77 Invasive Cardiac Procedures
 Percutaneous Transluminal Coronary Angioplasty
Percutaneous Transluminal Coronary Angioplasty
Chronic ischemic heart disease is usually due to obstruction of the coronary arteries by atherosclerosis. It is the leading cause of mortality and morbidity in economically developed countries. Percutaneous transluminal coronary angioplasty (PTCA) has emerged as a major therapeutic option in patients with coronary artery atherosclerosis. The first PTCA in a patient was performed by Andreas Grüntzig in Zurich in September 1977.1 PTCA was initially limited to the treatment of discrete stenoses in proximal segments of a coronary artery. Improvements in equipment and technique have increased the success rate and have led to its use in patients with complex stenoses or in high-risk clinical situations such as acute coronary syndromes (ACS)2,3 or cardiac arrest.4 PTCA is currently the most widely used coronary revascularization technique.
Procedure
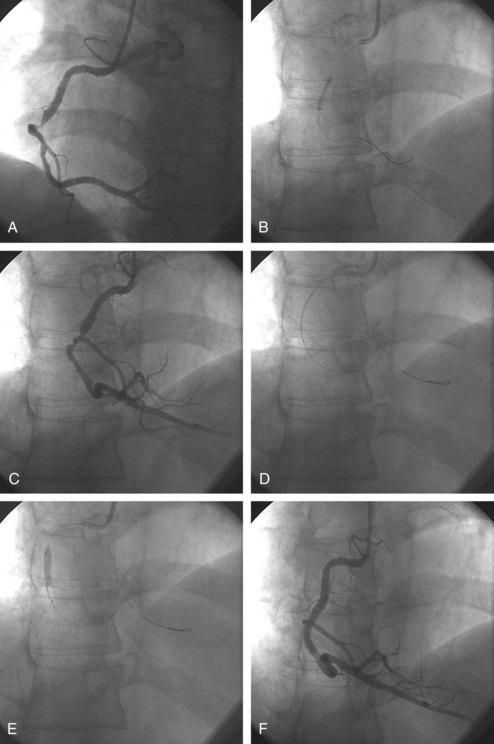
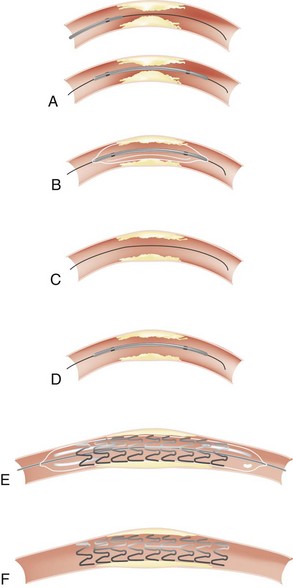
Vascular access is obtained either through the femoral or radial artery, where a sheath is introduced with the use of local anesthesia. A 5F to 8F guiding catheter is advanced through the sheath to the ostium of the coronary artery to be dilated. Once the guiding catheter is positioned in the coronary ostium, angiography of the diseased artery is performed to visualize the stenosis and the arterial segments proximal and distal to it; intracoronary infusion of nitrates is mandatory for the diagnosis and accurate sizing of the lesions and the choice of stent (Figure 77-1, A). A flexible guidewire is advanced through the guiding catheter, navigated across the stenosis by rotating and advancing its angulated tip, and positioned in the distal arterial segment. The deflated balloon angioplasty catheter is advanced over the wire and positioned at the stenosis. The positions of the guidewire and balloon catheter are confirmed periodically by injecting contrast medium into the coronary artery through the guiding catheter. Once it is positioned, the balloon is usually inflated with a mixture of saline and contrast medium so the inflation can be visualized (see Figure 77-1, B and C). Most often, a stent is implanted after balloon angioplasty (Figure 77-2). Balloon-expandable stents are most commonly used. The stent-balloon device is positioned on the predilated site (see Figure 77-1, D), and the stent is implanted in the coronary artery wall by a short balloon inflation (see Figure 77-1, E). The balloon catheter is deflated and pulled out. The result is evaluated by injecting contrast medium (see Figure 77-1, F). If the result is satisfactory, the guidewire is removed. If the angiographic result is unsatisfactory, the guidewire remains in place. The balloon catheter can be replaced by a larger one, or another stent can be implanted. At the end of the procedure, a final angiogram is obtained to confirm that the result is satisfactory.
