Chapter 7 Is Neuromonitoring Beneficial During Spinal Surgery?
Neurologic injury may follow even technically precise spinal surgery. Because neurologic complications after spinal surgery are potentially devastating, the development of preventative strategies, including intraoperative neurophysiologic monitoring, is of significant clinical relevance and great importance for enhancing patient safety.1 Intraoperative neurophysiologic monitoring is a method used to physiologically monitor the integrity of neural structures and to avoid surgical insults by enabling real-time response and action by the surgical team.
The ideal intraoperative monitoring modality should be highly sensitive and specific to spinal cord or nerve root injury and should also be “user friendly.” Currently, no such modality exists that fulfils all of these criteria. Somatosensory-evoked potential (SSEP) monitoring remains the standard test for intraoperative monitoring despite its suboptimal sensitivity to detect all types of neural injury.2 Despite the advances that have occurred in intraoperative spinal monitoring since the late 1990s, universal acceptance as to the benefit of intraoperative monitoring has not yet occurred.
MONITORING OPTIONS
Numerous neurophysiologic monitoring methods are now available including continuous free running electromyography (EMG), evoked EMG, compound muscle action potentials, rectal and urinary sphincter EMG, motor-evoked potentials (MEPs), SSEPs, and most recently, spinal cord mapping.1,3–5 None of these tests individually provides a global assessment of cord and root function. However, when multiple modalities are monitored, each one adds selective information that allows the surgical team to assess neural function with enhanced precision. Each of these electrophysiologic approaches has advantages and disadvantages. Hence, the decision regarding the optimal choice of approaches to monitor needs to be individually tailored for each surgery depending on what level of the spine is undergoing surgery and what aspect of neural function is most at risk.
Somatosensory-Evoked Potentials
Early attempts at monitoring relied solely on recording SSEPs. Reports of false-negative outcomes when using only SSEP monitoring illustrated the need for multimodality monitoring.6–8 SSEPs remain the standard technique for intraoperative monitoring. Newer techniques such as EMG and MEP have been developed and are used as an adjunct to SSEP monitoring.
Electromyography
Spontaneous electromyographic recordings provide real-time data that are sensitive to surgical manipulation or compression.2 Myotomes are selected for recording based on the operative level and the nerve roots most at risk. Burst or train activity is considered significant and is thought to represent ongoing compression or stretch. Spontaneous EMG is sensitive but not specific.9 As well as recording spontaneous electromyographic activity, a number of other applications have used electromyographic recordings to determine the proximity of nerve roots. Direct nerve root testing with EMG recording aids in the dissection of nerve roots off intradural tumors especially in the conus region. Triggered EMG has been used to aid in placement of pedicle screws.
EVIDENCE FOR MONITORING
Although the evidence for monitoring continues to evolve, there has been a vast increase in the body of evidence in the published literature that supports many aspects of current monitoring practices. Several authors have reported the results of good-quality studies that demonstrate a benefit when intraoperative monitoring is performed during spinal surgery. Relevant articles from the published literature were assigned a level of evidence as per the Journal of Bone and Joint Surgery (JBJS) guidelines.10 For the purposes of this review, only articles of Level II evidence were available and are discussed.
Evidence for Somatosensory-Evoked Potentials
Most of the earlier literature predominantly evaluated the role of SSEPs alone in spinal surgery. As far back as 1982, Grundy and colleagues11 reported a study showing that a wake-up test was not necessary provided SSEPs were monitored and stable. The authors prospectively studied the effects of moderate hypotension on 24 patients undergoing spinal fusion with Harrington rod instrumentation. Five of the 24 patients had alterations in their SSEPs and required an intraoperative wake-up test, all of which had normal results.
