4 Knee Injuries
Anterior Cruciate Ligament Injuries
Background
The anterior cruciate ligament (ACL) is the most frequently completely disrupted ligament in the knee; most of these injuries occur in athletes (Fig. 4-1). More than 100,000 ACL reconstructions are done each year in the United States.
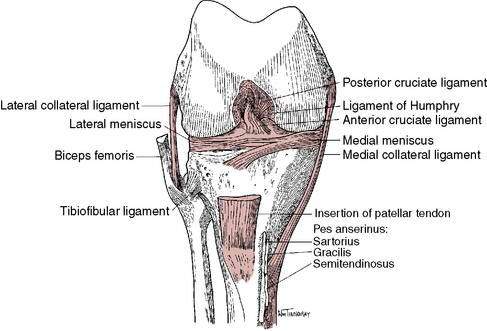
Figure 4-1 Anterior cruciate ligament and anatomic knee structures.
(Redrawn with permission from Miller MD, Howard RF, Planchar KD. Surgical Atlas of Sports Medicine. Philadelphia, 2003, Saunders, p. 74, Fig. 10-3.)
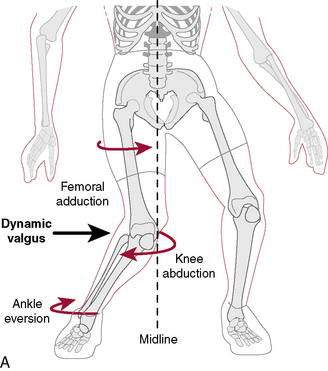
Hewett et al. (2005) in a level II study found that prescreened female athletes with subsequent ACL injury demonstrated increased dynamic knee valgus (Fig. 4-2) and high knee abduction loads on landing from a jump. Knee abduction moments, which directly contribute to lower extremity dynamic valgus and joint knee load, had a sensitivity of 78% and specificity of 73% for predicting future ACL injury. Neuromuscular training has been shown to decrease knee adduction moments at the knee (Hewitt et al. 1996), and this will be addressed at great length in the ensuing chapter.
Although a number of studies have suggested that OA eventually develops in 60% to 90% of individuals with ACL injuries (Beynnon 2005 Part 1, Andersson et al. 2009), a recent systematic review of the literature (Ojestad et al. 2009) concerning OA of the tibiofemoral joint more than 10 years after ACL injury suggests that these estimates are too high. The lack of a universal methodologic radiographic classification made it difficult to draw firm conclusions, but these investigators determined that in the highest-rated studies the reported prevalence of knee OA after isolated ACL injury was between 0% and 13%, and with meniscal injury, it was between 21% and 48% (level II evidence).
Treatment of ACL Injuries
Nonoperative Treatment (ACL-Deficient Knee)
Several authors have suggested criteria for nonoperative treatment in ACL tears: Fitzgerald et al. (2000) developed guidelines for selecting appropriate candidates for nonoperative ACL deficiency management (e.g., initiation of perturbation and strengthening program). The primary criteria were no concomitant ligament (e.g., medial collateral ligament) or meniscal damage and a unilateral ACL injury. Other criteria include the following:
Of four randomized controlled studies comparing nonoperative to operative treatment (level I evidence), one reported no difference in outcomes (Sandberg et al. 1987) and three reported superior results with operative treatment (Andersson et al. 1989 and 1991, Odensten et al. 1984).
Operative ACL Reconstruction
Timing of surgery. Because many patients had difficulty regaining full knee motion after acute or early reconstruction, delayed reconstruction has been suggested to minimize the possibility of postoperative arthrofibrosis. Good results have been reported after both acute and delayed reconstruction, mostly in retrospective case series. A prospective study compared outcomes in patients who had ACL reconstruction at four time points after injury (Hunter et al. 1996): within 48 hours, between 3 and 7 days, between 1 and 3 weeks, and more than 3 weeks. They found that restoration of knee motion and ACL integrity after ACL reconstruction was independent of the timing of surgery. Shelbourne and Patel (1995) suggested that the timing of ACL surgery should not be based on absolute time limits from injury. They reported that patients who had obtained an excellent range of motion (ROM), little swelling, good leg control, and an excellent mental state before surgery generally had good outcomes, regardless of the timing of surgery. Mayr et al. (2004) confirmed these observations in a retrospective review of 223 patients with ACL reconstructions: 70% of patients with a swollen, inflamed knee at the time of undergoing ACL reconstruction developed postoperative arthrofibrosis. It appears that the timing of reconstruction is not as important as the condition of the knee before surgery: full ROM, minimal effusion, and minimal pain are required (Beynnon et al. 2005, Part 1).
