CHAPTER 118 Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is an important disease entity because of its high prevalence, substantial morbidity, and enormous costs.1–3 In the United States, approximately 12% of patients seen by primary care physicians have IBS, but it is likely that this frequency is an underestimate.4–6 In gastrointestinal practices, more than one third of patients have functional gastrointestinal disorders, IBS being the most common diagnosis.7 Because a substantial proportion of gastroenterology practice comprises patients with IBS or other functional gastrointestinal disorders, it is essential that clinicians develop expertise in their diagnosis and treatment. The diagnosis of IBS rests on making a positive clinical diagnosis from the history; that tests often are not needed represents an important conceptual advance.8 There is increasing evidence that at least a subset of IBS has an organic basis in the gastrointestinal tract.9 Nonetheless, only symptom-directed therapy rather than disease-modifying treatments are available; the evidence base for current therapy has strengthened considerably with the publication of well-performed meta-analyses. In this chapter, current knowledge of the epidemiology and pathophysiology of IBS is reviewed to provide a rational basis for its diagnosis and therapy.
DEFINITIONS
IBS is characterized by the presence of abdominal discomfort or pain associated with disturbed defecation.3 Bloating or visible abdominal distention often is present in patients with IBS but are not considered essential symptoms for diagnosis.3 Furthermore, individual symptoms are neither sensitive nor specific enough on their own to diagnose IBS.10
In a classic study from the United Kingdom, Manning and associates first reported that six symptoms were more common in patients in whom IBS was subsequently documented, although only four were statistically significant in the initial report (Table 118-1).11 Later studies showed that these symptoms were specific, but not sensitive, for identifying IBS and were of greater diagnostic value in women.10,12 The Kruis scoring system is based on the presence and duration of symptoms, negative physical examination findings, and normal simple laboratory tests, and it has modest diagnostic utility (see Table 118-1).13
Table 118-1 Manning, Kruis, and Rome III Criteria for Irritable Bowel Syndrome
| Manning Criteria* |
Kruis criteria adapted from Kruis W, Thieme C, Weinzierl M, et al. A diagnostic score for the irritable bowel syndrome. Its value in the exclusion of organic disease. Gastroenterology 1984; 87:1-7.
Rome III criteria from Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. In: Drossman DA, editor. Rome III: The Functional Gastrointestinal Disorders. 3rd edition. McLean, VA: Dagnon Associates. 2006, pg 491. Used with permission from the Rome Foundation.
* Diagnostic cut-off: three or more of the six symptoms listed.
† If any abnormal physical findings or any of the laboratory parameters assessed by the physician are present, IBS is excluded.
‡ Criteria fulfilled for the previous three months, with symptom onset at least six months before diagnosis.
§ “Discomfort” means an uncomfortable sensation not described as pain. In pathophysiology research and clinical trials, a pain or discomfort frequency of at least two days a week during screening evaluation is recommended for subject eligibility.
Manning criteria adapted from Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. BMJ 1978; 2:653-4.
In an effort to build on the diagnostic utility of the Manning and Kruis criteria, the Rome (I, II, and III) criteria were created following a formal consensus process to provide a standard for clinical research (see Table 118-1).3 The Rome criteria are useful in clinical practice and can be used to make a positive clinical diagnosis.1,3 The sensitivity and specificity of the Rome I criteria have been reported to be 71% and 85%, respectively,14 and although adequate validation data for Rome III are lacking, the main criteria for Rome II and III are very similar.10 Comparisons of the criteria have shown that Rome I and II criteria are specific and identify similar patient populations, although, compared with Rome I criteria, the Rome II criteria appear to identify fewer cases in some studies.15–17 The Manning criteria identify additional patients with IBS-like symptoms who do not fulfill any of the Rome criteria but arguably also should be classified as having true IBS.15–17
CLINICAL FEATURES
HISTORY
Abdominal Discomfort or Pain
IBS should not be diagnosed in the absence of abdominal discomfort or pain.3 Distinguishing discomfort from pain can be problematic for both the patient and physician, however, because of the strong influence of cultural issues. In the United States, what a physician might label as mild pain often is considered discomfort by the patient. The pain or discomfort in IBS typically is relieved by defecation, or its onset is associated with an increase or decrease in stool frequency, or looser or harder stools. The pain often is poorly localized, waxes and wanes, may be aggravated by eating, and can occur in any part of the abdomen, although it more typically is located in the lower abdomen; it may be referred to different areas in the abdomen or to the chest or back. Exacerbation of pain by life events or difficult life situations is common. Abdominal discomfort or pain that is continuous or unrelated to defecation or induced by menstruation, urination, or physical activity is unlikely to be caused by IBS.
Constipation and Diarrhea
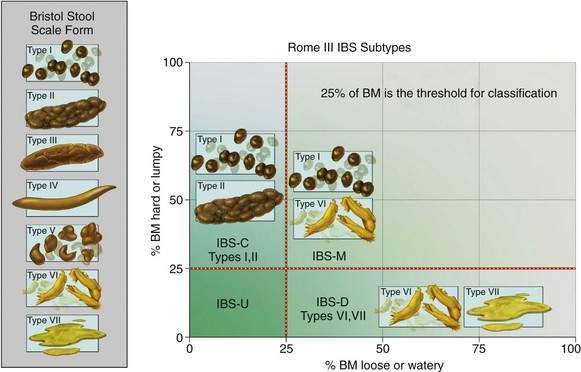
Patients with IBS experience constipation, diarrhea, or a mixture of these symptoms.3 Symptom predominance has led some authors to attempt to classify IBS patients by their predominant symptom: constipation (IBS-C), diarrhea (IBS-D), and mixed (IBS-M), although these symptoms often are variable and intermittent and patients can change their bowel habits from one pattern to another (see later). An irregular stool consistency (abnormal stool form) is characteristic. The terms “constipation” and “diarrhea” can reflect a wide variety of different symptom experiences to different patients, and so whenever a patient uses these terms, an exploration of their meaning is required.18 Any combination of infrequent defecation, passage of hard stools, excessive straining, feelings of incomplete rectal evacuation, or rectal discomfort may be referred to as constipation, whereas increased stool frequency, urgency, or the passage of liquid or watery stools, or even more frequent small hard stools, may be referred to as diarrhea by the patient. Stool form can be measured objectively and graded by patient or physician; the Bristol stool form scale (Fig. 118-1) now is routinely used in clinical trials, and changes in stool form (at the extreme ends of the scale) roughly correlate with colonic transit time.19,20
Bloating and Visible Distention
A feeling of bloating is almost universal in patients with IBS, and its site can be difficult for the patient to localize. Visible abdominal distention is characteristic but less common21; it can be objectively measured and usually is not imagined.22 Gas can mean excess bloating, belching, flatus, or even reflux symptoms to the patient, and so it is important to ask patients to explain the meaning of the terms they are using to describe their symptoms.
Noncolonic Symptoms
Other clinical features can help support the diagnosis of IBS but in themselves are not diagnostic. Nausea is common, and at least one third of patients with IBS have epigastric discomfort or pain (dyspepsia).1,8,23 Gastroesophageal reflux disease (GERD) occurs more commonly in IBS than would be expected by chance, affecting up to one in three persons with IBS.24 Extraintestinal symptoms including headache (and migraine), backache, impaired sleep, chronic fatigue, increased urinary frequency or urgency, pelvic pain, and dyspareunia are more common in patients with IBS but have no accepted diagnostic value.1,25 Musculoskeletal pain syndromes including fibromyalgia23 and temporomandibular joint disorder also are associated with IBS.2,23
Inflammatory Bowel Disease and Irritable Bowel Syndrome
Typical IBS symptoms are common in patients with documented inflammatory bowel disease (IBD) in remission; in one study, 33% with ulcerative colitis and 42% with Crohn’s disease fulfilled Rome II criteria for IBS.26 Clinically these conditions can be difficult to distinguish. IBS symptoms appear to be more prevalent before a diagnosis of IBD is made.27
Chronicity
For a confident diagnosis of IBS, symptoms should have been present for at least six months3; IBS may accompany other chronic disorders. For example, IBS is present in one third or more of patients with IBD in remission.26 A number of different conditions can cause transient bowel symptoms including pregnancy, dietary indiscretion, food poisoning, traveler’s diarrhea, bed rest, weight loss, and acute stress (nervous diarrhea); these must be distinguished from the chronic, recurrent symptoms of IBS.
PHYSICAL EXAMINATION
The physical examination in IBS usually is normal, although deep tenderness over the colon may be appreciated.10 Abdominal wall pain should be excluded clinically. Tensing the abdominal wall by flexing the chin on the chest or sitting up partially lessens tenderness that is caused by an intra-abdominal process. If tensing the abdominal wall muscles increases abdominal tenderness, a point of localized abdominal wall tenderness should be sought with a probing finger (Carnett’s test); identification of such a point might enable the tenderness to be treated with an injection of lidocaine and triamcinolone.28,29 The painful rib syndrome (point tenderness on springing the rib cage) also may be confused with IBS pain.30 Ovarian cancer needs to be considered in any middle-aged or older woman presenting with new-onset IBS-like symptoms.31 A pelvic examination therefore may be relevant to determine if there is any irregular, fixed pelvic mass.
EPIDEMIOLOGY
IBS is a common disorder all over the world.1,17,32 Epidemiologic studies have defined the prevalence and identified potential risk factors for IBS.
PREVALENCE
Prevalence estimates for IBS have varied anywhere from 3% to 20% in the United States, with similar results reported elsewhere; however, prevalence estimates are influenced substantially by the definition applied. For example, in Olmsted County, Minnesota, the prevalence of IBS varied from 8% to 22% depending on the criteria used.33
Younger people have a higher prevalence of IBS in the community. Generally, it is believed that IBS is uncommon in the elderly, but population-based studies indicate that IBS increases with advancing age. Thus, for example, using three or more of the Manning criteria to define IBS, the prevalence of IBS in Olmsted County ranged from 8% in those 65 to 74 years of age to more than 12% in those older than 85 years.34 Obviously, organic disease is more prevalent in elderly persons and could account for some of the reported IBS-like symptoms, but it seems likely that IBS in the elderly is often underdiagnosed or misdiagnosed, for example, as diverticular disease.35
GENDER AND RACE
Gender-specific prevalence rates for IBS are approximately two female to one male in most studies, and all population-based studies have reported a female predominance.17,36 Healthy women have greater rectal sensitivity, slower colonic transit, and smaller stool outputs than do men, which might explain why certain symptoms, such as straining and passage of hard stools, seem to be more common in women.37,38 In clinical practice in the United States, women outnumber men, which partly is explained by increased health care–seeking behavior among women; this appears to be culturally derived, because data from India indicate more men than women present for care of IBS in that part of the world.39
The prevalence of IBS generally is similar in whites and blacks, although some data have suggested it may be lower in Hispanics than in non-Hispanic whites in the United States.40,41 IBS is common in China, Japan, South America, and the Indian subcontinent. Indeed, IBS is common and its prevalence comparable in all countries where it has been studied.39,42–45
SUBGROUPS
Subdividing IBS based on the predominant symptom pattern is attempted commonly, but few data are available on IBS prevalence by symptom subgroup. Moreover, it is unclear if those with one predominant symptom—diarrhea or constipation—if followed long enough, eventually develop the other, namely, constipation in patients with IBS-D or diarrhea in patients with IBS-C; some data from primary care support this contention.46 The Rome III definition uses stool form to subclassify IBS, but definitions of IBS subgroups remain arbitrary, and different definitions have been used in different studies (see Fig. 118-1).3 Nonetheless, in a study from Olmsted County, Minnesota, 5.5% of the population had IBS-D and 5% IBS-C; both diarrhea and constipation-type symptoms occurred in 5% (IBS-M), and 4% did not meet strict criteria for either constipation or diarrhea.47 Another population-based study found higher rates of constipation in community subjects with IBS.48 In a study of 317 patients recruited for a clinical trial, at baseline 36% had IBS-D, 34% had IBS-C, and 31% had IBS-M49; more than 75% switched type over a year of follow-up, usually to and from the IBS-M pattern, but less than a third changed from IBS-D to IBS-C or vice versa.49
INCIDENCE AND DISAPPEARANCE OF SYMPTOMS
The onset rate of IBS was 67 per 1000 person-years by applying the Manning criteria to a cohort in Olmsted County that was surveyed at a 12- to 20-month interval.50 This study did not exclude people with a past history of IBS, however, and hence this is not the true incidence.50 Another study reported that the incidence of a clinical diagnosis of IBS in Olmsted County was 0.2% per year; this figure reflects the lower end of the incidence rate, because people with IBS symptoms who did not seek consultation could not be included in this calculation.51 Over a 10-year follow-up, 15% of community subjects free of baseline IBS symptoms developed the syndrome.32
In a follow-up study in Olmsted County, 38% of subjects meeting the definition of IBS at entry did not meet these criteria 12 to 20 months later50; they lost their symptoms. The actual prevalence of IBS did not change from year to year, however, because the disappearance of symptoms in some patients with IBS was balanced by others who developed IBS. Among those losing IBS in the community, there is a subset in whom symptoms evolve to reflect another functional gastrointestinal disorder52,53; hence, IBS usually is a chronic disorder, although symptoms often are variable.