Epstein and coworkers12 evaluated the role of SSEPs in cervical surgery by comparing the outcomes of patients who were monitored and operated on over a 3-year period (1989–1991) with those who were not monitored and had been operated on between 1985 and 1989. No instances of quadriplegia in the 100 patients who were monitored versus eight in the 218 who were not monitored were reported. The authors conclude that the reduction of neurologic deficit was attributed in part to early SSEP detection of vascular or mechanical compromise and to the immediate alteration of anesthetic or surgical technique in response to SSEP changes. Kombos and coworkers13 report similar findings in a prospective evaluation of the impact of SSEP monitoring during anterior cervical surgery. In a prospective study of 100 patients, they deduce that SSEP monitoring was easy to perform and helped to increase the safety during anterior cervical surgery. Monitoring of both cortical and subcortical sites for SSEP responses has been shown to increase the reliability of SSEPs during spinal surgery.14
Evidence for Motor-Evoked Potentials
Concerns over false-negative results when using SSEPs alone have led to the proposal of alternative strategies, with either monitoring of MEPs alone or used in combination with other modalities.6 Hilibrand and investigators15 analyzed the data of 427 patients who underwent anterior or posterior cervical spine surgery over a 2-year period. Twelve of their patients had loss of amplitude of MEPs, of which 10 had complete reversal of the loss after prompt intraoperative intervention and the remaining 2 had a new postoperative deficit. The sensitivity and specificity for MEPs was 100% in their series, whereas SSEP had only a sensitivity of 25%, although it was 100% specific. The authors conclude that transcranial MEPs appeared to be superior to conventional SSEPs for identifying evolving motor tract injury during cervical spine surgery.
Difficulties in obtaining and maintaining MEP responses with transcranial stimulation has led some authors to propose direct spinal cord stimulation to obtain neurogenic MEPs in certain pathologies.16 Komanetsky and colleagues17 compared two methods of stimulation when obtaining neurogenic MEPs. They prospectively compared spinous process stimulation with percutaneous stimulation in obtaining neurogenic MEPs in 184 patients. Both methods were found to be sensitive to neurologic deficit. When responses were obtained, the percutaneous method was found to be sufficiently reliable to obviate the need for the spinous process method.
Numerous studies have addressed the difficulties in obtaining reliable predictors for postoperative C5 palsy.18 Tanaka and colleagues18 evaluated the usefulness of transcranial MEPs for prediction of the occurrence of postoperative C5 palsy after cervical laminoplasty. They prospectively evaluated 62 consecutive patients, three of which developed postoperative transient C5 palsy. No critical decrease in amplitude occurred in any of the 62 patients. Because of this, the authors conclude that postoperative C5 palsy after cervical laminoplasty was not associated with an intraoperative injury (Table 7-1).
Evidence for Electromyography
There are fewer Level I or II evidence articles in the literature that validate the use of intraoperative EMG monitoring in spinal surgery. Dimopoulos and investigators19 conducted a prospective randomized trial to correlate the findings of intraoperative EMG with immediate postoperative pain in patients undergoing lumbar microdiscectomy (Level II). They found no correlation between intraoperative electromyographic findings and postoperative pain.
Krassioukov and coauthors20 examined the neurologic outcomes of 61 patients, most of whom were treated for spinal/spinal cord tumours (61%) or adult tethered cord syndrome (25%). Patients underwent multimodal neurophysiologic monitoring with EMG monitoring of the lower-limb muscles, external anal sphincter (EAS), external urethral sphincter (EUS), and lower-limb SSEPs. Spontaneous electromyographic activity was observed in the lower-extremity muscles and/or EAS and EUS in 51 cases (84%). In addition to spontaneously recorded electromyographic activity, electrically evoked EMG activity was also used as an intraoperative adjunct. The presence of electrically evoked EMG activity in structures encountered duringmicrodissection altered the plan of treatment in 24 cases (42%).
Similar findings were reported by Paradiso and colleagues,1 who examined the use of intraoperative monitoring in tethered cord syndrome. Posterior tibial nerve SSEPs were found to have high specificity, but low sensitivity, for predicting new neurologic deficits. In contrast, continuous EMG showed high sensitivity and low specificity. Evoked EMG accurately identified functional neural tissue.
