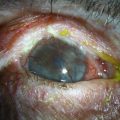
Keratoplasty in Ocular Surface Disease
Introduction
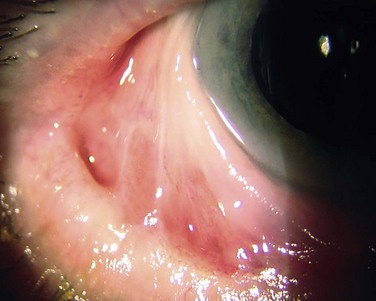
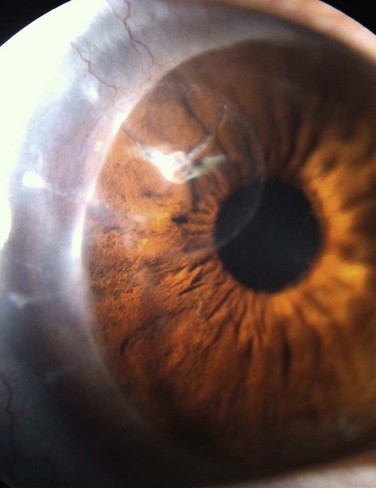
Approximately 50% of ocular surface reconstruction procedures will require keratoplasty for visual rehabilitation.1 The outcome of the corneal graft is dependent on several important factors, including the extent of the limbal stem cell deficiency (largely determined by the underlying etiology) and the presence or absence of conjunctival inflammation (Fig. 48.1).2 It is also important to consider whether other components of the ocular surface, besides limbal stem cells, are involved in the pathologic process. The consequences of ignoring any one of these items may result in non-healing epithelial defects, secondary ulceration, infectious keratitis, corneal vascularization, conjunctivalization of the cornea, and ultimate graft rejection or corneal melting. Even with perfect surgical technique and the best tissue available, poor outcomes may result without an adequate stem cell reserve, optimal tear function, and anatomically functional lids.1
Preoperative Considerations
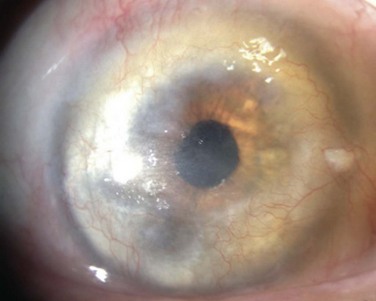
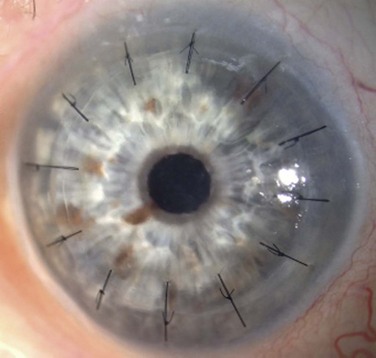
Prior to keratoplasty, all patients should undergo evaluation for ocular co-morbidities, such as eyelid malposition, eyelid disorders and glaucoma. In addition, the ocular surface should be screened for dry eye and dysfunctional tear syndrome states, limbal stem cell deficiency, conjunctival dysfunction, scarring and keratinization of the cornea, corneal vascularization, corneal sensation, and the status of the corneal epithelium (Fig. 48.2). If the conditions mentioned above are not addressed prior to keratoplasty, postoperative outcomes can be disappointing and potentially devastating.
Preoperative punctal occlusion, punctal cautery, entropion or ectropion repair, treatment of lagophthalmos, and trichiasis correction remain important preoperative treatment options for patients with concurrent eyelid disorders and dry eye conditions. Preservative-free artificial tears, gels, and lubricant ointments, as well as use of topical antiinflammatory agents can also be important adjuncts to management of preoperative conditions. Anterior and posterior blepharitis must also be controlled prior to keratoplasty in ocular surface disease, as detailed in Chapter 10. Glaucoma can also impact the outcome of keratoplasty, whether from the toxicity of glaucoma drops, high pressure exerting optic nerve damage and poor visual outcomes, or compromised endothelial dysfunction from certain glaucoma drops, high intraocular pressure or prior glaucoma surgery.3,4 Intraocular pressure must be managed prior to keratoplasty, with care to minimize corneal complications from glaucoma treatment.