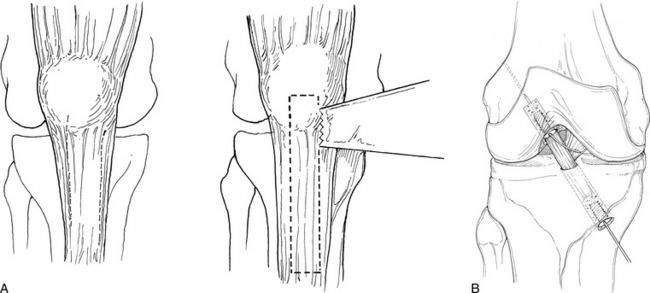
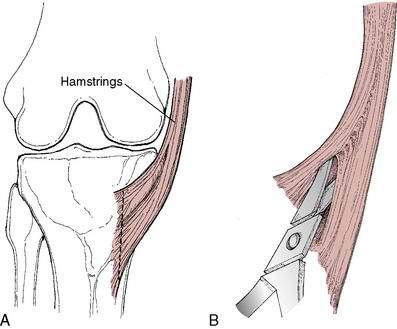
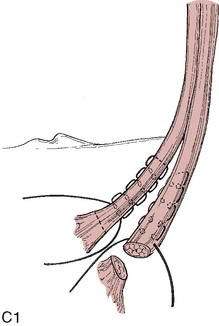
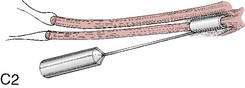
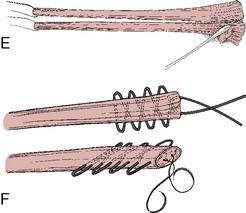
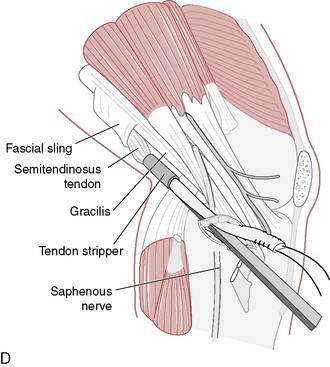
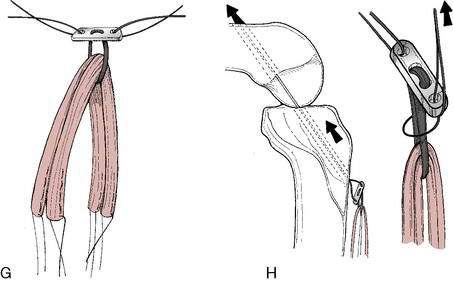
Graft choice. Bone-patellar tendon-bone (BPTB) autografts (Fig. 4-3) have been historically considered the “gold standard” for ACL reconstructions, although good outcomes have been reported with other graft choices, particularly hamstring grafts (Fig. 4-4 A–H). A number of studies have compared BPTB grafts with four-strand hamstring grafts, with most reporting no significant difference in functional outcomes, although difficulty with kneeling was more commonly reported by those with BPTB grafts.
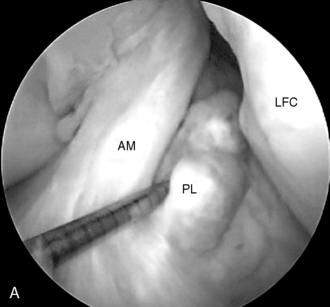
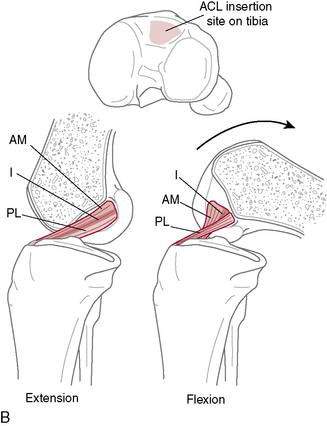
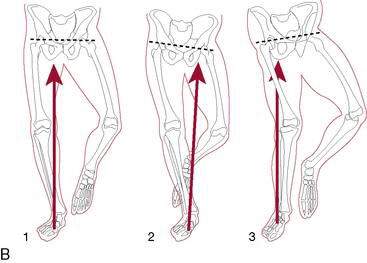
Single-or double-bundle reconstruction. The rationale for two-bundle reconstruction is based on the identification of two distinct ACL bundles: the anteromedial (AM) and the posterolateral (PL) bundle (Fig. 4-5). The femoral insertion sites of both bundles are oriented vertically with the knee in extension, but they become horizontal when the knee is flexed 90 degrees, placing the PL insertion site anterior to the AM insertion site. When the knee is extended, the bundles are parallel; when the knee is flexed, they cross. In flexion, the AM bundle tightens as the PL bundle becomes lax, while in extension the PL bundle tightens and the AM bundle relaxes.
Cited advantages of single-bundle techniques include proven success, less technical difficulty, less tunnel widening, fewer complications, easier revision, lower graft cost when allograft is used, lower implant cost, and shorter surgical time (Prodromos et al. 2008).
Method of fixation. A variety of fixation devices are used for ACL reconstruction, with no consensus as to what is best. Generally, fixation can be classified as interference screw-based, cortical, or cross-pin (Prodromos et al. 2008). Interference screw and cortical fixation can be used in both the femur and the tibia. Interference screw fixation functions by generating frictional holding power between the graft and the bone tunnel wall (Prodromos et al. 2008). Cortical fixation can be direct, compressing the graft against the cortex, or indirect, connecting the graft to the cortex with some sort of interface, often a fabric or metal loop through which the graft is passed. Cross-pinning is a relatively new fixation technique for which advocates cite the advantage of being closer to the tunnel opening than cortical fixation. This advantage, however, has not been proved. A meta-analysis showed that cortical fixation provided more stability than aperture fixation (Prodromos et al. 2005), and a prospective comparison of three fixation devices, including cross-pin fixation, found no statistically or clinically relevant differences in results at 2-year follow-up (Harilainen and Sandelin 2009). All currently used fixation techniques appear to provide adequate stability to allow early aggressive rehabilitation after ACL reconstruction (Hapa and Barber 2009).