Multimodality Monitoring
To overcome the limitations of individual monitoring modalities, many teams have explored the value of multimodal monitoring to obtain a more robust real-time assessment of intraoperative neural function. Pelosi and researchers21 investigated the combined monitoring of MEPs and SSEPs in 126 spinal operations. Combined monitoring was successfully achieved in 104 operations; it was possible only to monitor a single modality in 18 patients (16 SSEPs, 2 MEPs). No response to either modality could be recorded in two patients. The authors report that combined monitoring was superior to single-modality techniques both for increasing the number of patients in whom satisfactory monitoring could be achieved and for improving the sensitivity and predictivity of monitoring.
Gunnarsson and coauthors9 correlate the clinical and electrophysiologic findings in a prospective, consecutive series of 213 patients. Intraoperative electromyographic activation had a sensitivity of 100% and a specificity of 23.7% for the detection of a new postoperative neurologic deficit. SSEPs had a sensitivity of 28.6% and specificity of 94.7%. The authors conclude that combined intraoperative neurophysiologic monitoring with EMG and SSEP is helpful for predicting and possibly preventing neurologic injury during thoracolumbar spine surgery.
Costa and colleagues’22 prospective observational study reports on the use of combined multimodal monitoring in a cohort of patients undergoing a variety of spinal procedures. Combined SSEP and MEP monitoring was successfully obtained in 38 of 52 patients (73%), whereas MEPs from at least 1 target muscle were obtained in 12 patients (23%); both SSEPs and MEPs were absent in 2 patients (3.8%). Significant intraoperative changes occurred in one or both modalities in five patients, two of which were transient, whereas three had persistent changes associated with new deficits or worsening of the preexisting neurologic disability. The authors suggested that intraoperative combined monitoring is a safe, reliable, and sensitive method to detect and reduce neurologic injury to the spinal cord. However, there was no comparative group that did not use neuromonitoring.
After comparing transcranial MEPs and SSEP monitoring in a large cohort of patients who underwent cervical spine surgery, Hilibrand and investigators15 highlight the fact that, although SSEPs were specific, they remain relatively insensitive. In addition, they highlight the need for multimodal monitoring in cervical spine surgery.
Case Example
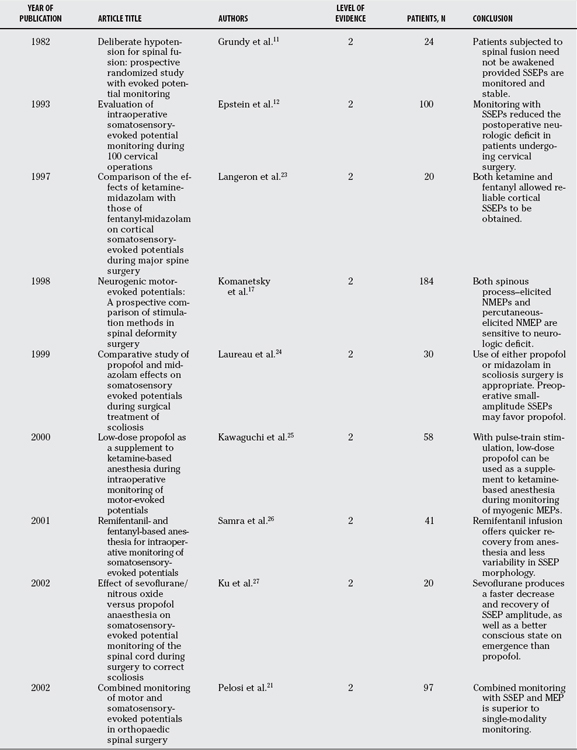
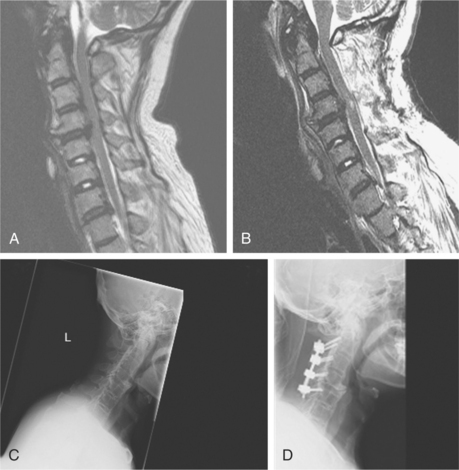
A 64-year-old man with known ankylosing spondylitis presented with a 6-month history of progressive gait difficulties and impairment of upper-limb fine motor function. His clinical examination confirmed that he had cervical myelopathy. Preoperative imaging (Fig. 7-1 A and C) showed that he had marked cord compression on the T2-weighted magnetic resonance imaging. He was electively booked for surgery where he underwent a multilevel cervical laminectomy and instrumented fusion. During the decompression stage of the operation, the surgeon was alerted that the patient had developed spontaneous electromyographic train activity in an upper-limb muscle (see Fig. 7-2A). Shortly after this, the patient’s SSEP responses