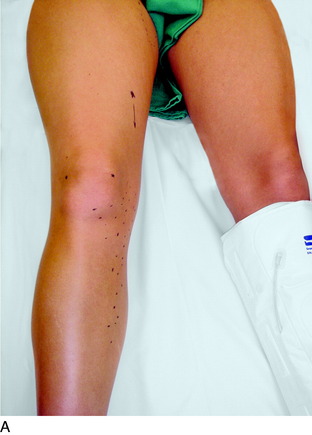
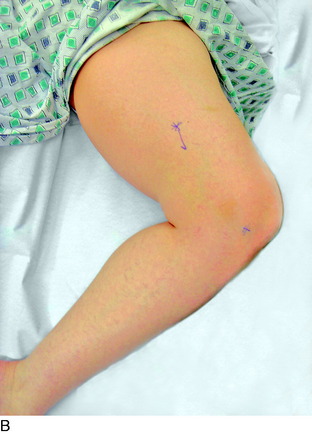
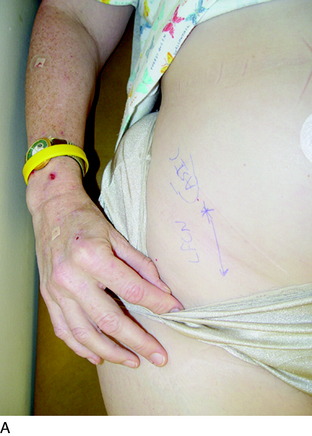
Finally, the groin should be examined to search for a Tinel sign over the lateral femoral cutaneous nerve. The patient will be noted to sit with the lower extremity extended at the hip joint (Fig. 42-7). The midthigh should be examined to search for a Tinel sign over Hunter’s adductor canal. This is most effectively done with the patient supine, and the effected leg externally rotated at the hip, leaving the knee bent. This stretches the adductor muscle group over the saphenous nerve. Then, gentle palpation over the canal produces a distal radiating painful response (Fig. 42-8).
PREOPERATIVE PLANNING
The fourth consideration is the existence of a neuropathy, either diabetic or idiopathic. If the patient has complaints related to the feet, the presence of neuropathy should be evaluated. In addition to the history and physical examination, the author’s preferred approach is to obtain nonpainful and noninvasive neurosensory testing with the Pressure-Specified Sensory Device (PSSD) (Sensory Management Services, LLC, Baltimore, MD).4,7,38 The PSSD documents the presence of a neuropathy and a nerve entrapment, providing a basis for decompression of the common or superficial peroneal nerve or both (dorsomedial and dorsolateral foot sensibility sites). This device also documents the presence of tibial nerve dysfunction by evaluating the medial calcaneal and medial plantar nerves (heel and hallux pulp skin test sites). This is especially helpful in the patient with postarthroplasty palsy, in whom the PSSD evaluation can document whether the involved nerves demonstrate a nerve regeneration pattern and warrant further observation or, in the absence of that pattern, whether neurolysis is indicated at the 3-month timeframe.9
Critical Points PREOPERATIVE PLANNING
The sixth consideration is the vascular status of the limb. The peripheral nerve surgery is done with the use of a tourniquet. Currently, it is recommended not to use a tourniquet in the presence of a leg that has had a bypass grafting procedure.21
The seventh consideration is to inform the patient that after a denervation procedure, a neurectomy of a peripheral nerve, or a decompression, there may be unusual and sometimes painful postoperative sensations. These are due to either nerve regeneration in the case of a nerve decompression or collateral sprouting in the case of a resection of a cutaneous nerve. Some patients perceive that the knee area is swollen, although no visible effusion is present. Postoperatively, the patient should be prepared to participate in aquatic therapy pool sessions two to three times a week for 3 weeks and perform water-walking to desensitize the skin and help reorganize the cortical maps.10
OPERATIVE TECHNIQUES
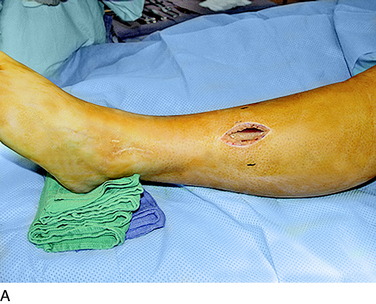
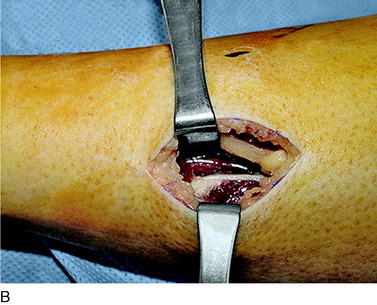
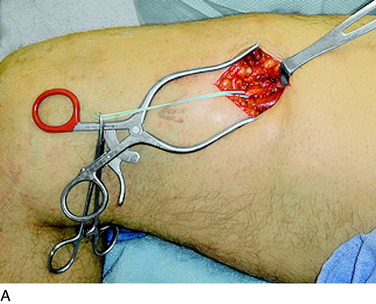
The operative techniques for partial knee denervation described later have been published previously.17–19 For each patient, a tourniquet is used and set at a pressure of 300 mg Hg. If the leg is quite large or exceptionally muscular, this pressure can be increased to 325 mm Hg. It is critical to note that the leg is not completely exsanguinated. Deliberately, some venous blood is left in the region around the knee by not wrapping the Ace bandage tightly. The small amount of blood left in all the recurrent geniculate artery and veins is helpful in identifying the small joint afferents that lie next to these structures and the larger nerves such as the infrapatellar branch of the saphenous nerve. A bipolar coagulator and Loupe magnification (3.5x) are used. Intravenous antibiotic prophylaxis is given prior to inflating the tourniquet. A femoral nerve block is not done owing to the risk of injury to the femoral nerve, but rather bupivicaine (Marcaine) 0.5% is infiltrated into the skin edges of the incisions at the end of the surgical procedure. The dressing consists of Xeroform gauze over the incision, followed by sterile 4- x 4-inch gauze, which is held in place with Kling and covered with a stockinette. Then, an Ace wrap is applied firmly and left in place for 30 minutes to counteract the reactive hyperemia attendant to use of the tourniquet.
