K
Kallikreins. Serine protease enzymes in tissue and plasma, performing diverse physiological functions. Produce kinins from kininogens and may also catalyse formation of renin from prorenin. Plasma kallikrein is produced from prekallikrein by various activating substances, including part of activated coagulation factor XII. Plasmin, a fibrinolytic enzyme, catalyses the reaction, as does kallikrein itself. Inhibited by aprotinin. Prostate-specific antigen (PSA) is a kallikrein used as a biomarker for prostatic cancer.
Katharometer. Device used for gas analysis, following separation, e.g. by gas chromatography. A heated wire is placed in the gas flow; different gases have different thermal conductive properties and therefore produce different changes in electrical resistance of the wire. Particularly useful in detecting inorganic gases, e.g. helium, CO2, N2O and O2. Used to quantify the amount of a known gas, not to identify unknown ones.
Kawasaki disease (Musculocutaneous lymph node syndrome). Autoimmune systemic vasculitis of unknown aetiology, usually affecting children < 5 years old. Incidence is 3.4 per 100 000 in the UK; three and 30 times more common in the USA and Japan respectively. Important because arteritis can lead to formation of coronary artery aneurysms in 15% of cases if untreated, with mortality of up to 4%. May present with fever (typically for more than 5 days), conjunctivitis, reddened lips, mouth and tongue, desquamating rash, peripheral oedema and cervical lymphadenopathy. Diagnosis may be difficult because not all these features may be present at once, other non-specific symptoms may be present (e.g. cough, abdominal pain, vomiting, arthralgia) and there is no diagnostic test. Treatment is with iv immunoglobulin 2 g/kg over 10 h and aspirin 30 mg/kg/day in 3–4 divided doses.
[Tomisaku Kawasaki, Japanese paediatrician]
Harnden A, Takahashi M, Burgner D (2009). Br Med J 2009; 338: 1133–8
Kelvin. SI unit of temperature. One kelvin (K) = 1/273.16 of the thermodynamic scale temperature of the triple point of water (point at which solid, liquid and gaseous water are at equilibrium). nK = (n – 273.16)°C.
[William Thomson (1824–1907), Irish-born Scottish physicist; became Lord Kelvin in 1892]
Kerley’s lines. Opaque markings seen on the CXR:
Ketamine hydrochloride. Phencyclidine derivative (Fig. 94), first used as an iv anaesthetic agent in 1965. Increasingly used for postoperative analgesia and sedation on ICU. An antagonist at NMDA receptors; also interacts with opioid, monoamine and cholinergic receptors. Recently described anti-inflammatory effects may be via reduced expression of cytokines (e.g. interleukin-6) by activated macrophages.
 induction of anaesthesia, traditionally in shocked patients. Produces a state of ‘dissociative anaesthesia’: intense analgesia with light sleep. Increased thalamic and limbic activity occurs, with dissociation from higher centres.
induction of anaesthesia, traditionally in shocked patients. Produces a state of ‘dissociative anaesthesia’: intense analgesia with light sleep. Increased thalamic and limbic activity occurs, with dissociation from higher centres.
 maintenance of anaesthesia as the sole agent, especially in burns and trauma, or short procedures (e.g. radiotherapy and radiological investigations). Widely used in battlefield and pre-hospital anaesthesia due to its relative preservation of upper airway reflexes. Its routine use at anaesthetic doses in adults is limited by psychomimetic emergence phenomena (see below).
maintenance of anaesthesia as the sole agent, especially in burns and trauma, or short procedures (e.g. radiotherapy and radiological investigations). Widely used in battlefield and pre-hospital anaesthesia due to its relative preservation of upper airway reflexes. Its routine use at anaesthetic doses in adults is limited by psychomimetic emergence phenomena (see below).
 perioperative analgesia: when given perioperatively, has a potent opioid-sparing effect, with reduced PONV and minimal side effects.
perioperative analgesia: when given perioperatively, has a potent opioid-sparing effect, with reduced PONV and minimal side effects.
 treatment of acute (e.g. postamputation) and chronic neuropathic pain.
treatment of acute (e.g. postamputation) and chronic neuropathic pain.