In noncalcified lesions, direct stent implantation without prior balloon dilation is often performed. Direct stenting shortens the duration of the procedure and reduces costs and is used in approximately 30% to 50% of cases.5
Preprocedure and Postprocedure Management and Medications
The combination of low-dose aspirin (75-325 mg) and clopidogrel has been shown to reduce the incidence of acute stent occlusion after PTCA and is considered essential therapy before coronary interventions.6,7 If patients are not treated chronically or if there is doubt about medication compliance, a dose of aspirin (500 mg orally) should be given more than 3 hours prior, or at least 300 mg intravenously (IV) directly prior to the procedure. For chronic use, there is no need for doses higher than 100 mg daily.8 Current guidelines recommend a loading dose of clopidogrel, 300 mg, administered at least 6 hours before the procedure. Higher loading doses (600 mg) lead to more rapid (around 2 hours) and long-lasting inhibition of platelet aggregation. The CURRENT-OASIS 7 trial compared 300-mg and 600-mg clopidogrel loading doses and demonstrated fewer adverse events with a loading dose of 600 mg of clopidogrel, followed by 150 mg daily for a week, and 75 mg daily for at least 3 weeks.9
Despite administration of dual antiplatelet therapy combining aspirin and clopidogrel, a small percentage (0.4-1.1%) still suffer subacute stent thrombosis. Variability in response to clopidogrel may account for some of these events,10–12 and more potent antiplatelet agents have been investigated. Prasugrel, a novel oral thienopyridine, has a more rapid onset and predictable and potent antiplatelet effects.13,14 In the TRITON-TIMI 38 trial,15 13,608 moderate to high-risk ACS patients were randomized to prasugrel (60-mg loading dose followed by 10 mg daily) or clopidogrel (300-mg loading dose followed by 75 mg daily). Follow-up was up to 15 months. Prasugrel was associated with a 19% reduction in the primary efficacy endpoint of cardiovascular death, nonfatal myocardial infarction (MI), and nonfatal stroke. This was accompanied by a 32% increase of major bleeding, especially in subgroups of patients with cerebrovascular accident, weight less than 60 kg, and older than 75 years of age. Ticagrelor, a reversible and direct-acting oral antagonist of the adenosine diphosphate receptor P2Y12, provides faster, greater, and more consistent platelet inhibition than clopidogrel. PLATO,16 a multicenter double-blind, randomized trial compared ticagrelor (180-mg loading dose, 90 mg twice daily thereafter) and clopidogrel (300-mg to 600-mg loading dose, 75 mg daily thereafter) for the prevention of cardiovascular events in 18,624 patients with an ACS. At 12 months, ticagrelor significantly reduced the occurrence of the primary endpoint, a composite of death from vascular causes, MI, or stroke—9.8% of patients receiving ticagrelor as compared with 11.7% of those receiving clopidogrel (hazard ratio, 0.84; 95% confidence interval [CI], 0.77 to 0.92; P < 0.001). No significant difference in the rates of major bleeding was found between the ticagrelor and clopidogrel groups (11.6% and 11.2%, respectively; P = 0.43), but ticagrelor was associated with a higher rate of major bleeding unrelated to coronary artery bypass grafting (CABG) (4.5% versus 3.8%, P = 0.03), including more instances of fatal intracranial bleeding and fewer fatal bleeding of other types.
