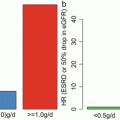
Fig. 15.1
Working group for the development of Clinical Guidelines for IgA Nephropathy, Progressive Renal Diseases Research by the Japanese Society of Nephrology, and Research on Intractable Disease by the Ministry of Health, Labour and Welfare of Japan
15.2 Characteristics of the Japanese Clinical Guidelines for IgA Nephropathy
The purpose of the Clinical Guidelines for IgA Nephropathy 2014 was to define evidence-based clinical guidelines that reflect the clinical situation of IgAN in Japan. This guideline is developed to provide answers to clinical questions (CQs) that nephrologists may encounter in the clinical practice for the treatment of IgAN. Each answer is shown as a statement, and recommendation grades based on the evidence-based levels are noted for each statement in the treatment section (Table 15.1). It was not aimed at creating an exhaustive textbook but at supporting clinical decisions by answering questions raised by nephrologists in clinical practice and establishing a standard treatment. With the aim of comprehensively supporting nephrologists in the treatment of IgAN in clinical settings, the Clinical Guidelines for IgA Nephropathy 2014 Advisory Committee independently evaluated the results of principal randomized parallel-group clinical trials published to date (Figs. 15.2 and 15.3) and presented the scheme of indications for preventive intervention of renal dysfunction progression in this guideline (Fig. 15.4). Now, the indications of therapeutic interventions of IgAN are mainly based on GFR and urinary protein level in this guideline and also the evidence-based practice guideline for the treatment of chronic kidney disease (CKD) [4]. The Clinical Guidelines for IgA Nephropathy 2014 also describe the characteristics and treatment of pediatric IgAN.
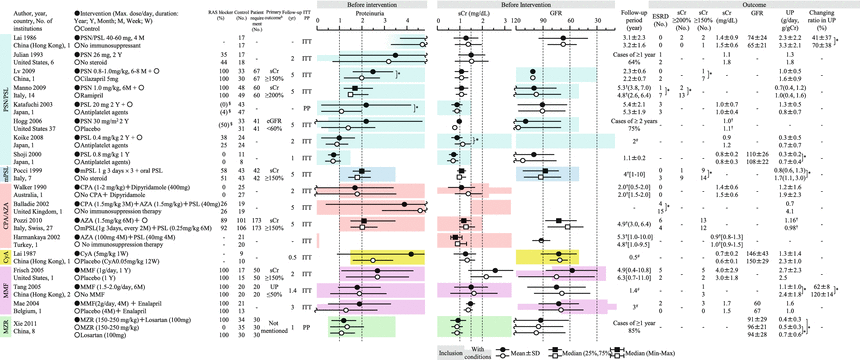
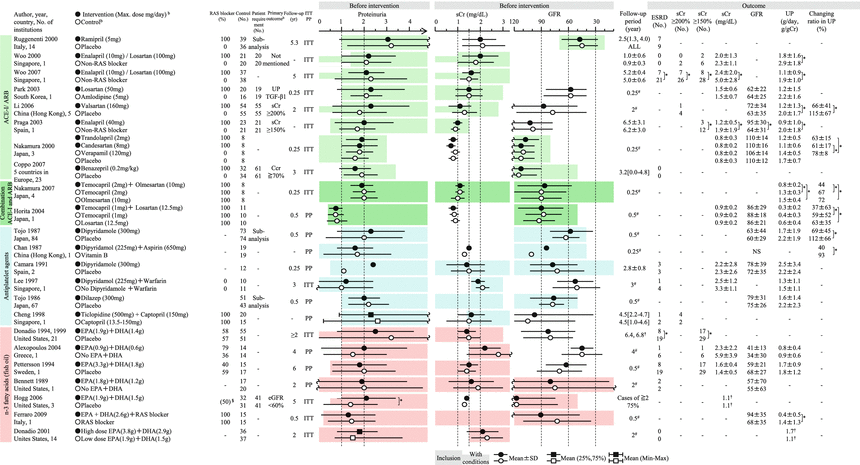
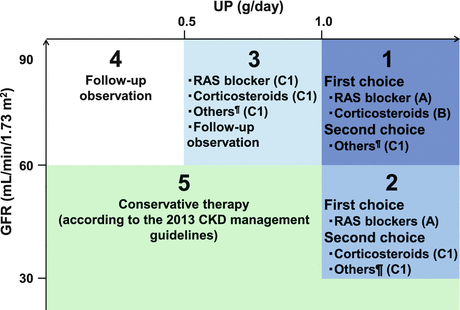
Table 15.1
Rating description of the guideline recommendations
|
Recommendation grade
|
|---|
|
A. Strongly recommended for implementation in routine clinical practice because of sufficient evidence
|
|
B. Recommended for implementation in routine clinical practice because of some evidence
|
|
C1. Might be implemented in routine clinical practice despite insufficient evidence
|
|
C2. Not recommended for implementation in routine clinical practice because of insufficient evidence
|
|
D. Not recommended for implementation in routine clinical practice because of evidence that it might be harmful to patients
|

Fig. 15.2
Summary of the randomized controlled trials of corticosteroids and immunosuppressive agents in adult IgAN patients. AZA azathioprine, CPA cyclophosphamide, CyA cyclosporin, ITT intention to treat, MMF mycophenolate mofetil, mPSL methylprednisolone, MZR mizoribine, PP pet protocol, PSL prednisolone, PSN prednisone. Mean ± SD, median (25 %, 75 %), mean or median [range]. -NA, *P < 0.05, §administration rate before intervention, #scheduled follow-up period, †median. aIndicated when the administration period is limited. bIndicated only when the number needed to treat is calculated

