Keratolimbal Allograft
Background
Keratolimbal allograft (KLAL) is a technique in which allogeneic cadaveric limbal stem cells are transplanted to a recipient eye with severe ocular surface disease using peripheral donor cornea as a carrier.1 A number of techniques for KLAL have been reported, many of which describe strategies to facilitate harvesting the donor limbal grafts and donor tissue dissection. One of the earliest techniques, termed ‘keratoepithelioplasty,’ was to dissect the limbal tissue from a whole globe.2 Tsubota and co-workers reported the use of stored corneoscleral rims for limbal stem cell transplantation, which allowed for better coordination of surgery after suitable donor tissue was retrieved.3 Holland and Schwartz further modified the technique to use two stored corneoscleral rims instead of one, in order to fashion a contiguous ring of KLAL lenticules around the recipient limbus, which doubled the quantity of limbal stem cell supply and created a barrier to conjunctivalization.4 Djajilian reported a conjunctival-based thin KLAL technique which employed fibrin glue alone to secure the limbal allografts using minimal or no peripheral scleral skirt.5
Optical keratoplasty, either deep anterior lamellar keratoplasty (DALK) or penetrating keratoplasty (PK), may be required after KLAL, if deeper corneal stromal scarring is present. Sundmacher et al. have described a procedure termed homologous penetrating central limbokeratoplasty, in which a stored corneoscleral rim is intentionally trephined off-center to create a penetrating keratoplasty button.6 Using this technique, approximately 30–40% of the graft circumference contains limbal stem cells, and the patient benefits from receiving a clear penetrating graft along with limbal stem cells in a single operation. However, in severe cases of LSCD, it may be more beneficial to have limbal stem cells through 360 degrees. Performing optical keratoplasty as a staged procedure at least 3–6 months after KLAL allows for decreased ocular surface inflammation and stabilization of the corneal epithelium.
Indications
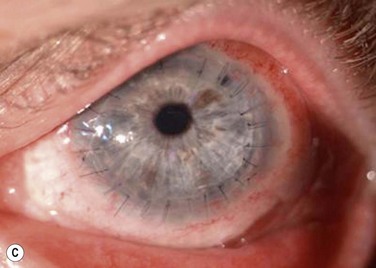
Keratolimbal allograft surgery is performed in patients who have no suitable living-related donor and have either bilateral LSCD or unilateral LSCD and fear damage to the healthy fellow eye (Fig. 42.1). Patients need to be suitable candidates for systemic immunosuppression, which is crucial to the success of KLAL in stabilizing the ocular surface and restoring a normal corneal epithelium phenotype.7–9
KLAL is ideal for diseases, such as aniridia, contact lens wear-related LSCD, and iatrogenic LSCD that affect mainly the limbus with minimal to no involvement of the conjunctiva.1,7,10 Patients with total LSCD require 360-degree KLAL, while those with sectoral LSCD may require only sectoral KLAL.