ACL Rehabilitation Rationale
Protocols for rehabilitation after ACL reconstruction follow several basic guiding principles:
REHABILITATION PROTOCOL 4-1 Criteria-Based Postoperative ACL Reconstruction Rehabilitation Protocol
Exercises
Phase III (Weeks 2–4)
Exercises
Phase IV (Weeks 4–8)
Precautions
Exercises
Phase V (Weeks 8–12)
Exercises
Phase VII (Weeks 16–24)
For Revision ACL Reconstructions
Per specific physician recommendation, follow typically similar protocol until 12 weeks, then extend weeks 12 to 16 through to 5- to 6-month timeline, when patients can then begin running and progress to functional sports activities. See Figure 4-90 for an illustration of abduction contrakicks/steamboats (flexion, extension, and adduction contrakicks can be performed by rotating patient 90 degrees at a time).
Single-leg triple hop for distance: 80% for running, 90% for return to sport
Triple crossover hop for distance: 80% for running, 90% for return to sport
Timed 10-m single-leg hop: 80% for running, 90% for return to sport
Timed vertical hop test: 60 seconds with good form and steady rhythm considered passing
Patient should have no knee pain following run.
Week 1: Run: walk 30 seconds: 90 seconds every other day (qod) (10–15 minutes)
Week 2: Run: walk 60:60 qod (10–20 minutes)
Week 3: Run: walk 90:30 qod (15–20 minutes)
Open and Closed Kinetic Chain Exercise
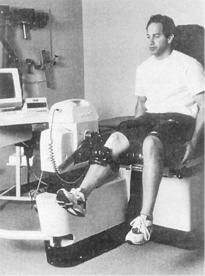
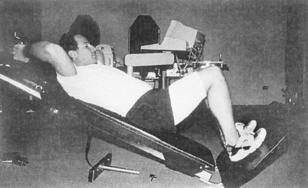
Considerable debate has occurred in recent years regarding the use of closed kinetic chain activity versus open kinetic chain activity after ACL reconstruction. An example of an open kinetic chain exercise is the use of a leg extension machine (Fig. 4-6). An example of closed kinetic chain exercise is the use of a leg press machine (Fig. 4-7). In theory, closed kinetic chain exercises provide a more significant compression force across the knee with activating co-contraction of the quadriceps and hamstring muscles. It has been suggested that these two factors help decrease the anterior shear forces in the knee that would otherwise be placed on the maturing ACL graft. Because of this, closed kinetic chain exercises have been favored over open kinetic chain exercises during rehabilitation after ACL reconstruction. However, the literature supporting this theory is not definitive. Many common activities cannot be clearly classified as open or closed kinetic chain, which adds to the confusion. Walking, running, stair climbing, and jumping all involve a combination of open and closed kinetic chain components to them.
Jenkins and colleagues (1997) measured side-to-side difference in anterior displacement of the tibia in subjects with unilateral ACL-deficient knees during open kinetic chain exercise (knee extension) and closed kinetic chain exercises (leg press) at 30 and 60 degrees of knee flexion and concluded that open chain exercises at low flexion angles may produce an increase in anterior shear forces, which may cause laxity in the ACL.
| 30 degrees knee flexion (mm) | 60 degrees knee flexion (mm) | |
| Open kinetic chain (knee extension) | 4.7 | 1.2 |
| Closed kinetic chain (leg press) | 1.3 | 2.1 |
(3–5 mm = abnormal; 5 mm = arthrometric failure)
(From Jenkins WL, Munns SW, Jayaraman G. A measurement of anterior tibial displacement in the closed and open kinetic chain. J Orthop Sports Phys Ther 25;49-56, 1997.)
Yack and colleagues (1993) also found increased anterior displacement during open kinetic chain exercise (knee extension) compared with closed kinetic chain exercise (parallel squat) through a flexion range of 0 to 64 degrees. Kvist and Gillquist (1999) demonstrated that displacement occurs with even low levels of muscular activity: Generation of the first 10% of the peak quadriceps torque produced 80% of the total tibial translation seen with maximal quadriceps torque. Mathematic models also have predicted that shear forces on the ACL are greater with open chain exercises. Jurist and Otis (1985), Zavetsky and coworkers (1994), and Wilk and Andrews (1993) all noted that changing the position of the resistance pad on isokinetic open kinetic chain devices could modify anterior shear force and anterior tibial displacement. Wilk and Andrews also found greater anterior tibial displacements at slower isokinetic speeds.