RISK FACTORS
The best-accepted risk factor for IBS is bacterial gastroenteritis.54–57 The risk of postinfection IBS has been reported to be increased with depression,58 adverse life events and hypochondriasis,59 female gender, younger age, and prolonged duration of diarrhea following the initial attack.60 Bacterial virulence factors also may be important,61 but IBS can follow nonbacterial enteritis, including Norovirus gastroenteritis, or infection with trichinella.62
Other risk factors for IBS include an affluent childhood environment,63 estrogen use, postmenopausal estrogen use,64 recent antibiotic use,65 food intolerance,66,67 extraintestinal somatic symptoms,67 and poor quality of life.32 IBS runs in families,68 and low birth weight is also a risk factor for IBS, even after controlling for genetic influences.69 In contrast, oral glucocorticoid users may be at a lower risk for IBS.70 IBS is associated with an approximately three-fold increased risk of ischemic colitis71; however, a cause-and-effect relationship has not been established and the absolute risk remains very small (43 per 100,000 person-years).
HEALTH CARE SEEKING
Understanding why a patient is presenting for care is important in terms of planning appropriate management strategies. Burden of illness studies estimate that there are 3.6 million physician visits for IBS in the United States annually.72 The rate of health care seeking for IBS may, in part, be affected by health care access; consulting rates in the United States have varied between 25% and 46%, but up to 40% of patients do not have easy access to health care in this country.73 In Australia, where health care access essentially is universal, consulting rates have been 73%.73
Drivers of health care seeking remain poorly documented.73 The severity and chronicity of symptoms, in particular abdominal pain, partly promote health care seeking.74,75 IBS patients are more concerned about their health and more fearful of illness, suggesting that anxiety about their illness may be another factor.73 Children of a parent with IBS may see physicians more often than those who do not have a parent with IBS.76 As adults, they also are more likely to report poorer health in childhood as well as having received greater parental attention and gifts or rewards for being ill, suggesting there may have been early childhood programming of abnormal illness behavior.77 Those who seek medical attention tend to be more disturbed psychologically than those who do not seek such consultation,78–81 and those who consult for IBS also are more likely to consult about relatively minor complaints as well as other nongastrointestinal somatic symptoms.73 There remain other unknown factors that must be important, however, because these psychological factors still seem to poorly explain observed health care–seeking rates.73
EXCESS ABDOMINAL SURGERY
There is evidence that patients with IBS are at risk for undergoing excess surgery.82–84 In a large health maintenance organization study, patients with IBS, compared with controls, reported having had more cholecystectomies (12% vs. 4%), appendectomies (21% vs. 12%), and hysterectomies (33% vs. 17%); IBS was associated independently with these operations.82 A full explanation for these findings is uncertain, but presumably, some of this excess surgery reflects misdiagnosis and inappropriate intervention.85 It also is possible that IBS could predispose to an excess of certain diseases that lead to surgery. For example, constipation is associated with an increased risk of gallstones,86 but whether this association applies to IBS-C is uncertain. Alternatively, a history of a biliary event—identification of gallstones or a cholecystectomy—is associated with an increased risk of new-onset IBS.87 Some surgeons continue to believe that patients with IBS-type symptoms respond favorably to intra-abdominal surgery, although the evidence is anecdotal and probably reflects a placebo response.85
IMPACT ON QUALITY OF LIFE AND COSTS
A systematic review concluded that there was good evidence for a decrease in health-related quality of life in patients with moderate to severe IBS88 and that the quality of life in IBS is impaired to a degree comparable with other chronic disorders such as depression or GERD. Rather than IBS causing impaired quality of life, an alternative explanation for this association is that poor quality of life predisposes to a higher risk of IBS, and some evidence supports this contention.32 Regardless, the presence of impaired quality of life in IBS indicates that IBS deserves serious attention and therapeutic intervention.88
IBS is associated with substantial costs because of days lost from work, excess physician visits, diagnostic testing, and use of medications.89–91 Patients with IBS miss three times as many days from work as do those without bowel symptoms.92 IBS is the sixth leading physician diagnosis in outpatients in the United States, and this is likely an underestimate.93 A comprehensive burden-of-illness study in the United States estimated that IBS cost $1.6 billion in direct costs and a staggering $19.2 billion in indirect costs.72 Moreover, patients with IBS consume over 50% more health care resources than do matched controls without IBS.89
PATHOPHYSIOLOGY
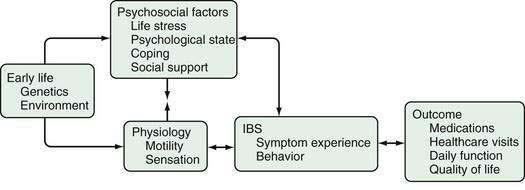
A number of different mechanisms have been implicated in the pathogenesis of IBS, including abnormal motility, visceral hypersensitivity, low-grade inflammation, and stress.1,8,94 Genetic factors could modulate the processing of gastrointestinal signals centrally and the inflammatory and immune responses locally, possibly predisposing to IBS. It seems reasonable to postulate that for IBS to manifest, several abnormalities—multiple hits—might need to occur. Some authors, therefore, conceptualize IBS as “a discrete collection of organic bowel diseases,”8 whereas other experts are concerned about “organification” of IBS because it might reduce the emphasis on the biopsychosocial model1,95 and imply that biological factors are sufficient to cause the disease. It seems likely that in IBS, an understanding of the individual, including his or her psychosocial nature and response to environmental factors, influences the expression of any biological determinants (Fig. 118-2). Regardless, further major therapeutic advances in the field seem unlikely to occur until the specific biological basis for symptoms is better identified.
ALTERED COLONIC AND SMALL BOWEL MOTILITY
In IBS, diarrhea can occur from multiple colonic mechanisms including increased high-amplitude propagated contractions (HAPCs), an enhanced gastrocolic response (prolonged rectosigmoid motor activity in response to a meal), or rectal hypersensitivity.96–98 Constipation may be secondary to increased segmental (nonpropulsive) contractions, decreased HAPCs, or reduced rectal sensation.99–101 Colonic and small bowel transit has been documented to be delayed in IBS-C and accelerated in IBS-D.1,101,102
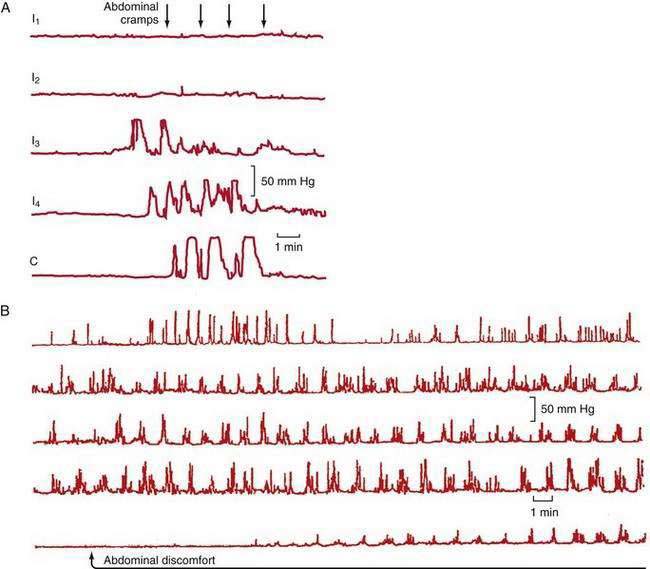
Abdominal pain in IBS also may be associated with HAPCs.103 A greater increase in phasic contractions in the terminal ileum and colon has been observed following distention, fatty meals, and cholecystokinin in patients with IBS (Fig. 118-3A).104 Discrete clusters of jejunal contractions also have been noted with increased frequency and duration in IBS (Fig. 118-3B), and they have been associated with pain in limited numbers of patients with IBS.104
Colonic motility in IBS can be increased by stress, anger, or instillation of deoxycholic acid, but this increase, although greater than in controls, is not specific for IBS1; a greater increase in colonic phasic contractions has been observed after administration of corticotropin-releasing hormone (CRH),105 and this increase is reduced by a CRH antagonist.106 Patients with IBS also have greater small bowel motor stimulation than do controls after cholecystokinin infusion, a fatty meal, or ileal distention.107 Autonomic dysfunction also has been reported in IBS patients with sympathetic adrenergic dysfunction associated with diarrhea and vagal dysfunction with constipation.108
VISCERAL HYPERSENSITIVITY
In the 1970s, balloon distention in the rectum was shown to induce pain at lower volumes in patients with IBS109; this has been confirmed in multiple studies using the barostat balloon that controls for changes in compliance, leading to the suggestion that colonic hypersensitivity is a useful biological marker of IBS.110,111 Visceral hypersensitivity might explain the fact that IBS patients seem more likely than controls to be aware of the presence of gas or intestinal contractions after meals or stress. Visceral hypersensitivity probably is confined to the intestine because somatic pain thresholds are normal, although not all studies agree on this.112–114
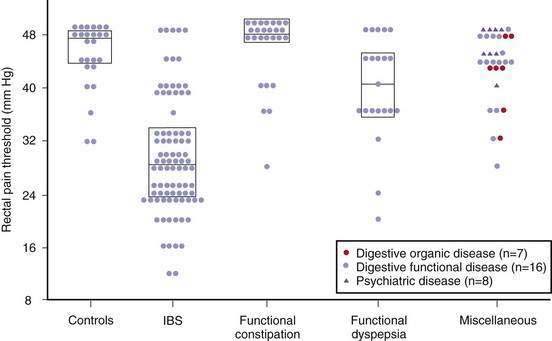
Visceral hypersensitivity is not a universal finding in patients with IBS and affects about 60% of patients (Fig. 118-4).115 In contrast to control subjects, patients with IBS and normal baseline visceral hypersensitivity might have rectal hypersensitivity induced by repeated distention of the sigmoid colon.116 This suggests that in IBS there is abnormal sensitization within the dorsal horn of the spinal cord or higher up in the central nervous system.
Putative neurotransmitters that are of relevance to visceral hypersensitivity include serotonin, neurokinins, and calcitonin gene-related peptide.117 The capsaicin (red pepper) receptor on nerve fibers, also called transient receptor potential vanilloid-1 (TRPV1), appears to be increased in the rectosigmoid colon in IBS and might mediate visceral pain.118 The N-methyl-d-aspartate (NMDA) receptor also may be important, because it modulates central (spinal cord) neuronal excitability.119 Visceral sensitivity, at least in the esophagus, can be reduced by an NMDA receptor antagonist.119
Serine proteases are thought to act as signaling molecules via the activation of proteinase-activated receptors (PARs). Extracts derived from colonic mucosal biopsies of patients with IBS (but not controls) have been observed to sensitize murine nerves in culture; this was blocked by a serine protease inhibitor.120 A significant increase in serine proteases has been observed in the stools of patients with IBS-D.121 Serine proteases could potentially damage tight junctions and increase intestinal permeability via PAR activation.122 The origin of stool serine proteases is uncertain, but they might derive from mast cells or the fecal microbiota.122
It is possible that inflammation is responsible for the sensitization in a subset of patients with IBS, as discussed later; however, some of this decreased threshold to balloon distention may be attributed to hypervigilance or excessive attention to, or fear of, a painful stimulus.123
ABNORMAL GAS PROPULSION AND EXPULSION
Ambulatory monitoring of abdominal girth has revealed that the abdomen normally swells during the day, peaking in the late evening, but decreasing on lying down; this phenomenon often is exaggerated in IBS.124 Retention of gas following gas infusion into the small intestine is greater in patients with IBS than it is in healthy controls.125 Furthermore, in those with IBS, intestinal gas infusion induces more discomfort than it does in controls when subjects are asked to voluntarily suppress passing the gas.125 During gas infusion, IBS patients, in contrast to healthy controls, involuntarily suppress their abdominal wall muscle contraction, which might explain their tendency to become distended; this could reflect an abnormal intestinal-somatic reflex response.126
Small intestinal bacterial overgrowth (SIBO) has been speculated to contribute to bloating in IBS, but this is not established with certainty.127,128 Although modest increases of bacteria have been documented in the proximal small intestine in IBS, SIBO rates do not differ between patients with IBS and controls.129 Similarly, lactulose breath testing, a surrogate marker of SIBO, often is abnormal in IBS using published criteria, but careful studies in controls suggest the rates of abnormality do not differ.130 Methane gas production by methagenic fecal flora, which occurs in the minority of the population, is now well established to be associated with constipation,128,131 possibly via slowing of intestinal transit in predisposed persons.132
Intravenous neostigmine has been demonstrated to clear retained intestinal gas and to reduce abdominal symptoms in patients with IBS and functional bloating.133 Physical activity might also enhance gas transit,134 and thus is to be encouraged.