 adjunct in regional anaesthesia: has local anaesthetic properties, and has been used for spinal and epidural anaesthesia within clinical trials; concerns over direct neurotoxicity have restricted its use beyond the trial setting.
adjunct in regional anaesthesia: has local anaesthetic properties, and has been used for spinal and epidural anaesthesia within clinical trials; concerns over direct neurotoxicity have restricted its use beyond the trial setting.
 possible neuroprotective effects via its NMDA receptor antagonism.
possible neuroprotective effects via its NMDA receptor antagonism.
 abuse as a recreational drug (‘K’, ‘Special K’), leading to its classification as a Class C drug under the Misuse of Drugs Act in 2006.
abuse as a recreational drug (‘K’, ‘Special K’), leading to its classification as a Class C drug under the Misuse of Drugs Act in 2006.
Ketamine is commercially available as a racemic mixture of two isomers or as the pure S(+) enantiomer. The latter is 2–4 times as potent as the R(–) enantiomer and less psychoactive, with a higher clearance and thus more rapid recovery.
Presented in 10, 50 and 100 mg/ml solutions, containing benzethonium chloride (the 10 mg/ml solution since 1997). pH is 3.5–5.5; pKa 7.5. 12% bound to plasma albumin. Elimination half-life is about 3 h. Metabolised in the liver to weakly active metabolites, excreted in urine.
• Effects:
 iv induction: smooth, but slower than thiopental (about 30 s).
iv induction: smooth, but slower than thiopental (about 30 s).
– BP and heart rate usually increase; cardiac output is maintained. Changes are probably via direct myocardial and central sympathetic stimulation, and blockade of noradrenaline uptake by sympathetic nerve endings. Contraindicated in severe ischaemic heart disease and hypertension.
– respiratory depression after rapid iv injection, but less than with other agents.
– bronchodilatation via a direct action and sympathomimetic effects.
– analgesia at subanaesthetic doses.
– increased muscle tone, twitching, blinking and nystagmus.
– increased cerebral blood flow and ICP; contraindicated in pre-eclampsia and severe intracranial pathology.
– anterograde amnesia in some cases.
– at anaesthetic doses may cause vivid dreams and emergence phenomena, e.g. unpleasant hallucinations. Usually less distressing in patients < 15 or > 65 years. Hallucinations may be reduced by benzodiazepines, and delirium by butyrophenones. physostigmine has also been used. Preoperative counselling, opioid/hyoscine premedication and recovery in a quiet, darkened room may also reduce their incidence.
– may cause nausea and vomiting. Salivation is increased.
– increases intraocular pressure; contraindicated in open eye operations.
– does not cause histamine release.
• Dosage (for racemic ketamine; dosages for S-ketamine are approximately 50% lower):
 iv induction: 1–2 mg/kg. Effects last for 5–10 min. Supplementary doses 0.5 mg/kg.
iv induction: 1–2 mg/kg. Effects last for 5–10 min. Supplementary doses 0.5 mg/kg.
 im induction; 10 mg/kg. Acts in 3–5 min, lasting 20–30 min.
im induction; 10 mg/kg. Acts in 3–5 min, lasting 20–30 min.
 sedation (e.g. whilst performing regional techniques or on ICU): 2 mg/kg im, 10 mg increments iv, or 1–2 mg/kg/h infusion.
sedation (e.g. whilst performing regional techniques or on ICU): 2 mg/kg im, 10 mg increments iv, or 1–2 mg/kg/h infusion.
 to treat severe asthma: 0.5–2.5 mg/kg/h infusion.
to treat severe asthma: 0.5–2.5 mg/kg/h infusion.
Himmelseher S, Durieux ME (2005). Anesthesiology; 102: 211–20
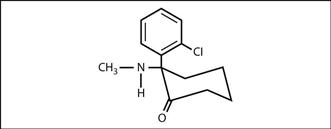
Fig. 94 Structure of ketamine
Ketanserin. 5-HT antagonist, and weak α1–adrenergic receptor antagonist. Also has class III antiarrhythmic properties. Has been used to prevent vasoconstriction and bronchospasm in carcinoid syndrome, and also to provide vasodilatation and hypotension in cardiac surgery.
