CHAPTER 74 Kidney and ureter
KIDNEY
In the fresh state, the kidneys are reddish-brown. They are situated posteriorly behind the peritoneum on each side of the vertebral column and are surrounded by adipose tissue. Superiorly they are level with the upper border of the 12th thoracic vertebra, inferiorly with the third lumbar vertebra. The right is usually slightly inferior to the left, reflecting its relationship to the liver. The left is a little longer and narrower than the right and lies nearer the median plane (Fig. 74.1). The long axis of each kidney is directed inferolaterally and the transverse axis posteromedially, which means that the anterior and posterior aspects usually described are in fact anterolateral and posteromedial. An appreciation of this orientation is important in percutaneous and endo-urologic renal surgery.
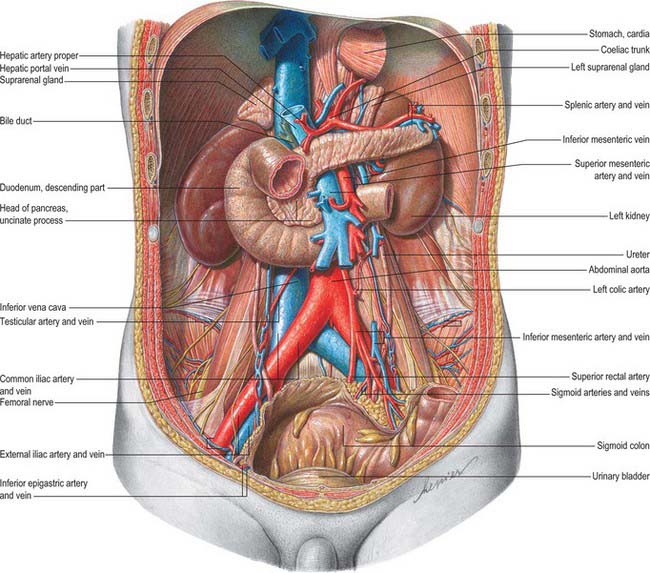
Fig. 74.1 Relationships of the kidneys and ureters in the male retroperitoneum.
(From Sobotta 2006.)
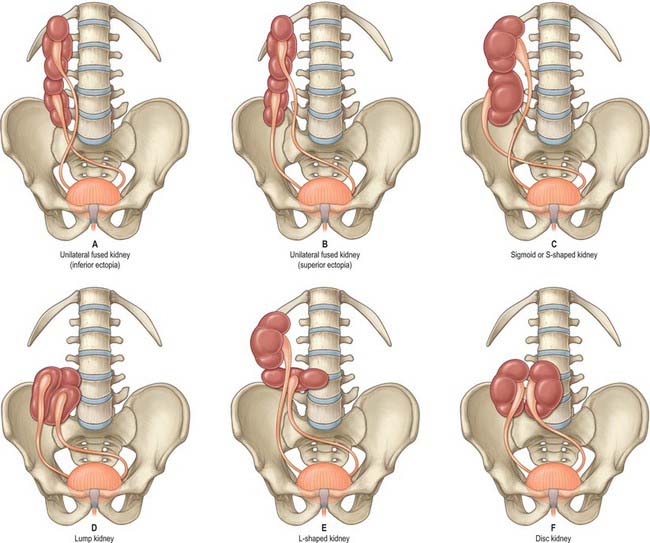
Very rarely, and despite the normal location of the ureteric orifices within the bladder, the two renal masses may be on the same side. This is termed crossed renal ectopia and usually the two renal masses are fused in such circumstances. A solitary crossed renal ectopia may be associated with skeletal and other genitourinary anomalies. A number of different anatomical patterns can result, all of which are extremely rare (Fig. 74.2).
Horseshoe kidneys are found in 1 in 400 individuals. A transverse bridge of renal tissue, the isthmus, which usually but not invariably contains functioning renal substance, connects the two renal masses. The isthmus lies between the inferior poles, most commonly anterior to the great vessels. The ureters curve anterior to the isthmus and often have a high insertion into the renal pelvis (Fig. 74.3A,B).
PERIRENAL FASCIA
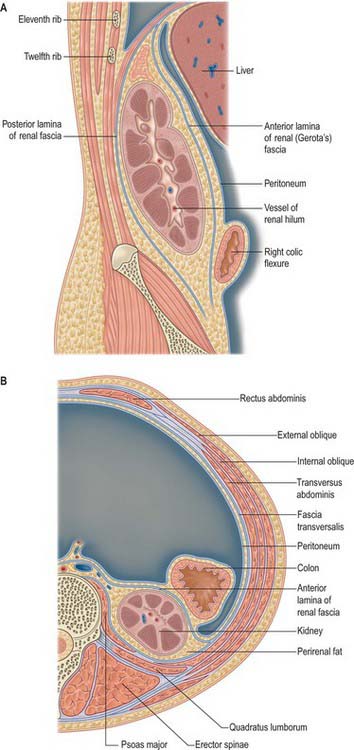
The perirenal fascia is a dense, elastic connective tissue sheath which envelops each kidney and suprarenal gland together with a layer of surrounding perirenal fat (Fig. 74.4A,B; see Fig. 62.1). The kidney and its vessels are embedded in perirenal fat, which is thickest at the renal borders and extends into the renal sinus at the hilum.
The perirenal fascia was originally described as being made up of two separate entities, the posterior fascia of Zuckerkandl and the anterior fascia of Gerota, which fused laterally to form the lateral conal fascia. According to this view, the lateral conal fascia continued anterolaterally behind the colon to blend with the parietal peritoneum. However, work by Mitchell (1950) showed that the perirenal fascia is not made up of distinct fused fasciae, but is in fact a single multilaminated structure which is fused posteromedially with the muscular fasciae of psoas major and quadratus lumborum. It then extends anterolaterally behind the kidney as a bilaminated sheet, which at a variable point divides into a thin anterior lamina, passing around the front of the kidney as the anterior perirenal fascia, and a thicker posterior lamina which continues anterolaterally as the lateral conal fascia, fusing with the parietal peritoneum.
There is some debate concerning the inferior fusion of the perirenal fascia. Many investigators believe that inferiorly the anterior and posterior leaves of the perirenal fascial fuse to produce an inverted cone which is open to the pelvis at its apex. Laterally the anterior and posterior leaves fuse with the iliac fascia, and medially they fuse with the periureteric connective tissue. The inferior apex of the cone is open anatomically towards the iliac fossa but rapidly becomes sealed in inflammatory disease. An alternative view is based on the dissection of recently deceased cadavers after injections of coloured latex into the perirenal space: these have shown that the anterior and posterior perirenal fasciae merge to form a single multilaminar fascia which contains the ureter in the iliac fossa. Anteriorly this common fascia is loosely connected to the parietal peritoneum, and so denies free communication between the perirenal space and the pelvis, and also denies communication between the perirenal and pararenal spaces.