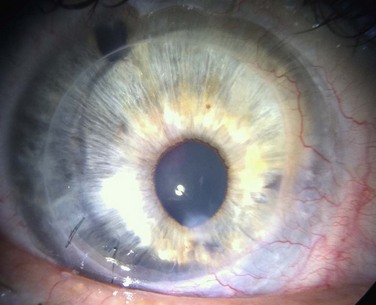
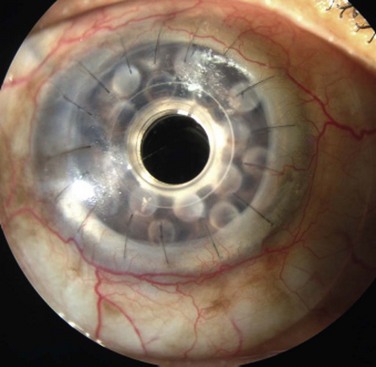
Once the tear film and lid function have been optimized and ocular co-morbidities addressed, the limbal stem cell reserve must be replenished in the presence of limbal stem cell deficiency. Chapters 40–45 detail surgical techniques for limbal stem cell transplantation. Given the scientific evidence and clinical experience, the authors prefer to perform keratoplasty as a staged procedure at least 3 months following stem cell transplantation (Fig. 48.3).
Surgical Technique and Considerations
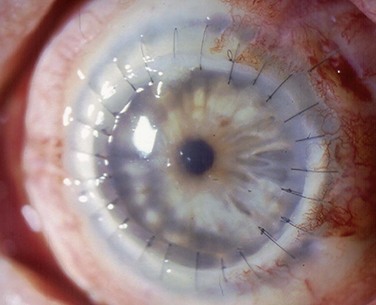
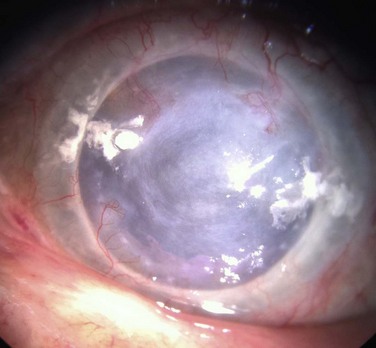
Penetrating keratoplasty (PK) in conjunction with or following stem cell transplant is performed with several unique considerations, compared to routine keratoplasty. Larger-diameter grafts are preferred to provide better apposition to the keratolimbal graft segments and lessen the risk of epithelial ingrowth.5 Same-size recipient trephination is often performed to prevent overlap between the peripheral donor cornea and stem cell segments. One important exception to same size trephination is chemical injury patients, who are oversized 0.5–0.75 mm, because the recipient bed in chemical burn cases often contracts following trephination. Because there is often asymmetric wound healing at the graft–host junction, interrupted sutures are preferred over running sutures to allow for selective removal to manage astigmatism (Fig. 48.4). Interrupted sutures also allow for easier suture removal and better wound stability after removal with a decreased risk of dehiscence, compared to running suture removal.6,7 Ocular surface disease patients must be watched closer for loose or vascularized sutures after keratoplasty as they are at increased risk of developing suture-related complications (Fig. 48.5).