Ketoacidosis. Metabolic acidosis due to accumulation of ketone bodies in plasma. Occurs when there is reduced availability of glucose (e.g. starvation, commonly seen in chronic alcoholism) or reduced ability to utilise glucose (e.g. diabetic ketoacidosis). The latter is associated with hyperglycaemia; the former is not.
 of acidosis, including hyperventilation and hyperkalaemia.
of acidosis, including hyperventilation and hyperkalaemia.
 characteristic sweet smell on the patient’s breath due to ketosis.
characteristic sweet smell on the patient’s breath due to ketosis.
Treatment includes rehydration, correction of electrolyte imbalance and hypoglycaemia if present. Diabetic ketoacidosis is treated with insulin.
Ketobemidone hydrochloride. Opioid analgesic drug, with similar potency and properties to morphine. Mainly used in Scandinavia.
Ketoconazole. Imidazole antifungal drug, active against a wide range of fungi and yeasts. Should not be given for superficial infections because of the risk of fatal hepatotoxicity. Suppresses synthesis of glucocorticoids and thus has been used for treatment of Cushing’s disease.
Ketone bodies. Acetone, acetoacetic acid and hydroxybutyric acid; formed from hepatic metabolism of free fatty acids released from adipose tissue. Normally utilised by brain, heart and other tissues as an energy source, keeping blood levels low. Levels increase when fat metabolism increases, especially when intracellular glucose is deficient, e.g. in diabetes mellitus, chronic alcoholism, starvation, and high fat/low carbohydrate diet. Increased levels may lead to ketoacidosis.
Ketorolac trometamol/tromethamine. NSAID, licensed for short-term postoperative analgesia. Has a marked analgesic effect with little anti-inflammatory effect. Maximal plasma levels occur within 60 min of im injection and 5 min of iv injection, with half-life of 5–6 h. Pain relief may not occur until 30 min after injection. Over 99% protein-bound, with hepatic metabolism (reduced in the elderly). Over 95% of metabolites are excreted in urine, with ~6% in the faeces. Recommended parenteral doses have been reduced from those originally proposed, following adverse events, including death from GIT perforation/haemorrhage, asthma, anaphylaxis and renal failure. The datasheet specifies intraoperative and prophylactic use before surgery as contraindications because of the increased risk of bleeding.
• Side effects: as for NSAIDs. CNS effects, including drowsiness, dizziness, psychological changes and convulsions. Contraindications include peptic ulcer disease, coagulation abnormalities/anticoagulant drugs (including low-dose heparin), asthma, concurrent treatment with or allergy to any other NSAID, renal impairment, hypovolaemia, CVA, pregnancy and lactation.
Kety–Schmidt technique. Method of measuring cerebral blood flow using the Fick principle, using N2O. 10% N2O is inhaled for 10–15 min, and the jugular venous concentration is assumed to be equal to the brain concentration. Cerebral N2O uptake is therefore calculated.
[Seymour S Kety (1915–2000) and Carl F Schmidt (1893–1988), US neuroscientists]
Key filling system. System for filling modern vaporisers with volatile anaesthetic agent, preventing filling with incorrect agent and supposedly reducing spillage and pollution by using closely fitting interlocking components.
Collar, filler tube base and block are colour-coded for the particular agent (halothane – red; enflurane – orange; isoflurane – purple; trichloroethylene – blue; methoxyflurane – green) and the name of the agent is written on the filler tube base.
Vaporisers for desflurane and sevoflurane do not utilise the key filling system, their bottles fitting to the vaporiser by an agent-specific attachment already on the bottle (desflurane) or fitted to it (sevoflurane).
See also, COSHH regulations; Environmental safety of anaesthetists
Kidney. Situated on the posterior abdominal wall, with the diaphragm and 11th and 12th ribs posteriorly. The renal hila are approximately level with the pylorus. Each kidney is about 10 cm long, 5 cm wide and 3 cm thick. Nerve supply is via the coeliac ganglion from T12 and L1. Blood supply is from the renal arteries that arise from the descending aorta, and venous drainage is via the renal veins into the inferior vena cava. Renal blood flow is 1200 ml/min (22% of cardiac output), about 400 ml/100 g/min. O2 consumption is 20 ml/min, or 6 ml/100 g/min.