Critical Points OPERATIVE TECHNIQUES
Finally, the concept of minimizing the risk of recurrent neuroma pain must be understood. Because the sensory nerves remain alive once divided owing to their intact nucleus in the dorsal root ganglion, nerve regeneration will occur in every joint afferent or cutaneous afferent that is divided. Alteration of the microenvironment of the nerve by implanting the proximal end of a divided sensory nerve into an innervated motor environment results in the absence of formation of a true neuroma.32 Implanting a sensory nerve into an innervated muscle after resecting the painful neuroma or after interruption of that nerve function by division of that nerve results in predictable pain relief without recurrent neuroma for upper15,30 and lower extremity12,28,29 peripheral nerves. The location chosen to place the divided nerve is explained for each operation described later.
Lateral Denervation of the Knee Joint
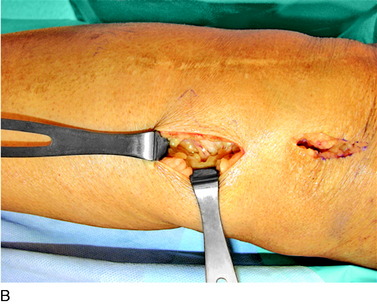
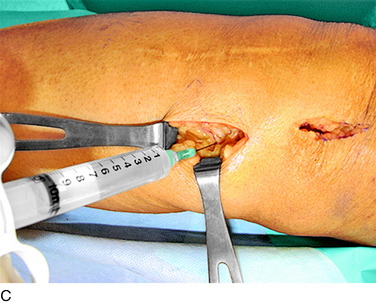
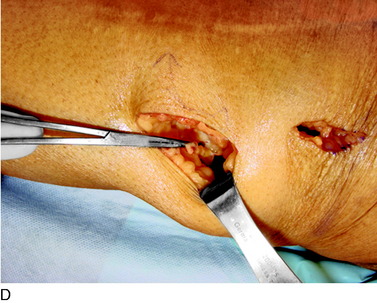
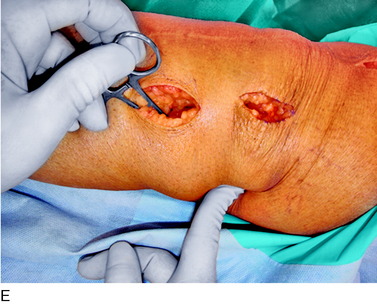
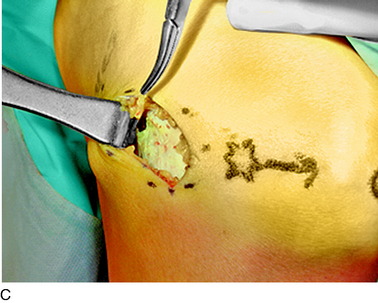
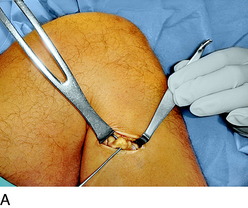
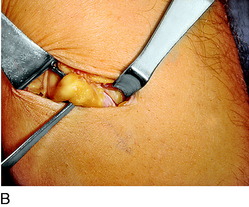
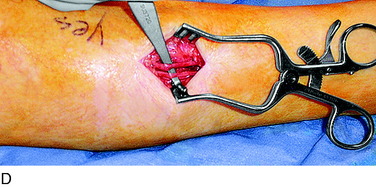
An incision is outlined lateral to the patella, beginning over the distal muscle belly of the vastus lateralis. This muscle sometimes has a slight distal split, and the surgeon must be sure to remain distal to the most distal portion of the vastus lateralis muscle. The iliotibial band (lateral retinaculum) is divided longitudinally for approximately 1.5 cm. Immediately adjacent to the muscle belly are small recurrent vessels and a 1- to 1.5-mm nerve that go from beneath the biceps tendon and the iliotibial band and across the synovium into the lateral joint and infrapatellar structures. This nerve is infiltrated with bupivacaine 0.5% down beneath the iliotibial band and the nerve and vessels are cauterized toward the patella to prevent bleeding and placed under traction and cauterized deep beneath the iliotibial band to prevent bleeding. The portion between the two cauterized sites is sent to pathology. The iliotibial band (lateral retinaculum) is repaired with two figure-eight sutures of 2-0 braided nonabsorbable material (Fig. 42-9).