RELATIONS
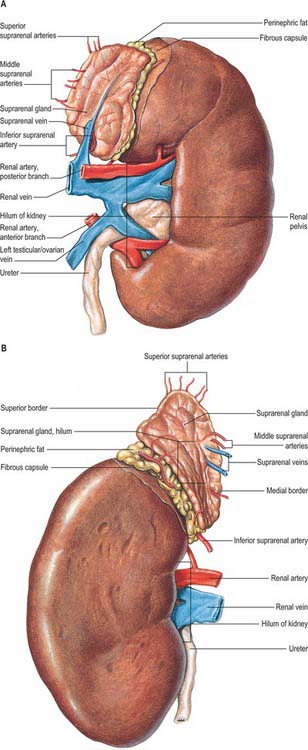
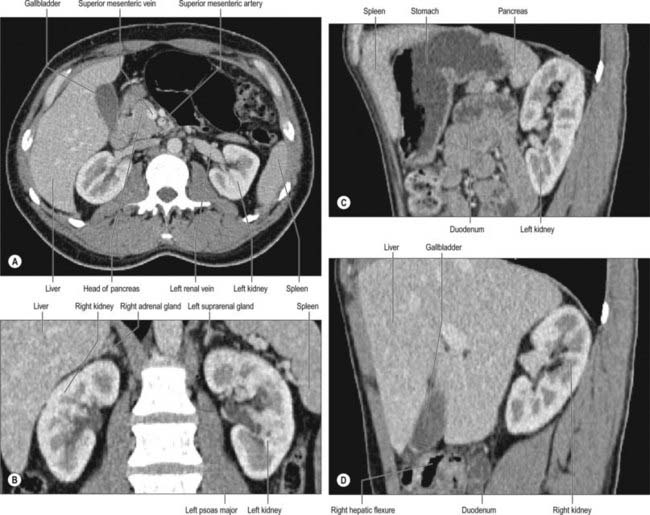
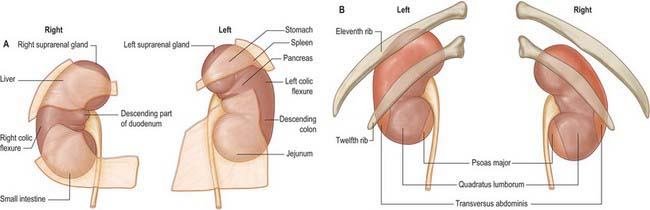
The superior poles of both kidneys are thick and round and each is related to its suprarenal gland (Fig. 74.5A,B). The inferior poles are thinner and extend to within 2.5 cm of the iliac crests. The lateral borders are convex. The medial borders are convex adjacent to the poles, concave between them and slope inferolaterally. In each a deep vertical fissure opens anteromedially as the hilum, which is bounded by anterior and posterior lips and contains the renal vessels and nerves and the renal pelvis. The relative positions of the main hilar structures are the renal vein (anterior), the renal artery (intermediate) and the pelvis of the kidney (posterior). Usually an arterial branch from the main renal artery runs over the superior margin of the renal pelvis to enter the hilum on the posterior aspect of the pelvis, and a renal venous tributary often leaves the hilum in the same plane. Above the hilum the medial border is related to the suprarenal gland and below to the origin of the ureter.
The convex anterior surface of the kidney actually faces anterolaterally and its relations differ on the right and left. Likewise the posterior surface of the kidneys in reality faces posteromedially. Its relations are similar on both sides of the body (Fig. 74.6A–D).
A small medial area of the superior pole is related to the left suprarenal gland. The lateral half of the anterior surface is related to the spleen from which it is separated by a layer of peritoneum. A central quadrilateral area lies in direct contact with the retroperitoneal pancreas and the splenic vessels. Above this a small variable triangular region, between the suprarenal and splenic areas, is in contact with the stomach separated by a layer of peritoneum. Below the pancreatic and splenic areas, a narrow lateral strip which extends to the lateral border of the kidney is directly related to the retroperitoneal left colic flexure and the beginning of the descending colon. An extensive medial area is related to intraperitoneal loops of jejunum. The gastric area is covered with the peritoneum of the lesser sac (omental bursa) and the splenic and jejunal areas are covered by the peritoneum of the greater sac. Behind the peritoneum covering the jejunal area, branches of the left colic vessels are related to the kidney (Fig. 74.7A,B).
INTERNAL MACROSTRUCTURE
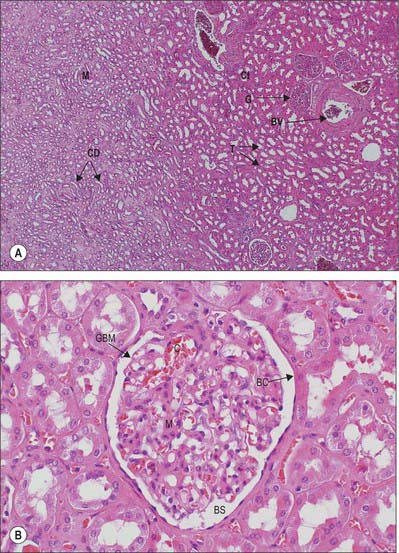
The postnatal kidney has a thin fibrous capsule composed of collagen-rich tissue with some elastic and smooth muscle fibres. In renal disease the capsule may become adherent (Fig. 74.8A,B).
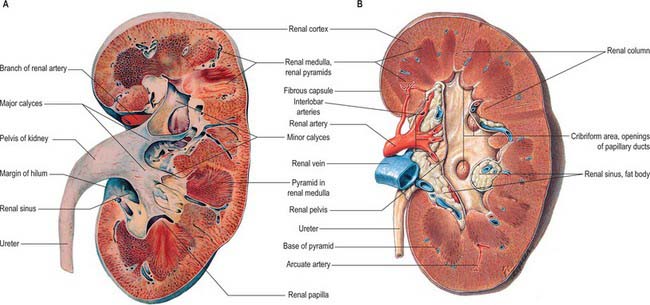
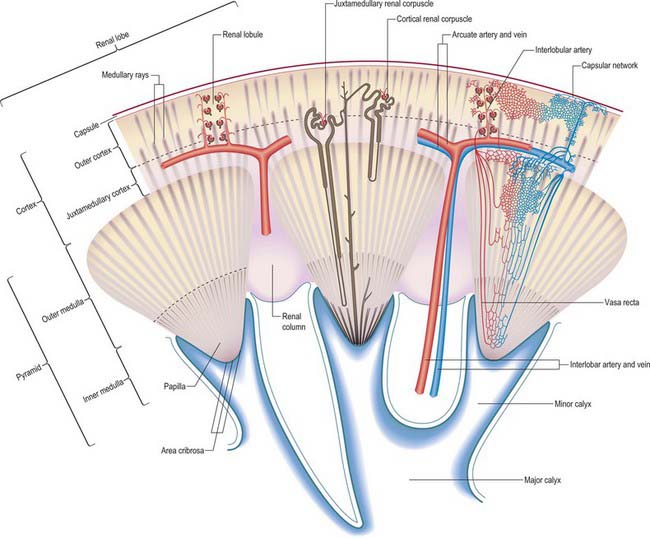
The renal cortex is subcapsular, arching over the bases of the pyramids and extending between them towards the renal sinus as renal columns. Its peripheral regions are termed cortical arches and are traversed by radial, lighter-coloured medullary rays, separated by darker tissue, the convoluted part. The rays taper towards the renal capsule and are peripheral prolongations from the bases of renal pyramids. The cortex is histologically divisible into outer and inner zones. The inner zone is demarcated from the medulla by tangential blood vessels (arcuate arteries and veins), which lie at the junction of the two, however a thin layer of cortical tissue (subcortex) appears on the medullary side of this zone. The cortex close to the medulla is sometimes termed the juxtamedullar cortex.