in all four limbs deteriorated (see Fig. 7-2B). A diagnosis of a presumptive cord injury was made and treatment was instituted with blood pressure elevation and administration of steroids. The patient’s SSEP traces in his upper limbs began to recover toward the end of the case. Postoperative imaging showed new high signal changes in the spinal cord (Fig. 7-1B and D). Immediately after surgery, the patient was quadriparetic, legs worse than arms, but his deficit had improved substantially by the time of discharge. The immediate diagnosis and institution of treatment potentially prevented a complete injury and a suboptimal clinical outcome for this patient.
Anesthetic Regimen for Monitoring
Some of the best evidence available in the intraoperative monitoring literature pertains to the optimal anesthetic regimen to use. In 1997, a randomized trial of 20 patients was undertaken to compare the effects of ketamine with those of fentanyl (both combined with midazolam) on cortical SSEP monitoring during major spinal surgery.23 Cortical SSEP latencies were not significantly affected in either group. The authors conclude that both ketamine and fentanyl allowed the recording of reliable cortical SSEPs, but a longer delay for voluntary postoperative motor assessment was observed in the ketamine group (Level II).
Laureau and colleagues24 report a prospective, randomized trial comparing the effects of propofol and midazolam in 2 groups of 15 patients undergoing surgery for idiopathic scoliosis. The amplitude of the cortical SSEP responses decreased after induction in both groups; in the midazolam-treated group, the amplitudes were smaller. The most significant finding in the study was that both propofol and midazolam seemed to be acceptable hypnotic agents for total intravenous anesthesia during intraoperative monitoring in the surgical treatment of scoliosis. This study has limitations in that it is difficult to correlate postoperative neurologic deficits and intraoperative monitoring data because no SSEP changes occurred and also because cortical SSEPs were recorded only unilaterally (Level II).
The effects of low-dose propofol as a supplement to ketamine-based anesthesia during intraoperative mo-nitoring of MEPs has been reported by Kawaguchi and coauthors.25 Intraoperative monitoring of MEPs was performed in 58 patients who underwent elective spinal surgery. Anesthesia was maintained with nitrous oxide-fentanyl-ketamine with or without low-dose propofol. The authors found that low-dose propofol could be effectively used as a supplement to ketamine-based anesthesia during intraoperative monitoring of myogenic MEPs as long as a train of pulses was used for transcranial stimulation. Addition of propofol significantly reduced the ketamine-induced psychedelic effects.
In 2001, Samra and colleagues’26 study was published comparing remifentanil- and fentanyl-based anesthesia for intraoperative monitoring of SSEPs with special attention to the speed of recovery from the anesthetic. They studied 41 patients who were randomized into 2 groups to receive either remifentanil- or fentanyl-based anesthesia. The authors report that both remifentanil and fentanyl infusion allowed satisfactory monitoring of SSEPs. Remifentanil infusion offers the advantage of quicker recovery from anesthesia and less variability of SSEP morphology.