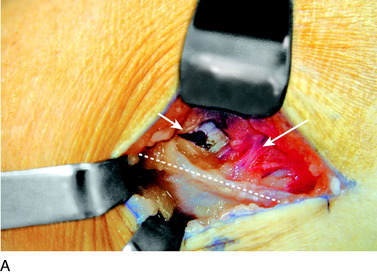
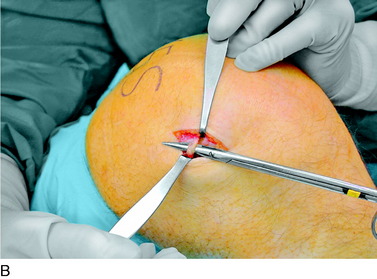
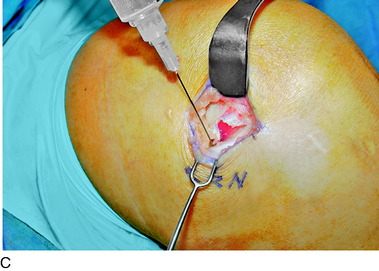
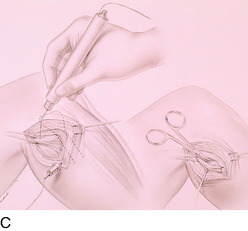
FIGURE 42-9 Lateral knee denervation. A, Incision is shown, lateral to the patella, centered over the tender site shown in Figure 42-5. The short arrow indicates the vastus lateralis. The iliotibial tract is incised (dotted line) to demonstrate the lateral retinacular nerve (long arrow) adjacent to the superior lateral geniculate vessels. B, The lateral retinacular nerve. C, The nerve is first infiltrated with local anesthetic, cauterized distally and proximally, and then divided proximally below the level of the iliotibial band so that the nerve drops into the popliteal fossa.
Medial Denervation of the Knee Joint
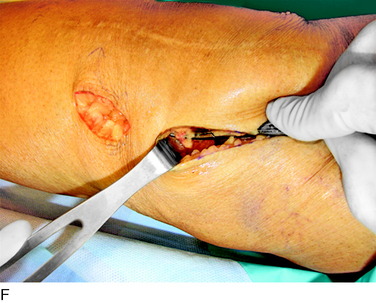
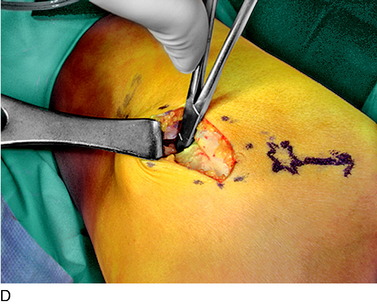
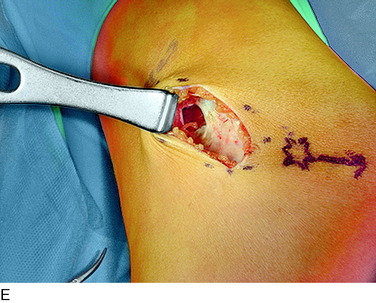
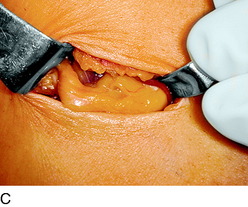
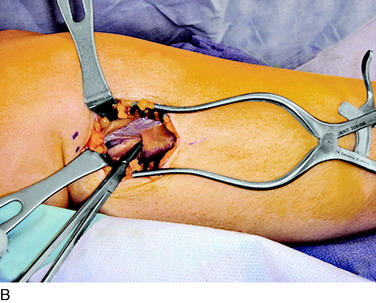
An incision is outlined medial to the patella beginning over the distal muscle belly of the vastus medialis. The thin medial retinaculum is divided longitudinally for approximately 1.5 cm. Immediately adjacent to the muscle belly are the small recurrent vessels and a 1- to 1.5-mm nerve that goes from beneath the vastus medialis and across the synovium into the medial joint and infrapatellar structures. This nerve is dissected proximally beneath the retinaculum until it exits from beneath the muscle. This nerve is infiltrated with bupivacaine 0.5% down beneath the vastus medialis and the nerve and vessels are cauterized toward the patella to prevent bleeding and placed under traction and cauterized deep beneath the vastus medialis to prevent bleeding. The portion between the two cauterized sites is sent to pathology. The medial retinaculum is repaired with two figure-eight sutures of 3-0 braided nonabsorbable material (Fig. 42-10).
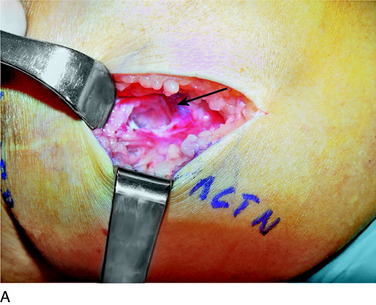
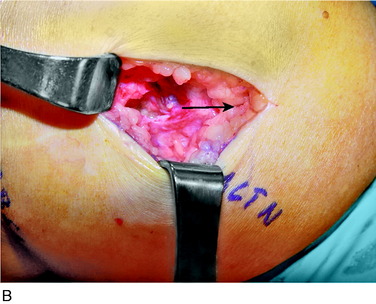
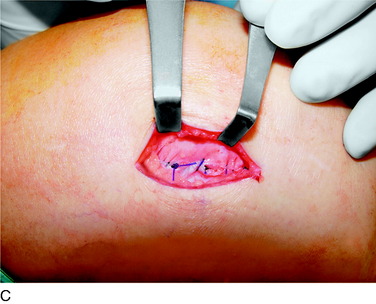
FIGURE 42-10 Medial knee denervation. A, Incision is shown, medial to the patella, centered over the tender site shown in Figure 42-5. The medial retinaculum is opened. The arrow points to the medial retinacular nerve adjacent to the recurrent medial recurrent geniculate vessels. B, The nerve has been resected. The arrow points to the direction in which, beneath the medial retinaculum, the nerve has been implanted into the vastus medialis muscle. C, Closure of the medial retinaculum.