Fig. 15.3
Summary of the randomized controlled trials of RAS blockers, antiplatelet agents, and fish oils in adult patients with IgAN. EPA eicosapentaenoic acid, DHA docosahexaenoic acid, ITT intention to treat, NS not significant, PP pet protocol, SI selectivity index. Mean ± SD, median (25 %, 75 %), mean or median [range]. -NA, *P < 0.05, §administration rate before intervention, # scheduled follow-up period, †median. aIndicated only when administration period is limited. bThe approved maximum daily dose (mg) of antihypertensive drug in Japan/JNC7 recommended dose in the United States (mg): amlodipine (10/10), enalapril (10/40), olmesartan (40/40), captopril (150/100), candesartan cilexetil (12/32), temocapril hydrochloride (4/−), trandolapril (2/4), valsartan (160/320), benazepril hydrochloride (10/40), verapamil (360/360), ramipril (−/10), and losartan potassium (100/100). cIndicated only when the number needed to treat is calculated

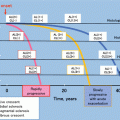
Fig. 15.4
Outline of the treatment of IgAN in adults, with a focus on the prevention of renal dysfunction. Others: tonsillectomy (combined with high-dose pulse corticosteroid therapy) and therapy with nonsteroidal immunosuppressive agents, antiplatelet agents, and n-3 fatty acids (fish oil). This figure shows the indications for treatment intervention, based mainly on the results (Figs. 15.2 and 15.3) of RCTs, often focusing on renal function and amount of urinary protein excreted as patient inclusion/exclusion criteria. In actual clinical practice, besides renal function and urinary protein level, other factors such as renal histopathological findings and age should also be considered to carefully decide the indications for these treatment interventions. Hypertension (CKD Guideline, Chap. 4), salt intake (Chaps. 3 and 4), lipid disorders (Chap. 14), glucose intolerance (Chap. 9), obesity (Chap. 15), smoking (Chap. 2), anemia (Chap. 7), CKD mineral and bone disorders (CKD-MBD, Chap. 8), and metabolic acidosis (Chap. 3) should also be managed as necessary
The Clinical Guidelines for IgA Nephropathy 2014 comprehensively covers the concept, diagnosis, pathology, epidemiology, and adverse effects of treatment, as well as the standard treatment of IgAN (Table 15.2), actively presenting the data obtained from Japanese patients in figures and tables. IgAN can be definitely diagnosed when kidney biopsy results show IgA deposition within the renal glomerular mesangium. However, glomerular IgA deposition can occur in normal kidneys. Thus, Japanese guidelines define IgAN as follows: IgAN is a disease characterized by urinary findings suggestive of glomerulonephritis; predominantly, IgA is deposited in the glomeruli, with no evidence of other underlying diseases. Glomerular hematuria and proteinuria are urinary findings that suggest glomerulonephritis.