Renal pelvis and calyces
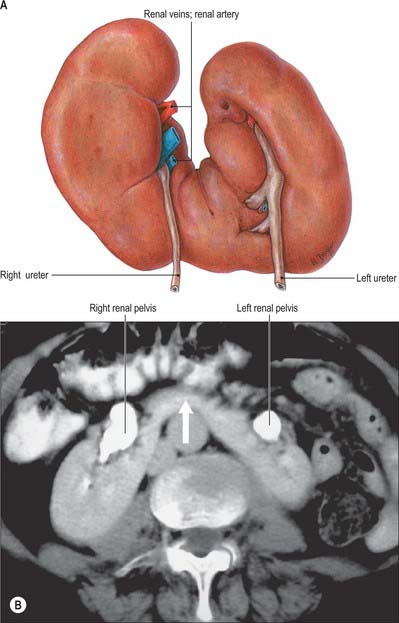
The hilum of the kidney leads into a central renal sinus, lined by the renal capsule and almost filled by the renal pelvis and vessels, the remaining space being filled by fat. Dissection into this plane can be challenging but is important in surgery on the renal pelvis, particularly open stone surgery. Within the renal sinus, the collecting tubules of the nephrons of the kidney open onto the summits of the renal papillae to drain into minor calyces, which are funnel-shaped expansions of the upper urinary tract. The renal capsule covers the external surface of the kidney and continues through the hilum to line the sinus and fuse with the adventitial coverings of the minor calyces. Each minor calyx surrounds either a single papilla or, more rarely, groups of two or three papillae. The minor calyces unite with their neighbours to form two or possibly three larger chambers, the major calyces. There is wide variation in the arrangement of the calyces. As the posterior aspect of the kidney rotates laterally during its ascent in utero, the calyces which were lateral in utero become positioned anteriorly, and the medial calyces move more posteriorly. The calyces drain into the infundibula. The renal pelvis is normally formed from the junction of two infundibula, one from the upper and one from the lower pole calyces, but there may be a third, which drains the calyces in the mid-portion of the kidney. The calyces are usually grouped so that three pairs drain into the upper pole infundibulum and four pairs into the lower pole infundibulum. If there is a middle infundibulum, the distribution is normally three pairs at the upper pole, two in the middle, and two at the lower pole. There is considerable variation in the arrangement of the infundibula and in the extent to which the pelvis is intrarenal or extrarenal. The funnel-shaped renal pelvis tapers as it passes inferomedially, traversing the renal hilum to become continuous with the ureter (Fig. 74.8A,B; see Fig. 74.9). It is rarely possible to determine precisely where the renal pelvis ceases and the ureter begins: the region is usually extrahilar and normally lies adjacent to the lower part of the medial border of the kidney. Rarely, the entire renal pelvis has been found to lie inside the sinus of the kidney so that the pelviureteric region occurs either in the vicinity of the renal hilum or completely within the renal sinus.
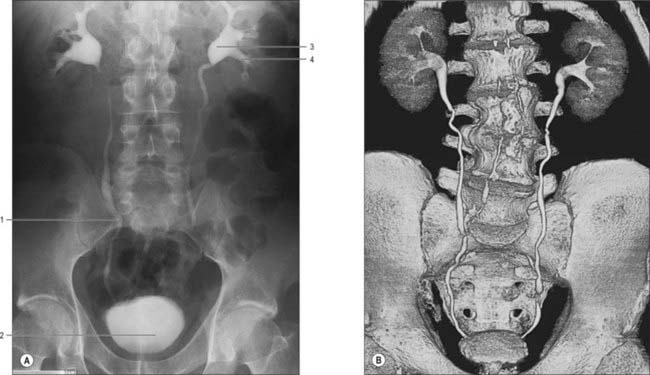
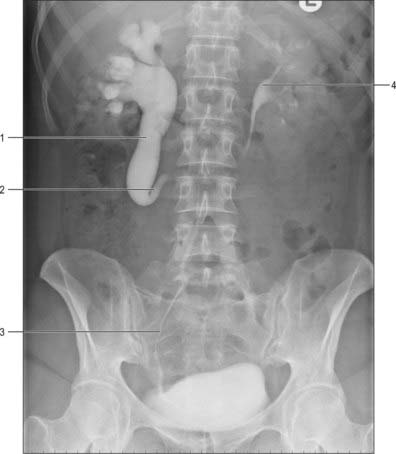
The calyces, renal pelvis and ureter are well-demonstrated radiologically following an intravenous injection of radio-opaque contrast which is excreted in the urine (intravenous urography – IVU) (Fig. 74.9A,B); or after the introduction of radio-opaque contrast into the ureter by catheterization through a cystoscope (ascending or retrograde pyelography. Normal cupping of the minor calyces by projecting renal papillae may be obliterated by conditions that cause hydronephrosis, chronic distension of the ureter and renal pelvis due to upper or lower urinary tract obstruction resulting in elevated intrapelvic pressure. An appreciation of the rotation of the kidneys which results in the posterior calyces lying relatively medially and the anterior calyces lying laterally is essential when interpreting contrast imaging of the collecting system of the kidneys.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Renal arteries
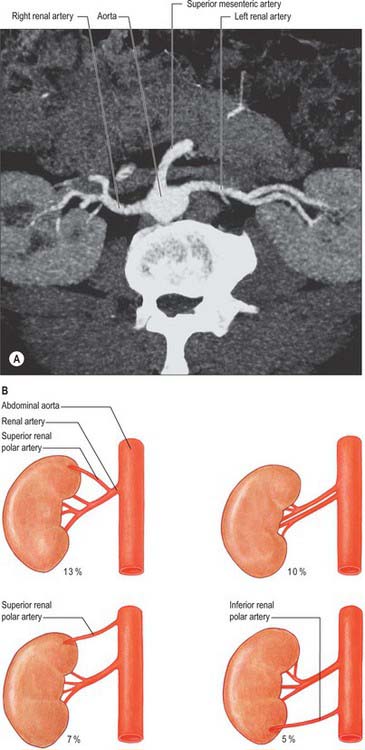
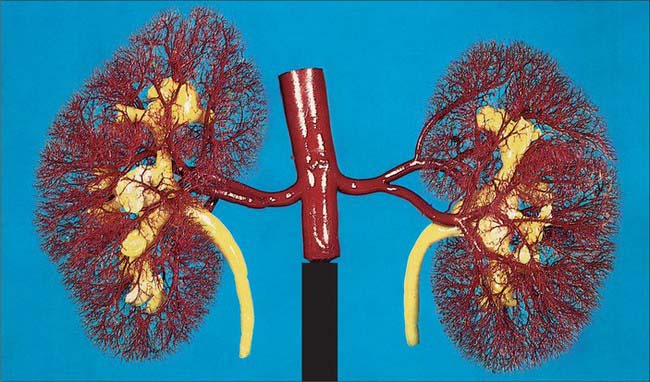
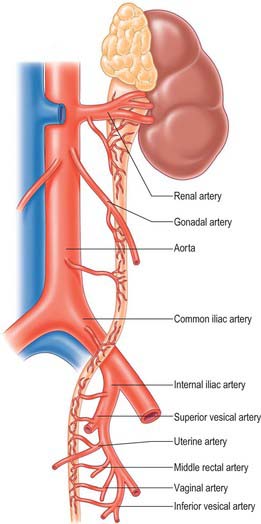
The paired renal arteries take about 20% of the cardiac output to supply organs that represent less than one-hundredth of total body weight. They branch laterally from the aorta just below the origin of the superior mesenteric artery (see Fig. 62.8; Fig. 74.10A). Both cross the corresponding crus of the diaphragm at right angles to the aorta. The right renal artery is longer and often higher, passing posterior to the inferior vena cava, right renal vein, head of the pancreas, and descending part of the duodenum. The left renal artery is a little lower and passes behind the left renal vein, the body of the pancreas, and splenic vein. It may be crossed anteriorly by the inferior mesenteric vein.