Ku and coworkers27 have reported the effect of sevoflurane/nitrous oxide versus propofol anesthesia on SSEP monitoring during scoliosis surgery. They randomized 20 patients into 2 groups to receive either sevoflurane or propofol. Changes in anesthetic concentrations produced little effect on the latency of SSEP, but the effect on the variability of amplitude was significant. The authors conclude that sevoflurane produced a faster decrease and recovery of SSEP amplitude, as well as a better conscious state on waking, than did propofol (Level II).
A number of studies have compared the effects of propofol versus isoflurane on monitoring SSEPs and MEPs intraoperatively.28–30 Clapcich and investigators29 randomized 12 patients into 4 groups receiving either variable doses of isoflurane-nitrous oxide combinations or a propofol combination. Chen28 randomized a group of 35 patients into 2 groups to receive either an isoflurane or a propofol infusion. Liu and coauthors30 reported a randomized trial of 60 patients who were randomized to receive similar types of infusions. All of the groups reported that both propofol and isoflurane decreased SSEP amplitude while the anesthetic took effect. Propofol caused less suppression of the cortical SSEP responses with better preservation of SSEP amplitude. Chen28 reports that MEPs were recordable in all patients receiving propofol, but in only 50% of patients receiving isoflurane28 (Level II).
In 2006, Lo and coauthors’31 study was published comparing a desflurane-nitrous oxide combination with propofol total intravenous anesthetic regimen. They prospectively randomized 20 patients undergoing scoliosis correction surgery into 2 groups. Reproducible MEP responses were always obtained throughout the procedure. The authors conclude that both desflurane and total intravenous anesthetic with propofol allow successful monitoring. They suggest using abductor hallucis muscle for recording MEPs when using desflurane (Level II).
AREAS OF UNCERTAINTY
Which Spinal Patients Should Be Monitored?
Currently, no universal agreement exists as to which patients should undergo intraoperative monitoring during spinal surgery. The published literature would indicate that patients with a preexisting cord deficit have a greater risk for having a major neurophysiologic alert and are at greater risk for an intraoperative injury.2,32, 33 Given that these patients appear to be at greater risk, it would seem prudent to monitor them.
MEPs are reliable in predicting clinical motor outcome, and their use has altered the surgical approach; for example, gross total resections of intramedullary tumors are more readily attempted as long as MEP data indicate the intact functional integrity of the corticospinal tract.34
What Modalities Are Optimal to Monitor?
Which set of tests is optimal to monitor needs to be individually tailored for each surgery. The choice of what modalities to use is dependent on the preference of the surgeon, the nature and localization of the pathology involved, and the technical feasibility of the modality in the specific context. A good way to select which tests to use is to choose a combination of modalities most specific to the neural tissue at risk during the procedure—MEPs for anterior cord, SSEPs for posterior cord, and EMGs for roots at risk, respectively.
All current electrophysiologic approaches have limitations. Currently, no all-encompassing test exists that can adequately monitor all facets of neural function during spinal surgery. The approach in our unit has been to combine highly specific, though relatively insensitive, electrophysiologic modalities (e.g., SSEPs with highly sensitive but less specific techniques) (e.g., free-running spontaneous EMG activity).1,9, 20 Monitoring of MEPs is reserved for high-risk cases—for example, for patients with a preexisting cord deficit or those undergoing surgery for intramedullary lesions.
Based on our expert opinion, attention to such quantitative intraoperative monitoring data may help to minimize postoperative motor deficits by avoiding or correcting excessive spinal cord manipulation, intraoperative hypotension to the spinal cord, and modifying surgical technique during tumor resection (Level V). We also advocate the use of free-running and evoked EMGs when operating in the cervical or lumbar areas. Table 7-2 provides recommendations for intraoperative spinal monitoring (graded as per JBJS guidelines35: grade B = fair evidence, Level II or III studies with consistent findings).
