Chapter 3 Irritable bowel syndrome
constipation-predominant (C-IBS)
AETIOLOGY
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterised by altered bowel habit and abdominal pain.1 It is a chronic disorder of unclear aetiology.2 Recent research, however, has brought to light a number of possible aetiological factors:
RISK FACTORS
CONVENTIONAL TREATMENT
Many agents are currently used for the treatment of IBS and no one agent has proven particularly effective and/or free from side effects.25–27 Key classes of medications used to treat C-IBS include antispasmodics, antidepressants, laxatives and 5-hydroxytryptamine type 4 receptor agonists (such as tegaserod). Therapeutic gains (the difference in treatment response between placebo and active therapy) have often been minimal with these agents28 and adverse events severe.29
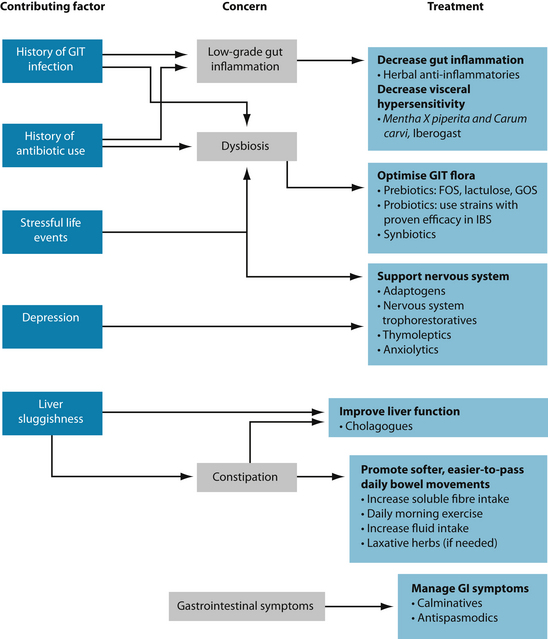
KEY TREATMENT PROTOCOLS
GIT microflora: overview
The microflora of the GIT represents an ecosystem of the highest complexity.30 The microflora is believed to be composed of over 50 genera of bacteria,31 accounting for over 500 different species.32 The adult human GIT is estimated to contain 1014 viable microorganisms, which is 10 times the number of eukaryotic cells found within the entire human body.33 Some researchers have called this microbial population the ‘microbe’ organ – an organ that is similar in size to the liver (1–1.5 kg in weight).34 Indeed, this ‘microbe’ organ is now recognised as rivalling the liver in the number of biochemical transformations and reactions in which it participates.35
The microflora plays many critical roles in the body; thus, there are many areas of host health that can be compromised when the microflora is altered. The GIT microflora is involved in the stimulation of the immune system, synthesis of vitamins (B group and K), enhancement of GIT motility and function, digestion and nutrient absorption, inhibition of pathogens (colonisation resistance), metabolism of plant compounds (for example, phytoestrogens and glycosides), and production of short-chain fatty acids and polyamines.30,36,37
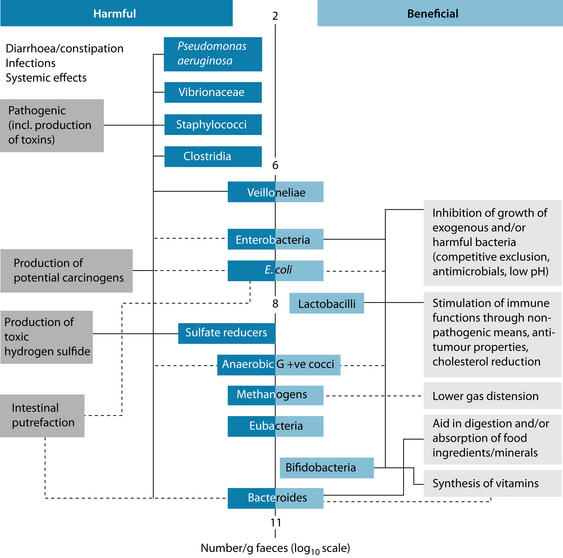
The colon is the most heavily colonised area of the GIT and this microbial ecosystem is believed to play the greatest role in human health. The colonic microflora is composed almost entirely of anaerobic bacteria, with the largest two genera being Bacteroides (accounting for up to 30% of all organisms) and Bifidobacterium (which can constitute up to 25% of total faecal counts). Other numerically important anaerobes include eubacteria, lactobacilli, clostridia and gram-positive cocci. Existing in smaller proportions are coliforms, methanogens, enterococci and dissimilatory sulfate-reducing bacteria. These important groups of bacteria have been divided into species that exert either beneficial or harmful effects on the host, as outlined in Figure 3.1.37 When the beneficial species are present in insufficient numbers or when concentrations of potentially harmful bacteria are relatively high, then dysbiosis is said to result. Dysbiosis is a state in which the microflora produces harmful effects through one or more of the following factors: (1) qualitative and quantitative changes in the intestinal flora itself; (2) changes in their metabolic activities; and (3) changes in their local distribution.38
Optimise the GIT microflora
Probiotics
The term ‘probiotic’ is derived from the Greek and literally means ‘for life’. It was first coined in 1965 by Lilley and Stillwell to describe substances secreted by one microorganism that stimulate the growth of another.39 In 1974, Parker modified this definition to ‘organisms and substances which contribute to intestinal microbial balance’.40 The current World Health Organization definition of probiotics is ‘live microorganisms which when administered in adequate amounts confer a health benefit on the host’.41 Probiotic organisms can be found in fermented foods (such as yoghurt, sauerkraut and kefir), as well as supplements. The microorganisms found in these products are typically lactobacilli and bifidobacteria.42
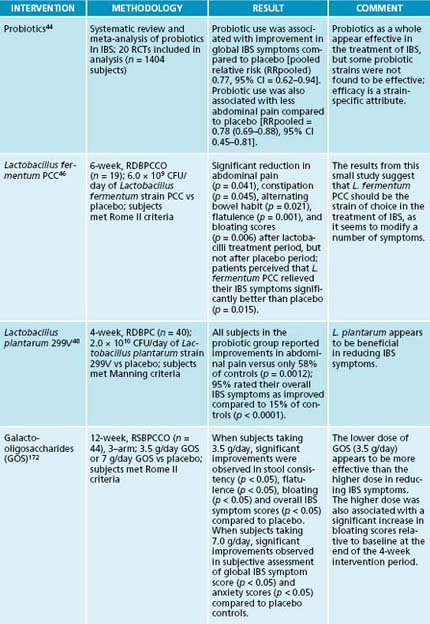
Probiotics have a long history of successful use in the treatment of IBS. In fact the first case series detailing the efficacy of a Lactobacillus acidophilus supplement in IBS was published in 1955.43 A recent systematic review and meta-analysis of randomised, controlled trials found probiotic use to be associated with improvements in global IBS symptoms compared to placebo and reductions in abdominal pain.44 It is well-known, however, that efficacy in this condition, as it is in all conditions, is strain dependent. Commercially available probiotic strains that have shown efficacy in the treatment of IBS include L. fermentum PCC45,46 and L. plantarum 299V.47,48 Commercially-available strains found to be ineffective in the management of IBS include L. acidophilus NCFM49 and L. rhamnosus GG.50,51