Beynnon and associates (1997) used implanted transducers to measure the strain in the intact ACL during various exercises and found no consistent distinction between closed kinetic chain and open kinetic chain activities. This finding contradicts the previous studies and indicates that certain closed chain activities, such as squatting, may not be as safe as the mathematic force models would predict, particularly at low flexion angles.
In summary, closed chain exercises can be used safely during rehabilitation of the ACL because they appear to generate low anterior shear force and tibial displacement through most of the flexion range, although some evidence now exists that low flexion angles during certain closed kinetic chain activities may strain the graft as much as open-chain activities and may not be as safe as previously thought. At what level strain becomes detrimental and whether some degree of strain is beneficial during the graft healing phase are currently unknown. Until these answers are realized, current trends have been to recommend activities that minimize graft strain, so as to put the ACL at the lowest risk for developing laxity. Open chain flexion that is dominated by hamstring activity appears to pose little risk to the ACL throughout the entire flexion arc, but open chain extension places significant strain on the ACL and the patellofemoral joint and should be avoided. An assessment of randomized controlled trials found that closed kinetic chain exercises produced less pain and laxity while promoting better subjective outcome than open kinetic chain exercises (Andersson et al. 2009).
From Beynnon BD, Fleming BC. Anterior cruciate ligament strain in-vivo: A review of previous work. J Biomech 31:519–525, 1998.
| Rehabilitation Activity | Peak Strain (0%) | Number of Subjects |
|---|---|---|
| Isometric quads contraction at 15 degrees (30 Nm of extension torque) | 4.4 | 8 |
| Squatting with Sport Cord | 4.0 | 8 |
| Active flexion–extension of the knee with 45-N weight boot | 3.8 | 9 |
| Lachman test (150 N of anterior shear load) | 3.7 | 10 |
| Squatting | 3.6 | 8 |
| Active flexion–extension (no weight boot) of the knee | 2.8 | 18 |
| Simultaneous quads and hams contraction at 15 degrees | 2.8 | 8 |
| Isometric quads contraction at 30 degrees (30 Nm of extension torque) | 2.7 | 18 |
| Anterior drawer (150 N of anterior shear load) | 1.8 | 10 |
| Stationary bicycling | 1.7 | 8 |
| Isometric hamstring contraction at 15 degrees (to 10 Nm of flexion torque) | 0.6 | 8 |
| Simultaneous quadriceps and hamstring contraction at 30 degrees | 0.4 | 8 |
| Passive flexion–extension of the knee | 0.1 | 10 |
| Isometric quadriceps contraction at 60 degrees (30 Nm of extension torque) | 0.0 | 8 |
| Isometric quadriceps contraction at 90 degrees (30 Nm of extension torque) | 0.0 | 18 |
| Simultaneous quadriceps and hamstring contraction at 60 degrees | 0.0 | 8 |
| Simultaneous quadriceps and hamstring contraction at 90 degrees | 0.0 | 8 |
| Isometric hamstring contraction at 30, 60, and 90 degrees (to 10 Nm of flexion torque) | 0.0 | 8 |
Perturbation Training for Postoperative ACL Reconstruction and Patients Who Were Nonoperatively Treated and ACL Deficient
Michael Duke, PT, CSCS, and S. Brent Brotzman, MD
Nonoperative management of ACL rupture has had limited success in patients who wish to return to high levels of activity. Evidence supports surgical intervention for these patients if they plan to return to their high-level sport (Daniel et al. 1994, Engstrom et al. 1993). For some individuals, however, circumstances may warrant a delay in or avoidance of surgical intervention. Such individuals might include an athlete who needs to demonstrate his or her abilities for scholarship or desires to finish the competitive season, seasonal workers who want to postpone surgery until after the busy work season, or individuals for whom life circumstances or stage of life make surgery undesirable but who want to remain active until they are able to undergo surgery.
Noncopers
Noncopers are those who are not able to return to full activity and tend to demonstrate a joint-stiffening strategy or a nonadaptive generalized co-contraction of the muscles that stabilize the knee. The noncoper strategy of joint stiffening is commonly seen with early motor learning of unfamiliar activities, and as the task becomes more familiar to the individual, the individual is able to demonstrate more complex motor patterns. Those who are able to return to high functional levels demonstrate alterations in muscle activity that improve stability of the knee joint (Ciccotti et al. 1994, Gauffin and Tropp 1992, Rudolph et al. 1998). Pertubation training has also been shown to improve knee function in noncopers (Logerstedt et al. 2009) with ACL injuries.
Several theories have been proposed to explain the ability to stabilize the knee and other joints. Johansson and Sjolander suggested that an increase in sensitivity of mechanoreceptors in joint structures may result in a higher state of “readiness” of muscles to respond to challenges to joint stability (Fitzgerald et al. 2000, Johansson and Sjolander 1993). The implication is that if the therapist can provide progressively destabilizing challenges to the knee during rehabilitation, the neuromuscular patterns can be altered in a way that improves joint stability despite a lack of passive restraints.
Hartigan et al. (2009) found that those who participated in a perturbation training protocol before ACL reconstruction showed no difference in knee excursion (knee flexion during gait) between the involved and uninvolved knees 6 months after ACL reconstruction. In contrast, a group who participated in only a standard strength ACL program showed significant side-to-side asymmetries. This finding indicates that some form of neuromuscular training, in particular perturbation training, is essential to restore normal movement patterns.