A single renal artery to each kidney is present in approximately 70% of individuals (Fig. 74.11). The arteries vary in their level of origin and in their calibre, obliquity and precise relations (Fig. 74.10B). In its extrarenal course each renal artery gives off one or more inferior suprarenal arteries, a branch to the ureter and branches which supply perinephric tissue, the renal capsule, and the pelvis. Near the renal hilum, each artery divides into an anterior and a posterior division, and these divide into segmental arteries supplying the renal vascular segments. Accessory renal arteries are common (30% of individuals), and usually arise from the aorta above or below (most commonly below) the main renal artery and follow it to the renal hilum. They are regarded as persistent embryonic lateral splanchnic arteries. Accessory vessels to the inferior pole cross anterior to the ureter and may, by obstructing the ureter, cause hydronephrosis. Rarely, accessory renal arteries arise from the coeliac or superior mesenteric arteries near the aortic bifurcation or from the common iliac arteries.
The subdivisions of the renal arteries are described sequentially as segmental, lobar, interlobar, arcuate and interlobular arteries and afferent and efferent glomerular arterioles (see Fig. 74.14).
Segmental arteries
Renal vascular segmentation was originally recognized by John Hunter in 1794, but the first detailed account of the primary pattern was produced in the 1950s from casts and radiographs of injected kidneys. Five arterial segments have been identified (Fig. 74.12). The apical segment occupies the anteromedial region of the superior pole. The superior (anterior) segment includes the rest of the superior pole and the central anterosuperior region. The inferior segment encompasses the whole lower pole. The middle (anterior) segment lies between the anterior and inferior segments. The posterior segment includes the whole posterior region between the apical and inferior segments. This is the pattern most commonly seen, and although there can be considerable variation it is the pattern that clinicians most frequently encounter when performing partial nephrectomy. Whatever pattern is present, it must be emphasized that vascular segments are supplied by virtual end arteries. In contrast, larger intrarenal veins have no segmental organization and anastomose freely.
Brödel (1911) described a relatively avascular longitudinal zone (the ‘bloodless’ line of Brödel) along the convex renal border, which was proposed as the most suitable site for surgical incision. However, many vessels cross this zone, and it is far from ‘bloodless’: planned radial or intersegmental incisions are preferable. Knowledge of the vascular anatomy of the kidney is important when undertaking partial nephrectomy for renal cell cancers. In this surgery the branches of the renal artery are defined so that the surgeon may safely excise the renal substance containing the tumour while not compromising the vascular supply to the remaining renal tissue.
Lobar, interlobar, arcuate and interlobular arteries
The initial branches of segmental arteries are lobar, usually one to each renal pyramid. Before reaching the pyramid they subdivide into two or three interlobar arteries, extending towards the cortex around each pyramid. At the junction of the cortex and medulla, interlobar arteries dichotomize into arcuate arteries which diverge at right angles. As they arch between cortex and medulla, each divides further, ultimately supplying interlobular arteries which diverge radially into the cortex. The terminations of adjacent arcuate arteries do not anastomose but end in the cortex as additional interlobular arteries. Though most interlobular arteries come from arcuate branches, some arise directly from arcuate or even terminal interlobar arteries (see Fig. 74.14). Interlobular arteries ascend towards the superficial cortex or may branch occasionally en route. Some are more tortuous and recurve towards the medulla at least once before proceeding towards the renal surface. Others traverse the surface as perforating arteries to anastomose with the capsular plexus (which is also supplied from the inferior suprarenal, renal and gonadal arteries).
Afferent and efferent glomerular arterioles
Afferent glomerular arterioles are mainly the lateral rami of interlobular arteries. A few arise from arcuate and interlobar arteries when they vary their direction and angle of origin: deeper ones incline obliquely back towards the medulla, the intermediate pass horizontally, and the more superficial approach the renal surface obliquely before ending in a glomerulus (see Fig. 74.14). Efferent glomerular arterioles from most glomeruli (except at juxtamedullary and, sometimes, at intermediate cortical levels) soon divide to form a dense peritubular capillary plexus around the proximal and distal convoluted tubules: there are thus two sets of capillaries, glomerular and peritubular, in series in the main renal cortical circulation, linked by efferent glomerular arterioles. The vascular supply of the renal medulla is largely from efferent arterioles of juxtamedullary glomeruli, supplemented by some from more superficial glomeruli, and ‘aglomerular’ arterioles (probably from degenerated glomeruli). Efferent glomerular arterioles passing into the medulla are relatively long, wide vessels, and contribute side branches to neighbouring capillary plexuses before entering the medulla, where each divides into 12–25 descending vasa recta. As their name suggests, these run straight to varying depths in the renal medulla, contributing side branches to a radially elongated capillary plexus (see Fig. 74.14) applied to the descending and ascending limbs of renal loops and to collecting ducts. The venous ends of capillaries converge to the ascending vasa recta, which drain into arcuate or interlobular veins. An essential feature of the vasa recta (particularly in the outer medulla) is that both ascending and descending vessels are grouped into vascular bundles, within which the external aspects of both types are closely apposed, bringing them close to the limbs of renal loops and collecting ducts. As these bundles converge centrally into the renal medulla they contain fewer vessels: some terminate at successive levels in neighbouring capillary plexuses. This proximity of descending and ascending vessels with each other and adjacent ducts provides the structural basis for the countercurrent exchange and multiplier phenomena (see countercurrent exchange mechanism later in chapter (see Fig. 74.17)). These complex renal vascular patterns show regional specializations which are closely adapted to the spatial organization and functions of renal corpuscles, tubules and ducts (see below).