Resection of the Infrapatellar Branch of the Saphenous Nerve
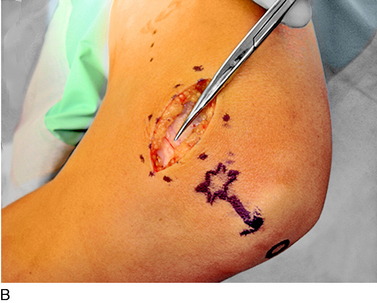
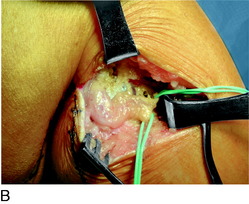
The saphenous nerve exits Hunter’s canal in the distal thigh to become the medial cutaneous nerve of the thigh, the infrapatellar branch of the saphenous nerve, and the distal saphenous nerve. The infrapatellar branch crosses the insertion of the adductor tendons into Gerdy’s tubercle beneath the deep fascia. There may already be two branches at this level. Ultimately, several terminal branches cross from medial to lateral across the region of the tibial tuberosity to innervate the lateral knee skin. The skin proximal to this laterally is the terminal zone of innervation of the lateral femoral cutaneous nerve. Whereas the numbness is lateral, the damage to the nerve usually occurs in the axial line of the knee from an incision or medially from a scope portal. The site of the Tinel sign medially is around Gerdy’s tubercle. An incision is made longitudinally across the Tinel sign. The dissection is carried deep to the fascia where one or more branches are noted by the blood in the vein that accompanies the nerve branch. A thorough search proximally and distally must be done to identify more than one branch. This is the most common location for a missed remaining painful nerve. The nerve is infiltrated with bupivacaine 0.5% proximally, the distal end cauterized to minimize bleeding, a segment resected for pathology, and the proximal end dissected. There is usually a clear tunnel where this nerve has transversed along or through the sartorius or other adductor muscle or tendon. The proximal end of the divided nerve, after cauterization to prevent bleeding, is turned blindly into these muscles and implanted there, proximal to the popliteal crease (Fig. 42-11).
Resection of the Medial Cutaneous Nerve of the Thigh
The medial cutaneous nerve goes to the skin overlying the patella. This skin is traditionally shown as being innervated by an anterior femoral cutaneous nerve coming vertically down the leg. However, the skin is innervated by a branch of the saphenous and approaches this region medially. Indeed, the same tender location for the medial retinacular nerve is the location of this nerve. The clinical clue is that the skin of the patella is dysesthetic. The nerve block medially will block both of these nerves. An incision is used to approach the medial retinacular nerve. There will be blood in the immediate subcutaneous tissue from the small vein that accompanies this nerve. The nerve is cauterized distally, and the proximal end is dissected medially across the surface of the vastus medialis. The nerve is injected with bupivacaine 0.5% proximally. A small window is opened into the fascia of the vastus medialis. The proximal end of the nerve is implanted loosely into this muscle (Fig. 42-12).
Neurolysis of the Common Peroneal Nerve
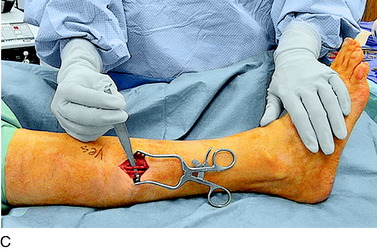
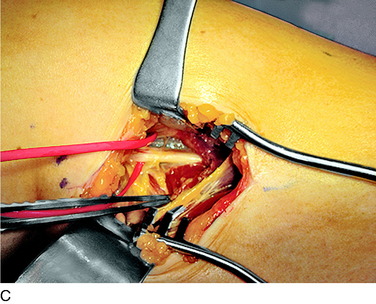
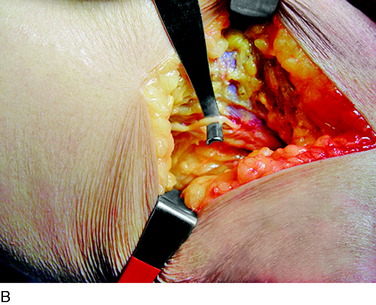
The deep fascia is opened. In patients who do not have a neuropathy but who sustained trauma, this fascia will be adherent to the common peroneal nerve, which will be white with an area of inflammation and swelling as the peroneus longus muscle is approached. In patients with neuropathy, and particularly those with glucose intolerance or actual diabetes, the common peroneal nerve will be yellow and resemble a neuroma. Be aware of these cases and handle the fat beneath the fascia with great care because it is usually the nerve. The dissection must release the nerve into the popliteal fossa, especially in the case of trauma or joint replacement. The usual compression site, however, is beneath the peroneus muscles. As the surgeon evaluates the common peroneal nerve approaching the fibular head and crossing the fibular neck, two or more tiny branches will be observed that go around the fibular head to innervate the proximal tibiofibular joint. Because the peroneus fascia is the main site of compression, divide the peroneus longus fascia transversely and from the fibula head distally, cauterizing the edges. If there is a septum between muscle groups, divide this septum, being careful on its deep surface. Retract the muscle; dividing the muscle is not necessary. In 20% of cadavers, but in 80% of patients, there will be another band of varying width and thickness deep to the muscle and overlying the nerve across the fibular neck.12 This is the site of compression, and this band is resected. Then, look beneath the nerve for fibrous bands on the lateral gastrocnemius muscle; if they are present, cauterize and resect them. Check the passageway of the common peroneal nerve beneath the muscles and into the leg. If this is tight, divide the fascial origins of the muscle at the fibula by cautery. This completes the neurolysis of the common peroneal nerve (Fig. 42-13).