LOCAL INFLAMMATION
The normal intestine is chronically in a state of inflammation, which occurs because of the balance between commensal enteric organisms and the host immune system. Inflammatory cells, including mast cells,135–138 and activated T lymphocytes139,140 are increased above normal in the mucosa in a subset of patients with IBS, suggesting that a low-grade inflammatory bowel disease may be present. Furthermore, lymphocytic infiltration of the myenteric plexus associated with neuron degeneration has been observed in severe IBS,141 as have increased mast cells in the muscularis externa.142 The cause of these abnormalities is unknown, but infections, abnormal bacterial flora, bile, or food antigens all could be contributors.
From 7% to 30% of patients who have recovered from a proved episode of bacterial enteritis develop IBS.54–56,58,60,61 One study, however, has suggested that those with pre-existing IBS who develop gastroenteritis may be more likely to seek medical consultation, thereby inflating the apparent risk estimates of this group.143 If the illness lasts more than three weeks or there are organisms involved that are toxigenic, then the risk of postinfection IBS is increased.61 Furthermore, those with psychological distress might have a further increased risk of postinfection IBS.55,58,59 In those who develop postinfection IBS, there are increases in CD3, CD4, and CD8 T lymphocytes, macrophages, and enteroendocrine (enterochromaffin) cells (Fig. 118-5).58,139 Increased small intestinal permeability as demonstrated by the lactulose-mannitol test also has been reported to occur in postinfection IBS61,139; however, this test is confounded by intestinal transit and bacterial overgrowth, and whether abnormal intestinal permeability occurs remains speculative.
Mast cells may play a central role in IBS. Activated mast cells release tryptase and histamine and have been observed to lie in close proximity to colonic nerves in patients with IBS; this finding has been correlated with abdominal pain.144 Supernatants prepared from colonic mucosal biopsies of patients with IBS have been shown to excite rat nociceptive visceral sensory nerves, suggesting that mast cell mediators, including tryptase, histamine, and prostaglandin E2, might represent another mechanism inducing visceral hypersensitivity in IBS.145
Colonic inflammation is associated with the production of a number of important mediators including 5-hydroxytryptamine (5-HT), prostaglandins, bradykinins, adenosine, and nerve growth factors.8 Abnormal release of 5-HT might have a central role in the manifestations of IBS.146 More than 95% of 5-HT is located in the enteroendocrine cells of the intestine and 5-HT is released from these cells following stroking or increased pressure, such as after a meal; 5-HT then acts on primary intrinsic afferent neurons to initiate the peristaltic reflex by activation of ascending excitation and descending inhibition.8,147 5-HT is taken up again by a specific serotonin transporter (SERT) expressed in the enterocytes. There is some evidence that an exaggerated release of 5-HT in IBS can occur after a meal.148
It has been observed in rectal biopsy specimens that 5-HT molecular signaling may be abnormal in IBS. In one study, 5-HT reuptake was reduced in IBS and also in ulcerative colitis compared with controls, although 5-HT release was unaffected and the numbers of enteroendocrine cells were unchanged.149 The findings were similar in IBS patients with constipation or diarrhea, leading to the hypothesis that in IBS there is increased availability of mucosal 5-HT that can induce diarrhea, but if there is desensitization of 5-HT receptors, this leads to constipation or an alternating bowel pattern149; confirmatory data currently are not available.150
ROLE OF FOOD
Many patients with IBS attribute their symptoms to certain foods, with wheat, dairy products, citrus fruits, potatoes, onions, and chocolate most commonly implicated.151,152 In an uncontrolled trial, one half of the patients with IBS reported improvement with elimination diets.66
Wheat Intolerance or Allergy
Substantial amounts of wheat are eaten in Western countries, 10% to 15% of which is not digested by human enzymes.8 Furthermore, subtle forms of gluten intolerance may be present in some people with IBS who do not have any overt evidence of celiac disease (see Chapter 104).153 A six-month gluten-free diet improved symptoms in 70% of patients with IBS-D who were HLA DQ2 positive,154 compared with 20% who were negative. In the absence of a definitive diagnosis of celiac disease, however, a gluten-free diet remains of unproven benefit.8
Sugar Malabsorption
Symptoms of IBS can be confused with those of lactose intolerance.155 Lactose intolerance occurs with varying prevalence depending on one’s ethnic group and is seen in 10% of populations of northern European descent, 40% to 60% of those of Asian descent, 90% of Chinese, and 60% to 80% of Africans (see Chapter 101). In acquired hypolactasia, there is some residual ability to digest small amounts of lactose and because most people do not ingest more than 12.5 g of lactose a day, they do not suffer from this ingestion.156 Unless a lactose-intolerant patient regularly ingests substantial amounts of lactose, lactose intolerance cannot be the explanation for the symptoms.157
Fructose and sorbitol malabsorption might contribute to IBS symptoms in some patients; however, fructose and sorbitol malabsorption, with a prevalence of 30% in those with IBS, may be no more common in IBS than in the background population.158–162 In a double-blind rechallenge trial, 25 patients who had responded to fructose withdrawal were challenged with fructose or fructans; nearly 80% developed symptoms compared with less than 15% who were given glucose.161
ABNORMAL COLONIC FLORA AND SMALL INTESTINAL BACTERIAL OVERGROWTH
It has been suggested that the colonic flora could be abnormal in a subset of patients with IBS, resulting in increased colonic fermentation, production of excess gas, and development of symptoms.163 This has led to an interest in pre- and probiotic therapy for IBS.
Others have reported that there is a high prevalence of SIBO in IBS, based on hydrogen breath testing and the clinical response to nonabsorbable antibiotics.164,165 Abnormal hydrogen breath test results, however, can occur because of transit abnormalities, and any association with IBS remains to be established.130,166,167
CENTRAL DYSREGULATION
Visceral afferent signals from the intestine reach the brainstem and thalamus and are consciously perceived only occasionally, although there may be some subliminal registration of low-intensity signals.3 Abnormal modulation of visceral afferent signals can occur at multiple levels in visceral, spinal, and central regions. Based on cerebral blood flow changes, functional brain imaging studies (functional magnetic resonance imaging or positron-emission tomography [PET]) have suggested that there are alterations in the brain response to visceral stimuli in IBS. In IBS patients, greater activation of the mid-cingular cortex, an area that processes visceral signals, has been reported following delivered or anticipated rectal distention.168–170 These observations could explain why anxiety or stress can enhance perception of visceral pain, whereas relaxation or distraction decreases pain in IBS. Using hypnosis to selectively alter noxious stimuli, PET scanning revealed significant changes in anterior cingulate cortex activity.171 Sex differences in brain networks concerned with antinociceptive and autonomic responses following rectal distention in IBS also have been observed.172
PSYCHOLOGICAL FACTORS (see Chapter 21)
Depression, anxiety, and somatization are the most common psychiatric conditions that coexist in IBS; in referral practice, 40% to 94% of patients with IBS are so affected.173 Some have suggested that consultation (referral) bias explains the higher rates of psychological and psychiatric comorbidity in IBS compared with controls,1,79–81,174–176 but other data suggest the association is real.174–176 In patients with IBS, a history of sexual, physical, or emotional abuse also is reported more often than in those without IBS.177–179 Abuse has not been shown to alter rectal sensation180 but it might modulate central brain responses to pain.181,182 Patients with IBS are more likely to report greater lifetime and daily stressful events than are those with organic disease or healthy controls and may be more susceptible to stress-altering gastrointestinal function.1
Childhood stress may be particularly important.94 Gastric suction at birth was associated with a subsequent three-fold increased risk of having a hospital admission for unexplained abdominal pain (or GI symptoms) compared with sibling controls who had not undergone gastric suction.183 In rats, maternal separation in the perinatal period induces an anxiety state and is associated with visceral hypersensitivity.94 Furthermore, in rats, moderately severe stress leads to the release of corticotropin-releasing factor and acceleration of colonic transit.184 Stress in healthy volunteers changes intestinal secretion and permeability responses.185 Sustained stress could, therefore, be important in both the onset and persistence of IBS.
Anxiety and depression, rather than being a primary problem, might occur secondary to production of proinflammatory cytokines.186 In IBS, immune activation of the intestine has been linked to elevated TNF-α levels and anxiety, suggesting that anxiety in IBS might occur secondary to intestinal inflammation in some cases.187 Mast cells communicate with the enteric and central nervous systems; an excess of mast cells in the colon appears to be associated with depression and fatigue in IBS.138
GENETIC FACTORS
Limited, but increasing, evidence points to at least a small hereditary component of IBS. There is clustering of IBS in families.68,188,189 Twin studies generally have shown that there is a greater concordance of IBS in monozygotic compared with dizygotic twins, suggesting a modest genetic component, although the environmental component probably is much greater.69,190–193
Potential candidate genes have been reported to be associated with IBS, but further confirmatory evidence is required and their functional significance must be unraveled.194 For example, patients with IBS have been reported to have significantly lower frequencies of the high-producer genotypes of interleukin (IL)-10. A lower amount of this anti-inflammatory cytokine might predispose to greater inflammation in response to an infectious insult in IBS195; others have failed to confirm these observations.196 A specific sodium channel mutation has been identified in IBS (SCN5A).197 Associations with polymorphisms of the promoter region of the serotonin transporter gene in IBS have not been consistent.197–199 A functional variant in the serotonin type III receptor gene may be associated with IBS-D in women.200 Common genetic factors do not appear to explain the association of IBS with depression.201
DIAGNOSIS
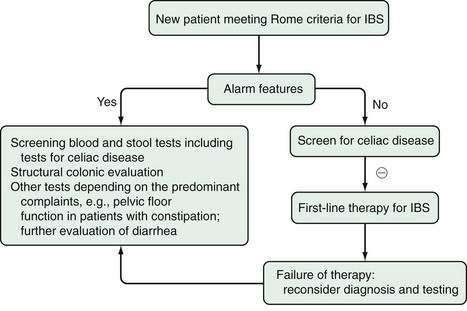
In patients who present with IBS-like symptoms, there are a number of alarm features (or red flags) that clearly warrant prompt investigation. These include any history of bleeding or evidence of anemia, a history of unexplained weight loss, unexplained vomiting, progressive dysphagia, a family history of malignancy, and new-onset symptoms in older age (Table 118-2).155,202,203 The traditional alarm features, however, have been documented to have poor diagnostic utility.1 For example, night-time symptoms are common in IBS and appear not to discriminate IBS from organic disease,202 although most physicians would still investigate patients who were being awakened in the middle of the night by pain or those with nocturnal diarrhea. Any patient 50 years of age or older requires a structural evaluation of the colon, if one has not been previously performed. The preference remains colonoscopy to exclude other disease and, in particular, colon cancer. Although older persons can develop IBS, risk of organic disease increases with age. Furthermore, colon cancer screening in those 50 years of age and older, even with no symptoms, currently is recommended in the United States.
Table 118-2 Alarm Features Considered Potentially Relevant in the Diagnosis of Organic Disease as Opposed to Irritable Bowel Syndrome
| History |
Adapted from Olden KW. Diagnosis of irritable bowel syndrome. Gastroenterology 2002; 122:1701-14.
Patients who meet the Rome criteria for IBS and who have no alarm features are less likely to have a cause for their presentation other than IBS. For example, among 98 patients who met the Rome I criteria and had no alarm features, of whom 50% had been referred because of diagnostic uncertainty, the Rome I criteria had a positive predictive value of up to 100% in this setting, although their sensitivity was only 65%.204
Systematic reviews have evaluated the value of diagnostic tests in IBS.9 The results, based on limited numbers of referred patients, suggested that IBS patients do not have an increased risk of most organic diseases compared with non-IBS controls (Table 118-3). Extensive investigations to exclude most possibilities are expensive and carry the danger of reinforcing abnormal illness behavior. There also is the real risk of uncovering findings that are irrelevant to the diagnosis but that precipitate yet more expensive and even dangerous investigations.