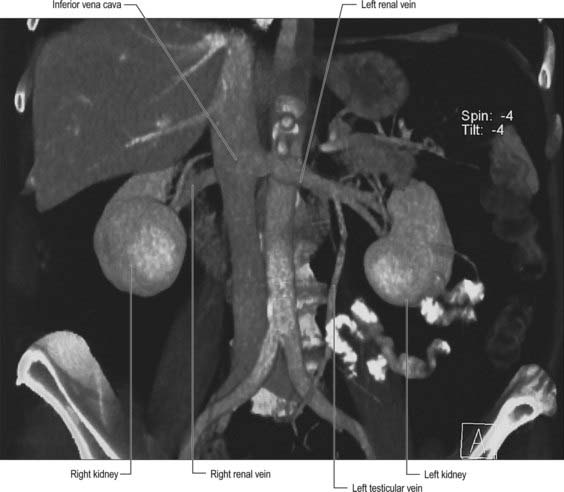
Renal veins
The large renal veins lie anterior to the renal arteries and open into the inferior vena cava almost at right angles. The left is three times longer than the right (7.5 cm and 2.5 cm respectively) and for this reason, the left kidney is the preferred side for live donor nephrectomy. The left renal vein runs from its origin in the renal hilum, posterior to the splenic vein and the body of pancreas, and then across the anterior aspect of the aorta, just below the origin of the superior mesenteric artery. The left gonadal vein enters it from below and the left suprarenal vein, usually receiving one of the left inferior phrenic veins, enters it above but nearer the midline. The left renal vein enters the inferior vena cava a little superior and to the right. The right renal vein is behind the descending duodenum and sometimes the lateral part of the head of the pancreas. It can be extremely short (<1 cm) such that safe nephrectomy may require excision of a cuff of the inferior vena cava (Fig. 74.13).
MICROSTRUCTURE
The kidney is composed of many tortuous, closely packed uriniferous tubules, bounded by a delicate connective tissue in which run blood vessels, lymphatics and nerves. Each tubule consists of two embryologically distinct parts, the nephron, which produces urine, and the collecting duct, which completes the concentration of urine and through which urine passes out into the calyces of the kidney, the renal pelvis, the ureter and urinary bladder (see p. 1305).
Nephron
The nephron consists of a renal corpuscle, concerned with filtration from the plasma, and a renal tubule, concerned with selective resorption from the filtrate to form the urine (Fig. 74.14). Collecting ducts carry fluid from several renal tubules to a terminal papillary duct, opening into a minor calyx at the apex of a renal papilla. Papillary surfaces show numerous minute orifices of these ducts and pressure on a fresh kidney expresses urine from them.
Renal corpuscle
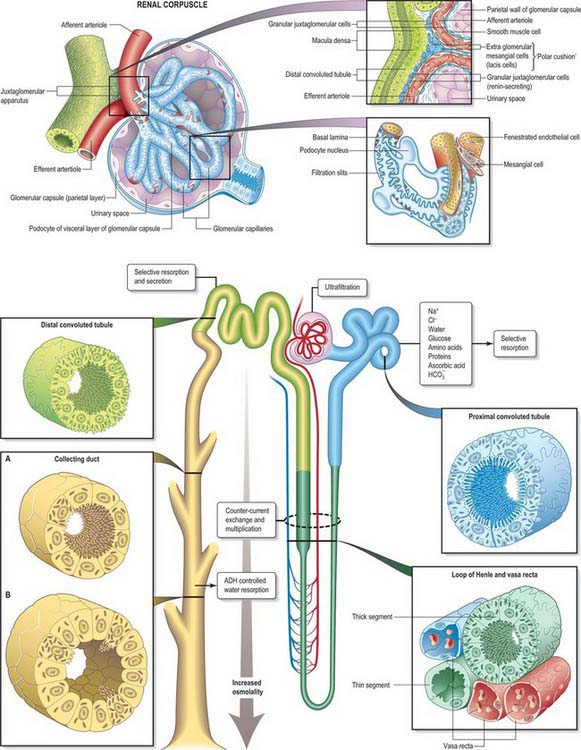
Renal corpuscles are small rounded structures averaging 0.2 mm in diameter, visible in the renal cortex deep to a narrow peripheral cortical zone (Fig. 74.15A,B). Each has a central glomerulus of vessels and a glomerular (Bowman’s) capsule, from which the renal tubule originates.
Glomerulus
A glomerulus is a collection of convoluted capillary blood vessels, united by a delicate mesangial matrix and supplied by an afferent arteriole which enters the capsule opposite the urinary pole, where the filtrate enters the tubule. (The term glomerulus is used most frequently to describe the entire renal corpuscle.) An efferent arteriole emerges from the same point, the vascular pole of the corpuscle. Glomeruli are simple in form until late prenatal life; some remain so for about 6 months after birth, the majority maturing by 6 years and all by 12 years (see p. 1305).
Bowman’s capsule
Bowman’s capsule is the blind expanded end of a renal tubule, and is deeply invaginated by the glomerulus. It is lined by a simple squamous epithelium on its outer (parietal) wall; its glomerular, juxtacapillary (visceral) wall is composed of specialized epithelial podocytes. Between the two walls of the capsule is a flattened urinary (Bowman’s) space, continuous with the proximal convoluted tubule (Fig. 74.15B; see Fig. 74.17).
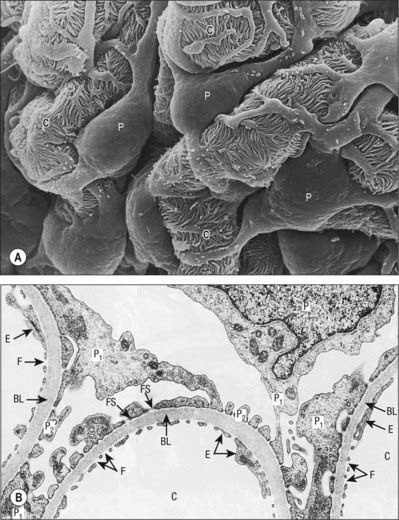
Podocytes are stellate cells. Their major (primary) foot processes curve around the capillary loops and branch to form secondary processes which are applied closely to the basal lamina; secondary or tertiary processes give rise to terminal pedicels (Fig. 74.16A). Pedicels of one cell alternate with those of an adjacent cell and interdigitate tightly with each other, separated by narrow (25 nm) gaps, the filtration slits (Fig. 74.16B). The latter are covered by a dense, membranous slit diaphragm, through which filtrate must pass to enter the urinary space. The differentiation of the adult podocyte phenotype is associated with the presence of several specific proteins, including nephrin, podocin, synaptopodin and GLEPP-1. Mutations in these proteins can cause important functional problems, e.g. the classical Finnish form of congenital nephritic syndrome is caused by a mutation of NHPS1 coding for nephrin. The luminal membrane and the slit diaphragm are covered by a dense surface coat rich in sialoglycoproteins, which gives this surface a very high negative charge and is one of the key characteristics of the perm-selectivity barrier. Differentiated podocytes cannot replicate.