In terms of donor tissue selection, some authors prefer to use pediatric or neonatal limbal tissue to allow for the greatest density of limbal stem cells for transplantation.8 Since pediatric donor corneal tissue is not commonly available, performing staged procedures allows for use of adult donor cornea tissue at a later date for keratoplasty. Optimizing keratoplasty donor tissue quality, especially relative to the donor ocular surface, can help prevent postoperative epithelial defects that could become persistent.9
Another consideration with keratoplasty in ocular surface disease is the choice of technique. The majority of reports in the literature on keratoplasty in ocular surface disease discuss PK; however, advances in deep anterior lamellar keratoplasty (DALK) and keratoprosthesis (KPro) make these procedures advantageous in selected circumstances. Femtosecond lasers and Anwar’s big bubble technique allow for deeper tissue dissections with DALK and decreased risk of interface haze/opacity, compared to lamellar techniques abandoned in the mid-twentieth century.10–13 The main advantages of DALK over PK include elimination of endothelial tissue rejection, extraocular rather than intraocular surgery, minor endothelial cell damage, faster potential suture removal, and the potential for increased tectonic wound strength (Fig. 48.6).10–14
The keratoprosthesis provides another viable alternative to limbal stem cell rehabilitation and cadaveric keratoplasty in this patient population. The advantages of a KPro over cadaveric tissue include elimination of endothelial tissue rejection, decreased dependence on limbal stem cell function and epithelial status, and less concern over conjunctival and eyelid status (Fig. 48.7).15,16 In particular, patients with Stevens–Johnson syndrome may do better with a KPro, as long as the inflammation is controlled to avoid melt and extrusion and the surface protected from trauma by a bandage contact lens.17,18 Patients who are elderly and/or cannot tolerate systemic immunosuppression risks or those who are not candidates for autologous tissue transplantation may also be better suited for KPro (Fig. 48.8). However, a KPro carries continued risks of endophthalmitis, glaucoma, extrusion after melt, and retroprosthetic membranes.15–18 Although the stem cell transplant patient will have a period of systemic immunosuppression typically lasting 6 to 24 months, the KPro patient must adhere indefinitely to post-perative measures, such as bandage contact lens wear, lifetime topical antibiotic prophylaxis, and lifetime glaucoma management. The surgeon must weigh these factors before deciding to proceed with KPro surgery.
Adjunct Procedures
Additional intraoperative and postoperative adjuvant treatments may enhance the outcomes of keratoplasty in ocular surface disease. Amniotic membrane can be placed over a newly placed graft to enhance epithelial recovery while still affording topical medication penetration into the cornea and anterior chamber. Amniotic membrane can be fixated with fibrin glue, sutures or by the sutureless ProKera® (Biotissue Inc., Miami, FL) following DALK or PK.19,20 Lateral tarsorrhaphy placement is another adjunct procedure that can enhance epithelial recovery after keratoplasty in concurrent severe dry eye disease and neurotrophic keratopathy. A therapeutic bandage lens may also be useful for short-term protection of the epithelial and limbal stem cells with close observation for avoidance of secondary infectious keratitis.21 Topical antibiotic prophylaxis should occur while the lens is in place. Postoperative medications that can enhance graft success in addition to topical corticosteroids include autologous serum, topical nerve growth factor, topical cyclosporine, and systemic immunosuppression in high-risk graft failure cases.22–24
Timing and Outcomes of Keratoplasty: Review of the Literature
Over the past quarter century, different authors have advocated different methods for ocular surface rehabilitation and keratoplasty. The primary point of debate is whether stem cell transplantation should be performed simultaneously with keratoplasty or as a staged procedure. There are advantages and disadvantages to both approaches. Advocates for the combined surgery argue that by obtaining limbal and corneal tissue from the same donor, the surgeon can avoid excessive use of donor tissue, avoid a second surgical procedure, and limit the antigenic challenge to the patient, thereby minimizing graft rejection. However, this procedure is technically more difficult, involves transplanting donor cornea into a less stable ocular surface, and likely incites more inflammation.25
Alternatively, keratoplasty may be performed sequentially, once the ocular surface has become more stable and less inflamed. This allows for a less technically challenging keratoplasty into a more optimized surface environment. The disadvantages include the need for two separate operations, exposure of the patient to two separate antigenic challenges, and a longer period of visual rehabilitation. Most published studies focus on one approach or the other, although several have compared sequential versus simultaneous procedures.25 Although the sample sizes are relatively small, evidence at this time favors sequential surgery, due to lower rates of corneal endothelial rejection and better survival of limbal grafts.