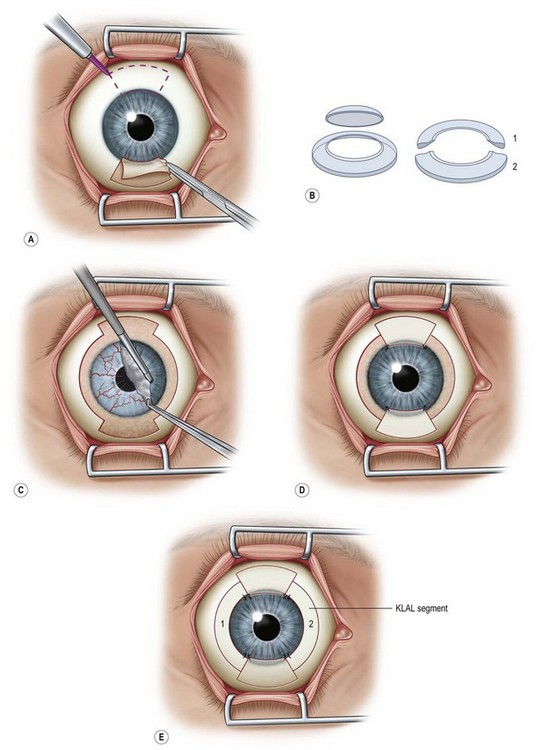
In patients with mild chemical injuries, Stevens – Johnson syndrome (SJS) or ocular cicatricial pemphigoid (OCP) and who have LSCD with mild to moderate conjunctival inflammation, it is best if the eye is allowed to quiet for at least a year or more prior to KLAL surgery in order to increase the chances of graft survival. The success rate of KLAL decreases with increasing conjunctival inflammation, such as in severe cases of chemical injuries, SJS, or OCP.1 In these cases, there is chronic conjunctival inflammation and scarring, decreased mucin and aqueous tear deficiency, and great potential for keratinization of the ocular surface. Since KLAL alone does not provide any healthy conjunctiva, for these severe cases of ocular surface disease, one can use in conjunction with two KLAL segments from one donor corneoscleral rim, living-related conjunctival limbal allograft (lr-CLAL) for bilateral disease and conjunctival limbal autograft (CLAU) for unilateral disease (Fig. 42.2).11,12
Preoperative Considerations
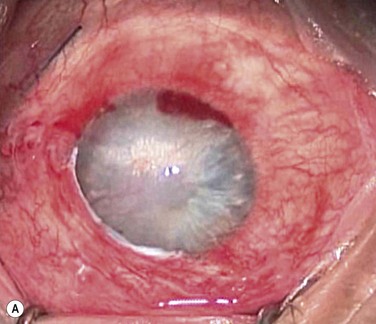
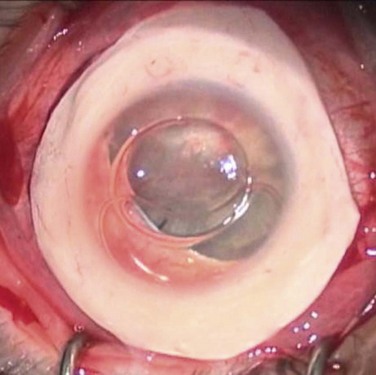
Patients, such as those after chemical injury, who have glaucoma or uncontrolled intraocular pressure, should undergo glaucoma drainage device implantation prior to KLAL, since multiple glaucoma medications may contribute to additional ocular surface toxicity (Fig. 42.3). Long-term use of topical corticosteroids to prevent graft rejection will also further aggravate any pre-existing glaucoma.
KLAL is contraindicated in patients with significant keratinization of the ocular surface.1 Uncontrolled severe inflammation is another poor prognostic factor for KLAL. Amniotic membrane grafts may be used in conjunction with KLAL to suppress inflammation and facilitate epithelialization.9 Finally, systemic immunosuppression medications plays an important role and patient compliance must be assured prior to KLAL, since noncompliance has been shown to be the main reason for graft rejection and failure.13
Donor Tissue Considerations
Eye Bank Tissue Preparation
Another difference from tissue preparation for PK or EK is that a 3–4-mm skirt of peripheral conjunctiva along with a 4–5-mm scleral rim is left on the corneoscleral rim for KLAL transplantation (Fig. 42.4). Leaving both this conjunctival and scleral rim minimizes damage to the limbal area and provides some goblet cells which are often deficient in recipient eyes. There has been no evidence to indicate any increased risk of infection by leaving the conjunctival rim intact.