Denervation of the Proximal Tibiofibular Joint
In the preceding section, the small branches to the proximal tibiofibular joint were observed. The safest way to denervate this joint is to perform a complete neurolysis of the common peroneal nerve. Then, using a disposable nerve stimulator, stimulate the deep and the superficial peroneal nerves to demonstrate a “positive control.” Stimulate the small branches purported to go the proximal tibiofibular joint. If no muscle twitches, these are indeed the correct nerves and they may be resected back to the common peroneal nerve and submitted to pathology. Do not inject bupivacaine into these nerves because this is likely to create a motor block and cause worry in the recovery room until it wears off (Fig. 42-14).
Neurolysis of the Superficial Peroneal Nerve
The most important concept here is that the superficial peroneal nerve in at least 25% of patients will have a branch in the anterior compartment.2,39 Therefore, in addition to opening the lateral compartment, the anterior compartment must be opened. The incision is made approximately 4 cm long, centered on the site of the positive Tinel sign. The most common location for the incision is 10 to 15 cm proximal to the lateral malleolus. In the subcutaneous tissue, look for a small bulge in the fascia or fat protruding from the fascia because this may be the transit site of the superficial peroneal nerve from deep to superficial to the fascia. There may be a small branch to the skin right at this point that must be preserved. Create a longitudinal opening in the lateral compartment. Even if a nerve is found, a longitudinal opening in the anterior compartment must be made. In addition, transversely cut the fascia to visualize all the way anteriorly. The fascial edges must be cauterized because they are well vascularized and can be a source of postoperative bleeding. The septum between the two compartments is another site in which a branch or the entire superficial peroneal nerve may be located. Do not just simply cut across this region.40 The goal is to leave the superficial peroneal nerves surrounded only by muscle, because this guides the proximal and distal extent of the fascial releases (Fig. 42-15).
Neurolysis of the Proximal Tibial Nerve
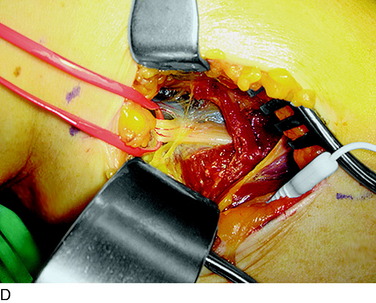
An incision approximately 12 cm long is made medially in the calf just anterior to the medial head of the gastrocnemius muscle. The center of the incision is made at the level of the tender proximal tibial nerve, approximately 9 cm distal to the popliteal crease. The deep fascia is opened and the medial gastrocnemius muscle is dissected bluntly from the soleus muscle. The plantaris tendon and the popliteus muscle will be seen. The tibial nerve and the popliteal vein will be observed to pass anterior to the fascial origin or arch or sling of the soleus muscle. A large clamp placed deep to this arch demonstrates the tight area. The soleus muscle fibers may require dissection to reveal the fibrous arch. If there has been bleeding in this area, this dissection can be difficult. The soleal arch must be completely divided. Usually, a notch in the tibial nerve will be identified (Fig. 42-16).
Neurolysis of the Lateral Femoral Cutaneous Nerve
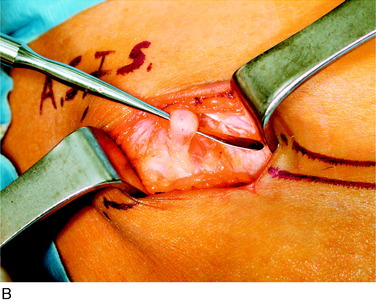
A tender spot exists just anterior to the anterior superior iliac crest. An incision approximately 4 cm long is made just cephalad to this area and deepened into the subcutaneous tissue. The inguinal ligament is identified and carefully divided, millimeter by millimeter, adjacent to the anterior superior iliac crest and the surgeon looks for the compressed lateral femoral cutaneous nerve. The nerve is narrowed, yellow, and inflamed and often does not look like a nerve. The nerve must be released into the thigh and then proximally until it enters the pelvis. Proximally, this entails dividing portions of the internal oblique fascia. Where this dissection stops, the surgeon should take care not to divide the deep circumflex iliac artery and vein. If the lateral femoral cutaneous nerve is too badly damaged by the compression, it may need to be resected with the proximal end allowed to lie within the pelvis (Fig. 42-17).