Table 118-3 Frequency of Organic Disease in Patients Meeting Symptom-Based Criteria for Irritable Bowel Syndrome
| ORGANIC DISEASE | IBS PATIENTS (%) | GENERAL POPULATION (%) |
|---|---|---|
| Inflammatory bowel disease | 0.51-0.98 | 0.3-1.2 |
| Colorectal cancer | 0-0.51 | 0-6 (varies with age) |
| Celiac sprue | 4.7 | 0.25-0.5 |
| Gastrointestinal infection | 0-1.5 | NA |
| Thyroid dysfunction | 6 | 5-9 |
| Lactose malabsorption | 22-26 | 25 |
NA, not applicable.
Data from Cash B, Schoenfeld P, Chey W. The utility of diagnostic tests in irritable bowel syndrome patients: A systematic review. Am J Gastroenterol 2002; 97:2812-19.
Traditional screening tests that have low yields in IBS patients include a full blood count, renal and liver biochemical testing, thyroid function testing, and evaluation of three fresh stool samples for parasites; these are inexpensive tests and they can be reassuring for both patient and physician if they are negative or normal.8 Crohn’s disease can be missed. An elevated C-reactive protein, although nonspecific, can indicate the presence of undiagnosed Crohn’s disease but often is negative in this clinical setting. Fecal calprotectin has been shown to discriminate IBS from Crohn’s disease with excellent sensitivity and specificity.14,205 In contrast, evaluation of the small intestine either radiologically or via capsule endoscopy has a very low yield in the setting of typical IBS symptoms without alarm features; only if there is concern should such investigations be considered. Hydrogen breath testing to identify lactose intolerance or SIBO cannot be endorsed routinely.8 Bile salt malabsorption causing diarrhea in the setting of IBS occurs uncommonly, and a therapeutic trial of cholestyramine is probably more useful than a 75-seleno-homocholic acid-taurine (Se-CAT) test.8,206,207 If diarrhea is persistent, colonoscopy with biopsy may be considered, although the yield of colonic biopsy remains low, and this test is probably not cost-effective.9 In a setting of severe constipation, exclusion of pelvic outlet obstruction with anorectal manometry including balloon expulsion testing should be considered because the result might alter management.1,2,8
Routine testing for celiac disease is now recommended in patients with typical symptoms of IBS-D.1 Data from the United Kingdom have shown a seven-fold increased risk of celiac disease in patients with IBS; up to 5% of patients with symptoms consistent with IBS had celiac disease compared with 0.5% of controls,208 but much lower rates have been observed in the United States.209 Decision analysis suggests that testing is cost-effective unless the prevalence of celiac disease falls to less than 1% in those with IBS-like symptoms.210 A screening test (tissue transglutaminase antibody) in those consuming a normal diet, followed by duodenal biopsies in those with a positive test, should be considered unless the background prevalence of celiac disease is very low in the patient population being seen. Latent celiac disease (antibody positive, biopsy normal) might respond to a gluten-free diet, but its prevalence in IBS is unclear.153 Celiac disease can manifest with atypical features including bloating and constipation without diarrhea (see Chapter 104).211
In summary, the diagnosis of IBS can be made based on the history (with particular attention to presence or absence of the Rome criteria) and in the absence of any red flags. A normal physical examination can be reassuring for patient and physician. In this setting, the patient who responds to an empiric trial of therapy for IBS does not require any further diagnostic evaluation (Fig. 118-6).203 Those who fail to respond should undergo more-extensive evaluation, depending on the predominant symptoms.
TREATMENT
EDUCATION AND SUPPORT
IBS tends to be a life-long disorder, and establishment of a strong physician-patient relationship is key to providing the best clinical care (Table 118-4).8,212 Indeed, patients with IBS often perceive their physician as having a highly negative medical belief about the disorder, and this perception itself impedes best care.213,214 Other common perceptions of the care they receive include being mislabeled as psychologically disturbed and that they have not been provided with adequate medical information or support.213,214 A good physician-patient relationship has been associated with reduced use of medical services.215
Table 118-4 Management Recommendations for Irritable Bowel Syndrome
Adapted from Talley N, Spiller RC. Irritable bowel syndrome: A little understood organic bowel disease? Lancet 2002; 360:555-64.
It is important to discover why the patient has decided to visit the health care provider at the time he or she did. The reasons can vary: new life stressors, exacerbating factors in the diet or changes in medications, increased fear of serious disease, and the development of treatable psychiatric comorbidity. A hidden agenda such as seeking disability or new narcotic abuse sometimes explains the consulting behavior. In terms of providing optimal reassurance, it is important first to educate patients and then to actively reassure them. Patients typically want to understand why their symptoms have occurred; they also want to obtain validation that their symptoms are real. Specific education classes designed for those with IBS appear to be useful therapeutic interventions,216,217 although randomized, controlled trials have not been done to prove the effectiveness of these interventions.
A stepped-care approach depending on the severity of the presenting symptoms provides a useful guide for considering therapies (Tables 118-5 and 118-6).218
Table 118-6 Suggested Sequence of Treatment of the Irritable Bowel Syndrome
| PREDOMINANT SYMPTOM | FIRST STEP | SECOND STEP |
|---|---|---|
| Bloating |
SSRIs, selective serotonin reuptake inhibitors.
DIET
The standard of care for IBS typically has been a high-fiber diet.219–225 Data from available randomized, controlled trials indicate that fiber is of global benefit, and the number needed to treat [NNT] is 11.226,227 NNT is used to assess the effectiveness of a health-care intervention and is the number of patients who need to be treated to prevent one additional bad outcome. Fiber supplements often are better tolerated than introduction of dietary fiber. The best evidence for a benefit of fiber supplements comes from the studies of ispaghula (psyllium hydrophilic mucilloid; ispaghula husk).1 Wheat bran has been no better than placebo in IBS.226,227 Fiber is not helpful for pain, but it can benefit constipation and can sometimes firm up loose stools.
Many patients with IBS suspect that food intolerance may be relevant to their symptoms. It is useful to determine the amounts of milk and milk products being consumed to decide whether lactose intolerance testing should be considered. Clinical experience suggests that even in the setting of a diagnosis of lactose intolerance and typical IBS symptoms, more often than not, IBS symptoms persist despite withdrawal of all lactose in the diet, indicating that this is the chance overlap of common conditions. Fructose consumption has increased dramatically in the United States and in other developed countries; excess fructose could lead to some IBS-like symptoms that might be relieved by exclusion of this sugar.158–161 Reducing fatty foods, gas-producing foods, caffeine, or alcohol also may be helpful for some patients, but randomized, controlled trials to support these recommendations are lacking.
Exclusion diets can be useful in some cases. One randomized trial measured IgG antibodies to foods and then excluded those IgG positive foods from the diet in the active arm of the trial; in the sham arm, other foods from the diet were excluded in a blinded fashion.228 The exclusion of foods with a positive IgG antibody response provided therapeutic benefit in patients with IBS-C and IBS-D. A systematic review of food-exclusion diets has suggested that in IBS, 12% to 67% of patients will respond, but most of the data are uncontrolled.229 Cromoglycate did show some benefit in one study of patients with positive skin prick tests to foods, but these results remain to be confirmed.230
MEDICATION
Antispasmodics and Anticholinergics
In the United States, anticholinergics (dicyclomine, propantheline, belladonna, and hyoscyamine) continue to be used commonly for IBS.223,224 A meta-analysis of randomized, controlled trials concluded that antispasmodics were superior to placebo in the treatment of IBS.224 Overall, there was an improvement of abdominal pain and IBS global symptoms in the pooled analyses224; however, the quality of most of these trials was low, the results were mixed, and publication bias could not be excluded. Moreover, only the anticholinergic antispasmodics are available in the United States.1 Non-anticholinergic antispasmodics, unavailable in the United States, that appear to be efficacious include otilonium (imetropium) and certain selective calcium channel blockers (e.g., pinaverium).224
Anticholinergics clinically seem most useful for patients with postprandial pain when taken 30 minutes before eating. No advantage of sublingual or suppository over oral anticholinergic preparations has been documented in patients with IBS. Peppermint oil does appear to be efficacious in IBS for abdominal pain, and it is usually well tolerated; the NNT is 2.5.1 The usual dose is 0.2 mL three times a day 30 minutes before meals (swallowed not chewed). Hot peppermint tea also may be useful if there are associated upper GI symptoms, but no controlled trial data exist regarding this agent.231
Laxatives
The efficacy of this class of drugs for IBS-C is uncertain. No randomized, controlled trials of laxatives in IBS are available. Osmotic laxatives often are prescribed but can aggravate bloating and pain. Polyethylene glycol, although not approved for this indication, does seem useful in practice, particularly if constipation is troublesome despite other therapy.232,233 Stimulant laxatives are probably safer than has been appreciated, but they often induce abdominal cramping or pain and generally seem unsatisfactory for patients with IBS.234
Lubiprostone is a chloride channel activator that stimulates intestinal fluid secretion. In IBS a lower dose of lubiprostone is currently approved for women with IBS-C than is used for chronic constipation (8 µg twice daily); the global benefit over placebo in IBS-C, however, is modest.235
Antidiarrheals
Loperamide is established to be efficacious based on randomized, controlled trials in IBS-D, but it does not improve abdominal pain or bloating.226,236–238 Loperamide is most effective when taken prophylactically, rather than being taken after diarrhea has occurred; doses of loperamide range from 2 to 16 mg/day, and high doses seem safe. Diphenoxylate has not been tested in IBS but may be similarly efficacious. Codeine phosphate, because of its side effects (dizziness, nausea, sedation) and high risk of inducing dependence, should be avoided in IBS.
Serotonin-Receptor Drugs
Alosetron is a 5-HT3 antagonist that is efficacious in women with severe IBS-D.239 The NNT is 8,239 and it has been shown to improve quality of life.240 The starting dose is 0.5 to 1 mg daily. In the United States, it is available only via a restricted prescribing program because of concerns about ischemic colitis and severe constipation.241 The dose can be increased to 1 mg twice daily after four weeks if symptoms are not controlled and there have been no side effects. Ischemic colitis occurs in 0.1% of alosetron-treated patients and is drug-related but dose independent; the ischemia is usually transient and without irreversible consequence, although up to 50% of patients with alosetron-associated ischemic colitis required hospitalization.242 Constipation occurs in one third of alosetron-treated patients. The prescription of the drug is absolutely contraindicated in IBS patients with any history of constipation, thrombotic tendency, or ischemic colitis. Pharmacogenomic data suggest that alosetron may be more efficacious in those with the long homozygous polymorphism of the SERT transporter gene, but this needs confirmation.243
Antidepressants and Anxiolytics
The tricyclic antidepressants appear to be efficacious in IBS but might improve global well-being more than symptoms.244 Meta-analyses have reported the NNT to be between 3 and 4, although these studies included a number of low-quality and potentially flawed trials.1,223,245 A large, high-quality randomized, controlled trial of desipramine (at a dose of 50 to 150 mg) versus placebo in female patients showed that 60% were responders to the TCA versus 47% to placebo; this difference failed to reach significance in the intention-to-treat analysis but was significant in the per-protocol analysis.246
When using a TCA in IBS, it is recommended to start it at a low dose (e.g., 10 to 25 mg of desipramine or nortriptyline) and increase the dose by 10 to 25 mg weekly, aiming for a dose of 50 mg initially. Many patients do not require full antidepressant dosing, unless comorbid depression is present. TCAs tend to be constipating, and therefore they may be of most benefit in IBS-D, although the data supporting this postulate are unclear. Adverse events with TCAs are a problem. Approximately one in three to one in four treated patients develops side effects, with one in 22 having potentially serious reactions; up to 40% discontinue use or change therapy because of intolerance.247
The selective serotonin reuptake inhibitors (SSRIs) cause fewer side effects than the TCAs, and a meta-analysis of the randomized, controlled trials in IBS has reported a global benefit of SSRIs with an NNT of 3.5.1 In one trial that lacked a placebo arm, paroxetine was compared with usual care in IBS patients and shown to improve quality of life but not abdominal pain.248 Other studies showed some benefit for pain or no effect on pain249,250; the findings remain inconsistent. It is possible that SSRIs may be more beneficial in IBS-C because they accelerate small intestinal transit.251
Benzodiazepines might have a small benefit over placebo in IBS, but the evidence for this observation is very weak.221 Because of habituation, this class of drugs generally should be avoided.