Table 15.2
Table of contents of the Japanese Clinical Guidelines for IgA Nephropathy
|
I. Introduction
|
|
1. Definition and background
|
|
2. Pathogenesis and pathophysiology
|
|
1. Overview
|
|
2. Genetics
|
|
3. Abnormal IgA molecules
|
|
4. Mucosal immunity
|
|
5. IgA1 glomerular deposition
|
|
6. Glomerular damage
|
|
II. Diagnosis
|
|
1. Diagnosis
|
|
2. Clinical manifestations and laboratory findings
|
|
1. Clinical symptoms and physical examination findings
|
|
2. Urinalysis findings
|
|
3. Blood biochemistry findings
|
|
4. Indications for renal biopsy
|
|
5. Features of childhood IgA nephropathy
|
|
3. Pathological findings
|
|
4. Classification
|
|
5. Atypical forms of IgA nephropathy
|
|
1. Minimal change nephrotic disease (MCD) with mesangial IgA deposits
|
|
2. Acute kidney injury (AKI) associated with macroscopic hematuria
|
|
3. Crescentic IgA nephropathy
|
|
III. Epidemiology, prognosis, and follow-up
|
|
1. Incidence and prevalence
|
|
2. Natural course
|
|
3. Changes in prognosis with changes in treatment guidelines
|
|
4. Clinical predictors of progression at the time of initial examination or renal biopsy
|
|
5. Clinical predictors of progression during follow-up
|
|
6. Remission of urinary findings and its significance
|
|
7. Follow-up
|
|
IV. Treatment
|
|
1. A summary of the management of IgAN in adults, with a focus on prevention of renal dysfunction
|
|
2. Clinical questions (CQs) about immunosuppressive therapy (adults)
|
|
CQ1. Are corticosteroids recommended in IgA nephropathy?
|
|
CQ2. Is tonsillectomy combined with steroid pulse therapy recommended?
|
|
CQ3. Is tonsillectomy (alone) recommended?
|
|
CQ4. Are nonsteroidal immunosuppressive agents recommended?
|
|
3. CQs about immunosuppressive therapy (children)
|
|
CQ1. Is immunosuppressive therapy recommended in childhood IgA nephropathy?
|
|
CQ2. Is combination “cocktail” therapy recommended in childhood IgA nephropathy?
|
|
4. CQs about supportive therapy (adults)
|
|
CQ1. Are RAS blockers recommended in IgA nephropathy?
|
|
CQ2. Are antiplatelet agents recommended in IgA nephropathy?
|
|
CQ3. Are n-3 fatty acids (fish oil) recommended in IgA nephropathy?
|
|
5. CQs about lifestyle and dietary guidance in IgA nephropathy
|
|
CQ1. Should limitation of salt intake be recommended?
|
|
CQ2. Should restricted protein intake be recommended?
|
|
CQ3. Should weight loss be recommended?
|
|
CQ4. Should exercise restriction be recommended?
|
|
CQ5. Should smoking cessation be recommended?
|
|
6. Adverse events associated with steroid therapy and immunosuppressive agents
|
Pathological lesions which are commonly seen in IgAN are shown in the figure panels. Differences in pathological classification between the Japanese severity classification and the Oxford classification are described in detail. The Clinical Guidelines for IgA Nephropathy 2014 has a supplemental material section that contains structured abstracts of the clinical trials published to date.
15.3 Summary of the Management of IgA Nephropathy in Adults, with a Focus on the Prevention of Renal Dysfunction
In Japan, the major potential treatment modalities for adult IgAN are the use of renin-angiotensin system (RAS) blockers, corticosteroids, nonsteroidal immunosuppressive agents, antiplatelet agents, and n-3 fatty acids (fish oil) and tonsillectomy (with corticosteroid pulse therapy). Based on the results of several randomized controlled trials (RCTs), we evaluated the reduction of proteinuria and preservation of kidney function in response to the therapeutic interventions (Figs. 15.2 and 15.3). Because the entry criteria of the majority of the RCTs included GFR and urinary protein levels, the indications of these therapeutic interventions are mainly based on GFR and urinary protein level in this guideline. Age and renal histological lesions may potentially provide clinically useful information on indications of these therapeutic interventions. Interventions to optimize blood pressure, salt intake, lipid and glucose metabolism, body weight, and smoking habits should be considered, if necessary (Fig. 15.4).
15.3.1 Adult IgA Nephropathy Patients with CKD Stages G1–G2 and Urinary Protein Levels >1.0 g/day
The first-line therapy includes RAS blockers and/or corticosteroid therapy. The second-line therapy includes immunosuppressive agents, antiplatelet agents, tonsillectomy (plus high-dose pulse corticosteroids), fish oil, and others.
These patients are the most common candidates for RCTs and given RAS blockers (grade A) and corticosteroids (grade B) as first-line therapy. These patients are predicted to have poor prognosis of renal function. Therefore, active intervention with the first-line therapy should be considered. The second-line therapy should be considered as a combination therapy with the first-line therapy or if the first-line therapy is unavailable for some reason.