Irregular mesangial cells, with phagocytic and contractile properties, lie within and secrete the glomerular mesangium, a specialized connective tissue which binds the loop of glomerular capillaries and fills the spaces between endothelial surfaces that are not invested by podocytes (Fig. 74.15B; see Fig. 74.17). Mesangial cells are related to vascular pericytes and are concerned with the turnover of glomerular basement membrane. They clear the glomerular filter of immune complexes and cellular debris, and their contractile properties help to regulate blood flow. Similar cells, the extraglomerular mesangial (lacis) cells, lie outside the glomerulus at the vascular pole and form part of the juxtaglomerular apparatus.
Renal tubule
A renal or uriniferous tubule consists of a glomerular capsule that leads into a proximal convoluted tubule connected to the capsule by a short neck and continuing into a sinuous or coiled convoluted part (Fig. 74.17). This straightens as it approaches the medulla, and becomes the descending thick limb of the loop of Henle and then the ascending limb by an abrupt U-turn. The limbs of the loop of Henle are narrower and thin-walled within the deeper medullary tissue where they become the descending and ascending thin segments. The ascending thick limb continues into the distal tubule. The tubule wall shows a focal thickening, the macula densa, where it comes close to the vascular pole of its parent glomerulus at the start of the convoluted part of the distal tubule. The nephron finally straightens once more as the connecting tubule, which ends by joining a collecting duct.
Collecting ducts originate in the cortical medullary rays and join others at intervals. They finally open into wider papillary ducts which open on to a papilla, their numerous orifices forming a perforated area cribrosa on the surface at its tip (Fig. 74.14).
Renal tubules are lined throughout by a single-layered epithelium (Figs 74.17, 74.18). The type of epithelial cell varies according to the functional roles of the different regions, e.g. active transport and passive diffusion of various ions and water into and out of the tubules; reabsorption of organic components such as glucose and amino acids; uptake of any proteins which leak through the glomerular filter.
Cells of the distal tubule are cuboidal and resemble those in the proximal tubule. They have few microvilli, and so the tubular lumen has a more distinct outline. The basolateral folds containing mitochondria are deep, almost reaching the luminal aspect (Fig. 74.17). Enzymes concerned in active transport of sodium, potassium and other ions are abundant. At the junction of the straight and convoluted regions the distal tubule comes close to the vascular pole of its parent renal corpuscle. Here, tubular cells form the macula densa, a sensory structure which is concerned with the regulation of blood flow and thus filtration rate. Cells in the terminal part of the distal tubule have fewer basal folds and mitochondria and constitute a connecting duct formed from metanephric mesenchyme during embryogenesis. Collecting ducts are lined by simple cuboidal or columnar epithelium. This increases in height from the cortex, where the ducts receive the contents of distal tubules, to the wide papillary ducts which discharge at the area cribrosa. The pale-staining principal cells have relatively few organelles or lateral interdigitations and only occasional microvilli. A second cell type, intercalated or dark cells (also present in smaller numbers in the distal convoluted tubule), have longer microvilli and more mitochondria and secrete H+ into the filtrate; they function in the maintenance of acid–base homeostasis.
PRODUCTION OF URINE
Glomerular filtration
Glomerular filtration (see Figs 74.14, 74.17) is the passage of water containing dissolved small molecules from the blood plasma to the urinary space in the glomerular capsule. Larger molecules, e.g. plasma proteins above 70 kilodaltons and those with a net negative charge, polysaccharides lipids and cells, are largely retained in blood by the selective permeability of the glomerular basal lamina.
Filtration occurs along a steep pressure gradient between the large glomerular capillaries and the urinary space, the principal structure separating the two being the glomerular basal lamina (Fig. 74.16B). This gradient far exceeds the colloid osmotic pressure of blood which opposes the outward flow of filtrate. In the peripheral renal cortex the arteriolar pressure gradient is enhanced because afferent glomerular arterioles are wider than efferent glomerular arterioles. In all glomeruli the rate of filtration can be altered by changes in the tone of the glomerular arterioles. When first formed, the glomerular filtrate is isotonic with glomerular blood and has an identical concentration of ions and small molecules.
Selective resorption
Selective resorption from the filtrate is an active process and occurs mainly in the proximal convoluted tubules, which resorb glucose, amino acids, phosphate, chloride, sodium, calcium and bicarbonate; they also take up small proteins (e.g. albumin) by endocytosis. Cells of the proximal tubules are permeable to water, which passes out of the tubules passively, so that the filtrate remains locally isotonic with blood. The rest of the tubule reabsorbs most of the water (to a variable extent up to 95%), so that when it reaches the calyces, urine is generally much reduced in volume and hypertonic to blood. The process depends on the establishment of high osmolality in the medullary interstitium, in order to exert sufficient osmotic pressure on water-permeable regions of the tubule, and is achieved by a countercurrent multiplier mechanism (see Figs 74.14, 74.17).
Countercurrent exchange mechanism
Rapid removal of ions from the renal medulla by the circulation of blood is minimized by another looped countercurrent system. This is the countercurrent exchange mechanism, in which arterioles entering the medulla pass for long distances parallel to the venules leaving it, before ending in capillary beds around tubules. This close apposition of oppositely flowing blood allows the direct diffusion of ions from outflowing to inflowing blood, so that the vasa recta (Fig. 74.14) conserve the high osmotic pressure in the medulla.
Concentration of urine
Control of hydrogen and ammonium ion concentrations is essential to the regulation of acids and bases in the blood. Secretion of various ions occurs at several sites. Over 91% of ingested potassium is excreted in urine, largely through secretion by cells of the distal tubule and collecting duct. (For further details of renal physiology, see Madias & Adroque, 2005; Tannen & Hallows, 2005.)
Juxtaglomerular apparatus
The afferent and efferent arterioles at the vascular pole of a glomerulus and the macula densa of the distal tubule of the same nephron lie in close proximity, enclosing a small cone of tissue populated by extraglomerular mesangial (lacis) cells (Fig. 74.17). The cells of the tunica media of afferent and, to a lesser extent, efferent, arterioles differ from typical smooth muscle cells. These juxtaglomerular cells are large, rounded myoepithelioid cells and their cytoplasm contains many mitochondria and dense, renin-containing vesicles, 10–40 nm in diameter.
URETER
The ureters are two muscular tubes whose peristaltic contractions convey urine from the kidneys to the urinary bladder (Fig. 74.9). Each measures 25–30 cm in length, is thick-walled and narrow, and is continuous superiorly with the funnel-shaped renal pelvis. Each descends slightly medially, anterior to psoas major, and enters the pelvic cavity where it curves initially laterally, then medially, to open into the base of the urinary bladder. The diameter of the ureter is normally 3 mm, but is slightly less at its junction with the renal pelvis, at the brim of the lesser pelvis near the medial border of psoas major, and where it runs within the wall of the urinary bladder, which is its narrowest part. These are the commonest sites for renal stone impaction.