TABLE 7-2 Recommendations for Intraoperative Spinal Monitoring
| STATEMENT | LEVEL OF EVIDENCE/GRADE OF RECOMMENDATION |
|---|---|
Graded as per Journal of Bone and Joint Surgery (JBJS) guidelines35: grade B = fair evidence, Level II or III studies with consistent findings.
EMG = electromyography; MEP = motor-evoked potential; SSEP = somatosensory-evoked potential.
1 Paradiso G, Lee GY, Sarjeant R, et al. Multimodality intraoperative neurophysiologic monitoring findings during surgery for adult tethered cord syndrome: Analysis of a series of 44 patients with long-term follow-up. Spine. 2006;31:2095-2102.
2 Padberg AM, Thuet ED. Intraoperative electrophysiologic monitoring: Considerations for complex spinal surgery. Neurosurg Clin N Am. 2006;17:205-226.
3 Kothbauer KF, Novak K. Intraoperative monitoring for tethered cord surgery: An update. Neurosurg Focus. 2004;16:E8.
4 Quinones-Hinojosa A, Gulati M, Lyon R, et al. Spinal cord mapping as an adjunct for resection of intramedullary tumors: Surgical technique with case illustrations. Neurosurgery. 2002;51:1199-1206.
5 Shi YB, Binette M, Martin WH, et al. Electrical stimulation for intraoperative evaluation of thoracic pedicle screw placement. Spine. 2003;28:595-601.
6 Wiedemayer H, Sandalcioglu IE, Armbruster W, et al. False negative findings in intraoperative SEP monitoring: Analysis of 658 consecutive neurosurgical cases and review of published reports. J Neurol Neurosurg Psychiatry. 2004;75:280-286.
7 Ben-David B, Haller G, Taylor P. Anterior spinal fusion complicated by paraplegia. A case report of a false-negative somatosensory-evoked potential. Spine. 1987;12:536-539.
8 Lesser RP, Raudzens P, Luders H, et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol. 1986;19:22-25.
9 Gunnarsson T, Krassioukov AV, Sarjeant R, et al. Real-time continuous intraoperative electromyographic and somatosensory evoked potential recordings in spinal surgery: Correlation of clinical and electrophysiologic findings in a prospective, consecutive series of 213 cases. Spine. 2004;29:677-684.
10 Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85:1-3.
11 Grundy BL, Nash CLJr, Brown RH. Deliberate hypotension for spinal fusion: Prospective randomized study with evoked potential monitoring. Can Anaesth Soc J. 1982;29:452-462.
12 Epstein NE, Danto J, Nardi D. Evaluation of intraoperative somatosensory-evoked potential monitoring during 100 cervical operations. Spine. 1993;18:737-747.
13 Kombos T, Suess O, Da SC, et al. Impact of somatosensory evoked potential monitoring on cervical surgery. J Clin Neurophysiol. 2003;20:122-128.
14 He JM, Tang Y. Combined application of two somatosensory evoked potential techniques at various recording points for monitoring the onset of stretch spinal injury during rhachial orthomorphia. Zhongguo Linchuang Kangfu. 2005;9:164-165.
15 Hilibrand AS, Schwartz DM, Sethuraman V, et al. Comparison of transcranial electric motor and somatosensory evoked potential monitoring during cervical spine surgery. J Bone Joint Surg Am. 2004;86:1248-1253.
16 Accadbled F, Henry P, de Gauzy JS, et al. Spinal cord monitoring in scoliosis surgery using an epidural electrode. Results of a prospective, consecutive series of 191 cases. Spine. 2006;31:2614-2623.
17 Komanetsky RM, Padberg AM, Lenke LG, et al. Neurogenic motor evoked potentials: A prospective comparison of stimulation methods in spinal deformity surgery. J Spinal Disord. 1998;11:21-28.