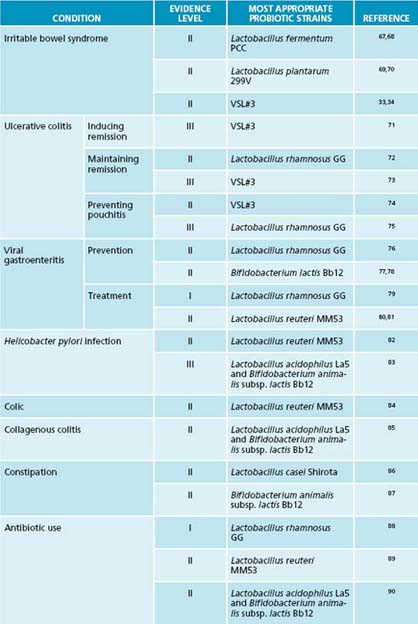
To achieve the desired therapeutic results, it is imperative to prescribe the precise probiotic strains that have demonstrated therapeutic and clinical efficacy in the condition in question. Strains that work in one condition will not necessarily be effective in other conditions. For example, Lactobacillus rhamnosus GG appears to be effective in the prevention of antibiotic-associated side effects,65 but not of any demonstrable benefit in urinary tract infections.66 Tables 3.2 and 3.3 outline the most appropriate probiotic strains and prebiotic to use for specific disease conditions, as determined by human trials.
Table 3.1 Mechanisms of delivery of probiotics, and their advantages and disadvantages
| DELIVERY SYSTEM | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Fermented foods |
Prebiotics
A prebiotic is defined as ‘a nondigestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon.’53 For food ingredients to be classified as prebiotics, they must:
Most emphasis to date has been on finding and trialling food sources that are used by lactic acid-producing bacteria. This is due to the health-promoting properties of these organisms.42 The best known lactic acid-producing bacteria belong to the genera Lactobacillus and Bifidobacterium. Commonly used prebiotics include lactulose, fructo-oligosaccharides (FOS), and galacto-oligosaccharides (GOS). The most appropriate prebiotics to use for specific health conditions are highlighted in Table 3.3.
| DISEASE CONDITIONS | PREBIOTIC |
|---|---|
| Prevention of UTIs | Lactulose (25 g/day) |
| Lowered immunity: decreased rates of infection | FOS (2 g/day in infants) |
| Poor calcium absorption | FOS (8 g/day) |
| Atopic eczema: prevention in infants | GOS and FOS (0.8 g/100 mL formula) |
| Constipation |
One trial has been performed investigating the efficacy of FOS in IBS with disappointing results.54 However, the dose of FOS used in the study (20 g/day in a single dose) is known to cause significant gastrointestinal side effects (such as bloating, distension, borborygmi and increased flatulence).55 Thus it is not surprising that FOS failed
PREBIOTICS VERSUS COLONIC FOODS
There are a number of other substances that are frequently referred to as prebiotics. Many of these, however, fail to meet the criteria outlined above. Slippery elm, psyllium husks, guar gum and pectin would more accurately be described as colonic foods rather than prebiotics as they appear to lack the selectivity of fermentation that is required of prebiotics.56–58 Other substances, such as polydextrose and larch arabinogalactans, have been shown to increase the growth of beneficial bacteria in human trials.59–60 However, they have thus far been the subject of inadequate research to determine if they meet all of the prebiotic requirements.
to reduce these same GIT symptoms in trial participants. It is possible that administration of lower doses of FOS (for example, 3 g/day) or other prebiotic agents (lactulose or GOS) will result in better clinical outcomes.
Synbiotics
Synbiotics are products that contain both probiotic and prebiotic agents.61 The combination is theorised to enhance the survival of the probiotic bacteria through the upper GIT, improve implantation of the probiotic in the colon, and have a stimulating effect on the growth and/or activities of both the exogenously provided probiotic strains and the endogenous inhabitants of the bowel.62 Synbiotics are a promising treatment avenue in C-IBS with two recently published, open-label trials finding a synbiotic preparation (containing a daily dose of 5 × 109 CFU Bifidobacterium longum strain W11 and 2.5 g fructo-oligosaccharides) to significantly decrease abdominal pain and bloating in patients with C-IBS, as well as increasing stool frequency.63,64
Promote daily, easy-to-pass bowel movements
Epidemiological studies have consistently found correlations between dietary fibre intake and improved bowel function.107,108 As the amount of dietary fibre in the diet is increased, mean gastrointestinal transit time decreases, stool frequency increases and stools become softer and easier to pass.107 Clinical trials of fibre supplementation have also consistently found increased bowel movement frequency and improved stool consistency.109
HOW DOES DIETARY FIBRE WORK?