Intracoronary nitrates are given at the beginning of and during the procedure to prevent vasospasm. Unfractionated heparin (typically 5000 to 10,000 units) is administered IV during PTCA to decrease the incidence of coronary artery thrombosis,17 but it is usually not continued after the procedure. Low-molecular-weight heparin has been suggested as an accepted alternative to unfractionated heparin.18
Although the mainstay of antiplatelet and anticoagulation therapy for PTCA is the combination of clopidogrel and aspirin before and after the procedure and heparin during it, the use of platelet glycoprotein IIb/IIIa inhibitors or the direct thrombin inhibitor, bivalirudin, have emerged as powerful adjunctive therapies. The platelet glycoprotein IIb/IIIa receptor binds fibrinogen to cross-link platelets and can be blocked irreversibly by inhibitors such as abciximab, eptifibatide, and tirofiban. Several multicenter randomized studies have compared heparin and aspirin to an additional treatment with platelet glycoprotein IIb/IIIa receptor inhibitors in patients undergoing PTCA and showed a significant reduction in major clinical events.19–21 The greatest treatment benefit of platelet glycoprotein IIb/IIIa inhibitors appears to be in procedures on high-risk lesions or in patients with severe clinical patterns, such as ACS with ST-segment changes or elevation of biological markers of myocardial necrosis.22–24 Bivalirudin, when used instead of heparin plus glycoprotein IIb/IIIa inhibitors, has been shown in large-scale randomized trials to reduce major and minor bleeding and thrombocytopenia while resulting in similar rates of ischemia after percutaneous intervention (PCI) in patients with stable angina or ACS.25
Although the femoral artery remains the most widely used approach in the United States for diagnostic and therapeutic procedures, the radial artery is used increasingly to reduce the local complication rate and increase the patient’s comfort. The sheath is pulled out immediately after the procedure, and hemostasis is obtained by applying a pressure dressing for several hours.26 Immediate ambulation is feasible, and hospital discharge on the same day is possible in selected cases.27
Efficacy of the Procedure
PTCA of a nonoccluded coronary artery is successful in more than 97% of patients. In the remaining patients, PTCA is unsuccessful because the stenosis cannot be crossed with either the guidewire or the balloon catheter, or because the stenosis is not adequately dilated despite the use of an appropriately sized balloon and stents. In 2% to 3% of cases, the vessel abruptly occludes (abrupt closure) during or immediately after the procedure. Reopening of the artery is attempted with repeat balloon inflations and multiple stent implantations. Stenting for abrupt closure (bailout stenting) has virtually eliminated the need for urgent coronary bypass surgery after failed PTCA. The most challenging lesions (long, angulated, calcified, or associated with intraluminal thrombus) carry a lower success rate.28 PTCA also has a lower initial success rate (50%-70%) in patients with chronically occluded arteries, because it may be difficult to manipulate the guidewire through a chronically occluded region.29,30 In patients with recurrent angina after bypass surgery, the success rate of PTCA performed for properly selected stenosis of saphenous and arterial bypass grafts is close to that of native arteries, but the incidence of late events (MI, repeat PTCA, or other surgery) is higher.31,32
Mechanisms of Coronary Artery Dilatation
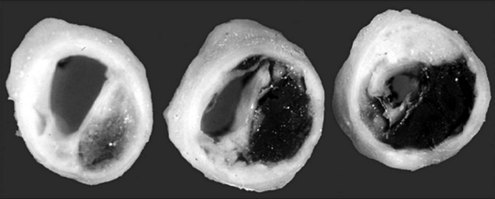
The mechanisms by which PTCA increases the size of the arterial lumen have been studied in animals and cadavers.33–36 Balloon-induced barotrauma causes endothelial denudation, cracking and disruption of the atherosclerotic plaque, and stretching or tearing of the media and adventitia (Figure 77-3). These brutal and profound changes account for the postballoon inflation angiographic features of intraluminal haziness, intimal flap, or dissection (Figure 77-4). Intracoronary ultrasound imaging, which provides a cross-sectional view of the artery within the lumen, detects dissection of the arterial wall—sometimes extensive—in 50% to 80% of patients who have undergone successful PTCA.37,38 These morphologic alterations open up new pathways for blood flow, leading to an increased luminal size. Balloon inflation may be deleterious, however, causing plaque hemorrhage, extensive dissection resulting in luminal compromise, platelet deposition, and thrombus formation.