In reconstructing the ACL, one of the main purposes is to restore passive restraint to anterior translation of the tibia on the femur. Beard et al. (2001) studied tibial translation both preoperatively and postoperatively in patients with ACL deficiency and found that tibial translation actually transiently increased after reconstruction, which the authors attributed to reduction of the protective hypertonicity of the hamstring group, making them less able to restrain tibial movement. Given this finding and the transient loss of the stabilizing effect of the hamstrings, it becomes even more critical to retrain the neuromuscular system to prevent “giving way” episodes with resultant meniscal damage. Perturbation training has been shown to be effective at this.
Once these criteria are met, the screening test is administered as described in Table 4-1. Patients who pass the screening test are considered good candidates for nonoperative rehabilitation.
Table 4-1 Screening Tests for Nonoperative Treatment of ACL Injury
| Test | Passing Score |
|---|---|
| Single, crossover, triple, and timed hop tests (Noyes et al. 1991, Reid et al. 2007) | 80% or more of uninvolved limb |
| Reported number of giving-way episodes from the time of injury to the time of testing | No more than one episode |
| The Knee Outcome Survey Activities of Daily Living Scale (Irrgang et al. 1998) | 80% or more |
| Subjective global rating of knee function (self-assessed 0%–100%) | 60% or more |
Augmenting a standard rehabilitation protocol with perturbation training has been shown to greatly increase the likelihood of returning to the competitive season with no episodes of giving way (Fitzgerald et al. 2000). Perturbation training generally is performed in 2 or 3 sessions a week for a total of 8 to 10 sessions, with the patient returning to sport during the last week of training.
Perturbation training consists of three techniques:
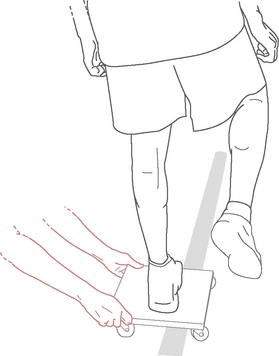
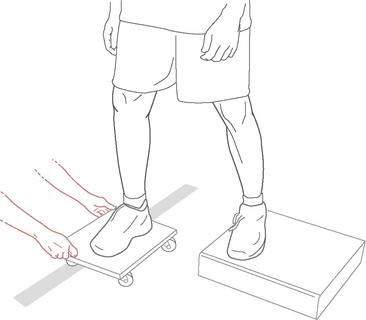
Roller board translations consist of the patient standing with both feet on a rolling platform while the therapist applies translational perturbations to the platform (Fig. 4-8). Initially, safety precautions should be used, such as placing the patient in parallel bars or in a doorway, but these can be discontinued once the therapist believes there are no safety issues. The therapist instructs the patient to maintain balance on the board. Progression of the exercise can have various forms, such as the following:
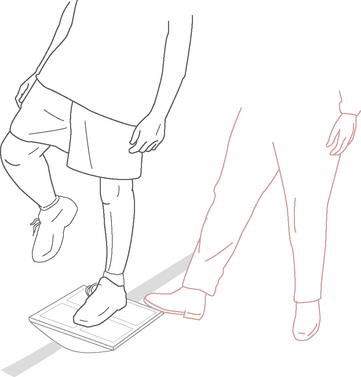
Tilt board perturbations consist of the patient standing on a tilt board while the therapist taps or steps on the edge of the board, causing the board to suddenly tip (Fig. 4-9). The patient is instructed to maintain balance and return to a neutral position after the therapist applies the perturbations. The patient can stand with the board tilting anterior and posterior, medial and lateral, or diagonally in either direction. Progression of the exercise can include all of the aforementioned challenges, with the addition of upright posture progression to progressively deeper squat positions.
Roller board and stationary platform perturbations consist of the patient standing with one limb on the platform and one on the roller board and the therapist applying translational forces to the roller board (Fig. 4-10). The patient is instructed to “match my force” or to prevent the board from moving without co-contraction of the lower limbs. It is important for the therapist to watch for co-contractions and gauge the speed and force of response given by the patient. The patient is learning to selectively activate muscle groups in response to an external challenge. Both the response time and force should improve, indicating the need to further challenge the patient. The following progressions can be made in addition to those already mentioned:
Proprioceptive recovery after ACL reconstruction is critical to joint stability. An intact ACL is known to have mechanoreceptors (Schultz et al. 1984, Schutte et al. 1987), and it has been noted by various authors that some reinnervation occurs in ACL grafts after reconstruction, although timing and extent may vary considerably (Barrack et al. 1997, Barrett 1991, Fremerey et al. 2000, Risberg et al. 2001).