There appear to be at least five ways by which dietary fibre consumption improves laxation. First, plant cell walls that resist microfloral degradation are able to exert a physical bulking effect by retaining water within their cellular structure. This increased bulk stimulates colonic movement. Secondly, the vast majority of consumed dietary fibre is extensively broken down and metabolised by the colonic microflora. This stimulates microbial growth, leading to an increase in microbial products and microbes themselves in faeces – again leading to an increase in faecal bulk. Thirdly, the increase in faecal bulk speeds up the faeces’ rate of passage through the bowel. As transit time decreases, the efficiency with which the bacteria grow improves – further bulking the stools. Shortened transit time also leads to reduced water absorption by the colon and, hence, moister stools. Fourthly, fermentation of dietary fibre by the colonic microflora results in the production of hydrogen, methane and carbon dioxide gas. When trapped within the gut contents they further add to stool bulk.116 Lastly, other end-products of fermentation (short-chain fatty acids, particularly acetate) enhance muscular contraction in the colon.117,118
A recent systematic review and meta-analysis found fibre to significantly improve global IBS symptoms (see Table 3.4, the review of evidence table) and IBS-related constipation. However, fibre supplementation was found to significantly worsen abdominal pain. When soluble fibre was examined in isolation, it was found to induce a greater reduction in global IBS symptoms and to improve constipation in C-IBS subjects. Conversely, insoluble fibre supplementation was found to have no significant effect upon global IBS symptoms, although it did improve constipation. The authors concluded that fibre supplementation appears to be of benefit in improving global IBS symptoms and particularly constipation; it does not, however, improve IBS-related abdominal pain.110
The findings from this systematic review suggest that sources of soluble fibre would be more appropriate therapeutic tools than sources of insoluble fibre in the treatment of C-IBS. Good sources of supplemental soluble fibre include ground flaxseeds,111 slippery elm powder,112 psyllium husks,113 oat bran114 and pectin.115
Fluid intake
Ensuring the intake of adequate fluid is also a vital, although under-researched, therapeutic tool. Positive associations have been observed between bowel movement frequency and fluid intake in epidemiological research.108 Stool frequency and stool weight have also been found to be significantly decreased during enforced periods of low fluid intake in prospective, human research.119 Adequate fluid intake is usually described as 2100–2600 mL daily, although this would need to be increased in warmer climates.120
Exercise
Epidemiological research has found an association between level of physical activity and frequency of bowel habit, with lower levels of physical activity being linked with impaired bowel habit.121 This link between physical activity levels and bowel health is widely known amongst the general populace, with even Roald Dahl remarking in his Revolting Rhymes:122
In support of this connection, prospective human research has found that daily moderate exercise is capable of significantly accelerating gastrointestinal transit time, which should equate to softer, easier-to-pass stools.123 Additionally, a recently published, randomised, controlled trial found daily exercise to significantly improve constipation-related symptoms in IBS subjects.124
Manage GIT symptoms
Although it is a benign disorder, IBS is associated with significant impairments in quality of life.134 Recent research has found that three gastrointestinal symptoms (straining at stool, abdominal pain and abdominal bloating) have the greatest negative impact upon quality of life in IBS sufferers.135 Providing relief from these symptoms should thus be at the forefront of naturopathic management of this condition.
Straining at stool is usually addressed with the agents and interventions discussed in the ‘Promote daily, easy-to-pass bowel movements’ section above. Occasionally, however, laxative herbs are needed at the onset of treatment. Sometimes gentle laxatives like Glycyrrhiza glabra are adequate. In more severe cases of constipation, the anthraquinone-containing laxatives can be used: Senna spp., Rhamnus purshiana,
WHAT IS A ‘NORMAL’ BOWEL PATTERN?
This question should really be broken up into two questions: what is a ‘normal’ bowel habit and what is a ‘healthy’ or ‘optimal’ bowel habit? A ‘normal’ bowel habit has been defined by conventional medicine as between three bowel movements per day to three bowel movements per week. These frequencies are based on epidemiological studies conducted on Western populations which have found the vast majority of individuals to have a bowel frequency within this range, with the most common frequency being once daily.125,126 Constipation is thus defined as less than three bowel movements per week.127
Naturopaths would generally not consider patients who experience three bowel movements per week to have a ‘normal’ bowel habit. Naturopath Henry Lindlahr stated in his 1919 classic Natural Therapeutics that ‘normally a person should have a copious movement of the bowels once in twenty-four hours – twice is better’.128 Studies looking at non-Western populations eating traditional fibre-rich diets found defecation frequencies to average two or three times daily.129 Other studies looking at vegans eating high-fibre diets (about 47 g fibre daily) have found a mean bowel movement frequency of ≥ 1.5 per day.107,108 From these data we can gather that individuals consuming a high-fibre, plant-based diet pass stools more frequently than individuals consuming the typical low-fibre, Western diet (about 11–16 g fibre daily).130 Optimal bowel frequency should be considered to be one, two or three movements daily. Naturopaths generally consider patients as being constipated if they experience less than one bowel movement daily.
Bowel habit: men versus women
Studies have consistently found men to have an increased frequency of daily bowel movements, to produce a greater quantity of faeces daily, to have shorter gastrointestinal transit times and to have softer, less-formed faeces than women.107,108,125 Stool form and bowel movement frequency also appears to vary in women according to the menstrual cycle. During the luteal phase of the cycle, gastrointestinal transit time significantly slows, resulting in more formed, harder stools.107
What is the ‘perfect’ bowel movement?
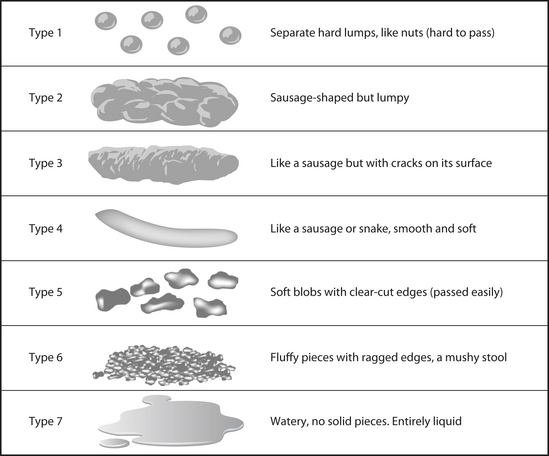
In terms of consistency, the most common stool passed by typical Western populations is well-formed (sausage or snake-like) with either cracks on its surface or a smooth and soft surface (types 3 and 4 in Figure 3.2).125 Populations consuming a predominantly plant-based, high-fibre diet, on the other hand, have softer, bulkier stools that tend to be less formed and more ‘mushy’ (type 5). These stools are associated with shorter gastrointestinal transit times, whereas harder stools are associated with longer transit times.107 So, what is the perfect bowel movement? Optimal stool form should vary between well-formed, sausage-like stools with cracks on its surface (type 3) through to softer blobs with clear-cut edges (type 5). In addition, there should be little-to-no straining at stool, little urgency and a feeling of complete evacuation after the event.131
What stimulates the bowels to move?