Antibiotics
Nonabsorbable antibiotics such as rifaximin appear to be superior to placebo in IBS in short-term treatment studies, especially for bloating and diarrhea,1 but long-term results are unavailable.164,165,252 The daily dose of rifaximin typically is 400 mg for 10 days. Any benefit can last at least 10 weeks, but treating a recurrence of IBS symptoms with another course cannot currently be recommended.1
Probiotics
It has been suggested that abnormal colonic flora could be relevant in the pathogenesis of IBS, which has led to great interest in using probiotics to try to naturally alter the flora. Some initial small trials produced promising results with Bifidobacterium infantis and combination products,253,254 but the findings have been variable.194 Such variation might reflect the strain and dose used, whether live or dead organisms were given, and other unknown factors. A capsule containing live organisms (more than 1 billion) given once a day is the usual dosing recommendation.
Other Drugs
Anticonvulsants have analgesic properties. Phenytoin was not shown to be beneficial in a randomized trial.255 Gabapentin might have some therapeutic value for pain of IBS anecdotally. Pregabalin is approved for neuropathic pain and fibromyalgia; anecdotally, some patients with IBS respond.256 Beta blockers can inhibit colonic motor function, but neither atenolol nor timolol was better than placebo in small trials.257,258 Leuprolide is a gonadotropin-releasing hormone analog that was superior to placebo in terms of reducing abdominal pain and nausea,259,260 but the entry criteria for these studies were vague and side effects of chemical castration were significant; this drug cannot be routinely recommended.
Colchicine increases spontaneous bowel movements and decreases colonic transit time,261 but its role in IBS with constipation is unknown. Misoprostol may be helpful for refractory constipation,262 but no controlled trials in IBS are available. Domperidone and erythromycin are prokinetic agents used predominantly to treat upper gastrointestinal tract disorders and probably are not efficacious in IBS.221 Pyridostigmine is a parasympathomimetic that enhances intestinal motility and might benefit patients with slow-transit constipation, but data in IBS are lacking.222
Octreotide reduces intestinal transit time, secretion, and sensation in IBS263 but is impractical to use for diarrhea in IBS. Clonidine, an α2-receptor agonist, may be useful in IBS-D because it enhances rectal compliance and reduces fasting colonic motor activity264; however, significant side effects limit its use, particularly postural hypotension, sedation, fluid retention, and depression.
There is increasing interest in testing anti-inflammatory drugs for IBS. Three weeks of oral prednisone (30 mg/day) failed to improve postinfection IBS symptoms in one placebo-controlled trial.265 The role of 5-aminosalicylic acid compounds in IBS is unknown, but these agents might have antinociceptive and mast cell-stabilizing actions.266
There is no convincing evidence that either simethicone267 or charcoal works to relieve the symptoms of excessive gas in IBS.268,269 Beano (α-galactosidase) might reduce flatus but not other IBS symptoms.270 Malodorous flatus might respond to zinc acetate, bismuth subsalicylate, a bulky charcoal cushion device, or peppermint oil.271–273
PSYCHOLOGICAL TREATMENTS
Psychotherapy, hypnotherapy, and cognitive behavioral therapy (CBT) have been proposed as useful treatments for IBS.1 A systematic review concluded that these therapies were superior to wait-list controls (who are followed similarly but are not treated).1 Hypnotherapy can improve cognition in IBS.274 Excellent efficacy data exist for CBT,95,248 albeit not in all controlled trials.275 There are no head-to-head studies comparing the different psychological interventions or combination therapy. Based on the available literature, IBS patients with abdominal pain, diarrhea, and psychological distress appear most likely to have a beneficial response to such intervention, particularly if the symptoms have been of short duration and have waxed and waned.1 Patients with constant abdominal pain do poorly with psychological treatment.1 Indeed, symptoms tend not to improve; rather, the ability to cope with IBS seems to drive any global benefit. The major advantage of psychological treatment is that despite the initial expense, long-term benefits might offset the cost.250
ALTERNATIVE TREATMENTS
Many different alternative remedies are used by patients with IBS.233,276 In one high-quality randomized, controlled trial, Chinese herbal medicine (comprising a combination of 20 herbs) was superior to placebo, although this requires confirmation277 and the risks of using multiple herbal concoctions continue to be of concern.278 An ayurvedic preparation also was superior to placebo,279 but the data here remain limited. Ginger (especially for nausea) and aloe are used often, but there are no randomized, controlled trials in IBS.276 St. John’s wort probably is not efficacious. Whether pancreatic enzymes help remains unknown, although a small double-blind cross-over study suggests some benefit for postprandial bloating.280 Acupuncture might reduce rectal hypersensitivity,281,282 but efficacy is not established in randomized, controlled trials.
PROGNOSIS
There is no evidence for even a small increase in mortality in IBS, despite referrals for invasive testing, excess abdominal and other surgery rates, and a link to ischemic colitis.283 In clinical practice, once a diagnosis of IBS has been made, it usually requires no revision despite prolonged follow-up. In 112 consecutive IBS patients followed through their medical records for a median of 29 years, survival in IBS was not different from expected, although 9% developed organic disease a median of 15 years after diagnosis.215 Among 75 patients with a clinical diagnosis of IBS followed up for 10 to 13 years, none had another explanation uncovered for their symptoms, yet symptoms did not resolve in 92% and 47% had undergone a repeat structural colonic evaluation to no avail.284 Some IBS patients have spontaneous improvement over time, but IBS usually is a relapsing disorder. The presence of excessive psychological distress or anxiety, as well as a long duration of complaints, tends to indicate a poorer prognosis.1
American College of Gastroenterology Task Force on IBS. Systematic review of the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2009;104(Suppl 1):S1-35. (Ref 1.)
Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702. (Ref 144.)
Bengtson MB, Ronning T, Vatn MH, et al. Irritable bowel syndrome in twins: Genes and environment. Gut. 2006;55:1754-9. (Ref 69.)
Bijkerk CJ, de Witt NJ, Muris JW, et al. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ. 2009;339:b3154.
Camilleri M, Atanasova E, Carlson PJ, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425-32. (Ref 245.)
Drossman DA, Corrazziari E, Delvaux M, et al. Rome III: the functional Gastrointestinal Disorders, 3rd ed. McLean, Va: Degnon Associates; 2006. (Ref 3.)
Drossman D, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19-31. (Ref 248.)
Ford AC, Talley NJ, Spiegel BM, et al. Efficacy of fibre, antispasmodics and peppermint oil in irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313. (Ref 224.)
Ford A, Talley NJ, Veldhuyzen van Zanten S. Will the history and physical examination reveal the cause of my patient’s lower gastrointestinal symptoms? JAMA. 2008;300:1793-805. (Ref 10.)
Gecse K, Roka R, Ferrier L, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: A colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591-9. (Ref 121.)
Halder SL, Locke GRI, Schleck CD, et al. Natural history of functional gastrointestinal disorders: A 12-year longitudinal population-based study. Gastroenterology. 2007;133:799-807. (Ref 53.)
Hammer J, Eslick G, Howell S, et al. Diagnostic yield of alarm features in irritable bowel syndrome and functional dyspepsia. Gut. 2004;53:666-72. (Ref 202.)
Hiatt RB, Katz L. Mast cells in inflammatory conditions of the gastrointestinal tract. Am J Gastroenterol. 1962;37:541-5. (Ref 142.)
Pimentel M, Lin HC, Enayati P, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1089-95. (Ref 132.)
Pimentel M, Park S, Mirocha J, et al. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: A randomized trial. Ann Intern Med. 2006;145:557-63. (Ref 254.)
Ringel Y, Drossman DA, Leserman JL, et al. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: An FMRI study. Gastroenterology. 2008;134:396-404. (Ref 182.)
Saito YA, Talley NJ. Genetics of irritable bowel syndrome. Am J Gastroenterol. 2008;103:2100-4. (Ref 194.)
Spiegel BM, DeRosa VP, Gralnek IM, et al. Testing for celiac sprue in irritable bowel syndrome with predominant diarrhea: A cost-effectiveness analysis. Gastroenterology. 2004;126:1721-32. (Ref 210.)
Talley N, Spiller RC. Irritable bowel syndrome: A little understood organic bowel disease? Lancet. 2002;360:555-64. (Ref 8.)
1. American College of Gastroenterology Task Force on IBS. Systematic review of the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2009;104:S1-35.
2. Eslick GD, Talley NJ. Natural history and predictors of outcome for non-cardiac chest pain: A prospective 4-year cohort study. Neurogastroenterol Motil. 2008;20:989-97.
3. Drossman DA, Corrazziari E, Delvaux M, et al. Rome III: the functional gastrointestinal disorders, 3rd ed. McLean, Va: Degnon Associates; 2006.
4. Longstreth GF, Burchette RJ. Family practitioners’ attitudes and knowledge about irritable bowel syndrome: effect of a trial of physician education. Family Practice. 2003;20:670-74.
5. Thompson WG, Heaton KW, Smyth GT, et al. Irritable bowel syndrome: the view from general practice. Eur J Gastroenterol Hepatol. 1997;97:689-92.
6. Wilson S, Roberts L, Roalfe A, et al. Prevalence of irritable bowel syndrome: A community survey. Br J Clin Pract. 2004;54:495-502.
7. Russo MW, Gaynes BN, Drossman DA. A national survey of practice patterns of gastroenterologists with comparison to the past two decades. J Clin Gastroenterol. 1999;29:339-43.
8. Talley N, Spiller RC. Irritable bowel syndrome: a little understood organic bowel disease? Lancet. 2002;360:555-64.
9. Cash B, Schoenfeld P, Chey W. The utility of diagnostic tests in irritable bowel syndrome patients: a systematic review. Am J Gastroenterol. 2002;97:2812-19.
10. Ford A, Talley NJ, Veldhuyzen van Zanten S. Will the history and physical examination reveal the cause of my patient’s lower gastrointestinal symptoms? JAMA. 2008;300:1793-805.
11. Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. BMJ. 1978;2:653-4.
12. Talley NJ, Phillips SF, Melton LJIII, et al. Diagnostic value of the Manning criteria in irritable bowel syndrome. Gut. 1990;31:77-81.
13. Kruis W, Thieme C, Weinzierl M, et al. A diagnostic score for the irritable bowel syndrome. Its value in the exclusion of organic disease. Gastroenterology. 1984;87:1-7.
14. Tibble JA, Sigthorsson G, Foster R, et al. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123:450-60.
15. Boyce PM, Koloski NA, Talley NJ. Irritable bowel syndrome according to varying diagnostic criteria: are the new Rome II criteria unnecessarily restrictive for research and practice? Am J Gastroenterol. 2000;95:3176-83.
16. Hillila MT, Farkkila MA. Prevalence of irritable bowel syndrome according to different diagnostic criteria in a non-selected adult population. Aliment Pharmacol Ther. 2004;20:339-45.
17. Cremonini F, Talley NJ. Irritable bowel syndrome: epidemiology, natural history, health-care seeking and emerging risk factors. Gastroenterol Clin North Am. 2005;34:189-204.
18. Talley NJ, Weaver AL, Zinsmeister AR, et al. Self-reported diarrhea: what does it mean? Am J Gastroenterol. 1994;89:1160-4.
19. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-24.
20. Degen LP, Phillips SF. How well does stool form reflect colonic transit? Gut. 1996;39:109-13.
21. Jiang X, Locke GR3rd, Choung RS, et al. Prevalence and risk factors for abdominal bloating and visible distention: a population-based study. Gut. 2008;57:756-63.
22. Reilly BP, Bolton MP, Lewis MJ, et al. A device for 24-hour ambulatory monitoring of abdominal girth using inductive plethysmography. Physiol Meas. 2002;23:661-70.
23. Riedl A, Schmidtmann M, Stengel A, et al. Somatic comorbidities of irritable bowel syndrome: a systematic analysis. J Psychosom Res. 2008;64:573-82.
24. Jung HK, Halder S, McNally M, et al. Overlap of gastro-oesophageal reflux disease and irritable bowel syndrome: prevalence and risk factors in the general population. Aliment Pharmacol Ther. 2007;26:453-61.
25. Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140-56.
26. Minderhoud IM, Itta M, Oldenburg B, et al. IBS-like symptoms in patients with inflammatory bowel disease in remission: relationships with quality of life and coping behavior. Dig Dis Sci. 2004;49:469-74.
27. Burgmann T, Clara I, Graff L, et al. The Manitoba Inflammatory Bowel Disease Cohort Study: prolonged symptoms before diagnosis—how much is irritable bowel syndrome? Clin Gastroenterol Hepatol. 2006;4:614-20.
28. Costanza CD, Longstreth GF, Liu AL. Chronic abdominal wall pain: clinical features, health care costs and long-term outcome. Clin Gastroenterol Hepatol. 2004;2:395-99.
29. Srinivasan R, Greenbaum DS. Chronic abdominal wall pain: a frequently overlooked problem. Practical approach to diagnosis and management. Am J Gastroenterol. 2002;97:824-30.