Composed of outer cortex and inner medulla, forming pyramids. Functional unit is the nephron, with accompanying arterioles and capillaries.
 filtration of plasma and excretion of waste products of metabolism, whilst maintaining water, osmolality, electrolyte and acid–base homeostasis.
filtration of plasma and excretion of waste products of metabolism, whilst maintaining water, osmolality, electrolyte and acid–base homeostasis.
 formation of 1,25-dihydroxycholecalciferol (important in calcium homeostasis).
formation of 1,25-dihydroxycholecalciferol (important in calcium homeostasis).
 metabolism and excretion of drugs.
metabolism and excretion of drugs.
• May be affected by anaesthesia/surgery:
 drug effects, e.g. methoxyflurane, diuretics.
drug effects, e.g. methoxyflurane, diuretics.
 alteration of renal blood flow, e.g. hypotension, aortic aneurysm repair.
alteration of renal blood flow, e.g. hypotension, aortic aneurysm repair.
 renal impairment following incompatible blood transfusion, severe jaundice, sepsis, obstetric emergencies or crush syndrome.
renal impairment following incompatible blood transfusion, severe jaundice, sepsis, obstetric emergencies or crush syndrome.
See also, Acid–base balance; Fluid balance; Renal failure; Renal transplantation; Renin/angiotensin system
Killian, Gustav (1860–1921). German laryngologist; published extensively on the structure and function of the larynx. A former student of Kirstein, he helped popularise direct laryngoscopy. Also performed the first translaryngeal removal of a foreign body from the right main bronchus in 1897, using a rigid oesophagoscope and cocaine local anaesthesia, thus pioneering bronchoscopy.
Kilogram. SI unit of mass. The standard kilogram is the mass of a cylindrical piece of platinum–iridium alloy kept at Sèvres, France.
Kilogram weight. Weight of a mass of 1 kilogram subjected to standard Earth gravity. Also called kilogram force.
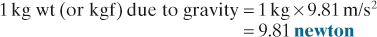
Kinins. Vasodilator peptides derived from precursor molecules (kininogens) by the action of kallikreins. Involved in many inflammatory and immune reactions, and possibly in shock. Also thought to be involved in the carcinoid syndrome. Bradykinin is the main plasma kinin, causing vasodilatation and increased vascular permeability. Glucocorticoids inhibit their release. Broken down by angiotensin converting enzyme, mainly in the lung. Elimination half-life is less than 15 s.
Kirstein, Alfred (1863–1922). German laryngologist; described the first direct laryngoscopy in 1895. His laryngoscopes could be placed anterior or posterior to the epiglottis. Stressed the importance of the ‘sniffing the morning air position’ for laryngoscopy. Also suggested translaryngeal removal of bronchial foreign bodies as being easier than via tracheostomy.
Hirsch NP, Smith GB, Hirsch PO (1986). Anaesthesia; 41: 42–5
Klippel–Feil syndrome. Inherited condition characterised by congenitally fused cervical vertebrae; subdivided according to the extent of vertebral involvement:
 type I: several vertebrae (cervical and upper thoracic) form a single unit.
type I: several vertebrae (cervical and upper thoracic) form a single unit.
 type II: one or two cervical vertebrae affected only.
type II: one or two cervical vertebrae affected only.
 type III: types I or II with thoracic or lumbar abnormalities too.
type III: types I or II with thoracic or lumbar abnormalities too.
 epidural and spinal anaesthesia may be technically difficult.
epidural and spinal anaesthesia may be technically difficult.
[Maurice Klippel (1858–1942) and Andre Feil (1884–1955), French neurologists]
Naguib M, Farag H, Ibrahim AEW (1986). Can Anesth Soc J; 33: 66–70
Knee, nerve blocks. Blockade of nerves at or near the knee joint, performed for surgery to the lower leg and foot. Blocks may be performed using ultrasound guidance or nerve stimulator techniques.
• Nerves that may be blocked (see Fig. 66; Femoral nerve block):
 tibial nerve (L4–S3): terminal branch of the sciatic nerve; supplies the anteromedial part of the sole of the foot via plantar branches. In the popliteal fossa, the nerve lies superficial and lateral to the popliteal vessels, with vein medially and artery most medial.
tibial nerve (L4–S3): terminal branch of the sciatic nerve; supplies the anteromedial part of the sole of the foot via plantar branches. In the popliteal fossa, the nerve lies superficial and lateral to the popliteal vessels, with vein medially and artery most medial.