In 1989, Kenyon and Tseng described a series of 26 consecutive patients who underwent conjunctival limbal autograft, including five that later required keratoplasty. Two were treated for acute alkali injuries, two for the sequelae of chronic acid burns, and one for stem cell deficiency from multiple prior surgeries. In four cases, keratoplasty was done 8–12 months following conjunctival autograft with final visual acuity 20/20–20/40. However, one patient with acute alkali injury had a PK 3 weeks after autograft and failed to improve, with visual acuity 20/400 at 2 months before being lost to follow-up. The authors conclude by recommending that keratoplasty be performed at least 1 year after limbal transplantation.26
Frucht-Pery and colleagues,27 in 1998, reported a small series of nine patients who received conjunctival limbal autografting, including three patients who required a PK 3–6 months later. Patients were treated with postoperative topical and oral steroids (1 mg/kg) and oral cyclosporine A (300 mg/day). Epithelialization was complete 7–12 days after surgery, and there were no episodes of graft rejection, although final visual acuity after PK was not reported. Patients were still on oral immunosuppression at the time of keratoplasty.27
In 1998, Croasdale et al.28 published a series of 36 cases of sequential keratolimbal allograft transplantation (KLAL) followed by PK approximately 3 months later. Two eyes from the same donor were used for limbal transplantation and a third donor used for keratoplasty. Patients were treated with topical corticosteroids and oral cyclosporine A for 12 to 18 months. In an unpublished expansion of the study to 54 patients with limbal allograft, 35 patients underwent keratoplasty 3–4 months later and were followed for at least 1 year. Sixty percent (21 of 35) had successful corneal grafts. Of the 14 failed grafts, three developed endothelial rejection and 11 failed, due to recurrent ocular surface disease.1,28
In 2005, Sangwan et al.29 presented four eyes of four patients who underwent PK 3–4.5 months after cadaveric keratolimbal allograft or living-related conjunctival–limbal allograft. Three patients had prior chemical injuries and one had xeroderma pigmentosum. Visual acuity improved in all patients in the early phase; however, two developed graft opacification, one from endothelial rejection and one following trans-scleral cyclophotocoagulation for uncontrolled glaucoma.29
In 2010, Baradaran-Rafii et al.30 published four eyes of four patients who had PK following limbal stem cell transplantation cultivated on amniotic membrane. All patients developed progressive sectorial conjunctivalization and one patient had primary graft failure, due to exposure. In 2011, Biber and colleagues31 described 24 eyes of 19 patients that underwent the ‘Cincinnati procedure’ with combined living-related conjunctival limbal allografts and keratolimbal allografts. Nineteen eyes (79.2%) then had sequential keratoplasty, of which 14 eyes had penetrating keratoplasty, four eyes had Boston type I Kpro, and one eye had DALK. Seventy-five percent had improvement in visual acuity with 70.8% achieving 20/125 or better.31
Simultaneous limbal allograft transplantation with PK was first described by Tsubota et al.32 in 1995 in eight patients, utilizing the same donor tissue for the stem cell transplant and the keratoplasty. Patients received oral and topical cyclosporine A, as well as intravenous dexamethasone postoperatively. Five of the eight grafts remained clear for a mean of 12.3 months. Although there were two episodes of graft rejection that were managed medically and two patients required a second limbal graft, all eight patients had an improvement in visual acuity.32
In 1997, Tseng and Tan33 presented a case report of simultaneous KLAL and PK in a patient with severe thermal burns. Again, a single donor was used for both tissues. Postoperative medication included oral cyclosporine A 5 mg/kg, topical prednisolone acetate 1% and topical tobramycin 0.3%. The cornea reepithelialized within 24 days, and the cornea remained clear for the brief follow-up period of 21 weeks.33
In 1998, Tseng et al.34 reported, as part of a larger series, 14 eyes that received combined KLAL and PK from a single donor, following a previously performed amniotic membrane transplant. Nine of 14 eyes (64%) developed corneal graft rejection, with two eyes requiring repeat PK, due to irreversible endothelial rejection and three developing persistent corneal epithelial defects. None experienced limbal graft rejection.34