18 Tanaka N, Nakanishi K, Fujiwara Y, et al. Postoperative segmental C5 palsy after cervical laminoplasty may occur without intraoperative nerve injury: A prospective study with transcranial electric motor-evoked potentials. Spine. 2006;31:3013-3017.
19 Dimopoulos VG, Feltes CH, Fountas KN, et al. Does intraoperative electromyographic monitoring in lumbar microdiscectomy correlate with postoperative pain? South Med J. 2004;97:724-728.
20 Krassioukov AV, Sarjeant R, Arkia H, et al. Multimodality intraoperative monitoring during complex lumbosacral procedures: Indications, techniques, and long-term follow-up review of 61 consecutive cases. J Neurosurg Spine. 2004;1:243-253.
21 Pelosi L, Lamb J, Grevitt M, et al. Combined monitoring of motor and somatosensory evoked potentials in orthopaedic spinal surgery. Clin Neurophysiol. 2002;113:1082-1091.
22 Costa P, Bruno A, Bonzanino M, et al. Somatosensory- and motor-evoked potential monitoring during spine and spinal cord surgery. Spinal Cord. 2007;45:86-91.
23 Langeron O, Lille F, Zerhouni O, et al. Comparison of the effects of ketamine-midazolam with those of fentanyl-midazolam on cortical somatosensory evoked potentials during major spine surgery. Br J Anaesth. 1997;78:701-706.
24 Laureau E, Marciniak B, Hebrard A, et al. Comparative study of propofol and midazolam effects on somatosensory evoked potentials during surgical treatment of scoliosis. Neurosurgery. 1999;45:69-74.
25 Kawaguchi M, Sakamoto T, Inoue S, et al. Low dose propofol as a supplement to ketamine-based anesthesia during intraoperative monitoring of motor-evoked potentials. Spine. 2000;25:974-979.
26 Samra SK, Dy EA, Welch KB, et al. Remifentanil- and fentanyl-based anesthesia for intraoperative monitoring of somatosensory evoked potentials. Anesth Analg. 2001;92:1510-1515.
27 Ku AS, Hu Y, Irwin MG, et al. Effect of sevoflurane/nitrous oxide versus propofol anaesthesia on somatosensory evoked potential monitoring of the spinal cord during surgery to correct scoliosis. Br J Anaesth. 2002;88:502-507.
28 Chen Z. The effects of isoflurane and propofol on intraoperative neurophysiological monitoring during spinal surgery. J Clin Monit Comput. 2004;18:303-308.
29 Clapcich AJ, Emerson RG, Roye DPJr, et al. The effects of propofol, small-dose isoflurane, and nitrous oxide on cortical somatosensory evoked potential and bispectral index monitoring in adolescents undergoing spinal fusion. Anesth Analg. 2004;99:1334-1340.
30 Liu EH, Wong HK, Chia CP, et al. Effects of isoflurane and propofol on cortical somatosensory evoked potentials during comparable depth of anaesthesia as guided by bispectral index. Br J Anaesth. 2005;94:193-197.
31 Lo YL, Dan YF, Tan YE, et al. Intraoperative motor-evoked potential monitoring in scoliosis surgery: Comparison of desflurane/nitrous oxide with propofol total intravenous anesthetic regimens. J Neurosurg Anesthesiol. 2006;18:211-214.
32 Thuet ED, Padberg AM, Raynor BL, et al. Increased risk of postoperative neurologic deficit for spinal surgery patients with unobtainable intraoperative evoked potential data. Spine. 2005;30:2094-2103.
33 Lee JY, Hilibrand AS, Lim MR, et al. Characterization of neurophysiologic alerts during anterior cervical spine surgery. Spine. 2006;31:1916-1922.
34 Sala F, Palandri G, Basso E, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: A historical control study. Neurosurgery. 2006;58:1129-1143.
35 Wright JG, Einhorn TA, Heckman JD. Grades of recommendation. J Bone Joint Surg Am. 2005;87:1909-1910.