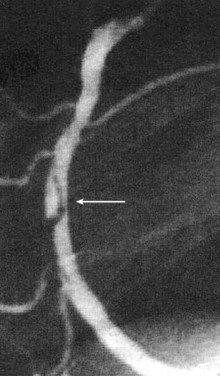
Figure 77-4 Arterial dissection (arrow) after balloon inflation in midsegment of a right coronary artery.
In the weeks after successful PTCA, favorable remodeling of the disrupted plaque and endothelialization at the sites of intimal injury result in an increased luminal size. Angiographic studies indicate that intimal disruption usually resolves within 1 month after successful PTCA.39
Restenosis
In patients who have undergone successful PTCA, recurrence of the stenosis, or restenosis, was the main limitation to long-term, event-free survival. Restenosis occurs in about 30% to 50% of patients in whom a coronary artery stenosis has been dilated by balloon alone.40–42 Restenosis typically occurs 1 to 6 months after PTCA.40
The process of restenosis is multifactorial. Injury of the vessel initiates release of thrombogenic, vasoactive, and mitogenic factors.43 Endothelial and deep-vessel injury leads to platelet aggregation, thrombus formation, inflammation, and activation of macrophages. These events induce the production and release of growth factors and cytokines, which in turn may promote their own synthesis and release from target cells.44 A self-perpetuating process is initiated that results in the migration of smooth muscle cells from their usual location in the arterial media to the intima, where they change to a synthetic phenotype, produce extracellular matrix, and proliferate, thereby resulting in a stenosis within the vessel lumen. Intimal thickening accounts for about 30% of the loss in lumen diameter 6 months after coronary interventions. In addition, arterial remodeling occurs in the weeks after PTCA and can be evaluated using serial intravascular ultrasound imaging to measure the reduction in the cross-sectional area of the vessel.45,46
Coronary stenting with bare metal stents significantly reduces the incidence of restenosis, because it produces large lumens and staves off pathologic remodeling.47,48 Several multicenter randomized trials showed that the incidence of restenosis is 25% to 50% lower after coronary stenting than after balloon angioplasty. Drug-eluting stents have been developed to further reduce the restenosis rate. Stents are covered with a polymer that allows progressive delivery of antiproliferative drugs such as sirolimus, paclitaxel, or everolimus that inhibit smooth muscle cell proliferation. The restenosis rate has been virtually abolished with drug-eluting stents, with no increase in the acute complication rate.49 Currently, drug-eluting stents are used in the majority of procedures.
An annual rate of 0.4 to 0.6% of late stent thrombosis has been noted after implantation of sirolimus- or paclitaxel-eluting stents, with no increase of death or MI.50 Incomplete endothelial coverage of the stent and a prolonged inflammatory reaction to the polymer have been suggested as causal factors. Current guidelines recommend dual antiplatelet therapy for 12 months after implantation of drug-eluting stents, although no randomized trial has proved the efficacy of this strategy to prevent late stent thrombosis.8,51 Interruption of clopidogrel administration after implantation of a drug-eluting stent is associated with acute stent thrombosis, especially during the first 6 months.52 Management of dual antiplatelet therapy in patients with drug-eluting stents in the setting of surgical procedures requires a clear consensus between surgeons, anesthesiologists, and cardiologists.53 Future developments to reduce the rate of late stent thrombosis include drug-eluting stents with bioabsorbable polymers (Figure 77-5) and nonmetallic stents which are completely bioabsorbed.54
 Comparison of Clinical Applications
Comparison of Clinical Applications
Percutaneous Transluminal Coronary Angioplasty Versus Medical Therapy
PTCA has been compared with medical therapy for stable angina in several studies. In the COURAGE trial,57 2287 patients who had stable angina with objective evidence of myocardial ischemia and significant coronary artery disease were randomized to PCI with optimal therapy or optimal medical therapy. The primary outcome was death from any cause and nonfatal MI. There was a statistically significant difference in the rates of freedom from angina throughout most of the follow-up period in favor of the PCI group. However, there was no difference at 4.6 years in the primary outcome, all-cause death, and nonfatal MI (19.0% in the PCI group and 18.5% in the medical therapy group; hazard ratio for the PCI group, 1.05; 95% CI, 0.87-1.27; P = 0.62). There were no significant differences between the PCI group and the medical therapy group in the composite of death, MI, and stroke (20.0% versus 19.5%; hazard ratio, 1.05; 95% CI, 0.87-1.27; P = 0.62); hospitalization for ACS (12.4% versus 11.8%; hazard ratio, 1.07; 95% CI, 0.84-1.37; P = 0.56); or MI (13.2% versus 12.3%; hazard ratio, 1.13; 95% CI, 0.89-1.43; P = 0.33). Therefore, in patients with stable angina, even though PCI provides more optimal relief of symptoms, optimal medical therapy can be administered safely and angioplasty performed only in patients with persistent symptoms.