Tsubota et al.35 (1999) described a series of 43 eyes of 39 patients that underwent keratolimbal allograft, of which 28 eyes had simultaneous PK. Similar to previous reports, the KLAL and the donor cornea tissue were obtained from the same donor, limiting antigenic challenge. Cyclosporine A was used systemically preoperatively and for 1 month postoperatively, along with IV dexamethasone, which was used for 4 days postoperatively. Fifty-four percent (15 of 28) of the penetrating grafts survived, while 46% (13 of 28) rejected. Nine of those 13 that rejected were regrafted, and seven of those rejected again. Four patients required a third PK. Etiology of stem cell deficiency had an effect on epithelialization and rejection, as patients with Stevens–Johnson syndrome or ocular cicatricial pemphigoid epithelialized only 41%, compared to 71% of patients with chemical or thermal burns. On the other hand, chemical and thermal burns had a 69% rejection rate, compared to 27% of pemphigoid and Stevens–Johnson patients. Thirty-seven percent developed significant ocular hypertension following simultaneous surgery.35
In 2001, Shimazaki et al.36 published a case series of 45 eyes of 43 patients that had simultaneous KLAL and PK. Endothelial rejection occurred in 16 eyes (35.6%), of which 10 eyes (62.5%) were clear following treatment. Overall, there was no evidence of clinical rejection in the limbal grafts during the periods of corneal graft rejection, and all corneas re-epithelialized following rejection episodes. The authors theorized different mechanisms of rejection for the two types of tissue, with no effect on limbal stem cell function associated with corneal graft rejection.36
Solomon et al.37 performed combined KLAL and PK in 23 eyes and published the results in 2002. Patients received topical corticosteroids and antibiotics postoperatively, as well as systemic cyclosporine A 5 mg/kg, with a taper to appropriate trough levels. Central corneal graft survival was 47.8% after the first year and only 13.7% after 3 years. Grafts with Stevens–Johnson syndrome had significantly worse outcomes, with only 20% surviving at 1 year, and none surviving after 3 years.37
More recently, two case series have highlighted simultaneous limbal stem cell transplantation with DALK. In 2005, Fogla and Padmanabhan38 reported a series of 7 eyes of 7 patients that underwent combined conjunctivo-limbal autograft and DALK for unilateral severe chemical injury. All eyes achieved successful re-epithelialization and retained a stable ocular surface for a mean of 16.5 months. Average best-corrected final visual acuity was 20/50 and all but one eye had remarkable improvement in visual acuity.38 In 2010, Omoto and colleagues39 published a series of six eyes of five patients that had simultaneous KLAL and DALK for various etiologies, including Stevens–Johnson syndrome, aniridia, and gelatinous drop-like dystrophy. Four of five eyes (80%) retained a smooth epithelial surface and had improvement in visual acuity of more than two lines.39
To date, only two published studies have compared staged versus simultaneous surgery. In 2004, Shimizaki et al.8 reported 32 eyes of 32 patients with limbal stem cell deficiency from chemical burns (27 eyes) and thermal burns (5 eyes). Twenty-one eyes received KLAL transplantation, of which 15 had simultaneous PK and six had staged keratoplasty at a mean of 7.7 months. Although not statistically significant, corneal epithelialization and clarity were better in the staged group. Endothelial rejection was significantly greater in the simultaneous group than in the staged group (53.3% versus 0%, p = 0.019), and the need for a repeat limbal stem cell transplant was more common in the simultaneous group (66.7% versus 16.7%, p = 0.06).8 Most recently, in 2011, Basu described 12 eyes with combined autologous cultivated limbal epithelial transplantation and PK, compared to 35 eyes performed in two stages.40 Corneal allograft survival at 1 year was significantly higher in the staged group versus the simultaneous group (80% versus 25%, p = 0.0003). Recurrence of limbal stem cell deficiency was significantly higher in the simultaneous group than the staged group (58.3% versus 14.3%, p = 0.008).40
References
1. Biber, JM, Neff, KD, Holland, EJ, et al, Corneal transplantation in ocular surface disease. Cornea: fundamentals, diagnosis, and management, 3rd ed. Krachmer, JH, Mannis, MJ, Holland, EJ, eds. Cornea: fundamentals, diagnosis, and management, vol. 2. Elsevier: Philadelphia, 2010.