For 360-degree limbal coverage using KLAL tissue, the corneoscleral rims from both eyes of the same donor are used for one recipient eye. In cases in which KLAL is performed in conjunction with living-related conjunctival limbal allograft (‘Cincinnati procedure’) or conjunctival limbal autograft (‘Modified Cincinnati procedure’) surgery, only one corneoscleral rim is needed.11,12
Surgical Technique
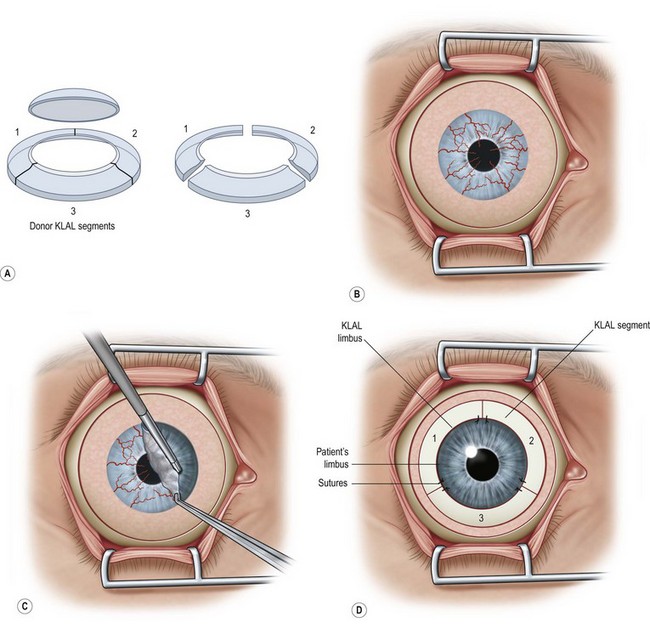
In general, retrobulbar anesthesia with a seven cranial nerve block or general anesthesia is administered. Figure 42.1 and the associated video footage demonstrate key steps of the KLAL procedure (Video 1), which has been further adapted from the original corneoscleral crescent technique of Holland and Schwartz.4,7
Preparation of the Donor Tissue
If the surgeon does not have an assistant to provide countertraction during tissue preparation, he or she may utilize cyanoacrylate glue to stabilize the KLAL scleral bed onto a sterile plastic platform followed by a thin strip of viscoelastic near the anterior edge of the lenticule during donor dissection of the anterior corneal stroma and donor conjunctival limbal tissue.5
Placement of the Donor Tissue
Each KLAL segment may be secured at the limbal edge using two 10-0 nylon sutures cut very short. The segments may then be trimmed as needed so that they do not overlap. Fibrin tissue glue is applied to secure the base of the KLAL lenticules to the recipient sclera and to ensure that the conjunctiva sits abutting the posterior edge of the KLAL lenticule. During suturing, the KLAL tissue should be protected from trauma with a viscoelastic coating. If the donor tissue is very thin, fibrin glue alone may be used to secure the lenticules (Fig. 42.5).
Postoperative Care
Local Management and Systemic Immunosuppression
Systemic immunosuppression must be used in patients after KLAL to prevent immune rejection of the allograft tissue and to manage the chronic inflammation present in eyes with severe ocular surface disease. Inflammation may lead to destruction of transplanted stem cells in the short and long term. A regimen consisting ideally of systemic steroids, tacrolimus, and mycophenolate mofetil has been used with good success to increase graft survival.5,8,9 Triple treatment consisting of systemic steroids, cyclosporine, and azathioprine has previously been used. Co-management with an organ transplant specialist with experience in the use of these immunosuppression medications is highly recommended.
Optical Keratoplasty
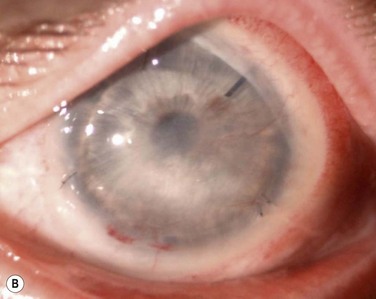
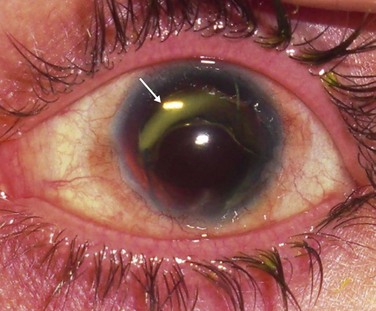
After achieving a stable ocular surface with KLAL (see Fig. 42.3Bb), subsequent optical keratoplasty may be performed to clear any deep stromal scarring (see Fig. 42.3C). It is advisable to wait at least 3–6 months to achieve a stable ocular surface before proceeding with PK or DALK. There are technical challenges in corneal transplantation after ocular surface stem cell transplantation. Usually, larger-diameter trephinations (approximately 9.5 to 11 mm) are sized to abut the border of the KLAL tissue. Donor buttons should be oversized by 0.5 mm, since eyes with chronic inflammation or cicatricial disease from chemical injury often contract after trephination of the host cornea. Deep bites should be employed with 24 interrupted 10-0 nylon sutures to ensure that the donor and host cornea are fully approximated, avoiding suturing just to the superficial KLAL segment.14
Outcomes
Graft survival rates for KLAL range from 33% to 84%.15 The use of systemic immunosuppression after KLAL is required to decrease the risk of graft failure secondary to rejection. This subject is discussed more extensively in Chapter 46.