Patients who have had ACL surgery demonstrate co-contraction patterns similar to those who are ACL deficient (Vairo et al. 2008). Considering the time of recovery of quadriceps strength and the need for healing of the hamstring after an autograft reconstruction, we recommend that perturbation training begin around 12 weeks after ACL reconstruction. Several criteria should be met before perturbation training is initiated after ACL reconstructive surgery:
Gender Issues in ACL Injury
ACL Injury in the Female Athlete
Overview
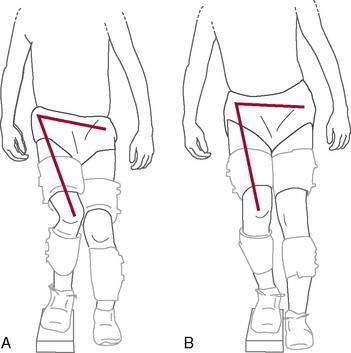
More than 70% of all ACL injuries occur via a noncontact mechanism during activities such as cutting and landing. Evidence has shown that females perform these activities with the knee positioned in maladaptive femoral adduction, femoral internal rotation, and tibial external rotation (referred to as dynamic valgus). These combined motions apply high valgus loads onto the knee, which can lead to ACL injury (Fig. 4-11). Another contributor to ACL injury is landing from a jump with the knee in a minimally flexed position (rather than the more desired flexed knee position). This position results in greater quadriceps activation relative to the hamstrings, leading to increased anterior tibial translation on the femur.
Of note, female athletes have been shown to perform athletic maneuvers with maladaptive variation from their male counterparts on landing including decreased knee and hip flexion, increased quadriceps activation, and greater dynamic knee valgus angles and moments (Powers 2010).
Intrinsic and extrinsic factors (Table 4-2) may account for the higher incidence of ACL injury in the female athlete. Intrinsic factors are anatomic or physiologic in nature and are not amenable to change. Extrinsic factors are biomechanical or neuromuscular in nature and are potentially modifiable. Clinicians have focused much attention on these extrinsic factors for the development and implementation of ACL injury prevention and rehabilitation programs.
| Intrinsic Factors Associated with Female ACL Injury |
Intrinsic Risk Factors
Recent attention has focused on ligament stiffness. Hashemi et al. (2008) reported that the ACL from female cadavers exhibited a decrease in length, cross-sectional area, and volume compared to males. They concluded that inherent ligament weakness, in combination with a smaller intercondylar notch size, might contribute to the ACL injury gender bias.
Physiologic laxity (e.g., general joint laxity and ligamentous laxity) represents another intrinsic factor. Because the ACL primarily limits excessive anterior tibial translation relative to the femur, injury can occur when joint movement exceeds ligamentous strength. Uhorchak et al. (2003) have reported that females with physiologic laxity have a 2.7 times higher risk for sustaining an ACL injury.
Extrinsic Risk Factors
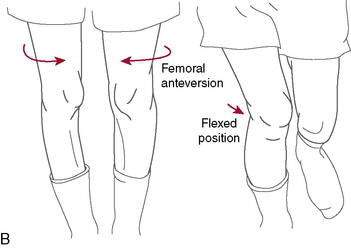
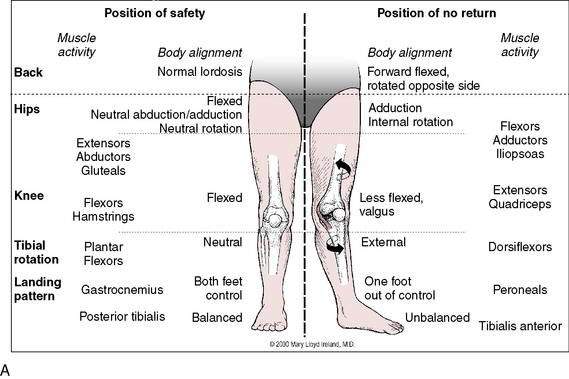
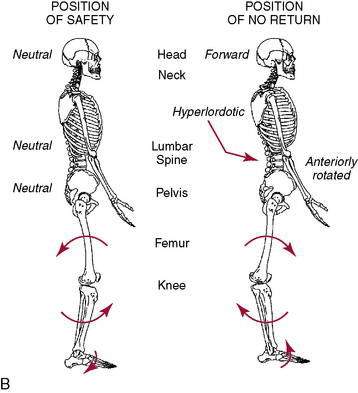
As mentioned previously, dynamic knee valgus applies high loads onto the ACL that can cause injury. During the past 10 years, researchers have ascertained that female athletes perform higher demanding activities in positions making them more vulnerable to ACL injury. It is important to note that structures both proximal and distal to the knee can influence ACL loading. Ireland (1999) has described the position of no return to explain gender differences regarding trunk and lower extremity kinematics and muscle activity (Fig. 4-12). The following summarizes extrinsic factors making the female athlete more vulnerable to ACL injury during running, cutting, and landing tasks:
(Reprinted with permission from Ireland M. The Female Athlete. Saunders, Philadelphia, 2002. Fig 43-4.)