15.3.2 Adult IgA Nephropathy Patients with CKD Stage G3 and Urinary Protein Levels >1.0 g/day
The first-line therapy includes RAS blockers. The second-line therapy includes corticosteroid therapy, immunosuppressive agents, antiplatelet agents, tonsillectomy (plus high-dose pulse corticosteroids), fish oil, and others.
These patients are predicted to have extremely poor prognosis of renal function. Therefore, active intervention with RAS blockers (grade A) as first-line therapy should be considered. Because the efficacy of corticosteroids has not been examined in these patients in RCTs, corticosteroids are classified as second-line therapy. The second-line therapy can be considered as a combination therapy with the first-line therapy or if the first-line therapy is unavailable for some reason.
15.3.3 Adult IgA Nephropathy Patients with CKD Stages G1–G3 and Urinary Protein Levels Between 0.50 and 0.99 g/day
We suggest therapeutic interventions as shown in Fig. 15.4 to prevent proteinuria at urinary protein levels >1.0 g/day, which is closely associated with accelerated decline in kidney function. Furthermore, urinary protein levels between 0.5 and 0.99 g/day are reportedly a high-risk factor of decline in kidney function.
Currently, the necessity of intervention for patients with IgAN whose urinary protein levels are between 0.50 and 0.99 g/day is less certain because the clinical significance for such patients has not been established yet as a predictor of prognosis of renal function and only a few RCTs have been conducted with these patients. However, some studies reported urinary protein levels of 0.50–0.99 g/day as a relative prognostic factor of renal function, and progression to proteinuria at urinary protein levels ≥1.00 g/day, which is a definite poor prognostic factor of renal function, should be prevented. Therefore, interventions should be examined in consideration of benefits and risks.
15.3.4 Adult IgA Nephropathy Patients with CKD Stages G1–G2 and Urinary Protein Levels <0.5 g/day
We recommend annual examination of patients because urinary protein levels <0.5 g/day with preserved renal function carry a favorable impact on long-term outcome.
The prognosis of renal function in patients with IgAN can be favorable if the urinary protein level is <0.50 g/day and the CKD stage is G1–G3. However, careful follow-up is required because gradual increase in proteinuria and decrease in renal function have been observed in some patients. When any finding indicating poor prognosis of renal function other than proteinuria/renal function is found on kidney biopsy or other examinations, intervention should be examined in consideration of benefits and risks.
15.3.5 Adult IgA Nephropathy Patients with CKD Stages G3–G5 and Urinary Protein Levels <1.0 g/day
We recommend supportive therapy according to the evidence-based Clinical Practice Guidelines for CKD 2013 [4].
15.4 How the Japanese Guidelines Differ from the KDIGO Guidelines
Although both the Japanese Clinical Guidelines for IgA Nephropathy 2014 and Chap. 10 in the KDIGO Clinical Practice Guidelines for Glomerulonephritis evaluated indications for intervention for IgAN based mainly on the results of RCTs, they have some differences. One of the major differences is the usage of different criteria to determine the strength of intervention recommendations. The KDIGO guidelines do not indicate an intervention if its efficacy has not been demonstrated in RCTs, whereas the Japanese guidelines indicate that such an intervention be used as second-line therapy if non-RCTs or cohort studies suggested the efficacy of the intervention.
Another major difference is the strength of the recommendations for RAS blockers and corticosteroids. The Japanese guidelines position both RAS blockers and corticosteroids as first-line therapy for IgAN patients with CKD stages G1–G2 and urinary protein levels >1.0 g/day. By contrast, the KDIGO guidelines indicate corticosteroids for patients with persistent proteinuria at urinary protein levels ≥1 g/day despite treatment with RAS blockers, because of a concern about adverse effects due to corticosteroid administration.
Tonsillectomy is not recommended for patients with IgAN, unless for tonsil-related diseases, in the KDIGO guidelines. The Japanese guidelines state that tonsillectomy combined with steroid pulse therapy may improve urinary findings in patients with IgAN and lower the progression of renal dysfunction. This may also be considered a treatment option, as the RCT conducted by the Progressive Renal Dysfunction Research Group of the MHLW reported that tonsillectomy combined with steroid pulse therapy was found to be more effective than steroid pulse therapy alone in reducing urinary protein [5]. To establish more amount of substantial evidence, the superiority of tonsillectomy combined with steroid pulse therapy should be further investigated. No RCT of tonsillectomy alone has been conducted. Previous studies may have shown the long-term efficacy of tonsillectomy in cases of IgAN. Therefore, according to the current clinical practices in Japan, tonsillectomy alone may also be considered as a treatment option.