The TIMI IIIB study addressed the benefit of PTCA for patients with unstable angina or non–Q wave MI.58 This study enrolled 2220 patients with unstable angina and MI without ST-segment elevation who had electrocardiographic (ECG) evidence of changes in the ST segment or T wave, elevated levels of cardiac markers, a history of coronary artery disease, or all three findings. Patients were randomly assigned to an early invasive strategy that included routine catheterization within 4 to 48 hours and revascularization as appropriate, or to a more conservative (selectively invasive) strategy, in which catheterization was performed only if the patient had objective evidence of recurrent ischemia or an abnormal stress test. At 6 months, the rate of the primary endpoint (a composite of death, nonfatal MI, and rehospitalization for ACS) was 15.9% with the early invasive strategy and 19.4% with the conservative strategy (odds ratio, 0.78; 95% CI, 0.62-0.97; P = 0.025). In the FRISC II trial,59,60 2457 patients were randomly assigned to invasive or noninvasive treatment and 3 months of dalteparin or placebo. After 1 year, in 100 patients, an invasive strategy saved 1.7 lives, prevented 2.0 nonfatal MIs and 20 readmissions, and provided earlier and better symptom relief at the cost of 15 more patients with CABG and 21 more with PTCA.59 An invasive approach with early (<48 hours) angiography is therefore the preferred strategy in patients with unstable coronary artery disease and signs of ischemia on ECG or raised levels of biochemical markers of myocardial damage.
In patients with acute myocardial infarction (AMI), PTCA performed without prior thrombolytic therapy (primary PTCA) by an experienced team results in a lower risk of death or reinfarction than thrombolytic therapy.2,3,61 In patients with AMI complicated by cardiogenic shock, emergency revascularization improves survival.62,63 PTCA performed after failed thrombolytic therapy reduces adverse cardiac events and improves left ventricular function at 1 month.64 In patients with right ventricular infarction, complete reperfusion of the right coronary artery by angioplasty results in dramatic recovery of right ventricular performance, as assessed by echocardiography, and an excellent clinical outcome.65 In cardiac arrest, a strategy of immediate coronary angiography followed by angioplasty if necessary may increase survival.4
Percutaneous Transluminal Coronary Angioplasty Versus Bypass Surgery
Several studies have compared PTCA with bypass surgery for patients with multivessel coronary artery disease. The SYNTAX trial randomly assigned 1800 patients with three-vessel or left main coronary artery disease to undergo CABG or PCI (in a 1 : 1 ratio).66 For all these patients, the local cardiac surgeon and interventional cardiologist determined that equivalent anatomic revascularization could be achieved with either treatment. A non-inferiority comparison of the two groups was performed for the primary endpoint—a major adverse cardiac or cerebrovascular event (death from any cause, stroke, MI, or repeat revascularization) during the 12-month period after randomization. Rates of major adverse cardiac or cerebrovascular events at 12 months were significantly higher in the PCI group (17.8%, versus 12.4% for CABG; P = 0.002), in large part because of an increased rate of repeat revascularization (13.5% versus 5.9%, P < 0.001); as a result, the criterion for non-inferiority was not met. At 12 months, the rates of death and MI were similar between the two groups; stroke was significantly more likely to occur with CABG (2.2%, versus 0.6% with PCI; P = 0.003) The SYNTAX score was designed in this study to predict outcomes related to anatomic characteristics. In the group of patients with high SYNTAX scores, the composite rate of death, nonfatal MI, and stroke was raised in the PCI group.
