Iron
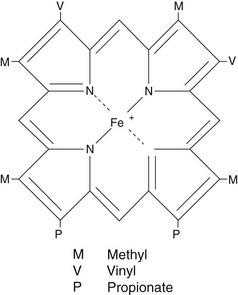
Iron is an essential element in humans, being the central ion in haem, the non-protein component of haemoglobin, myoglobin and the cytochromes (Fig 57.1). Iron deficiency causes a failure in haem synthesis and since haemoglobin is required for delivery of oxygen to the tissues, this leads to anaemia and tissue hypoxia. However, free iron is highly toxic to cells and must be bound to protein at all times.
Iron physiology
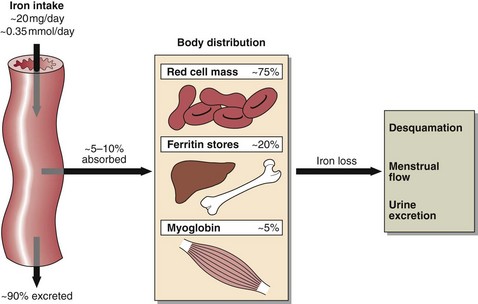
Iron levels are controlled by regulating iron uptake, since there is no mechanism for controlling its excretion. Dietary intake of iron is about 0.35 mmol (20 mg) per day and there are 50–70 mmol (3–4 g) of iron in the body, distributed as shown in Figure 57.2. Iron in the tissue stores is bound to the iron storage proteins ferritin (soluble) and haemosiderin (insoluble). The 1% of body iron in the plasma is associated with the iron binding glycoprotein, transferrin, each molecule of which binds two Fe2+ ions.
Laboratory investigation of iron disorders
 Serum iron determinations are of limited routine value, being of most assistance in the diagnosis of iron overload and acute iron poisoning.
Serum iron determinations are of limited routine value, being of most assistance in the diagnosis of iron overload and acute iron poisoning.
 Transferrin can be measured directly or indirectly as the total iron binding capacity (TIBC). Normally transferrin is about 30% saturated with iron. When saturation falls to 15%, iron deficiency is likely and some degree of clinical effect can be expected. A higher percentage saturation indicates iron overload. Transferrin and, therefore, also total serum iron, is decreased as part of the acute phase response. Protein energy malnutrition decreases transferrin synthesis and hence its serum concentration.
Transferrin can be measured directly or indirectly as the total iron binding capacity (TIBC). Normally transferrin is about 30% saturated with iron. When saturation falls to 15%, iron deficiency is likely and some degree of clinical effect can be expected. A higher percentage saturation indicates iron overload. Transferrin and, therefore, also total serum iron, is decreased as part of the acute phase response. Protein energy malnutrition decreases transferrin synthesis and hence its serum concentration.
 Serum ferritin is the best indicator of body iron stores. The concentration is normally greater than 12 µg/L. The acute phase response can result in increases in serum ferritin, making the diagnosis of marginal iron deficiency difficult or impossible in these circumstances.
Serum ferritin is the best indicator of body iron stores. The concentration is normally greater than 12 µg/L. The acute phase response can result in increases in serum ferritin, making the diagnosis of marginal iron deficiency difficult or impossible in these circumstances.
 Zinc protoporphyrin (ZPP) is markedly increased in iron deficiency and sometimes used as a screening test in children; it is expressed as µmol ZPP/mole haem and is usually <60. Concentrations are also increased following chronic exposure to lead, though in children ZPP rise is a late phenomenon so less reliable than measurement of lead levels. For a full investigation of iron status, the haemoglobin concentration, the appearance of the erythrocytes (deficiency), and liver biopsy (excess) may be required.
Zinc protoporphyrin (ZPP) is markedly increased in iron deficiency and sometimes used as a screening test in children; it is expressed as µmol ZPP/mole haem and is usually <60. Concentrations are also increased following chronic exposure to lead, though in children ZPP rise is a late phenomenon so less reliable than measurement of lead levels. For a full investigation of iron status, the haemoglobin concentration, the appearance of the erythrocytes (deficiency), and liver biopsy (excess) may be required.
Iron deficiency
Iron deficiency anaemia develops in three stages:
1. Depletion of iron stores: confirmed by serum ferritin levels of less than 12 µg/L.
2. Deficient erythropoiesis with normal haemoglobin but increased zinc protoporphyrin. Iron concentration falls, the synthesis of transferrin is increased and the percentage saturation is decreased.
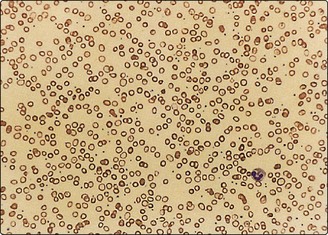
3. Iron deficiency anaemia, in which both iron and haemoglobin are low and there is a microcytic, hypochromic anaemia (Fig 57.3). Low stainable iron is seen in the bone marrow.
Iron overload
Iron poisoning
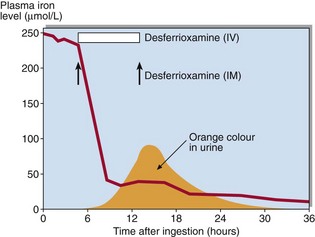
Iron poisoning in children is common and may be life-threatening. Symptoms include nausea and vomiting, abdominal pain and haematemesis. In severe cases, hypotension and coma can result. Serum iron is increased and transferrin is >70% saturated. Treatment is by chelation of the iron in the stomach and the plasma with desferrioxamine. Chelated iron is excreted in the urine as a deep orange coloured complex (Fig 57.4).