Neurolysis of the Saphenous Nerve in Hunter’s Canal
A 6-cm incision is made longitudinally at the site of the Tinel sign of the saphenous nerve in the adductor canal. The dissection goes deep until the fascia connecting the vastus medialis to the adductor longus is identified. The length of this tunnel is between 6 and 8 cm. Small nerves will be in this vicinity. They need to be stimulated, because some are not saphenous branches but motor branches. Once the fascia is released, the saphenous nerve will be present as one or more branches. Deep to them will be the superficial femoral artery (Fig. 42-18).
AUTHOR’S CLINICAL STUDIES
If a patient obtains relief of knee pain after a block of any combination of joint or cutaneous afferents, there is a 90% chance that he or she should also obtain good to excellent relief of his or her knee pain postoperatively.17–19 Patients who achieve less than a satisfactory result either have another nerve that requires removal or have confounding problems such as a central mechanism for their chronic pain, drug addiction, or legal issues related to work or an accident.
The first group of patients selected for partial knee denervation were chosen from a group who had undergone a TKA but had persistent pain for greater than 6 months that was unrelated to loosening, malalignment, or infection.19 In this study, the orthopaedic surgeons completed a preoperative Knee Society Function Score, range of motion, and pain assessment in 15 patients. The author independently performed the partial knee denervation, whereas the orthopaedic surgeons conducted the postoperative assessment. To be selected for surgery, each patient had to achieve a reduction of 5 points on a visual analog scale for pain after undergoing selective nerve blocks. A total of 45 nerves were resected in the 15 patients including (in each patient) both the medial and the lateral retinacular nerve and the infrapatellar branch of the saphenous nerve. All patients reported subjective improvement in the immediate postoperative period, which was maintained at a mean follow-up of 12 months (range, 6–16 mo). It was concluded that selective knee denervation is indicated in the management of intractable knee pain of neuroma origin after TKA.
The next series reported comprised 70 patients.18 Some of these patients had persistent knee pain after TKA, but the indications were extended to include those with chronic pain after knee trauma or tibial osteotomy. In patients with TKA, aseptic loosening, sepsis, ligamentous instability, malalignment, and polyethylene wear were systematically ruled out as the source of pain by the orthopaedic surgeons. In patients with non-TKA pain, arthrosis, synovitis, ligamentous instability, and meniscal derangement were excluded as a source of pain. All candidates for the procedure had a successful selective nerve block. Sixty of the 70 patients (86%) were satisfied with the denervation procedure as judged by direct questioning and a reduction in their preoperative pain visual analog score of 5 or more points. The average Knee Society Score improved from a preoperative mean of 51 points (range, 40–62 points) to a follow-up mean of 82 points (range, 15–100 points). Forty-nine patients (70%) had final Knee Society objective scores greater than 80 points. There was no difference in patient satisfaction whether the follow-up period was less than 2 years or more than 2 years. Selective knee denervation is indicated in the management of intractable knee pain after exhaustion of traditional approaches to any structural or infectious etiologies and after successful selective nerve block.
In 2000, a series of 344 patients were reviewed.17 Of these, 255 had a previous TKA and 89 had knee trauma. Most patients had several nerves removed; none had only one nerve resected. All required removal of the medial and the lateral retinacular nerves, and the majority also required removal of the medial cutaneous nerve of the thigh and the infrapatellar branch of the saphenous nerve. The nerves least often removed were the anterior femoral cutaneous and the distal saphenous nerve. Approximately one half of the patients, especially those who had sustained knee trauma, required a neurolysis of the common peroneal nerve. The proximal tibiofibular joint required denervation in patients with fractures of the fibula or tibial plateau and who had undergone a Maquet procedure or a high tibial osteotomy. The results for the entire series were 70% excellent (no remaining pain), 20% good (some pain remained, but no medication was required), 5% some improvement (surgery helped, but pain medication still required), and 5% no improvement. No patient was neurologically downgraded or made worse. No knee implant or hardware was exposed. No patient had to be hospitalized to treat infection. The abnormal sensibility, which is usually decreased sensibility, remains to some degree in everyone after a cutaneous nerve is removed.
A review of knee denervation patients on our office computer from January of 2000 through December of 2006 includes 405 patients. Whereas this large group of patients has not been reviewed at this time, the overall experience with the diagnosis, intraoperative management, and postoperative outcomes remains the same as that obtained in our 2000 review data.16
OTHER AUTHORS’ CLINICAL STUDIES
In contrast to the vast literature on relief of acute knee pain with femoral, obturator, or even lateral femoral cutaneous nerve blocks (more than 120 references cited in Pubmed between 2006 and 2008), with the exception of one surgeon in Germany,23,24 there have been no reported studies of partial knee denervation. Between May of 1995 and June of 1999, Fromberg and Hempfling24 performed partial denervation using the “Dellon technique” described in this chapter in 45 knees. The investigations of Fromberg and Hempfling included patients with direct knee injury as well as those who had knee joint replacement. Thirty-four patients were followed, 11 with bilateral knee pain, whose ages ranged from 25 to 86 years (mean, 34 yr). At follow-up, 6 to 18 months postoperatively, 70% of the patients reported “a reduction in pain,” and after 4 years, 50% “still confirmed a positive result.” Complications included 1 hematoma and 2 seromas, which resolved with conservative management.
