Chapter 6 Isomers
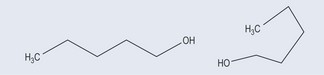
If two compounds have the same molecular formulae (in other words, the same number and types of atoms) but the atoms are arranged differently, they are classed as isomers. Care has to be taken with this definition, however, as the two examples in Figure 6.1 are not isomers but the same molecule bent into a different shape because the mobility that the single bond allows it. In this example, the arrangement of atoms differs by their arrangement in space only.
Bear in mind that a single bond enables a molecule to rotate freely around it, so the apparent change in the shape in the molecule does not make it an isomer (compare this with the limited rotation around a double bond, see Figure 6.5).
There are several different types of isomer:
Structural Isomers
Chain isomerism
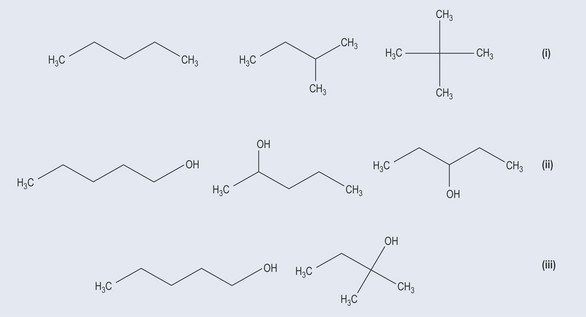
Chain isomers (Figure 6.2(i)) occur because functional groups can branch off from the main carbon backbone. The number of carbon and hydrogen atoms remains the same but the structures are very different.
Stereoisomers
Entaniomers
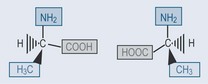
Enantiomers are stereoisomers that are mirror images of each other and all thecomponents of the compound radiating from one point are different. The term forthis is chiral. The position of the compound around which this occurs is a chiral centre (see Figures 6.3, and Figures 6.7). Take, for example, natural alanine, which is foundin only one isomeric form: L -alanine ( Figures 6.3). If alanine is produced synthetically, then both forms L and D alanine, are found. This is signifi cant when it comes togiving a patient nutritional supplementation, as the L isomer is more likely to fit intothe receptor site of an enzyme, which explains why natural products are favoured innutritional supplementation.
• What exactly do D and L mean?
Light normally vibrates in all directions, but if put through a polarizer it will vibrate in only one direction (Figure 6.4(i)). If another polarizer is placed at exactly 90°, no light will get through. You can test this yourself with two pairs of good-quality sunglasses. Line them up, and then turn one through 90°. The lens will appear black because no light is getting through (Figure 6.4 (ii)).
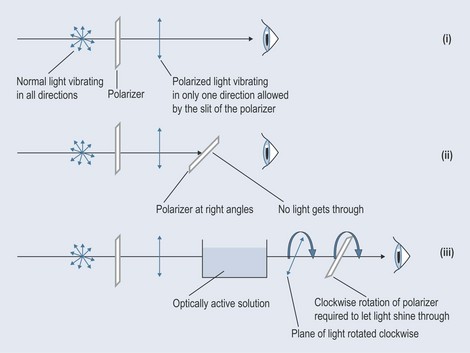
Figure 6.4 The principles of the polarization of light and how optically active solutions can affect it.
If a solution of an optically active isomer is placed in the path of monochrome (one wavelength only) polarized light, then the light will change orientation. To make it visible again, the next polarizer will have to be twisted either clockwise (dextro-rotation) or anticlockwise (laevo-rotation) to see light again (Figure 6.4 (iii)). This is where the notation dextrorotatory (D) and laevorotatory (L) comes from. The notation now being phased in is:
Disastereoisomers
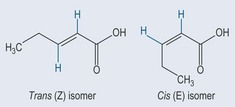
• E-Z (cis–trans isomerism, geometrical isomerism)
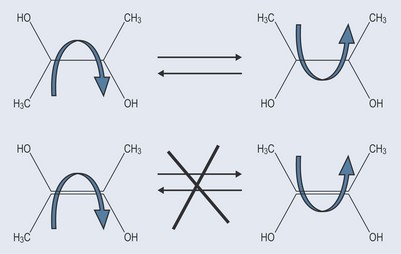
An example of E-Z isomerism is shown in Figure 6.5 in the case of a compound with a double bond (which has already been shown to prevent rotation; see Figure 4.2, p. 22). The only way to make these compounds identical is to break the bond and rotate it, before rejoining the bond again.
This orientation around a bond can have metabolic significance with regards to the structure of a compound and a patient’s health (see Chapter 10, ‘Lipids’, p. 76) because, as diastereoisomers, compounds possess different chemical properties.
In the literature, you will see these types of isomer referred to in two different ways (Figure 6.6). The older terminology of cis and trans is slowly being replaced by E (for cis) and Z (for trans). However, as cis and trans are well known, certainly in the area of fatty acids, as far as the general public is concerned it is useful to be conversant with both.
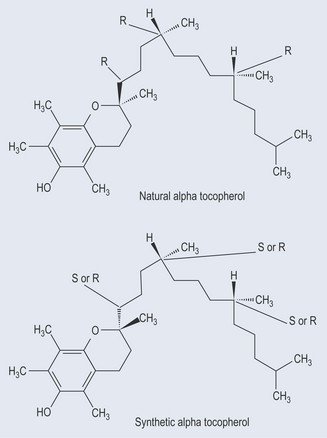
What Happens When a Compound has More Than One Chiral Centre?
Chemical compounds can have more than one chiral centre. Alpha tocopherol (Figure 6.7) is a good example of this. In this case, the nomenclature S or R is used to denote whether the methyl group is to the left (S) or right (L), respectively, counting from the aromatic end. The other notations of (+) or (−) can also be used for the various types of tocopherol (see Chapter 13 ‘Vitamins and minerals’, p. 107). All natural tocopherol is RRR. Synthetic tocopherol can have SRR, SSR, SRS, SSS, RSR, RRS, RSS and RRR forms, which is why nutritionists stress the use of natural tocopherol.