Colonic motility is the key factor determining the whens and whys of bowel movements. The greatest stimulant of colonic contractile activity is morning awakening. Colonic motility is greatly reduced and sometimes even completely abolished during sleep. Upon awakening contractile activity increases briskly.133 Not surprisingly, population studies have found that the vast majority of bowel movements occur between 6 and 9 a.m.125 The other main stimulus of colonic motility is eating – referred to as the colonic motor response to eating or the gastrocolonic reflex. Within minutes of consuming the first mouthful of food, colonic contractile activity begins, and lasts for at least 3 hours.133 In Western populations, a second peak of defecation frequency has been observed at 6–7 p.m., the time at which the largest meal of the day is typically consumed.125
Rumex crispus or Juglans cinerea. The use of these agents can result in griping abdominal pain; concurrent administration of carminatives and antispasmodics is thus highly recommended. If anthraquinone-containing laxatives are used, the aim should be to use them for a short-term period only.
Abdominal pain and bloating can be addressed using a combination of carminatives and antispasmodics. Useful carminatives include Mentha piperita, Mentha spicata, Carum carvi, Foeniculum vulgare, Citrus reticulata, Coriandrum sativum, Elettaria cardamomum, Origanum vulgare and Anethum graveolens. Commonly prescribed GIT antispasmodics include Matricaria recutita, Viburnum opulus, Dioscorea villosa, Melissa officinalis and Zingiber officinale.
Support the nervous system
Given that C-IBS is often associated with depression23,24 and given the link between perceived stress levels and IBS-symptom severity,21,22 support for the nervous system is also an important component of naturopathic management. Mood has also been shown to affect bowel habit, with depression significantly slowing gastrointestinal transit time and anxiety significantly speeding it.136
Decrease gut inflammation and diminish visceral hypersensitivity
Patients with IBS have been shown to have increased visceral sensation and may suffer from mild colonic mucosal inflammation.3,4 Ingestion of gastrointestinal anti-inflammatories such as Curcuma longa, Glycyrrhiza glabra and Matricaria recutita may help decrease this inflammation. Recent research has found a combination of peppermint (Mentha piperita) and caraway (Carum carvi) essential oils effective in decreasing visceral hypersensitivity in an animal model of IBS.137 A proprietary herbal preparation (Iberogast) has also been found to decrease visceral hypersensitivity in an animal model.138 Iberogast is an ethanolic extract containing Iberis amara, Angelica archangelica, Silybum marianum, Carum carvi, Glycyrrhiza glabra, Chelidonium majus, Matricaria recutita, Melissa officinalis and Mentha piperita.
Improve liver function
Poor liver function is viewed by some practitioners as an important contributing factor in IBS.139 Liver congestion has also long been seen by naturopaths as a common cause of constipation128 and bile salts are well-known laxative agents.140 Accordingly, patients with C-IBS can sometimes receive benefit from the ingestion of cholagogues (Cynara scolymus, Curcuma longa, Taraxacum officinale radix, Chelidonium majus, Berberis vulgaris or Juglans cinerea). See Chapter 19 for information on liver function and detoxification.
In support of the use of cholagogues in C-IBS, two clinical trials have been conducted on herbal cholagogues with both trials finding evidence of therapeutic effectiveness. In a post-marketing surveillance study Cynara scolymus extract was found to significantly decrease abdominal pain, bloating, flatulence and constipation in subjects with IBS over a 6-week period.141 In an open-label trial, two different doses of turmeric extract (Curcuma longa) were examined to assess their effects on IBS symptoms.142 Both extracts were found over an 8-week period to significantly reduce abdominal pain/discomfort scores from baseline. Additionally, bowel pattern was found to normalise. There were, however, no significant differences between the high and low dose groups.
INTEGRATIVE MEDICAL CONSIDERATIONS
Referral
In many ways, IBS is a clinical diagnosis – a diagnosis is based on the presence of typical signs and symptoms (see the box on the Rome III criteria for current diagnostic criteria). Specificity of using the Rome criteria for the diagnosis of IBS has been found to be about 98% in the absence of ‘alarm features’. Hence further investigations are typically not required to confirm the diagnosis and exclude organic disease. If ‘alarm features’ are present, however, referral for further investigation is recommended. ‘Alarm features’ include the presence of blood in the stools, weight loss, fever and family history of colon cancer.4 Any change in bowel pattern in individuals over 45 years of age is also an ‘alarm feature’ requiring further investigation.165
REDISCOVERING OUR HERBAL ROOTS
Traditional Chinese medicine
Initial research suggested that acupuncture may have a role to play in the management of IBS.166 Follow-up research using more rigorous designs has not backed this up, however167,168 and a recent systematic review of randomised, controlled trials failed to find acupuncture more effective than sham acupuncture in the management of IBS symptoms.169 The results of a recent systematic review of herbal medicines in IBS found Chinese herbal medicine formulas effective in relieving IBS symptoms, while being very well tolerated. Unfortunately many of the trials were of poor quality, preventing firm conclusions about efficacy from being drawn.170
STRAIN SELECTIVITY OF ACTION
Within each species of bacteria there is a multitude of strains. Some probiotic strains are resilient and strong, with a demonstrated capacity to survive passage through the upper GIT and inhibit pathogenic bacteria, whereas others are weak and cannot even survive transit through the stomach. It is vital to note that just because one strain of bacteria in a given species has a proven action or characteristic, it does not mean that another strain will too, even if they are closely related. Strains of bacteria within the same species can have significantly different actions, properties and characteristics, as these are all essentially strain-specific qualities.171
Example treatment
Iberogast was prescribed to address the visceral hypersensitivity seen in IBS and to reduce the main gastrointestinal symptoms of pain, bloating, distension and excessive flatulence. She was advised to cease taking Lactobacillus acidophilus NCFM, as this strain has been found to be ineffective in IBS.49 Instead L. fermentum PCC was prescribed – a probiotic strain clinically proven to reduce abdominal pain and bloating, and to decrease flatulence in IBS sufferers (see the box on strain specificity of action).46 Freshly ground flaxseeds were used as a source of soluble fibre to promote laxation. Slippery elm powder was prescribed to decrease the gastric irritation caused by the Celebrex. Additionally, she was put on a turmeric extract primarily to reduce the bursitis-related pain and inflammation, but also for
its GI anti-inflammatory and cholagogue actions. After 7 days of taking the turmeric extract and slippery elm she was advised to start weaning off the Celebrex and Nexium. She was advised to continue taking the B-complex and the Coversyl.