ACL Injury Prevention and Rehabilitation Programs in Female Athletes
ACL injury prevention programs should incorporate strengthening and neuromuscular training for the knee, hip, and trunk muscles on both stable and unstable surfaces (Figs. 4-13 through 4-16). The athlete should perform all plyometric-type exercises with the knees in a more varus, flexed position to reduce valgus loading and facilitate quadriceps/hamstring co-contraction (Fig. 4-17). Sport-specific drills that emphasize proper lower extremity alignment are another important consideration (Figs. 4-18 and 4-19). Throughout the process, the clinician should provide the athlete continual feedback regarding proper technique when performing cutting and landing activities. The female athlete should practice proper deceleration techniques during cutting maneuvers, with a special emphasis on the avoidance of pivoting on a fixed foot. She should perform landing activities with an emphasis on keeping the knees over the toes (to minimize knee valgus) and landing as soft as possible using increased knee flexion (to dampen ground reaction forces).
An important aspect of rehabilitation prior to ACL reconstruction is the restoration of knee ROM and strength. Although quadriceps strengthening is an important component, Hartigan et al. (2009) have reported on the importance of preoperative perturbation training on ACL reconstruction outcomes (see page 219). Perturbation training is a neuromuscular training program aimed at improving dynamic knee stability (Table 4-3).
Table 4-3 ACL Injury: Prevention and Rehabilitation Programs
(Adapted from Fitzgerald GK, Axe MJ, Snyder-Mackler L. The efficacy of perturbation training in nonoperative anterior cruciate ligament rehabilitation programs for physically active individuals. Phys Ther 80:128–140, 2000.)
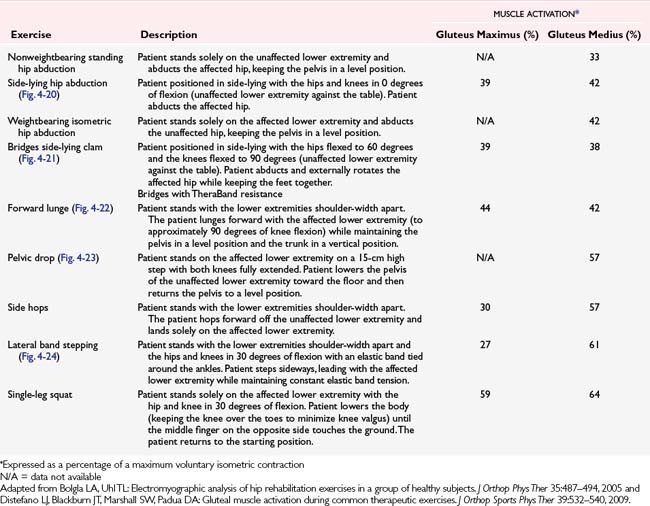
Regarding postoperative ACL rehabilitation, clinicians should continue to follow protocols that emphasize symmetric knee ROM, gait normalization, and controlled weightbearing exercises. Other considerations include hip strengthening exercises (Table 4-4). The clinician also should incorporate neuromuscular retraining as indicated throughout the rehabilitation process through use of single-leg stance exercises with a progression toward perturbation training. Later stages of rehabilitation should include plyometric-type exercises and sport-specific drills similar to those used in ACL injury prevention programs. As with ACL injury prevention programs, the clinician should provide the athlete continuous feedback regarding proper technique when performing cutting and landing tasks.
Anterior Cruciate Ligament Reconstruction with Meniscal Repair
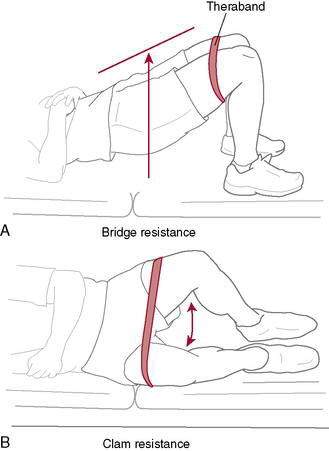
Figure 4-20 A, Bridge with Theraband resistance. B, Hip strengthening with clam and Theraband resistance.
Functional Testing, Functional Training, and Criteria for Return to Play After ACL Reconstruction
Mark V. Paterno, PT, MS, SCS, ATC, and Timothy E. Hewett, PhD, FACSM
Current Guidelines to Return to Sports
Current evidence designed to quantify rehabilitative factors indicates that temporal guidelines and measures such as isokinetic strength and functional hop performance are typically utilized to determine readiness to return to sport. However, these measures, when used in isolation, have limitations. Recommendations regarding return to sport based solely on temporal guidelines are somewhat arbitrary in the medical community and neglect to consider individual patient variability in healing and progression of impairments and function. In a survey of “experts” in the sports medicine community, inclusive of orthopedic surgeons and physical therapists, Harner et al. (2001) report that some practitioners release their patients to return to strenuous sports as early as 4 months postoperative, whereas others may delay up to 18 months. The wide variability in these recommendations is unsupported by current evidence.
Targeting End-Stage Rehabilitation
Despite the absence of a rigorous end-stage rehabilitation protocol and a lack of a specific cluster of validated objective measures to accurately determine an athlete’s readiness to safely return to sport, several authors have begun to address this topic. We attempted to specifically address these concerns related to a lack of objectivity in rehabilitation progression, optimal timing to release to activity, and an absence of a criteria-based progression by creating a program designed for patients after ACL reconstruction. The goal of this program was to target specific neuromuscular imbalances believed to increase risk for ACL injury. We developed an initial model of a criteria-based progression of end-stage rehabilitation (Rehabilitation Protocol 4-2) and an algorithmic approach of progression with the ultimate criteria for determination of readiness to return to sport (Rehabilitation Protocol 4-3). The intent of introducing principles of ACL prevention to the end stages of rehabilitation was to target neuromuscular imbalances and potentially reduce the risk of future ACL injury in this population. This program includes specific rehabilitation phases targeting core stability, functional strength, power development, and symmetry of sports performance. Each phase was designed to specifically target a neuromuscular imbalance previously identified as a potential risk factor for ACL injury.