 Mitral Valvuloplasty
Mitral Valvuloplasty
In patients with severe mitral stenosis, surgical mitral commissurotomy alleviates symptoms and improves mid- and long-term prognosis. Percutaneous mitral valvuloplasty was first applied in 1984 to young patients with rheumatic mitral stenosis, using a transseptal approach.67 The technique is widely accepted as a first-choice treatment in cases of severe but noncalcified mitral stenosis. Selection of patients is based on the echocardiographic features of the mitral valve.68
Overall procedure mortality is 1% to 2%. Long-term follow-up studies demonstrate preservation of the improved mitral orifice.69
 Aortic Valvuloplasty
Aortic Valvuloplasty
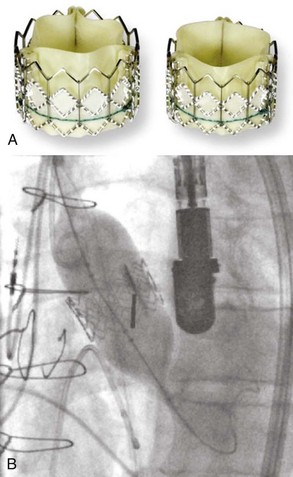
Calcific aortic stenosis in an adult is the most common indication for the more than 25,000 aortic valve replacements performed in the Unites States each year. Percutaneous aortic balloon valvuloplasty was proposed as an alternative to surgery. The balloon catheter is advanced retrogradely through the aortic stenosis using a femoral approach in most cases. Mid- and long-term results are disappointing; improvement in the orifice area is less than that obtained with a valve replacement, and echocardiographic follow-up shows recurrence of aortic stenosis in most cases.70 More recently, percutaneous transcatheter implantation of a heart valve prosthesis for aortic stenosis has been assessed in patients with contraindications to surgery71 (Figure 77-6). Outcomes are encouraging despite a high rate of local femoral complications due to the size of the sheaths used to introduce the system. New systems are currently being developed with smaller insertion sheaths. Future trials will determine whether the percutaneous approach is equivalent to surgical replacement in patients with aortic stenosis and no contraindication to surgery.
Key Points
Andersen HR, Nielsen TT, Rasmussen K, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733-742.
Bowers TR, O’Neill WW, Grines C. Effect of reperfusion on biventricular function and survival after right ventricular infarction. N Engl J Med. 1998;338:933-940.
Hochman JS, Sleeper LA, Webb JG, Dzavik V, Buller CE, Aylward P, et al. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. SHOCK Investigators. JAMA. 2006;295:2511-2515.
Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. SYNTAX Investigators. N Engl J Med. 2009;360:961-972.
Wallentin L, Lagerqvist B, Husted S, et al. Outcome at 1 year after an invasive compared with a non-invasive strategy in unstable coronary-artery disease: the FRISC II invasive randomised trial. Lancet. 2000;356:9-16.
1 Gruntzig A. Transluminal dilatation of coronary-artery stenosis. Lancet. 1978;1(8058):263.
2 Grines CL, Browne KF, Marco J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med. 1993;328(10):673-679.
3 Zijlstra F, de Boer MJ, Hoorntje JC, Reiffers S, Reiber JH, Suryapranata H. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med. 1993;328(10):680-684.
4 Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336(23):1629-1633.
5 Mehilli J, Kastrati A, Dirschinger J, et al. Intracoronary stenting and angiographic results: Restenosis after direct stenting versus stenting with predilation in patients with symptomatic coronary artery disease (ISAR-DIRECT trial). Catheter Cardiovasc Interv. 2004;61(2):190-195.
6 Barnathan ES, Schwartz JS, Taylor L, et al. Aspirin and dipyridamole in the prevention of acute coronary thrombosis complicating coronary angioplasty. Circulation. 1987;76(1):125-134.
7 Schomig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334(17):1084-1089.
8 Silber S, Albertsson P, Aviles FF, et al. Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur Heart J. 2005;26(8):804-847.