Expected outcomes and follow-up protocols
If treated with carminatives and GI antispasmodics (or, in this case, Iberogast), gastrointestinal symptoms such as cramping pain and bloating should improve rapidly over the next few days. In the author’s experience, the key to keeping C-IBS symptoms under control is to ensure daily, easy-to-pass bowel movements. If the bowels are kept moving regularly, this tends to prevent the other GI symptoms (for example, bloating and cramping) from occurring – the pain and bloating is actually secondary to the constipation. If patients are able to implement dietary changes that successfully increase their fibre intake, exercise regularly and ingest adequate amounts of fluid, then this is usually adequate to maintain daily bowel movements for most individuals suffering from C-IBS. The addition of a soluble-fibre source, such as ground flaxseeds, is usually needed in the short-term and is obviously healthy to take long-term if need be – the consumption of freshly milled flaxseeds has a number of ancillary health benefits. Initially, the carminative, anti-inflammatory and antispasmodic herbs should be taken throughout the day every day, but after a few weeks their use can be reserved as prophylaxis for situations that are known to cause symptom flare-ups and to treat flare-ups. Nervines and adaptogens provide restoration over the long term, so their use should be continued until both the practitioner and the patient are confident that they are no longer needed. Pro-, pre- and synbiotics can (in IBS) be considered disease-modifying agents that need to be taken long-term for their benefits to be truly felt.
KEY POINTS
Azpiroz F., et al. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19(Suppl. 1):62-88.
Hawrelak J.A., Myers S.P. The causes of intestinal dysbiosis: a review. Altern Med Rev. 2004;9(2):180-197.
Spiller R. Review article: probiotics and prebiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:385-396.
Ohman L., Simren M. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig Liver Dis. 2007;39(3):201-215.
1. American Gastroenterology Association. Irritable bowel syndrome: a technical review for practice guideline development. Gastroenterology. 2002;112:2120-2137.
2. Mayer E.A. Emerging disease model for functional gastrointestinal disorders. Am J Med. 1999;107(5A):12S-19S.
3. Delvaux M. Role of visceral sensitivity in the pathophysiology of irritable bowel syndrome. Gut. 2002;51(Suppl. 1):i67-i71.
4. Camilleri M., et al. Consensus report: clinical perspectives, mechanisms, diagnosis and management of irritable bowel syndrome. Aliment Pharmacol Ther. 2002;16:1407-1430.
5. Bercik P., et al. Is irritable bowel syndrome a low-grade inflammatory bowel disease? Gastroenterol Clin North Am. 2005;34:235-245.
6. Coulie B. Role of GI motor abnormalities in irritable bowel syndrome. Acta Gastroenterol Belg. 2001;64:276-280.
7. Serra J., et al. Impaired transit and tolerance of intestinal gas in irritable bowel syndrome. Gut. 2001;48:14-19.
8. King T.S., et al. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352:1187-1189.
9. Treem W.R., et al. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr. 1996;23:280-286.
10. Si J.M., et al. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J Gastroenterol. 2005;10:1802-1805.
11. Malinen E., et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373-382.
12. Balsari A., et al. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 1982;5:185-194.
13. Morris-Yates A.D., et al. Evidence of a genetic contribution to self reported symptoms of irritable bowel syndrome. Gastroenterol. 1995;108:A652.
14. Locke G.R.III, et al. The irritable bowel syndrome and functional dyspepsia: familial disorders? Gastroenterol. 1996;110:A26.
15. Rodriguez L.A.G., Ruigomez A. Increased risk of irritable bowel syndrome after bacterial gastroenteritis: cohort study. BMJ. 1999;318:565-566.
16. McKendrick W., Read N.W. Irritable bowel syndrome – post salmonella infection. J Infect. 1994;29:1-3.
17. Gwee K.A., et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400-406.
18. Mayer E.A. Emerging disease model for functional gastrointestinal disorders. Am J Med. 1999;107(5A):12S-19S.
19. Mendall M.A., Kumar D. Antibiotic use, childhood affluence and irritable bowel syndrome. Eur J Gastroenterol Hepatol. 1998;10:59-62.
20. Maxwell P.R., et al. Antibiotics increase functional abdominal symptoms. Am J Gastroenterol. 2002;97:104-108.
21. Bennett E.J., et al. Level of chronic stress predicts clinical outcome in irritable bowel syndrome. Gut. 1998;43:256-261.
22. Whitehead W.E., et al. Symptoms of psychological distress associated with irritable bowel syndrome. Gastroenterol. 1998;95:709-714.
23. Masand P.S., et al. Major depression and irritable bowel syndrome: is there a relationship? J Clin Psychiatry. 1995;56:363-367.
24. Hochstrasser B., Angst J. The Zurich study: XXII. Epidemiology of gastrointestinal complaints and comorbidity with anxiety and depression. Eur Arch Psychiatry Clin Neurosci. 1996;246:261-272.
25. Wysowski D.K., et al. Postmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actions. Am J Gastroenterol. 2001;96:1698-1703.
26. Klein K.B. Controlled treatment trials in the irritable bowel syndrome: a critique. Gastroenterology. 1988;95:232-241.
27. Friedel D., et al. Ischemic colitis during treatment with alosetron. Gastroenterology. 2001;120:557-560.
28. Schoenfeld P. Efficacy of current drug therapies in irritable bowel syndrome: what works and does not work. Gastroenterol Clin N Am. 2005;34:319-335.
29. DiBaise J.K. Tegaserod-associated ischemic colitis. Pharmacotherapy. 2005;25:620-625.
30. Holzapfel W.H., et al. Overview of gut flora and probiotics. Int J Food Microbiol. 1998;41:85-101.
31. Gibson G.R. Dietary modulation of the human gut microflora using prebiotics. Br J Nutr. 1998;80(Suppl. 2):S209-S212.
32. Moore W.E.C., Holdeman L.V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974;27:961-979.
33. Savage D.C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1997;3:107-133.
34. Bengmark S. Probiotics and prebiotics in prevention and treatment of gastrointestinal diseases. Gastroenterol Int. 1998;11(Suppl. 1):4-7.
35. Macfarlane G.T., Macfarlane S. Human colonic microbiota: ecology, physiology and metabolic potential of intestinal bacteria. Scand J Gastroenterol. 1997;32(Suppl. 222):3-9.
36. Noack J., et al. Dietary guar gum and pectin stimulate intestinal microbial polyamine synthesis in rats. J Nutr. 1998;128:1385-1391.
37. Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401-1412.
38. Hawrelak J.A., Myers S.P. Intestinal dysbiosis: a review of the literature. Altern Med Rev. 2004;9:180-197.
39. Lilley D.M., Stillwell R.H. Probiotics: growth promoting factors produced by microorganisms. Science. 1965;147:747-748.