RELATIONS
In the abdomen the ureter descends posterior to the peritoneum on the medial part of psoas major, which separates it from the tips of the lumbar transverse processes. During surgery on intraperitoneal structures, the ureter can be tented up as the peritoneum is drawn anteriorly, resulting in inadvertent ureteric injury. Anterior to psoas major it crosses in front of the genitofemoral nerve and is obliquely crossed by the gonadal vessels (Fig. 74.19). It enters the lesser pelvis anterior to either the end of the common iliac vessels or at the origin of the external iliac vessels (Fig. 74.20).
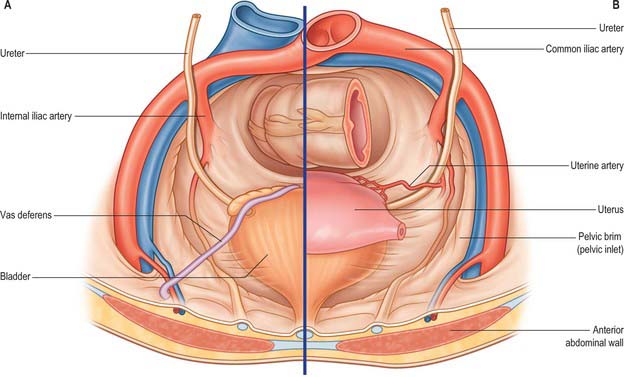
Fig. 74.19 Relations of lower ureter. A, Male pelvis. B, Female pelvis.
(From Drake, Vogl and Mitchell 2005.)
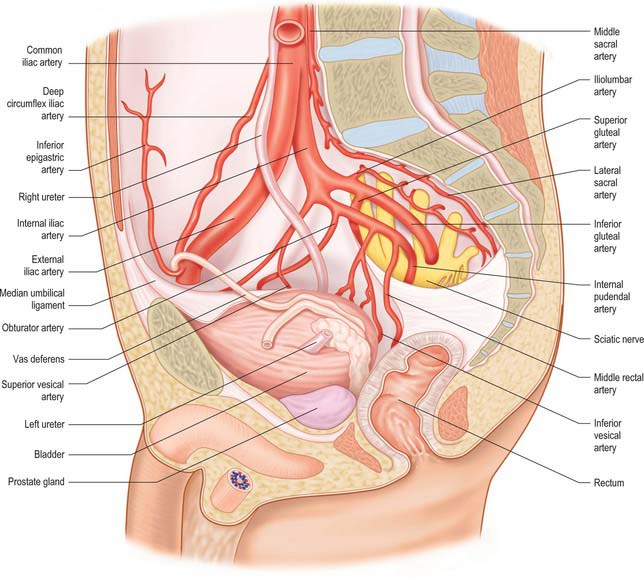
Fig. 74.20 Relations of male lower right ureter.
(By permission from Walsh PC, Retik AB, Vaughan ED et al (eds) 2002 Campbell’s Urology, 8th edn. Philadelphia: Saunders.)
The inferior vena cava is medial to the right ureter while the left ureter is lateral to the aorta. The inferior mesenteric vein has a long retroperitoneal course lying close to the medial aspect of the left ureter.
At its origin the right ureter is usually overlapped by the descending part of the duodenum. It descends lateral to the inferior vena cava, and is crossed anteriorly by the right colic and ileocolic vessels. Near the superior aperture of the lesser pelvis it passes behind the lower part of the mesentery and terminal ileum. The left ureter is crossed by the gonadal and left colic vessels (see Fig. 74.24). It passes posterior to loops of jejunum and sigmoid colon and its mesentery in the posterior wall of the intersigmoid recess.
In the pelvis the ureter lies in extraperitoneal areolar tissue (see Fig. 75.3). At first it descends posterolaterally on the lateral wall of the lesser pelvis along the anterior border of the greater sciatic notch. Opposite the ischial spine it turns anteromedially into fibrous adipose tissue above levator ani to reach the base of the bladder. On the pelvic side-wall it is anterior to the internal iliac artery and the beginning of its anterior trunk, posterior to which are the internal iliac vein, lumbosacral nerve and sacroiliac joint. Laterally it lies on the fascia of obturator internus. It progressively crosses to become medial to the umbilical, inferior vesical, and middle rectal arteries.
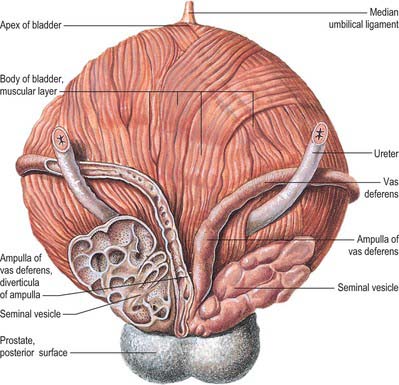
In males, the pelvic ureter hooks under the vas deferens (Fig. 74.21), then passes in front of and slightly above the upper pole of the seminal vesicle to traverse the bladder wall obliquely before opening at the ipsilateral trigonal angle. Its terminal part is surrounded by tributaries of the vesical veins. In females, the pelvic part at first has the same relations as in males, but anterior to the internal iliac artery it is immediately behind the ovary, forming the posterior boundary of the ovarian fossa (see Ch. 77). In the anteromedial part of its course to the bladder it is related to the uterine artery, uterine cervix and vaginal fornices. It is in extraperitoneal connective tissue in the inferomedial part of the broad ligament of the uterus where it may be damaged during hysterectomy. In the broad ligament, the uterine artery is anterosuperior to the ureter for 2.5 cm and then crosses to its medial side to ascend alongside the uterus. The ureter turns forwards slightly above the lateral vaginal fornix and is generally 2 cm lateral to the supravaginal part of the uterine cervix in this location. It then inclines medially to reach the bladder, with a variable relation to the anterior aspect of the vagina. As the uterus is commonly deviated to one side, one ureter, usually the left, may be more extensively apposed to the vagina, and may cross the midline.
The distal 1–2 cm of each ureter is surrounded by an incomplete collar of non-striated muscle, which forms a sheath (of Waldeyer). The ureters pierce the posterior aspect of the bladder and run obliquely through its wall for a distance of 1.5–2.0 cm before terminating at the ureteric orifices (see Fig. 75.5B). This arrangement is believed to assist in the prevention of reflux of urine into the ureter, since the intramural ureters are thought to be occluded during increases in bladder pressure at the time of micturition. There is no evidence of a classic ureteral sphincter mechanism in man. The longitudinally oriented muscle bundles of the terminal ureter continue into the bladder wall and at the ureteric orifices become continuous with the superficial trigonal muscle. In the distended bladder, in both sexes, the ureteric openings are usually 5 cm apart, and 2.5 cm apart when the bladder is empty.