The best results after limbal stem cell transplantation are achieved with adequate triple systemic immunosuppression. In a study of 31 eyes of 23 patients with aniridia after KLAL with follow-up of 12 to 117 months, 23 eyes (74.2%) achieved a stable ocular surface, and overall mean visual acuity improved from 20/1000 to 20/165.7 A study of 19 eyes from 16 patients demonstrated a KLAL survival rate of 76.9% after a follow-up of 31 months.5 Fifteen eyes (78.9%) showed an improvement in vision of two or more Snellen lines. Another study of 12 eyes from 10 patients with follow up of 36 to 91 months after KLAL reports a stable ocular surface achieved in 83%.9
References
1. Holland, EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc. 1996;94:677–743.
2. Thoft, RA. Keratoepithelioplasty. Am J Ophthalmol. 1984;97:1–6.
3. Tsubota, K, Toda, I, Saito, H, et al. Reconstruction of the corneal epithelium by limbal allograft transplantation for severe ocular surface disorders. Ophthalmology. 1995;102:1486–1496.
4. Croasdale, CR, Schwartz, GS, Malling, JV, et al. Keratolimbal allograft: recommendations for tissue procurement and preparation by eye banks, and standard surgical technique. Cornea. 1999;18:52–58.
5. Nassiri, N, Pandya, HK, Djalilian, AR. Limbal allograft transplantation using fibrin glue. Arch Ophthalmol. 2011;129:218–222.
6. Reinhard, T, Sundmacher, R, Spelsberg, H, et al. Homologous penetrating central limbo-keratoplasty (HPCLK) in bilateral limbal stem cell insufficiency. Acta Ophthalmol Scand. 1999;7:663–667.
7. Holland, EJ, Djalilian, AR, Schwartz, G. Management of aniridic keratopathy with keratolimbal allograft: a limbal stem cell transplantation technique. Ophthalmology. 2003;110:125–130.
8. Holland, EJ, Mogilishetty, G, Skeens, HM, et al. Systemic immunosuppression in ocular surface stem cell transplantation: results of a 10-year experience. Cornea. 2012;31:655–661.
9. Liang, L, Sheha, H, Tseng, SC. Long-term outcomes of keratolimbal allograft for total limbal stem cell deficiency using combined immunosuppressive agents and correction of ocular surface deficits. Arch Ophthalmol. 2009;127:1428–1434.
10. Schwartz, GS, Holland, EJ. Iatrogenic limbal stem cell deficiency. Cornea. 1998;17:31–37.
11. Biber, JM, Skeens, HM, Neff, KD, et al. The Cincinnati procedure: technique and outcomes of combined living-related conjunctival limbal allografts and keratolimbal allografts in severe ocular surface failure. Cornea. 2011;30:765–771.
12. Chan, CC, Biber, JM, Holland, EJ. The modified Cincinnati procedure: combined conjunctival limbal autografts and keratolimbal allografts for severe unilateral ocular surface failure. Cornea. 2012;31:655–661.
13. Andrea, AY, Chan, CC, Biber, JM, et al. Ocular surface stem cell transplantation rejection: incidence, characteristics, and outcomes. Cornea. 2012. [June 4].
14. Biber, JM, Neff, KD, Holland, EJ, et al, Corneal transplantation in ocular surface disease. Cornea, 3rd ed. Krachmer, JH, Mannis, MJ, Holland, EJ, eds. Cornea, vol. 2. Elsevier: London, 2010:1755–1758.
15. Cauchi, PA, Ang, GS, Azuara-Bianco, A, et al. A systematic literature review of surgical interventions for limbal stem cell deficiency in humans. Am J Ophthalmol. 2008;146:251–259.