A recent study evaluated the innervation of the patella in 30 knees of 15 formaldehyde-fixed cadavers.33 A nerve from the vastus medialis (which is the nerve described previously1) entering the patella “superomedially” and a nerve “from the vastus lateralis entering the patella superolaterally” were identified. The origin of these nerves was not described. The superolateral nerve “from the vastus lateralis” was most likely the nerve described previously that originates from the sciatic nerve and crosses in front of the vastus lateralis.25 This anatomic study in fixed cadavers confirms the author’s observations reported in 1994.25 Maralcan and coworkers33 confirmed that these nerves were patellar pain afferents by performing a local anesthesia block in 32 knees of 20 patients with patellofemoral pain. They observed a significant difference between the visual analog scale scores before and after local anesthetic injections (P < .01). Their observations appear to confirm that knee pain can be of neural origin from the medial and the lateral retinacular nerves, as described previously.17–19,25
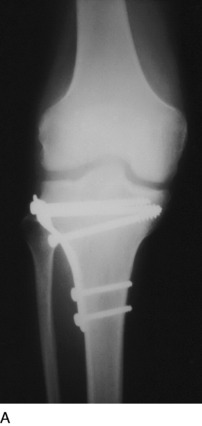
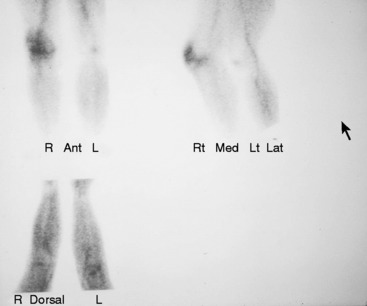
REFLEX SYMPATHETIC DYSTROPHY OF THE KNEE
In 1986, Katz and associates27 defined reflex sympathetic dystrophy of the knee as diffuse pain, often accompanied with skin color changes, in patients who did not allow their knee to be touched and exhibited all the characteristics of what today is termed complex regional pain syndrome. These authors reported on 5 patients from a series of 662 primary total knee arthroplasties (0.8%) who demonstrated, in addition to pain, marked limitation of flexion. They stated that the traditional bone scan and osteopenia that were used as criteria for diagnosing reflex sympathetic dystrophy in the hand were difficult to aid in diagnosing this disease in patients who had TKA (Fig. 42-19). A lumbar sympathetic block was believed to be more helpful in making the diagnosis.
With the vision of hindsight, the author now maintains that this condition is related to the combination of joint and cutaneous neuromas. However, instead of attempting to treat this problem by blocking the sympathetic outflow, the author’s preferred treatment is to interrupt the pain stimuli from entering the dorsal columns of the spinal cord using the techniques described previously. The author’s experience with this approach was reviewed in 40 upper and 30 lower extremity patients with complex regional pain syndrome.11 At a mean postoperative time interval of 15 months, and based upon decreased pain medication usage, recovery of function, repeat history and physical examination, outcome in the upper extremity patients was excellent in 55%, good in 35%, and failed in 10%. Outcome in the lower extremity patients was similar: excellent in 66%, good in 30%, and failed in 4%.
1 Aszmann O.C., Dellon A.L., Birely B.T., McFarland E.G. Innervation of the human shoulder joint and its implications for surgery. Clin Orthop Relat Res. 1996;330:202-207.
2 Barrett S.L., Dellon A.L., Rosson G.D., Walters L. Superficial peroneal nerve (superficial fibularis nerve): the clinical implications of anatomic variability. J Foot Ankle Surg. 2006;45:174-176.
3 Dellon A.L. Anterior shoulder denervation. Clin Exp Plast Surg. 2004;36:175-180.
4 Dellon A.L. Clinical grading of peripheral nerve problems. Neurosurg Clin N Am. 2001;12:229-240.
5 Dellon A.L. Deep peroneal nerve entrapment on the dorsum of the foot. Foot Ankle. 1990;11:73-80.
6 Dellon A.L. Denervation of the sinus tarsi for chronic post-traumatic lateral ankle pain. Orthopedics. 2002;25:849-851.
7 Dellon A.L. Diabetic neuropathy: review of a surgical approach to restore sensation, relieve pain, and prevent ulceration and amputation. Foot Ankle Int. 2004;25:749-755.
8 Dellon A.L. Partial dorsal wrist denervation: resection of the distal posterior interosseous nerve. J Hand Surg [Am]. 1985;10:527-533.
9 Dellon A.L. Postarthroplasty “palsy” and systemic neuropathy: a peripheral-nerve management algorithm. Ann Plast Surg. 2005;55:638-642.
10 Dellon A.L., editor. Somatosensory Testing and Rehabilitation. Bethesda, MD: American Occupational Therapy Association, 1997.
11 Dellon A.L., Andronain G., Rosson G.D. Successful surgical approach to complex regional pain syndrome. J Brachial Plexus Peripheral Nerve Inj. 2009. (in press)
12 Dellon A.L., Aszmann O.C. Treatment of superficial and deep peroneal neuromas by resection and translocation of the nerves into the anterolateral compartment. Foot Ankle Int. 1998;19:300-303.
13 Dellon A.L., Barrett S.L. Sinus tarsi denervation: clinical results. J Am Podiatr Med Assoc. 2005;95:108-113.
14 Dellon A.L., Ebmer J., Swier P. Anatomic variations related to decompression of the common peroneal nerve at the fibular head. Ann Plast Surg. 2002;48:30-34.