40. Parker R.B. Probiotics, the other half of the antibiotic story. Animal Nut Hlth. 1974;29:4-8.
41. Food and Agriculture Organization and World Health Organization Expert Consultation. Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Food and Agriculture Organization of the United Nations and World Health Organization. 2001.
42. Collins M.D., Gibson G.R. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69(Suppl):1052S-1057S.
43. Winkelstein A. Lactobacillus acidophilus tablets in the therapy of various intestinal disorders: a preliminary report. Am Pract Dig Treat. 1955;6:1022-1025.
44. McFarland L.V., Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14:2650-2661.
45. Conway P.L., et al. Modulation of faecal enteric bacteria and symptoms of irritable bowel syndrome using Lactobacillus fermentum and resistant starch. J Diet Suppl. 2005. In press
46. Amansec S., et al. Lactobacillus fermentum PCC™ relieves the symptoms of medically diagnosed irritable bowel syndrome. Unpublished. 2005.
47. Nobaek S., et al. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95(5):1231-1238.
48. Niedzielin K., et al. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001;13:1143-1147.
49. Newcomer A.D., et al. Response of patients with irritable bowel syndrome and lactase deficiency using unfermented acidophilus milk. Am J Clin Nutr. 1983;38:257-263.
50. O’Sullivan M.A., O’Morain C.A. Bacterial supplementation in the irritable bowel syndrome: a randomised double-blind placebo-controlled crossover study. Dig Liver Dis. 2000;32:294-301.
51. Bausserman M., Michail S. The use of Lactbacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J Pediatr. 2005;147:197-201.
52. Saxelin M. Colonization of the human gastrointestinal tract by probiotic bacteria (Lactobacillus GG). Nutrition Today. 1996;31:5S-9S.
53. Roberfroid M.B. Prebiotics and synbiotics: concepts and nutritional properties. Br J Nutr. 1998;80(Suppl. 2):S197-S202.
54. Olesen M., Gudmand-Hoyer E. Efficacy, safety, and tolerability of fructooligosaccharides in the treatment of irritable bowel syndrome. Am J Clin Nutr. 2000;72:1570-1575.
55. Bouhnik Y., et al. Short-chain fructo-oligosaccharide administration dose-dependently increases fecal bifidobacteria in healthy humans. J Nutr. 1999;129:113-116.
56. Salyers A.A., Leedle J.A.Z. Carbohydrate metabolism in the human colon. In: Hentges D.J., editor. Human intestinal microflora in health and disease. New York: Academic Press; 1983:129-146.
57. Bernalier A., et al. Biochemistry of Fermentation. In: Gibson G.R., Roberfroid M., editors. Colonic microbiota, nutrition and health. Dordoecht: Kluwer Academic Publishers; 1999:37-54.
58. Gibson S.A.W., Conway P.L. Recovery of a probiotic organism from human faeces after oral dosing. In: Gibson S.A.W., editor. Human health: the contribution of microorganisms. London: Springer-Verlag; 1994:119-121.
59. Robinson R.R., et al. Effects of dietary arabinogalactan on gastrointestinal and blood parameters in healthy human subjects. J Am Coll Nutr. 2001;20:279-285.
60. Jie Z., et al. Studies on the effects of polydextrose intake on physiologic functions in Chinese people. Am J Clin Nutr. 2000;72:1503-1509.
61. Schrezenmeir J., de Vrese M. Probiotics, prebiotics, and synbiotics – approaching a definition. Am J Clin Nutr. 2001;73:361-364.
62. Casiraghi M.C., et al. Effects of a synbiotic milk product on human intestinal ecosystem. J Appl Microbiol. 2007;103:499-506.
63. Dughera L., et al. Effects of symbiotic preparations on constipated irritable bowel syndrome symptoms. Acta Biomed. 2007;78:111-116.
64. Colecchia A., et al. Effects of a symbiotic preparation on the clinical manifestations of irritable bowel syndrome, constipation-variant. Results of an open, uncontrolled multicenter study. Minerva Gastroenterol Dietolo. 2006;52:349-358.
65. Arvola T., et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104(5):1-4.
66. Kontiokari T., et al. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ. 2001;322:1571.
67. Conway P.L., et al. Modulation of faecal enteric bacteria and symptoms of irritable bowel syndrome using Lactobacillus fermentum and resistant starch. Unpublished. 2005.
68. Amansec S., et al. Lactobacillus fermentum PCC™ relieves the symptoms of medically diagnosed irritable bowel syndrome (IBS). Unpublished. 2005.
69. Nobaek S., et al. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95(5):1231-1238.
70. Niedzielin K., et al. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eu J Gastroenterol Hepatol. 2001;13:1143-1147.
71. Tursi A., et al. Low-dose balsalazide plus a high-potency probiotic preparation is more effective than balsalazide alone or mesalazine in the treatment of acute mild-to-moderate ulcerative colitis. Med Sci Monit. 2004;10:126-131.
72. Zocco M.A., et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1567-1574.
73. Venturi A., et al. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1103-1108.
74. Mimura T., et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108-114.
75. Gosselink M.P., et al. Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Dis Colon Rectum. 2005;47:876-884.
76. Szajewska H., et al. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. J Pediatr. 2001;138:361-365.
77. Chouraqui J.P., et al. Acidified milk formula supplemented with Bifidobacteria lactis: impact on infant diarrhea in residential care settings. J Pediatr Gastroenterol Nutr. 2004;38:288-292.
78. Saavedra J.M., et al. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet. 1994;344:1046-1049.
79. Szajewska H., et al. Meta-analysis: Lactobacillus GG for treating acute diarrhoea in children. Aliment Pharmacol Ther. 2007;25:871-881.
80. Shomikova A.V., et al. Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. J Pediatr Gastroenterol Nutr. 1997;24:399-404.
81. Shornikova A.V., et al. Bacteriotherapy with Lactobacillus reuteri in rotavirus gastroenteritis. Pediatr Infect Dis. 1997;16:1103-1107.
82. Saggioro A., et al. Helicobacter pylori eradication with Lactobacillus reuteri. A double-blind placebo-controlled study. Dig Liv Dis. 2005;37:588.
83. Wang K.Y., et al. Effects of ingesting Lactobacillus– and Bifidobacterium-containing yogurt in subjects with colonized Helicobacter pylori. Am J Clin Nutr. 2004;80:737-741.
84. Savino F., et al. Lactobacillus reuteri ATCC 55730 versus simeticone in the treatment of infantile colic: a prospective randomised study. Paediatrics. 2005;119:124-130.