2. Holland, EJ, Schwartz, GS. The Paton Lecture: ocular surface transplantation: 10 year’s experience. Cornea. 2004;23:425–431.
3. Ayyala, RS. Penetrating keratoplasty and glaucoma. Surv Ophthalmol. 2000;45:91–105.
4. Alvarenga, LS, Mannis, MJ, Brandt, JD, et al. The long-term results of keratoplasty in eyes with a glaucoma drainage device. Am J Ophthalmol. 2004;138:200–205.
5. Skeens, HM, Holland, EJ. Large-diameter penetrating keratoplasty: indications and outcomes. Cornea. 2010;29:296–301.
6. Abou-Jaoude, ES, Brooks, M, Katz, DG, et al. Suture-related complications following keratoplasty: a 5-year retrospective study. Ophthalmology. 2002;109:1291–1296.
7. Lee, WB, Mannis, MJ. Corneal suturing techniques. In: Macsai MS, ed. Ophthalmic microsurgical suturing techniques. New York: Springer, 2006. [Ch. 6].
8. Shimazaki, J, Shimmura, S, Tsubota, K. Donor source affects the outcome of ocular surface reconstruction in chemical or thermal burns of the cornea. Ophthalmology. 2004;111:38–44.
9. Kim, T, Palay, DA, Lynn, M. Donor factors associated with epithelial defects after penetrating keratoplasty. Cornea. 1996;15:451–456.
10. Yoo, SH, Kymionis, GD, Koreishi, A, et al. Femtosecond laser-assisted anterior lamellar keratoplasty. Ophthalmology. 2008;115:1303–1307.
11. Anwar, M, Teichmann, KD. Big bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28:398–403.
12. Anwar, M, Teichmann, KD. Deep lamellar keratoplasty: surgical techniques for anterior lamellar keratoplasty with and without baring of Descemet’s membrane. Cornea. 2002;21:374–383.
13. Lee, WB, Mannis, MJ. The return of lamellar keratoplasty. Vision Panamerica. 2009;8:164–167.
14. Reinhart, WJ, Musch, DC, Jacobs, DS, et al. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty. Ophthalmology. 2011;118:209–218.
15. Zerbe, BL, Belin, MW, Ciolino, JB, et al. Results from the multicenter Boston Type 1 keratoprosthesis study. Ophthalmology. 2006;113:1779–1784.
16. Bradley, JC, Hernandez, EG, Schwab, IR, et al. Boston Type 1 Keratoprosthesis: the University of California Davis experience. Cornea. 2009;28:321–327.
17. Biber, JM, Skeens, HM, Neff, KD, et al. The Cincinnati procedure: technique and outcomes of combined living-related conjunctival limbal allografts and keratolimbal allografts in severe ocular surface failure. Cornea. 2011;30:765–771.
18. Sayegh, RR, Ang, LPK, Foster, S, et al. The Boston keratoprosthesis in Stevens–Johnson syndrome. Am J Ophthalmol. 2008;145:438–444.
19. Fernandes, M, Sridhar, MS, Sangwan, VS, et al. Amniotic membrane transplantation for ocular surface. Cornea. 2005;24:639–642.