REHABILITATION PROTOCOL 4-2 Criteria-Based Progression Through Four-Phase Return-to-Sport Rehabilitation After Anterior Cruciate Ligament Reconstruction (Myer GD, Paterno MV, Ford KR)
Myer et al. (2006) described a criteria-based progression through a four-stage rehabilitation program after ACL reconstruction. They suggested that return-to-sport rehabilitation progressed by quantitatively measured functional goals may improve the athlete’s integration back into sport participation. Their criteria-based protocol incorporates a dynamic assessment of baseline limb strength, patient-reported outcomes, functional knee stability, bilateral limb symmetry with functional tasks, postural control, power, endurance, agility, and technique with sport-specific tasks.
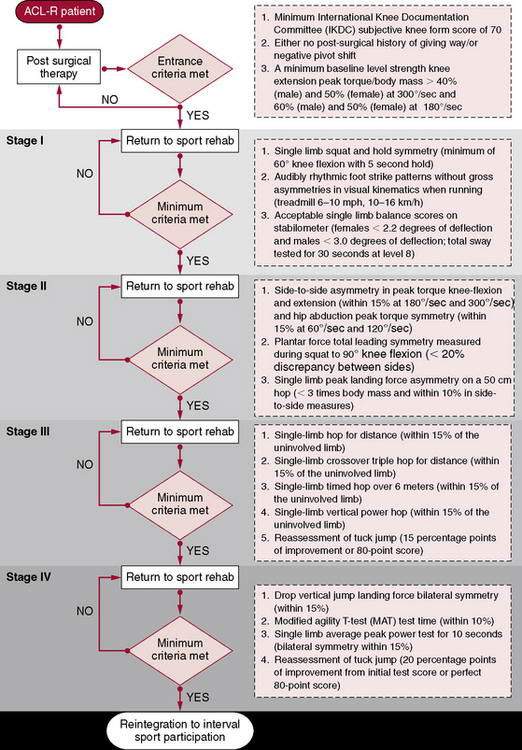
Criteria for entrance into the return-to-sport phase:
Stage 1
Stage 2
REHABILITATION PROTOCOL 4-3 Return-to-Sport Rehabilitation After ACL Reconstruction (Myer GD, Paterno MV, Ford KR)
Stage I
Stage II
Criteria for Progression to Stage III
Stage IV
Criteria for Progression to Return-to-Sport
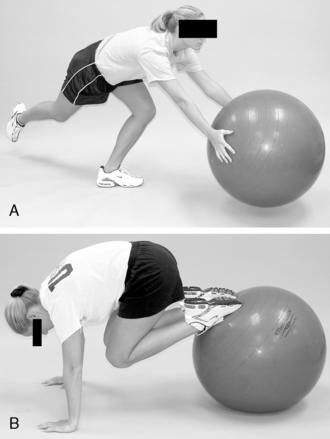
The ability to control the position and mobility of the center of mass during athletic maneuvers is critical for safe participation in sports. The authors have demonstrated deficits in trunk control and proprioception resulted in a greater incidence of knee and ACL injuries in collegiate female athletes. In addition, the authors noted that female athletes playing high-risk sports often land with a single limb outside of their base of support. Landing with the center of mass outside the base of support often increases load on the knee and thus risk of injury. Therefore, targeted rehabilitation to control trunk motion may help athletes safely progress back to sports. The authors utilized dynamic stabilization and core stability exercises to address these impairments (Figs. 4-25 through 4-29).
Finally, a functional reintegration phase is critical to return athletes to sports following lower extremity injury. The goal of this final phase is to ensure the athlete’s ability to symmetrically load lower extremity forces and introduce the sports-specific movements required for the athlete to return to their sport. Prior studies have shown asymmetries in balance, strength, and loading patterns persist after lower extremity injury. If these asymmetries are unresolved when clearance to return to sport is granted, abnormal movement patterns can develop. This may ultimately result in excessive loading on the uninvolved extremity lacking sufficient strength and motor control to absorb force when involved in a competitive, athletic situation. Resolution of these final impairments may not only lead to successful reintegration to sports, but also may begin to reduce the extraordinarily high incidence of reinjury after return to sports. The program that we developed and described attempted to utilize the best current available evidence and supplemented any deficits in the literature with expert clinical opinion. The final outcome was designed as a template and may stimulate future research attempting to develop more rigorous treatment progressions designed for the end stages of rehabilitation after any lower extremity injury, in addition to designing valid, reliable, and objective means to determine the athlete’s readiness to successfully and safely return to sport with minimal risk of reinjury (see Rehabilitation Protocols4-2 and 4-3).