9 Mehta SR, Bassand JP, Chrolavicius S, et al. Design and rationale of CURRENT-OASIS 7: a randomized, 2 × 2 factorial trial evaluating optimal dosing strategies for clopidogrel and aspirin in patients with ST and non-ST-elevation acute coronary syndromes managed with an early invasive strategy. Am Heart J. 2008;156(6):1080-1088. e1
10 Gurbel PA, Bliden KP. Durability of platelet inhibition by clopidogrel. Am J Cardiol. 2003;91(9):1123-1125.
11 Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45(2):246-251.
12 Steinhubl SR, Berger PB, Mann JT3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288(19):2411-2420.
13 Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation. 2007;116(25):2923-2932.
14 Montalescot G, Sideris G, Cohen R, et al. Prasugrel compared with high-dose clopidogrel in acute coronary syndrome. The randomised, double-blind ACAPULCO study. Thromb Haemost. 2010;103(1):213-223.
15 Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015.
16 Cannon CP, Harrington RA, James S, et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375:283-293.
17 McGarry TFJr, Gottlieb RS, Morganroth J, et al. The relationship of anticoagulation level and complications after successful percutaneous transluminal coronary angioplasty. Am Heart J. 1992;123(6):1445-1451.
18 Dumaine R, Borentain M, Bertel O, et al. Intravenous low-molecular-weight heparins compared with unfractionated heparin in percutaneous coronary intervention: quantitative review of randomized trials. Arch Intern Med. 2007;167(22):2423-2430.
19 Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. Lancet. 1998;352(9122):87-92.
20 Randomised placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina: the CAPTURE Study. Lancet. 1997;349(9063):1429-1435.
21 Effects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplasty. The RESTORE Investigators. Randomized Efficacy Study of Tirofiban for Outcomes and Restenosis. Circulation. 1997;96(5):1445-1453.
22 Topol EJ, Califf RM, Weisman HF, et al. Randomised trial of coronary intervention with antibody against platelet IIb/IIIa integrin for reduction of clinical restenosis: results at six months. The EPIC Investigators. Lancet. 1994;343(8902):881-886.
23 Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. The EPIC Investigation. N Engl J Med. 1994;330(14):956-961.
24 Kastrati A, Mehilli J, Schuhlen H, et al. A clinical trial of abciximab in elective percutaneous coronary intervention after pretreatment with clopidogrel. N Engl J Med. 2004;350(3):232-238.
25 Webster MW. Horizons-AMI. Lancet. 2010;375(9712):375.
26 Spaulding C, Lefevre T, Funck F, et al. Left radial approach for coronary angiography: results of a prospective study. Cathet Cardiovasc Diagn. 1996;39(4):365-370.
27 Kiemeneij F, Laarman GJ, Slagboom T, van der Wieken R. Outpatient coronary stent implantation. J Am Coll Cardiol. 1997;29(2):323-327.
28 Kimmel SE, Berlin JA, Strom BL, Laskey WK. Development and validation of simplified predictive index for major complications in contemporary percutaneous transluminal coronary angioplasty practice. The Registry Committee of the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1995;26(4):931-938.
29 Di Mario C, Werner GS, Sianos G, et al. European perspective in the recanalisation of Chronic Total Occlusions (CTO): consensus document from the EuroCTO Club. Eur Interv. 2007;3(1):30-43.
30 Stone GW, Kandzari DE, Mehran R, et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part I. Circulation. 2005;112(15):2364-2372.
31 Hanekamp CE, Koolen JJ, Den Heijer P, et al. Randomized study to compare balloon angioplasty and elective stent implantation in venous bypass grafts: the Venestent study. Catheter Cardiovasc Interv. 2003;60(4):452-457.
32 Keeley EC, Velez CA, O’Neill WW, Safian RD. Long-term clinical outcome and predictors of major adverse cardiac events after percutaneous interventions on saphenous vein grafts. J Am Coll Cardiol. 2001;38(3):659-665.