30. Scott EM, Scott BB. Painful rib syndrome—a review of 76 cases. Gut. 1993;34:1006-8.
31. Webb PM, Purdie DM, Grover S, et al. Symptoms and diagnosis of borderline, early and advanced epithelial ovarian cancer. Gynecol Oncol. 2004;92:232-39.
32. Ford AC, Forman D, Bailey AG, et al. Irritable bowel syndrome: A 10-yr natural history of symptoms and factors that influence consultation behavior. Am J Gastroenterol. 2008;103:1229-39.
33. Saito YA, Talley NJ, Melton LJIII, et al. The effect of new diagnostic criteria for irritable bowel syndrome on community prevalence estimates. Neurogastroenterol Motil. 2003;15:687-94.
34. Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
35. Bennett G, Talley NJ. Irritable bowel syndrome in the elderly. Best Pract Res Clin Gastroenterol. 2002;16:63-76.
36. Thompson WG. Gender differences in irritable bowel symptoms. Eur J Gastroenterol Hepatol. 1997;9:299-302.
37. Lee OY, Mayer EA, Schmulson M, et al. Gender-related differences in IBS symptoms. Am J Gastroenterol. 2001;96:2184-93.
38. Houghton LA, Lea R, Jackson N, et al. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471-4.
39. Gwee KA. Irritable bowel syndrome in developing countries—a disorder of civilization or colonization? Neurogastroenterol Motil. 2005;17:317-24.
40. Taub E, Cuevas JL, Cook EW3rd, et al. Irritable bowel syndrome defined by factor analysis. Gender and race comparisons. Dig Dis Sci. 1995;40:2647-55.
41. Zuckerman M, Guerra L, Drossman D, et al. Comparison of bowel patterns in Hispanics and non-Hispanic whites. Dig Dis Sci. 1995;40:1763-69.
42. Gwee KA, Wee S, Wong ML, et al. The prevalence, symptom characteristics, and impact of irritable bowel syndrome in an Asian urban community. Am J Gastroenterol. 2004;99:924-31.
43. Lau EM, Chan FK, Ziea ET, et al. Epidemiology of irritable bowel syndrome in Chinese. Dig Dis Sci. 2002;47:2621-24.
44. Lule GN, Amayo EO. Irritable bowel syndrome in Kenyans. East Afr Med J. 2002;79:360-63.
45. Olubuyide IO, Olawuyi F, Fasanmade AA. A study of irritable bowel syndrome diagnosed by Manning criteria in an African population. Dig Dis Sci. 1995;40:983-85.
46. Mearin F, Balboa A, Badia X, et al. Irritable bowel syndrome subtypes according to bowel habit: revisiting the alternating subtype. Eur J Gastroenterol Hepatol. 2003;15:165-72.
47. Mearin F, Baro E, Roset M, et al. Clinical patterns over time in irritable bowel syndrome: symptom instability and severity variability. Am J Gastroenterol. 2004;99:113-21.
48. Talley NJ, Zinsmeister AR, Melton LJIII. Irritable bowel syndrome in a community: symptom subgroups, risk factors and health care utilization. Am J Epidemiol. 1995;142:76-83.
49. Drossman DA, Morris CB, Hu Y, et al. A prospective assessment of bowel habit in irritable bowel syndrome in women: defining an alternator. Gastroenterology. 2005;128:580-9.
50. Talley N, Weaver A, Zinsmeister A, et al. Onset and disappearance of gastrointestinal symptoms and functional gastrointestinal disorders. Am J Epidemiol. 1992;136:165-77.
51. Locke GRIII, Yawn BP, Wollan PC, et al. Incidence of a clinical diagnosis of the irritable bowel syndrome in a United States population. Aliment Pharmacol Ther. 2004;19:1025-31.
52. Agreus L, Svardsudd K, Talley NJ, et al. Natural history of gastroesophageal reflux disease and functional abdominal disorders: a population-based study. Am J Gastroenterol. 2001;96:2905-14.
53. Halder SL, Locke GRI, Schleck CD, et al. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology. 2007;133:799-807.
54. Ilnyckyj A, Balachandra B, Elliott L, et al. Post–traveler’s diarrhea irritable bowel syndrome: a prospective study. Am J Gastroenterol. 2003;98:596-9.
55. Gwee KA, Graham JC, McKendrick MW, et al. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347:150-3.
56. Dunlop SP, Jenkins D, Neal KR, et al. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651-59.
57. Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome—a meta-analysis. Am J Gastroenterol. 2006;101:1894-9.
58. Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol. 2003;98:1578-83.
59. Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400-6.
60. Neal KR, Hebden JM, Spiller RC. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ. 1997;314:779-82.
61. Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662-71.
62. Soyturk M, Akpinar H, Gurler O, et al. Irritable bowel syndrome in persons who acquired trichinellosis. Am J Gastroenterol. 2007;102:1064-9.
63. Howell S, Talley NJ, Quine S, et al. The irritable bowel syndrome has origins in the childhood socioeconomic environment. Am J Gastroenterol. 2004;99:1572-8.
64. Ruigomez A, Garcia Rodriguez LA, Johansson S, et al. Is hormone replacement therapy associated with an increased irritable bowel syndrome? Maturitas. 2003;44:133-40.
65. Mendall MA, Kumar D. Antibiotic use, childhood affluence and irritable bowel syndrome (IBS). Eur J Gastroenterol and Hepatol. 1998;10:59-62.
66. Nanda R, James R, Smith H, et al. Food intolerance and the irritable bowel syndrome. Gut. 1989;30:1099-104.
67. Locke GRIII, Zinsmeister AR, Talley NJ, et al. Risk factors for irritable bowel syndrome: role of analgesics and food sensitivities. Am J Gastroenterol. 2000;95:157-65.
68. Saito YA, Zimmerman JM, Harmsen WS, et al. Irritable bowel syndrome aggregates strongly in families: a family-based case-control study. Neurogastroenterol Motil. 2008;20:790-7.
69. Bengtson MB, Ronning T, Vatn MH, et al. Irritable bowel syndrome in twins: genes and environment. Gut. 2006;55:1754-9.
70. Huerta C, Garcia Rodriguez LA, Wallander MA, et al. Users of oral steroids are at a reduced risk of developing irritable bowel syndrome. Pharmacoepidemiol Drug Saf. 2003;12:583-8.
71. Cole JA, Cook SF, Sands BE, et al. Occurrence of colon ischemia in relation to irritable bowel syndrome. Am J Gastroenterol. 2004;99:486-91.
72. Sandler R, Everhart J, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500-11.
73. Koloski N, Talley N, Boyce P. Predictors of health care seeking for irritable bowel syndrome and nonulcer dyspepsia: a critical review of the literature on symptom and psychosocial factors. Am J Gastroenterol. 2001;96:1340-49.
74. Koloski NA, Talley NJ, Huskic SS, et al. Predictors of conventional and alternative health care seeking for irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2003;17:841-51.
75. Talley N, Boyce P, Jones M. Predictors of health care seeking for irritable bowel syndrome: a population based study. Gut. 1997;41:394-98.
76. Levy RL, Whitehead WE, Von Korff MR, et al. Intergenerational transmission of gastrointestinal illness behavior. Am J Gastroenterol. 2000;95:451-6.
77. Whitehead WE, Winget C, Fedoravicius AS, et al. Learned illness behavior in patients with irritable bowel syndrome and peptic ulcer. Dig Dis Sci. 1982;27:202-8.
78. Hu WHC, Wong W-M, Lam CLK, et al. Anxiety but not depression determines health care-seeking behaviour in Chinese patients with dyspepsia and irritable bowel syndrome: a population-based study. Aliment Pharmacol Ther. 2002;16:2081-8.
79. Smith RC, Greenbaum DS, Vancouver JB, et al. Psychosocial factors are associated with health care seeking rather than diagnosis in irritable bowel syndrome. Gastroenterology. 1990;98:293-301.
80. Drossman DA, McKee DC, Sandler RS, et al. Psychosocial factors in the irritable bowel syndrome. A multivariate study of patients and nonpatients with irritable bowel syndrome. Gastroenterology. 1988;95:701-8.
81. Whitehead WE, Bosmajian L, Zonderman AB, et al. Symptoms of psychologic distress associated with irritable bowel syndrome. Gastroenterology. 1988;95:709-14.
82. Longstreth GF, Yao JF. Irritable bowel syndrome and surgery: a multivariate analysis. Gastroenterology. 2004;126:1665-73.
83. Kennedy TM, Jones RH. Epidemiology of cholecystectomy and irritable bowel syndrome in a UK population. Br J Surg. 2000;87:1658-63.
84. Hasler WL, Schoenfeld P. Systematic reivew: abdominal and pelvic surgery in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:997-1005.
85. Talley NJ. Unnecessary abdominal and back surgery in irritable bowel syndrome: time to stem the flood now? Gastroenterology. 2004;126:1899-903.
86. Veysey MJ, Thomas LA, Mallet AI, et al. Colonic transit influences deoxycholic acid kinetics. Gastroenterology. 2001;121:812-22.
87. McNally M, Locke GR, Zinsmeister AR, et al. Biliary events and an increased risk of new onset irritable bowel syndrome: A population-based cohort study. Aliment Pharmacol Ther. 2008;28:334-43.
88. El-Serag HB, Olden KW, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther. 2002;16:1171-85.
89. Inadomi JM, Fennerty MB, Bjorkman D. Systematic review: the economic impact of irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18:671-82.
90. Longstreth GF, Wilson A, Knight K, et al. Irritable bowel syndrome, health care use, and costs: a U.S. managed care perspective. Am J Gastroenterol. 2003;98:600-7.
91. Leong SA, Barghout V, Birnbaum HG, et al. The economic consequences of irritable bowel syndrome: a US employer perspective. Arch Intern Med. 2003;163:929-35.
92. Drossman D, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569-80.
93. Shaheen NJ, Hansen RA, Morgan DR, et al. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101:2128-38.
94. Mayer EA, Collins SM. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122:2032-48.
95. Drossman DA. The “organification” of functional GI disorders: implications for research. Gastroenterology. 2003;124:6-7.
96. Clemens CH, Samsom M, Van Berge Henegouwen GP, et al. Abnormalities of left colonic motility in ambulant nonconstipated patients with irritable bowel syndrome. Dig Dis Sci. 2003;48:74-82.
97. Chey WY, Jin HO, Lee MH, et al. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499-506.
98. Steens J, Van Der Schaar PJ, Penning C, et al. Compliance, tone and sensitivity of the rectum in different subtypes of irritable bowel syndrome. Neurogastroenterol Motil. 2002;14:241-8.
99. Vassallo MJ, Camilleri M, Phillips SF, et al. Colonic tone and motility in patients with irritable bowel syndrome. Mayo Clin Proc. 1992;67:725-31.
100. Cole SJ, Duncan HD, Claydon AH, et al. Distal colonic motor activity in four subgroups of patients with irritable bowel syndrome. Dig Dis Sci. 2002;47:345-55.
101. Camilleri MC, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastoenterol Hepatol. 2008;6:772-81.
102. Hutchinson R, Notghi A, Smith NB, et al. Scintigraphic measurement of ileocaecal transit in irritable bowel syndrome and chronic idiopathic constipation. Gut. 1995;36:585-9.
103. Clemens CH, Samsom M, Roelofs JM, et al. Association between pain episodes and high amplitude propagated pressure waves in patients with irritable bowel syndrome. Am J Gastroenterol. 2003;98:1838-43.
104. Kellow JE, Phillips SF. Altered small bowel motility in irritable bowel syndrome is correlated wtih symptoms. Gastroenterology. 1987;92:1885-93.
105. Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845-9.
106. Sagami Y, Shimada Y, Tayama J, et al. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958-64.
107. Kellow JE, Phillips SF, Miller LJ, et al. Dysmotility of the small intestine in irritable bowel syndrome. Gut. 1988;29:1236-43.
108. Aggarwal A, Cutts TF, Abell TL, et al. Predominant symptoms in irritable bowel syndrome correlate with specific autonomic nervous system abnormalities. Gastroenterology. 1994;106:945-50.
109. Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut. 1973;14:125-32.
110. Bouin M, Plourde V, Boivin M, et al. Rectal distention testing in patients wtih irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771-7.
111. Mertz H, Naliboff B, Munakata J, et al. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterol. 1995;109:40-52.
112. Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7-14.
113. Chang L, Mayer EA, Johnson T, et al. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain. 2000;84:297-307.
114. Cook IJ, van Eeden A, Collins SM. Patients with irritable bowel syndrome have greater pain tolerance than normal subjects. Gastroenterology. 1987;93:727-33.