15.5 Summary of the Guidelines
We summarize the recommendation grades of the Japanese guidelines in Table 15.3. The Clinical Guidelines for IgA Nephropathy 2014 was developed in accordance with the Japanese clinical practice for the treatment of IgAN. Publishing a comprehensive practice guideline suitable for Japanese clinical practice may provide an optimum treatment and encourage new clinical trials based on clinical questions.
Table 15.3
Summary of recommendation grades in the treatment of IgAN (adults)
|
Immunosuppressive therapy
|
|
Corticosteroids
|
|
[Recommendation grade B] To control the progression of renal dysfunction in patients with IgAN with urinary protein levels ≥1 g/day and CKD stages G1–2, a short course of high-dose oral steroid therapy (prednisolone at dose of 0.8–1.0 mg/kg for about 2 months, followed by gradual tapering over about 6 months) is recommended
|
|
[Recommendation grade B] To control the progression of renal dysfunction in patients with IgAN with urinary protein levels ≥1 g/day and CKD stages G1–2, steroid pulse therapy (methylprednisolone 1 g for 3 days by infusion [or intravenously] every other month, 3 times + prednisolone 0.5 mg/kg every other day for 6 months) is recommended
|
|
[Recommendation grade C1] Steroid therapy may reduce proteinuria in patients with IgAN with urinary protein levels of 0.5–1.0 g/day and CKD stages G1–2, and this may also be considered a treatment option
|
|
Tonsillectomy combined with steroid pulse therapy
|
|
[Recommendation grade C1] Tonsillectomy combined with steroid pulse therapy may improve urinary findings in patients with IgAN and inhibit renal dysfunction progression. This may also be considered a treatment option
|
|
Tonsillectomy (alone)
|
|
[Recommendation grade C1] Tonsillectomy may improve urinary findings in patients with IgAN and inhibit the renal dysfunction progression. This may also be considered as a treatment option
|
|
Nonsteroidal immunosuppressive agents
|
|
[Recommendation grade C1] Cyclophosphamide, azathioprine, cyclosporine, mycophenolate mofetil, and mizoribine may improve the renal prognosis in IgAN patients. They may also be considered treatment options (off-label use)
|
|
Supportive therapy
|
|
RAS blockers*
|
|
[Recommendation grade A] RAS blockers control the progression of renal dysfunction in IgAN patients with urinary protein levels ≥1.0 g/day and CKD stages G1–3b; therefore, their use is recommended
|
|
[Recommendation grade C1] RAS blockers may reduce proteinuria in IgAN patients with urinary protein levels of 0.5–1.0 g/day. They may be considered treatment options
|
|
*RAS blockers for IgAN patients without hypertension have off-label use in Japan
|
|
Antiplatelet agents
|
|
[Recommendation grade C1] Dipyridamole may be effective in reducing proteinuria and controlling the progression of renal dysfunction. This may be considered as a treatment option
|
|
[Recommendation grade C1] Dilazep hydrochloride (dilazep) may be effective in reducing proteinuria, and it may be considered as a treatment option
|
|
n-3 fatty acids (fish oil)
|
|
[Recommendation grade C1] n-3 fatty acids (fish oil) may improve renal prognosis in IgAN patients. They may be considered as a treatment option
|
Acknowledgment
This study was supported in part by a Grant-in-Aid for Progressive Renal Diseases Research and Research on Rare and Intractable Disease, from the Ministry of Health, Labour and Welfare of Japan. We appreciate all of the members in the working group for developing guidelines for their great effort and help.
Conflict of Interest The authors declare that they have no conflict of interest.
References
1.
Japanese Society of Nephrology. Clinical guides for immunoglobulin A (IgA) nephropathy in Japan, third version. Nihon Jinzo Gakkai shi. 2011;53(2):123–35.
2.
KDIGO Clinical Practice Guideline for Glomerulonephritis. Chapter 10: immunoglobulin A nephropathy. Kidney Int Suppl. 2012;2(2):209–17.
3.
Japanese Society of Nephrology. JSN and MHWL clinical practice guidelines for IgA nephropathy 2014. Nihon Jinzo Gakkai shi. 2015;57(1):5–137.
4.
Japanese Society of Nephrology. Special issue: evidence-based practice guideline for the treatment of CKD. Nihon Jinzo Gakkai shi. 2013;55(5):585–860.
5.
Kawamura T, Yoshimura M, Miyazaki Y, Okamoto H, Kimura K, Hirano K, et al. A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin A nephropathy. Nephrol Dial Transplant. 2014;29(8):1546–53.CrossRefPubMedPubMedCentral