20. Seitz, B, Das, S, Sauer, R, et al. Simultaneous amniotic membrane patch in high-risk keratoplasty. Cornea. 2011;30:269–272.
21. Lee, WB, Therapeutic hydrogel bandage lenses. Duane’s clinical ophthalmology. Tasman, W, Jaeger, EA, eds. Duane’s clinical ophthalmology, Vol 4. Lippincott Williams & Wilkins: Philadelphia, 2007. [Chapter 11].
22. Schrader, S, Wedel, T, Moll, R, et al. Combination of serum eye drops with hydrogel bandage contact lenses in the treatment of persistent epithelial defects. Graefes Arch Clin Exp Ophthalmol. 2006;244:1345–1349.
23. Matsumoto, Y, Dogru, M, Goto, E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111:1115–1120.
24. Lambiase, A, Manni, L, Bonini, S, et al. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci. 2000;41:1063–1069.
25. Mannis, MJ. Penetrating keratoplasty in ocular stem cell disease. In: Holland EJ, Mannis MJ, eds. Ocular surface disease: medical and surgical management. New York: Springer; 2002:253–256.
26. Kenyon, KR, Tseng, SCG. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–722.
27. Frucht-Pery, J, Siganos, CS, Solomon, A, et al. Limbal cell autograft transplantation for severe ocular surface disorders. Graefe’s Arch Clin Exp Ophthalmol. 1998;236:582–587.
28. Croasdale, CR, Schwartz, GS, Malling, JV, et al. Keratolimbal allograft: recommendations for tissue procurement and preparation by eye banks, and standard surgical technique. Cornea. 1999;18:52–58.
29. Sangwan, VS, Fernandes, M, Bansal, AK, et al. Early results of penetrating keratoplasty following limbal stem cell transplantation. Indian J Ophthalmol. 2005;53:31–35.
30. Baradaran-Rafii, A, Ebrahimi, M, Kanavi, MR, et al. Midterm outcomes of autologous cultivated limbal stem cell transplantation with or without penetrating keratoplasty. Cornea. 2010;29:502–509.
31. Biber, JM, Skeens, HM, Neff, KD, et al. The Cincinnati procedure: technique and outcomes of combined living-related conjunctival limbal allografts and keratolimbal allografts in severe ocular surface failure. Cornea. 2011;30:765–771.
32. Tsubota, K, Toda, I, Saito, H, et al. Reconstruction of the corneal epithelium by limbal allograft transplantation for severe ocular surface disorders. Ophthalmology. 1995;102:1486–1496.
33. Theng, JT, Tan, DT. Combined penetrating keratoplasty and limbal allograft transplantation for severe corneal burns. Ophthalmic Surg Lasers. 1997;28:765–768.
34. Tseng, SCG, Prabhasawat, P, Barton, K, et al. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431–441.
35. Tsubota, K, Satake, Y, Kaido, M, et al. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340:1697–1703.
36. Shimazaki, J, Maruyama, F, Shimmura, S, et al. Immunologic rejection of the central graft after limbal allograft transplantation combined with penetrating keratoplasty. Cornea. 2001;20:149–152.
37. Solomon, A, Ellies, P, Anderson, DF, et al. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology. 2002;109:1159–1166.
38. Fogla, R, Padmanabhan, P. Deep anterior lamellar keratoplasty combined with autologous limbal stem cell transplantation in unilateral severe chemical injury. Cornea. 2005;24:421–425.
39. Omoto, M, Shimmura, S, Hatou, S, et al. Simultaneous deep anterior lamellar keratoplasty and limbal allograft in bilateral limbal stem cell deficiency. Jpn J Ophthalmol. 2010;54:537–543.
40. Basu, S, Mohamed, A, Chaurasia, S, et al. Clinical outcomes of penetrating keratoplasty after autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol. 2011;152:917–924.