85. Wildt S., et al. Probiotic treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial with Lactobacillus acidophilus and Bifidobacterium animalis subsp lactis. Inflamm Bowel Dis. 2006;12:395-401.
86. Koebnick C., et al. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenterol. 2005;17:655-659.
87. Pitkala K.H., et al. Fermented cereal with specific bifidobacteria normalizes bowel movements in elderly nursing home residents. A randomized, controlled trial. J Nutr Health Aging. 2007;11:305-311.
88. Hawrelak J.A., et al. Is Lactobacillus rhamnosus GG effective in preventing the onset of antibiotic-associated diarrhea: a systematic review. Digestion. 2005;72:51-56.
89. Lionetti E, et al. The effect of oral administration of Lactobacillus reuteri on antibiotic-assocaited gastrointestinal side-effects during Helicobacter pylori eradication therapy. 12th National Congress of Italian Society of Pediatric Gastroenterology, 22–24 September 2005.
90. Wenus C., et al. Prevention of antibiotic-associated diarrhoea by a fermented probiotic milk drink. Eur J Clin Nutr. 2008;62:299-301.
91. Gorbach S.L., et al. Successful treatment of relapsing Clostridium diffcile colitis with Lactobacillus GG. Lancet. 1987;2:1519.
92. Bennett R.G., et al. Treatment of relapsing Clostridium difficile diarrhea with Lactobacillus GG. Nutr Today. 1996;31:35S-39S.
93. Klarin B., et al. Lactobacillus plantarum 299v reduces colonisation of Clostridium difficile in critically ill patients treated with antibiotics. Acta Anaesthesiol Scand. 2008;52:1096-1102.
94. Manley K.J., et al. Probiotic treatment of vancomycin-resistant enterococci: a randomised controlled trial. Med J Aust. 2007;186:454-457.
95. Delia P., et al. Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol. 2007;13:912-915.
96. Nase L., et al. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res. 2001;35:412-420.
97. Hatakka K., et al. Effect of long-term consumption of probiotic milk on infections in children attending day care centres: double-blind, randomised trial. BMJ. 2001;322:1327.
98. Weizman Z., et al. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. 2005;115:5-9.
99. Tubelius P., et al. Increasing work-place healthiness with the probiotic Lactobacillus reuteri: a randomised, double-blind placebo-controlled trial. Environ Health. 2005;4:25.
100. Leyer G.J., et al. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics. 2009;124:172-179.
101. Sazawal S., et al. Efficacy of milk fortified with a probiotic Bifidobacterium lactis (DR-10) and prebiotic galacto-oligosaccharides in prevention of morbidity and on nutritional status. Asia Pacific J Clin Nutr. 2004;13(Suppl):S28.
102. Kalliomaki M., et al. Probiotics in primary prevention of atopic disease: a randomised, placebo-controlled trial. Lancet. 2001;357:1076-1079.
103. Kalliomaki M., et al. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomised, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1019-1021.
104. Wickens K., et al. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2008;122:788-794.
105. Weston A., et al. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90:892-897.
106. Hilton E., et al. Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann Intern Med. 1992;116(5):353-357.
107. Davies G.J., et al. Bowel function measurements of individuals with different eating patterns. Gut. 1986;27:164-169.
108. Sanjoaquin M.A., et al. Nutrition and lifestyle in relation to bowel movement frequency: a cross-sectional study of 20,630 men and women in EPIC-Oxford. Public Health Nutr. 2004;7:77-83.
109. Tramonte S.M., et al. The treatment of chronic constipation in adults: a systematic review. J Gen Intern Med. 1997;12:15-24.
110. Bijkerk C.J., et al. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19:245-251.
111. Cunnane S.C., et al. High a-linolenic acid flaxseed (Linum usitatissimum): some nutritional properties in humans. Br J Nutr. 1993;69:443-453.
112. van Wyk B.E., Wink M. Medicinal plants of the world. Pretoria, South Africa: Briza; 2004.
113. Sierra M., et al. Therapeutic effects of psyllium in type 2 diabetic patients. Eur J Clin Nutr. 2002;56:830-842.
114. Knudsen K.E.B., Johansen H.N. Mode of action of oat bran in the gastrointestinal tract. Eur J Clin Nutr. 1995;49(Suppl 3):S163-S169.
115. Southgate D.A. Dietary fiber parts of food plants and algae. In: Spiller G.A., editor. CRC handbook of dietary fibre in human nutrition. Boca Raton: CRC Press; 2001:11-13.
116. Cummings J.H. The effect of dietary fiber on fecal weight and composition. In: Spiller G.A., editor. CRC handbook of dietary fibre in human nutrition. Boca Raton: CRC Press; 2001:183-252.
117. Cherbut C., et al. Effects of short-chain fatty acids on gastrointestinal motility. Scand J Gastroenterol. 1997;32(Suppl. 222):58-61.
118. Topping D.L. Short-chain fatty acids produced by intestinal bacteria. Asia Pacific J Clin Nutr. 1996;5(Suppl):15-19.
119. Klauser A.G., et al. Low fluid intake lowers stool output in healthy male volunteers. Z Gastroenterol. 1990;28:606-609.
120. National Health and Medical Research Council. Nutrient reference values for Australia and New Zealand. National Health and Medical Research Council. 2006.
121. Everhart J.E., et al. A longitudinal survey of self-reported bowel habits in the United States. Dig Dis Sci. 1989;34:1153-1162.
122. Dahl R. Revolting rhymes. London: Puffin Books; 1995.
123. Oettle G.J. Effect of moderate exercise on bowel habit. Gut. 1991;32:941-944.
124. Daley A.J., et al. The effects of exercise upon symptoms and quality of life in patients diagnosed with irritable bowel syndrome: a randomised controlled trial. Int J Sports Med. 2008;29:778-782.
125. Heaton K.W., et al. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. 1992;33:818-824.
126. Bassoti G., et al. An extended assessment of bowel habits in a general population. World J Gastroenterol. 2004;10:713-716.
127. Longstreth G.F., et al. Functional bowel disorders. Gastroenterol. 2006;130:1480-1491.
128. Lindlahr H. Natural therapeutics Volume 2: practice. Essex UK: CW Daniel Company Ltd,. 1981.
129. Walker A.R.P. Nutritionally related disorders/diseases in Africans. Highlights of half a century of research with special reference to unexpected phenomena. In: Kritchevsky D., Bonfield C., editors. Dietary fibre in health and disease. New York: Plenum Press; 1997:1-14.