With patient prone and knee extended, a needle is inserted in the midline of the popliteal fossa, level with the femoral condyles. 5–10 ml local anaesthetic agent is injected at a depth of 2–3 cm.
Koller, Carl (1857–1944). Austrian ophthalmologist; first described the use of local anaesthetic agent (cocaine) for a surgical operation (for glaucoma) in Vienna in 1884, having previously investigated the drug with Freud. This was reported by a colleague at an ophthalmological congress in Heidelberg on the following day. Disappointed with his subsequent career progress, he emigrated to New York in 1888.
Korotkoff sounds. Sounds heard during auscultation over the brachial artery, whilst a proximal cuff is slowly deflated from above systolic pressure. Thought to result from turbulent flow within the artery, causing vessel wall vibration and resonance. Used in arterial BP measurement. Three phases were originally described by Korotkoff; these were subsequently increased to five:
Krebs cycle, see Tricarboxylic acid cycle
Kuhn, Franz (1866–1929). German physician; wrote extensively on tracheal intubation for anaesthesia. Described tracheal insufflation in 1900 and nasotracheal intubation in 1902.
Kussmaul breathing (Air hunger). Hyperventilation originally described in diabetic hyperglycaemic ketoacidosis. Caused by stimulation of central chemoreceptors by hydrogen ions. Occurs in severe metabolic acidosis from any cause.
Kussmaul’s sign, see Cardiac tamponade
There may also be rotational deformity.
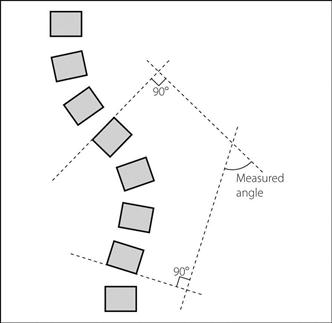
May be associated with congenital skeletal, muscular or neurological abnormalities and diseases. Idiopathic scoliosis develops in childhood and is the most common form. The angle of curvature is measured from X-rays of the spine (Fig. 95) and an angle > 10° is abnormal. There may be rotation of the vertebrae, with the spinous processes turned inward towards the concavity of the curve. Thoracic, rib and chest wall deformity may cause restriction of ventilation, especially if the curve exceeds 65°. Surgical fixation is increasingly performed early using metal Harrington rods which allow progressive distraction of the vertebrae as the child grows.
– assessment for associated disease.
– MH may be commoner in this group.
– assessment of RS: lung volumes and compliance are reduced; the main defect is restrictive.  mismatch may be present. Repeated aspiration may occur in neuromuscular disease. CXR is mandatory; lung function tests and arterial blood gas interpretation are useful.
mismatch may be present. Repeated aspiration may occur in neuromuscular disease. CXR is mandatory; lung function tests and arterial blood gas interpretation are useful.
– chronic hypoxaemia may lead to pulmonary hypertension and cor pulmonale.
– preoperative physiotherapy and antibiotics may be required.
– tracheal intubation may be difficult, especially if the patient is unable to lie flat.
– surgery may be prolonged, with risk of major blood loss and hypothermia.
– transthoracic surgery may involve anterior or posterior approaches.
– hypotensive anaesthesia is advocated by some, to reduce blood loss and ease surgery. Others argue that the risk of spinal cord ischaemia precludes this. Careful positioning of the patient is required to prevent pressure on the abdominal inferior vena cava.
– risk of cord damage during distraction of vertebrae may be assessed by the wake-up test or monitoring of evoked potentials.
– access to the patient may be restricted.
 postoperatively: ventilatory failure is possible; close monitoring is required. CXR is usual to exclude pneumothorax. An epidural catheter may be placed by the surgeon for postoperative analgesia.
postoperatively: ventilatory failure is possible; close monitoring is required. CXR is usual to exclude pneumothorax. An epidural catheter may be placed by the surgeon for postoperative analgesia.
[Paul R Harrington (1911–1980), Texas surgeon]
Raw DA, Beattie JK, Hunter JM (2003); Br J Anaesth; 91: 886–904