115. Delvaux M. Role of visceral sensitivity in the pathophysiology of irritable bowel syndrome. Gut. 2002;51:i67-71.
116. Munakata J, Naliboff B, Harraf F, et al. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterol. 1997;112:55-63.
117. Buéno L, Fioramonti J, Garcia-Villar R. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications. III. Visceral afferent pathways: a source of new therapeutic targets for abdominal pain. Am J Physiol Gastrointest Liver Physiol. 2000;278:G670-76.
118. Akbar A, Yiangou Y, Facer P, et al. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923-9.
119. Willert RP, Woolf CJ, Hobson AR, et al. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-d-aspartate receptor. Gastroenterology. 2004;126:683-92.
120. Cenac N, Andrews CN, Holzhausen M, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636-47.
121. Gecse K, Roka R, Ferrier L, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591-9.
122. Barbara G, Cremon C. Serine proteases: new players in diarrhoea-predominant irritable bowel syndrome. Gut. 2008;57:1035-7.
123. Whitehead WE, Palsson OS. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology. 1998;115:1263-71.
124. Quigley EMM. From comic relief to real understanding; how intestinal gas causes symptoms. Gut. 2003;52:1659-61.
125. Serra J, Azpiroz F, Malagelada JR. Mechanisms of intestinal gas retention in humans: impaired propulsion versus obstructed evacuation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G138-43.
126. Villoria A, Azpiroz F, Soldevilla A, et al. Abdominal accommodation: A coordinated adaptation of the abdominal wall to its content. Am J Gastroenterol. 2008;103:2807-15.
127. Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852-8.
128. Grover M, Kanazawa M, Palsson OS, et al. Small intestinal bacterial overgrowth in irritable bowel syndrome: association with colon motility, bowel symptoms, and psychological distress. Neurogastroenterol Motil. 2008;20:998-1008.
129. Posserud I, Agerforz P, Ekman R, et al. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102-8.
130. Bratten JR, Spanier J, Jones MP. Lactulose breath testing does not discriminate patients with irritable bowel syndrome from healthy controls. Am J Gastroenterol. 2008;103:958-63.
131. Chatterjee S, Park S, Low K, et al. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol. 2007;102:837-41.
132. Pimentel M, Lin HC, Enayati P, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1089-95.
133. Caldarella MP, Serra J, Azpiroz F, et al. Prokinetic effects in patients with intestinal gas retention. Gastroenterology. 2002;122:1748-55.
134. Dainese R, Serra J, Azpiroz F, et al. Effects of physical activity on intestinal gas transit and evacuation in healthy subjects. Am J Med. 2004;116:536-9.
135. Weston AP, Biddle WL, Bhatia PS, et al. Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci. 1993;38:1590-5.
136. O’Sullivan M, Clayton N, Breslin NP, et al. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449-57.
137. Talley NJ, Butterfield JH. Mast cell infiltration and degranulation in colonic mucosa in the irritable bowel syndrome. Am J Gastroenterol. 1996;91:1675-6.
138. Piche T, Saint-Paul MC, Dainese R, et al. Mast cells and cellularity of the colonic mucosa correlated with fatigue and depression in irritable bowel syndrome. Gut. 2008;57:468-73.
139. Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804-11.
140. Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778-83.
141. Tornblom H, Lindberg G, Nyberg B, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-9.
142. Hiatt RB, Katz L. Mast cells in inflammatory conditions of the gastrointestinal tract. Am J Gastroenterol. 1962;37:541-5.
143. Parry SD, Stansfield R, Jelley D, et al. Is irritable bowel syndrome more common in patients presenting with bacterial gastroenteritis? A community-based, case-control study. Am J Gastroenterol. 2003;98:327-31.
144. Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702.
145. Barbara G, Wang B, Stanghellini V, et al. Mast cell–dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26-37.
146. Crowell MD, Shetzline MA, Moses PL, et al. Enterochromaffin cells and 5-HT signaling in the pathophysiology of disorders of gastrointestinal function. Curr Opin Investig Drugs. 2004;5:55-60.
147. Gershon MD, Tack J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397-414.
148. Houghton LA, Atkinson W, Whitaker RP, et al. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut. 2003;52:663-70.
149. Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657-64.
150. Camilleri MC, Andrews C, Bharucha AE, et al. Alterations in expression of p11 and SERT in mucosal biopsy specimens of patients with irritable bowel syndrome. Gastroenterology. 2007;132:17-25.
151. Whorwell P, Lea R. Dietary treatment of the irritable bowel syndrome. Curr Treat Options Gastroenterol. 2004;7:307-16.
152. Niec AM, Frankum B, Talley NJ. Are adverse food reactions linked to irritable bowel syndrome? Am J Gastroenterol. 1998;93:2184-90.
153. Wahnschaffe U, Ullrich R, Riecken EO, et al. Celiac disease–like abnormalities in a subgroup of patients with irritable bowel syndrome. Gastroenterol. 2001;121:1329-38.
154. Wahnschaffe U, Schulzke JD, Zeitz M, et al. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:844-50.
155. Olden KW. Diagnosis of irritable bowel syndrome. Gastroenterology. 2002;122:1701-14.
156. Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med. 1995;333:1-4.
157. Parker TJ, Woolner JT, Prevost AT, et al. Irritable bowel syndrome: is the search for lactose intolerance justified? Eur J Gastroenterol Hepatol. 2001;13:219-25.
158. Nelis GF, Vermeeren MA, Jansen W. Role of fructose-sorbitol malabsorption in the irritable bowel syndrome. Gastroenterology. 1990;90:1016-20.
159. Ledochowski M, Widner B, Bair H, et al. Fructose- and sorbitol-reduced diet improves mood and gastrointestinal disturbances in fructose malabsorbers. Scand J Gastroenterol. 2000;35:1048-52.
160. Skoog SM, Bharucha AE. Dietary fructose and gastrointestinal symptoms: a review. Am J Gastroenterol. 2004;99:2046-50.
161. Shepherd SJ, Parker FC, Muir JG, et al. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765-71.
162. Skoog SM, Bharucha AE, Zinsmeister AR. Comparison of breath testing with fructose and high fructose corn syrups in health and IBS. Neurogastroenterol Motil. 2008;20:505-11.
163. Madden JA, Hunter JO. A review of the role of the gut microflora in irritable bowel syndrome and the effects of probiotics. Br J Nutr. 2002;88:S67-72.
164. Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. A double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412-19.
165. Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503-6.
166. Hasler WL. Lactulose breath testing, bacterial overgrowth, and IBS: just a lot of hot air? Gastroenterology. 2003;125:1898-900.
167. O’Leary C, Quigley EMM. Small bowel bacterial overgrowth, celiac disease, and IBS: what are the real associations? Am J Gastroenterol. 2003;98:720-2.
168. Mertz H, Morgan V, Tanner G, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterol. 2000;118:842-8.
169. Naliboff BD, Derbyshire SW, Munakata J, et al. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom Med. 2001;63:365-75.
170. Rapps N, van Oudenhove L, Enck P, et al. Brain imaging of visceral functions in healthy volunteers and IBS patients. J Psychosom Res. 2008;64:599-604.
171. Rainville P, Duncan GH, Price DD, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;1997:968-71.
172. Naliboff BD, Berman S, Chang L, et al. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738-47.
173. Palsson OS, Drossman DA. Psychiatric and psychological dysfunction in irritable bowel syndrome and the role of psychological treatments. Gastroenterol Clin North Am. 2005;34:281-303.
174. Locke GRIII, Weaver AL, Melton LJIII, et al. Psychosocial factors are linked to functional gastrointestinal disorders: a population based nested case-control study. Am J Gastroenterol. 2004;99:350-7.
175. Koloski NA, Talley NJ, Boyce PM. Does psychological distress modulate functional gastrointestinal symptoms and health care seeking? A prospective, community cohort study. Am J Gastroenterol. 2003;98:789-97.
176. Talley NJ, Boyce PM, Jones M. Is the association between irritable bowel syndrome and abuse explained by neuroticism? A population based study. Gut. 1998;42:47-53.
177. Drossman DA, Talley NJ, Leserman J, et al. Sexual and physical abuse and gastrointestinal illness. Review and recommendations. Ann Int Med. 1995;123:782-94.
178. Drossman DA, Leserman J, Nachman G, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann intern Med. 1990;113:828-33.
179. Talley NJ, Fett SL, Zinsmeister AR. Self-reported abuse and gastrointestinal disease in outpatients: association with irritable bowel-type symptoms. Am J Gastroenterol. 1995;90:366-71.
180. Ringel Y, Whitehead WE, Toner BB, et al. Sexual and physical abuse are not associated with rectal hypersensitivity in patients with irritable bowel syndrome. Gut. 2004;53:838-42.
181. Ringel Y, Drossman DA, Turkington TG, et al. Regional brain activation in response to rectal distension in patients with irritable bowel syndrome and the effect of a history of abuse. Dig Dis Sci. 2003;48:1774-81.
182. Ringel Y, Drossman DA, Leserman JL, et al. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: An FMRI study. Gastroenterology. 2008;134:396-404.
183. Anand KJ, Runeson B, Jacobson B. Gastric suction at birth associated with long-term risk for functional intestinal disorders in later life. J Pediatr. 2004;144:449-54.
184. Tache Y. Corticotropin releasing factor receptor antagonists: potential future therapy in gastroenterology? Gut. 2004;53:919-21.
185. Alonso C, Guilarte M, Vicario M, et al. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology. 2008;135:163-72. e1
186. Cyranowski JM, Marsland AL, Bromberger JT, et al. Depressive symptoms and production of proinflammatory cytokines by peripheral blood mononuclear cells stimulated in vitro. Brain Behav Immun. 2007;21:229-37.
187. Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913-20.
188. Locke GRIII, Zinsmeister AR, Talley NJ, et al. Familial association in adults with functional gastrointestinal disorders. Mayo Clin Proc. 2000;75:907-12.
189. Kalantar JS, Locke GRIII, Zinsmeister AR, et al. Familial aggregation of irritable bowel syndrome: a prospective study. Gut. 2003;52:1703-7.
190. Levy RL, Jones KR, Whitehead WE, et al. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology. 2001;121:799-804.
191. Morris-Yates A, Talley NJ, Boyce PM, et al. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol. 1998;93:1311-17.
192. Lembo A, Zaman M, Jones M, et al. Influence of genetics on irritable bowel syndrome, gastro-oesophageal reflux and dyspepsia: a twin study. Aliment Pharmacol Ther. 2007;25:1343-50.
193. Talley NJ. Genes and environment in irritable bowel syndrome: one step forward. Gut. 2006;55:1694-6.
194. Saito YA, Talley NJ. Genetics of irritable bowel syndrome. Am J Gastroenterol. 2008;103:2100-4.
195. Gonsalkorale WM, Perrey C, Pravica V, et al. Interleukin 10 genotypes in irritable bowel syndrome: evidence for an inflammatory component? Gut. 2003;52:91-3.
196. van der Veek P, van den Berg M, de Kroon YE, et al. Role of tumor necrosis factor-α and Interleukin-10 gene polymorphisms in irritable bowel syndrome. Am J Gastroenterol. 2005;100:2510-16.
197. Saito YA, Strege PR, Tester DJ, et al. Sodium channel mutation in irritable bowel syndrome: Evidence for an ion channelopathy. Am J Physiol Gastrointest Liver Physiol. 2009;296:G211-18.
198. Pata C, Erdal ME, Derici E, et al. Serotonin transporter gene polymorphism in irritable bowel syndrome. Am J Gastroenterol. 2002;97:1780-84.
199. Grudell AB, Camilleri M, Carlson P, et al. An exploratory study of the association of adrenergic and serotonergic genotype and gastrointestinal motor functions. Neurogastroenterol Motil. 2008;20:213-19.
200. Kapeller J, Houghton LA, Monnikes H. First evidence for an association of a functional variant in the micro RNA-510 target site of the serotonin receptor type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967-77.
201. Wojczynski MK, North KE, Pedersen NL, et al. Irritable bowel syndrome: a co-twin control analysis. Am J Gastroenterology. 2007;102:2220-9.
202. Hammer J, Eslick G, Howell S, et al. Diagnostic yield of alarm features in irritable bowel syndrome and functional dyspepsia. Gut. 2004;53:666-72.
203. Cash BD, Chey WD. Irritable bowel syndrome—an evidence-based approach to diagnosis. Aliment Pharmacol Ther. 2004;19:1235-45.
204. Vanner SJ, Depew WT, Paterson WG, et al. Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. Am J Gastroenterol. 1999;94:2912-17.