In 1 in 125 individuals, two ureters drain the renal pelvis on one side; this is termed a duplex system (Fig. 74.22). Bilateral duplex ureters occur in approximately 1 in 800 cases. The duplex ureters derive from two ureteric buds arising from the mesonephric duct. They are contained in a single fascial sheath and may fuse at any point along their course or may be separate until they insert through separate ureteric orifices into the bladder. Care must be taken not to compromise the blood supply of the second ureter when excising or reimplanting a single ureter of a duplex.
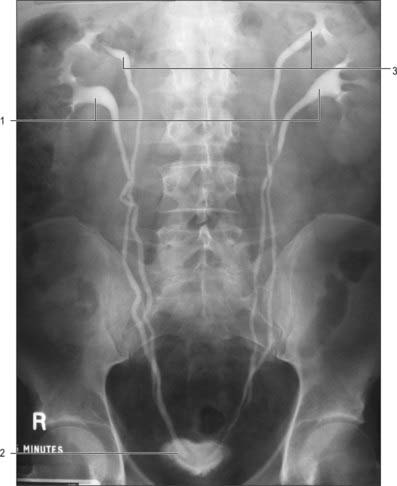
A ureterocele is a cystic dilatation of the lower end of the ureter: the ureteric orifice is covered by a membrane which expands as it is filled with urine and then deflates as it empties. Ureteroceles can vary in size, and although usually they have no influence on ureteric drainage they can be a cause of obstruction in the ureter and pelvicalyceal system more proximally. They usually do not cause bladder outflow obstruction except for the rare prolapsing ureterocele. Prolapsing ureteroceles, though small, prolapse from their position around the uretero-vesical junction region in to the urethra, causing intermittent bladder outflow obstruction. They are identified antenatally with ultrasound. In adults, ureteroceles tend to be bilateral and small and often found incidentally when the urinary tract is being imaged in the investigation of a coincidental pathology. Radiologically they classically result in a ‘cobra-head’ halo around the ureteric orifice following administration of contrast on intravenous urography (Fig. 74.22).
A persistence of the posterior cardinal vein, associated with high confluence of the right and left common iliac veins or a double inferior vena cava, may result in a retrocaval (or circumcaval) ureter which passes behind the inferior vena cava, usually at the level of the inferior edge of the third part of the duodenum, before it emerges in front of it to pass from medial to lateral. Retrocaval ureter occurs in 1 in 1500 individuals. Most commonly it has no clinical sequelae although it can result in upper ureteric obstruction (Fig. 74.23).
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Arteries
The ureter is supplied by branches from the renal, gonadal, common iliac, internal iliac, vesical and uterine arteries, and the abdominal aorta (Fig. 74.24). The pattern of distribution is subject to much variation. The abdominal ureter is supplied from vessels originating medial to the ureter, while the pelvic ureter is supplied by vessels lateral to the ureter. There is a good longitudinal anastomosis between these branches on the wall of the ureter, which means that the ureter can be safely transected at any level intraoperatively, and a uretero-ureterostomy performed, without compromising its viability. The branches from the inferior vesical artery are constant in their occurrence and supply the lower part of the ureter as well as a large part of the trigone of the bladder. The branch from the renal artery is also constant and is preserved whenever possible in renal transplantation to ensure good vascularity of the ureter.
MICROSTRUCTURE
Like the calyces and the renal pelvis, the wall of the ureter is composed of an external adventitia, a smooth muscle layer and an inner mucosal layer (Fig. 74.25). The mucosal layer consists of a urothelium and an underlying connective tissue lamina propria.
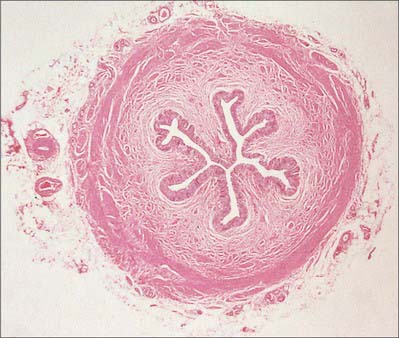
Fig. 74.25 Transverse section of ureter. The walls are muscular and are lined by a specialized urothelium.
(By permission from Stevens A, Lowe JS 1996 Human Histology, 2nd edn. London: Mosby.)
The ureteric adventitial blood vessels and connective tissue fibres are orientated parallel to the long axis of the ureter. Throughout its length, the muscle coat of the ureter is fairly uniform in thickness and in cross-section measures 750–800 μm in width. The muscle bundles which constitute this coat are frequently separated from one another by relatively large amounts of connective tissue. However, branches which interconnect muscle bundles are common and there is frequent interchange of muscle fibres between adjacent bundles. As a consequence of this extensive branching, individual muscle bundles do not spiral around the ureter, but form a complex meshwork of interweaving bundles. Moreover, unlike the gut (see Ch. 66), the muscle bundles are so arranged that morphologically distinct longitudinal and circular layers cannot be clearly distinguished. In the upper part of the ureter, the inner muscle bundles tend to lie longitudinally while those on the outer aspect have a circular or oblique orientation. In the middle and lower parts, there are additional outer longitudinally orientated fibres, and as the ureterovesical junction is approached, the muscle coat consists predominantly of longitudinally orientated muscle bundles.
Brodel M. The intrinsic blood-vessels of the kidney and their significance in nephrotomy. John Hopkins Hosp Bull. 1911;12:10-13.
Burkhill GJC, Healy JC. Anatomy of the retroperitoneum. Imaging. 2000;12:10-20.
Review of the imaging literature describing the contentious anatomy of the perirenal fascia..
Davies A, Blakeley AGH, Kidd C. The renal system. In: Human Physiology. Edinburgh: Churchill Livingstone; 2001:713-797. Chapter 8.
Davies JM. The role of the efferent arteriole in tubuloglomerular feedback. Kidney Int (Suppl). 1991;32:S71-S73.
Gosling JA, Dixon JS. Species variation in the location of upper urinary tract pacemaker cells. Invest Urol. 1974;11:418.
Early paper describing the identification of pacemaker cells in various species..
Merklin RJ, Michels NA. The variant renal and suprarenal blood supply with data on the inferior phrenic, ureteral, and gonadal arteries: a statistical analysis based on 185 dissections and a review of the literature. J Int Coll Surg. 1958;29:41-76.
A review of renal vascular anatomy in almost 11,000 kidneys..
Mitchell GAG. The renal fascia. Br J Surg. 1950;37:257-266.
Novick AC. Anatomic approaches in nephron-sparing surgery for renal cell carcinoma. Atlas Urol Clin North Am. 1998;6:39.
Madias NE, Adrogué HJ. Hypo-hypernatraemia: disorders of water balance. Davison AM, Cameron JS, Grunfeld J-P, et al, editors. Oxford Textbook of Clinical Nephrology, Volume 1. Oxford University Press, 2005. Chapter 2.1
Tannen RL, Hallows KR. Hypo-hyperkalaemia. Davison AM, Cameron JS, Grunfeld J-P, et al, editors. Oxford Textbook of Clinical Nephrology, Volume 1. Oxford University Press, 2005. Chapter 2.2;