15 Dellon A.L., Mackinnon S.E. Treatment of the painful neuroma by neuroma resection and muscle implantation. Plast Reconstr Surg. 1986;77:427-438.
16 Dellon A.L., Mackinnon S.E., Daneshvar A. Terminal branch of anterior interosseous nerve as source of wrist pain. J Hand Surg [Br]. 1984;9:316-322.
17 Dellon A.L., Mont M.A., Hungerford D.S. Partial denervation for the treatment of painful neuromas complicating total knee arthroplasty. In: Insall J.N., Scott W.N., editors. Surgery of the Knee. Philadelphia: W. B. Saunders; 2000:1772-1786.
18 Dellon A.L., Mont M.A., Krackow K.A., Hungerford D.S. Partial denervation for persistent neuroma pain after total knee arthroplasty. Clin Orthop Relat Res. 1995;316:145-150.
19 Dellon A.L., Mont M.A., Mullick T., Hungerford D.S. Partial denervation for persistent neuroma pain around the knee. Clin Orthop Relat Res. 1996;329:216-222.
20 Dellon A.L., Seif S.S. Anatomic dissections relating the posterior interosseous nerve to the carpus, and the etiology of dorsal wrist ganglion pain. J Hand Surg [Am]. 1978;3:326-332.
21 Ducic I., Chang S., Dellon A.L. Use of the tourniquet in reconstructive surgery in patients with previous ipsilateral lower extremity revascularization: is it safe? A survey. J Reconstr Microsurg. 2006;22:183-189.
22 Ducic I., Dellon L., Larson E.E. Treatment concepts for idiopathic and iatrogenic femoral nerve mononeuropathy. Ann Plast Surg. 2005;55:397-401.
23 Fromberg G. Die selective Denervation des Kniegelenks. In: Rabenseifner L., editor. Arthrosemanagement Knie. Darmstadt, Germany: Steinkopff; 2000:13-19.
24 Fromberg G., Hempfling H. Die selective Denervation des Kniegelenks: Technik nach Dellon. In: Hempfling H., editor. Neue Techniken der Arthroskopie. Hans Marseille: Munich; 2000:379-383.
25 Horner G., Dellon A.L. Innervation of the human knee joint and implications for surgery. Clin Orthop Relat Res. 1994;301:221-226.
26 Ji J.H., Shafi M., Kim W.Y., et al. Compressive neuropathy of the tibial nerve and peroneal nerve by a Baker’s cyst: case report. Knee. 2007;14:249-252.
27 Katz M.M., Hungerford D.S., Krackow K.A., Lennox D.W. Reflex sympathetic dystrophy as a cause of poor results after total knee arthroplasty. J Arthroplasty. 1986;1:117-124.
28 Kim J., Dellon A.L. Neuromas of the calcaneal nerves. Foot Ankle Int. 2001;22:890-894.
29 Kim J., Dellon A.L. Pain at the site of tarsal tunnel incision due to neuroma of the posterior branch of the saphenous nerve. J Am Podiatr Med Assoc. 2001;91:109-113.
30 Mackinnon S.E., Dellon A.L. Results of treatment of recurrent dorsoradial wrist neuromas. Ann Plast Surg. 1987;19:54-61.
31 Mackinnon S.E., Dellon A.L. Tarsal tunnel syndrome. In: Surgery of the Peripheral Nerve. New York: Thieme; 1988:305-318.
32 Mackinnon S.E., Dellon A.L., Hudson A.R., Hunter D.A. Alteration of neuroma formation by manipulation of its microenvironment. Plast Reconstr Surg. 1985;76:345-353.
33 Maralcan G., Kuru I., Issi S., et al. The innervation of patella: anatomical and clinical study. Surg Radiol Anat. 2005;27:331-335.
34 Mastaglia F.L., Venerys J., Stokes B.A., Vaughan R. Compression of the tibial nerve by the tendinous arch of origin of the soleus muscle. Clin Exp Neurol. 1981;18:81-85.
35 Mont M.A., Dellon A.L., Chen F., et al. The operative treatment of peroneal nerve palsy. J Bone Joint Surg Am. 1996;78:863-869.
36 Morganti C.M., McFarland E.G., Cosgarea A.J. Saphenous neuritis: a poorly understood cause of medial knee pain. J Am Acad Orthop Surg. 2002;10:130-137.
37 Nahabedian M.Y., Dellon A.L. Meralgia paresthetica: etiology, diagnosis, and outcome of surgical decompression. Ann Plast Surg. 1995;35:590-594.
38 Radoiu H., Rosson G.D., Andonian E., et al. Comparison of measures of large-fiber nerve function in patients with chronic nerve compression and neuropathy. J Am Podiatr Med Assoc. 2005;95:438-445.
39 Rosson G.D., Dellon A.L. Superficial peroneal nerve anatomic variability changes surgical technique. Clin Orthop Relat Res. 2005;438:248-252.
40 Williams E.H., Dellon A.L. Intraseptal superficial peroneal nerve. Microsurgery. 2007;27:477-480.
41 Williams E.H., Dellon A.L. Soleus arch as compression site for proximal tibial nerve: cadaver study. Ann Plast Surg. 2009. (in press)