205. Tibble J, Teahon K, Thjodleifsson B, et al. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000;47:506-13.
206. Sinha L, Liston R, Testa HJ, et al. Idiopathic bile acid malabsorption: qualitative and quantitative clinical features and response to cholestyramine. Aliment Pharmacol Ther. 1998;12:839-44.
207. Wildt S, Norby Rasmussen S, Lysgard Madsen J, et al. Bile acid malabsorption in patients with chronic diarrhoea: clinical value of SeHCAT test. Scand J Gastroenterol. 2003;38:826-30.
208. Sanders DS, Carter MJ, Hurlstone DP, et al. Association of adult coeliac disease with irritable bowel syndrome: a case-control study in patients fulfilling Rome II criteria referred to secondary care. Lancet. 2001;358:1504-8.
209. Locke GRIII, Murray JA, Zinsmeister AR, et al. Celiac disease serology in irritable bowel syndrome and dyspepsia: a population-based case-control study. Mayo Clin Proc. 2004;79:476-82.
210. Spiegel BM, DeRosa VP, Gralnek IM, et al. Testing for celiac sprue in irritable bowel syndrome with predominant diarrhea: A cost-effectiveness analysis. Gastroenterology. 2004;126:1721-32.
211. Catassi C, Kryszak D, Louis-Jacques O, et al. Detection of celiac disease in primary care: a multicenter case-finding study in North America. Am J Gastroenterol. 2007;102:1454-60.
212. Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. Bmj. 2008;336:999-1003.
213. Dixon-Woods M, Critchley S. Medical and lay views of irritable bowel syndrome. Fam Pract. 2000;17:108-13.
214. Bertram S, Kurland M, Lydick E, et al. The patient’s perspective of irritable bowel syndrome. J Fam Pract. 2001;50:521-5.
215. Owens DM, Nelson DK, Talley NJ. The irritable bowel syndrome: long-term prognosis and the physician-patient interaction. Ann Intern Med. 1995;122:107-12.
216. Saito YA, Prather CM, Van Dyke CT, et al. Effects of multidisciplinary education on outcomes in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2004;2:576-84.
217. Colwell LJ, Prather CM, Phillips SF, et al. Effects of an irritable bowel syndrome educational class on health-promotion behaviors and symptoms. Am J Gastroenterol. 1998;93:901-5.
218. Drossman DA, Thompson WG. The irritable bowel syndrome: review and a graduated multicomponent treatment approach. Ann Intern Med. 1992;116:1009-16.
219. Mertz HR. Irritable bowel syndrome. N Engl J Med. 2003;349:2136-46.
220. Thompson WG. Review article: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2002;16:1395-406.
221. Talley NJ. Evaluation of drug treatment in irritable bowel syndrome. Br J Clin Pharmacol. 2003;56:362-9.
222. Dhamija R, Pittock SJ, Foxx-Orenstein A, et al. Serological profiles aiding the diagnosis of autoimmune gastrointestinal dysmotility. Clin Gastoenterol Hepatol. 2008;6:988-92.
223. Brandt L, Bjorkman D, Fennerty M, et al. Systematic review on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S7-26.
224. Ford AC, Talley NJ, Spiegel BM, et al. Efficacy of fibre, antispasmodics and peppermint oil in irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313.
225. Fass R, Longstreth GF, Pimentel M, et al. Evidence- and consensus-based practice guidelines for the diagnosis of irritable bowel syndrome. Arch Intern Med. 2001;161:2081-8.
226. Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med. 2000;133:136-47.
227. Bijkerk CJ, Muris JWM, Knotterus JA, et al. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19:245-51.
228. Atkinson W, Gurney R, Sheldon TA, et al. Do food elimination diets improve irritable bowel syndrome? A double blind trial based on IgG antibodies to food. Gastroenterology. 2003;124:A29.
229. Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? A systematic review. Neurogastroenterol Motil. 2006;18:595-607.
230. Stefanini GF, Bazzocchi G, Prati E, et al. Efficacy of oral disodium cromoglycate in patients with irritable bowel syndrome and positive skin prick tests to foods. Lancet. 1986;1:207-8.
231. Talley NJ. Conquering Irritable Bowel Syndrome. Philadelphia: BC Decker; 2005.
232. DiPalma JA, DeRidder PH, Orlando RC, et al. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am J Gastroenterol. 2000;95:446-50.
233. Corazziari E, Badiali D, Bazzocchi G, et al. Long term efficacy, safety, and tolerabilitity of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution(PMF-100) in the treatment of functional chronic constipation. Gut. 2000;46:522-6.
234. Wald A. Is chronic use of stimulant laxatives harmful to the colon? J Clin Gastroenterol. 2003;36:386-9.
235. Johanson JF, Drossman DA, Panas R, et al. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2008;27:685-96.
236. Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol. 1996;31:463-8.
237. Lavo B, Stenstam M, Nielsen AL. Loperamide in treatment of irritable bowel syndrome—a double-blind placebo controlled study. Scan J Gastroenterol Suppl. 1987;130:77-80.
238. Cann PA, Read NW, Holdsworth CD, et al. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Dig Dis Sci. 1984;29:239-47.
239. Cremonini F, Delgado-Aros S, Camilleri M. Efficacy of alosetron in irritable bowel syndrome: a meta-analysis of randomized controlled trials. Neurogastroenterol Motil. 2003;15:79-86.
240. Watson ME, Lacey L, Kong S, et al. Alosetron improves quality of life in women with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;96:455-9.
241. Lembo A, Weber HC, Farraye FA. Alosetron in irritable bowel syndrome: strategies for its use in a common gastrointestinal disorder. Drugs. 2003;63:1895-905.
242. Miller DP, Alfredson T, Cook SF, et al. Incidence of colonic ischemia, hospitalized complications of constipation, and bowel surgery in relation to use of alosetron hydrochloride. Am J Gastroenterol. 2003;98:1117-22.
243. Camilleri M, Atanasova E, Carlson PJ, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425-32.
244. Talley NJ. Antidepressants in IBS: are we deluding ourselves? Am J Gastroenterol. 2004;99:921-3.
245. Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med. 2000;108:65-72.
246. Drossman D, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19-31.
247. Clouse RE, Lustman PJ, Geisman RA, et al. Antidepressant therapy in 138 patients with irritable bowel syndrome: a five-year clinical experience. Aliment Pharmacol Ther. 1994;8:409-16.
248. Creed F, Fernandes L, Guthrie E, et al. The cost-effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology. 2003;124:303-17.
249. Tabas G, Beaves M, Wang J, et al. Paroxetine to treat irritable bowel syndrome not responding to high-fiber diet: a double-blind, placebo-controlled trial. Am J Gastroenterol. 2004;99:914-20.
250. Kuiken SD, Tytgat GN, Boeckxstaens GE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: a double blind, randomized, placebo-controlled study. Clin Gastoenterol Hepatol. 2003;1:219-28.
251. Gorard DA, Libby GW, Farthing MJG. Influence of antidepressants on whole gut orocaecal transit times in health and irritable bowel syndrome. Aliment Pharmacol Ther. 1994;8:159-66.
252. Pimentel M, Park S, Mirocha J, et al. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557-63.
253. Nobaek S, Johansson ML, Molin G, et al. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231-8.
254. Kim HJ, Camilleri M, McKinzie S, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895-904.
255. Greenbaum DS, Ferguson RK, Kater LA, et al. A controlled therapeutic study of the irritable-bowel syndrome: effect of diphenylhydantoin. N Engl J Med. 1973;288:13-16.
256. Abeles M, Solitar BM, Pillinger MH, et al. Update on fibromyalgia therapy. Am J Med. 2008;121:555-61.
257. Fielding JF. Timolol treatment in the irritable bowel syndrome. Digestion. 1981;22:155-8.
258. McIntyre AS, Burnham WR, Thompson DG. Atenolol in irritable bowel syndrome. Lancet. 1988;1:8575-6.
259. Mathias JR, Clench MH, Abell TL, et al. Effect of leuprolide acetate in treatment of abdominal pain and nausea in premenopausal women with functional bowel disease: a double-blind, placebo-controlled, randomized study. Dig Dis Sci. 1998;43:1347-55.
260. Mathias JR, Clench MH, Reeves-Darby VG, et al. Effect of leuprolide acetate in patients with moderate to severe functional bowel disease. Double-blind, placebo-controlled study. Dig Dis Sci. 1994;39:1155-62.
261. Verne GN, Davis RH, Robinson ME, et al. Treatment of chronic constipation with colchicine: randomized, double-blind, placebo-controlled, crossover trial. Am J Gastroenterol. 2003;98:1112-16.
262. Roarty TP, Weber F, Soykan I, et al. Misoprostol in the treatment of chronic refractory constipation: Results of a long-term open label trial. Aliment Pharmacol Ther. 1997;11:1059-66.
263. Schewetz I, Naliboff B, Munakata J, et al. Anti-hyperalgesic effect of octreotide in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19:123-31.
264. Camilleri M, Kim DY, McKinzie S, et al. A randomized, controlled exploratory study of clonidine in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2003;1:111-21.
265. Dunlop SP, Jenkins D, Neal KR, et al. Randomized, double-blind, placebo-controlled trial of prednisolone in post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18:77-84.
266. Distrutti E, Sediari L, Mencarelli A, et al. 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H-[1,2]dithiol-3yl)-phenyl ester(ATB-429), a hydrogen sulfide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of postinflammatory hypersensitivity. J Pharmacol Exp Ther. 2006;319:447-58.
267. Friis H, Bode S, Rumessen JJ, et al. Effect of simethicone on lactulose-induced H2 production and gastrointestinal symptoms. Digestion. 1991;49:227-30.
268. Suarez FL, Furne J, Springfield J, et al. Failure of activated charcoal to reduce the release of gases produced by the colonic flora. Am J Gastroenterol. 1999;94:208-12.
269. Potter T, Ellis C, Levitt M. Activated charcoal: in vivo and in vitro studies of effect on gas formation. Gastroenterology. 1985;88:620-4.
270. Ganiats TG, Norcross WA, Halverson AL, et al. Does Beano prevent gas? A double-blind crossover study of oral α-galactosidase to treat dietary oligosaccharide intolerance. J Fam Pract. 1994;39:441-5.
271. Suarez FL, Furne JK, Springfield J, et al. Bismuth subsalicylate markedly decreases hydrogen sulfide release in the human colon. Gastroenterology. 1998;114:923-9.
272. Suarez FL, Springfield J, Levitt MD. Identification of gases responsible for the odour of human flatus and evaluation of a device purported to reduce this odour. Gut. 1998;43:100-4.
273. Fink RN, Lembo AJ. Intestinal gas. Curr Treat Options Gastroenterol. 2001;4:333-7.
274. Gonsalkorale WM, Toner BB, Whorwell PJ. Cognitive change in patients undergoing hypnotherapy for irritable bowel syndrome. J Psychosom Res. 2004;56:271-8.
275. Boyce PM, Talley NJ, Balaam B, et al. A randomized controlled trial of cognitive behavior therapy, relaxation training, and routine clinical care for the irritable bowel syndrome. Am J Gastroenterol. 2003;98:2209-18.
276. Spanier JA, Howden CW, Jones MP. A systematic review of alternative therapies in the irritable bowel syndrome. Arch Intern Med. 2003;163:265-74.
277. Bensoussan A, Talley NJ, Hing M, et al. Treatment of irritable bowel syndrome with Chinese herbal medicine: A randomized controlled trial. JAMA. 1998;280:1585-9.
278. Langmead L, Rampton DS. Review article: Herbal treatment in gastrointestinal and liver disease—benefits and dangers. Aliment Pharmacol Ther. 2001;15:1239-52.
279. Yadav SK, Jain AK, Tripathi SN, et al. Irritable bowel syndrome: therapeutic evaluation of indigenous drugs. Indian J Med Res. 1989;90:496-503.
280. Suarez F, Levitt MD, Adshead J, et al. Pancreatic supplements reduce symptomatic response of healthy subjects to a high fat meal. Dig Dis Sci. 1999;44:1317-21.
281. Xing J, Larive B, Mekhail N, et al. Transcutaneous electrical acustimulation can reduce visceral perception in patients with the irritable bowel syndrome: a pilot study. Altern Ther Health Med. 2004;10:38-42.
282. Xiao WB, Liu YL. Rectal hypersensitivity reduced by acupoint TENS in patients with diarrhea-predominant irritable bowel syndrome: a pilot study. Dig Dis Sci. 2004;49:312-19.
283. Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil. 2008;20:121-9.
284. Adeniji OA, Barnett CB, DiPalma JA. Durability of the diagnosis of irritable bowel syndrome based on clinical criteria. Dig Dis Sci. 2004;49:572-4.