130. Jones J.M. Consumption of dietary fibre 1992–2000. In: Spiller G.A., editor. CRC handbook of dietary fibre in human nutrition. Boca Raton: CRC Press; 2001:553-566.
131. Heaton K.W., et al. How bad are the symptoms and bowel dysfunction of patients with the irritable bowel syndrome? A prospective, controlled study with emphasis on stool form. Gut. 1991;32:73-79.
132. Lewis S.J., Heaton K.W. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924.
133. Bassoti G., et al. Human colonic motility: physiological aspects. Int J Colorectal Dis. 1995;10:173-180.
134. Gralnek I.M., et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654-660.
135. Spiegel B., et al. Predictors of patient-assessed illness severity in irritable bowel syndrome. Am J Gastroenterol. 2008;103:2536-2543.
136. Gorard D.A., et al. Intestinal transit in anxiety and depression. Gut. 1996;39:551-555.
137. Adam B., et al. A combination of peppermint oil and caraway oil attenuates the post-inflammatory visceral hyperalgesia in a rat model. Scan J Gastroenterol. 2006;41:155-160.
138. Muller M.H., et al. STW 5 (Iberogast) reduces afferent sensitivity in the rat small intestine. Phytomedicine. 2006;13:100-106.
139. Bone K. Phytotherapy and irritable bowel syndrome. Br J Phytotherapy. 1997;4:190-198.
140. Bretagne J.F., et al. Increased cell loss in the human jejunum induced by laxatives (ricinoleic acid, dioctyl sodium sulphosuccinate, magnesium sulphate, bile salts). Gut. 1981;22(264):269.
141. Walker A.F., et al. Artichoke leaf extract reduces symptoms of irritable bowel syndrome in a post-marketing surveillance study. Phytother Res. 2001;15:58-61.
142. Bundy R., et al. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: a pilot study. J Altern Complement Med. 2004;10:1015-1018.
143. Gils C.V., Cox P.A. Ethnobotany of nutmeg in the Spice Islands. J Ethnopharmacol. 1994;42:117-124.
144. Felter H.W. The eclectic materia medica, pharmacology and therapeutics. Cincinnati: John K Scudder; 1922.
145. Bensky D., et al. Chinese herbal medicine materia medica. Seattle: Eastland Press; 2004.
146. Khare C.P. Indian herbal remedies. Rational western therapy, ayurvedic and other traditional usage, botany. Heidelberg: Springer-Verlag; 2004.
147. Bennett A., et al. The biological activity of eugenol, a major constituent of nutmeg (Myristica fragrans): studies on prostaglandins, the intestine and other tissues. Phytother Res. 1988;2:124-130.
148. Dhingra D., Sharma A. Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. J Med Food. 2006;9:84-89.
149. Sonavane G.S., et al. Anxiogenic activity of Myristica fragrans seeds. Pharmacol Biochem Behav. 2002;71:247-252.
150. Felter H.W., Lloyd J.U. King’s American dispensatory. Cincinnati: Ohio Valley Company; 1898.
151. Ononiwu I.M., et al. Effects of piperine on gastric acid secretion in albino rats. Afr J Med Sci. 2002;31:293-295.
152. Vasudevan K., et al. Influence of intragastric perfusion of aqueous spice extracts on acid secretion in anesthetized albino rats. Indian J Gastroenterol. 2000;19:53-56.
153. Bhat B.G., Chandrasekhara N. Effect of black pepper and piperine on bile secretion and composition in rats. Food/Nahrung. 1987;31:913-916.
154. Platel K., Srinivassan K. Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Food/Nahrung. 2000;44(42):46.
155. Izzo A.A., et al. Effect of vanilloid drugs on gastrointestinal transit in mice. Br J Pharmacol. 2001;132:1411-1416.
156. Bajad S., et al. Antidiarrhoeal activity of piperine in mice. Planta Med. 2001;67:284-287.
157. Weiss R.F. Herbal medicine. Ab Arcanum: Beaconsfield; 1988.
158. May B., et al. Efficacy of a fixed peppermint oil/caraway oil combination in non-ulcer dyspepsia. Arzneimittel-Forschung. 1996;46:1149-1153.
159. May B., et al. Efficacy and tolerability of a fixed combination of peppermint oil and caraway oil in patients suffering from functional dyspepsia. Aliment Pharmacol Ther. 2000;14:1671-1677.
160. Madisch A., et al. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride. A multicenter, reference-controlled double-blind equivalence study. 1999;49:925-932.
161. Rios J.L., et al. An update review of saffron and its active constituents. Phytother Res. 1996;10:189-193.
162. Pierpoint Johnson C. The useful plants of Great Britain: a treatise upon the principal native vegetables capable of application as food, medicine, or in the arts and manufactures. London: R. Hardwicke; 1862.
163. Noorbala A.A., et al. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97:281-284.
164. Akhondzadeh S., et al. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial. BMC Complement Altern Med. 2004;4:12.
165. Jones J., et al. British Society of Gastroenterology guidelines for management of the irritable bowel syndrome. Gut. 2000;47(Suppl. 2):ii1-ii19.
166. Chan J., et al. The role of acupuncture in the treatment of irritable bowel syndrome: a pilot study. Hepatogastroenterology. 1997;44:1328-1330.
167. Forbes A., et al. Acupuncture for irritable bowel syndrome: a blinded placebo-controlled trial. World J Gastroenterol. 2005;11:4040-4044.
168. Schneider A., et al. Acupuncture treatment in irritable bowel syndrome. Gut. 2006;55:649-654.
169. Lim B., et al. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. (4):2006. CD005111
170. Shi J., et al. Effectiveness and safety of herbal medicines in the treatment of irritable bowel syndrome: a systematic review. World J Gastroenterol. 2008;14:454-462.
171. Lewis S.J., Freedman A.R. The use of biotherapeutic agents in the prevention and treatment of gastrointestinal disease. Aliment Pharmacol Ther. 1998;12:807-822.
172. Silk D.B., et al. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:508-518.
173. Kline R.M., et al. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr. 2001;138:125-128.
174. Sallon S., et al. A novel treatment for constipation-predominant irritable bowel syndrome using Padma®Lax, a Tibetan herbal formula. Digestion. 2002;65:161-171.
175. Vejdani R., et al. The efficacy of an herbal medicine, Carmint, on the relief of abdominal pain and bloating n patients with irritable bowel syndrome: a pilot study. Dig Dis Sci. 2006;51:1501-1507.