33 Faxon DP, Weber VJ, Haudenschild C, Gottsman SB, McGovern WA, Ryan TJ. Acute effects of transluminal angioplasty in three experimental models of atherosclerosis. Arteriosclerosis. 1982;2(2):125-133.
34 Steele PM, Chesebro JH, Stanson AW, et al. Balloon angioplasty. Natural history of the pathophysiological response to injury in a pig model. Circ Res. 1985;57(1):105-112.
35 Farb A, Virmani R, Atkinson JB, Kolodgie FD. Plaque morphology and pathologic changes in arteries from patients dying after coronary balloon angioplasty. J Am Coll Cardiol. 1990;16(6):1421-1429.
36 Soward AL, Essed CE, Serruys PW. Coronary arterial findings after accidental death immediately after successful percutaneous transluminal coronary angioplasty. Am J Cardiol. 1985;56(12):794-795.
37 Tenaglia AN, Buller CE, Kisslo KB, Stack RS, Davidson CJ. Mechanisms of balloon angioplasty and directional coronary atherectomy as assessed by intracoronary ultrasound. J Am Coll Cardiol. 1992;20(3):685-691.
38 Potkin BN, Keren G, Mintz GS, et al. Arterial responses to balloon coronary angioplasty: an intravascular ultrasound study. J Am Coll Cardiol. 1992;20(4):942-951.
39 Waller BF. Early and late morphologic changes in human coronary arteries after percutaneous transluminal coronary angioplasty. Clin Cardiol. 1983;6(8):363-372.
40 Nobuyoshi M, Kimura T, Nosaka H, et al. Restenosis after successful percutaneous transluminal coronary angioplasty: serial angiographic follow-up of 229 patients. J Am Coll Cardiol. 1988;12(3):616-623.
41 Does the new angiotensin converting enzyme inhibitor cilazapril prevent restenosis after percutaneous transluminal coronary angioplasty? Results of the MERCATOR study: a multicenter, randomized, double-blind placebo-controlled trial. Multicenter European Research Trial with Cilazapril after Angioplasty to Prevent Transluminal Coronary Obstruction and Restenosis (MERCATOR) Study Group. Circulation. 1992;86(1):100-110.
42 Hirshfeld JWJr, Schwartz JS, Jugo R, et al. Restenosis after coronary angioplasty: a multivariate statistical model to relate lesion and procedure variables to restenosis. The M-HEART Investigators. J Am Coll Cardiol. 1991;18(3):647-656.
43 Lange RA, Willard JE, Hillis LD. Southwestern internal medicine conference: restenosis: the Achilles heel of coronary angioplasty. Am J Med Sci. 1993;306(4):265-275.
44 Libby P, Schwartz D, Brogi E, Tanaka H, Clinton SK. A cascade model for restenosis. A special case of atherosclerosis progression. Circulation. 1992;86(6 Suppl):III47-III52.
45 Mintz GS, Popma JJ, Pichard AD, et al. Intravascular ultrasound predictors of restenosis after percutaneous transcatheter coronary revascularization. J Am Coll Cardiol. 1996;27(7):1678-1687.
46 Mintz GS, Popma JJ, Pichard AD, et al. Arterial remodeling after coronary angioplasty: a serial intravascular ultrasound study. Circulation. 1996;94(1):35-43.
47 Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331(8):496-501.
48 Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331(8):489-495.
49 Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346(23):1773-1780.
50 Spaulding C, Daemen J, Boersma E, Cutlip DE, Serruys PW. A pooled analysis of data comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356(10):989-997.
51 Smith SCJr, Feldman TE, Hirshfeld JWJr, et al. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention–Summary Article: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention) 10.1161/CIRCULATIONAHA.105.170815. Circulation. 2006;113(1):156-175.
52 Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126-2130.
53 Patrono C, Bachmann F, Baigent C, et al. Expert consensus document on the use of antiplatelet agents. The task force on the use of antiplatelet agents in patients with atherosclerotic cardiovascular disease of the European society of cardiology. Eur Heart J. 2004;25(2):166-181.
54 Serruys PW, Ormiston JA, Onuma Y, et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373(9667):897-910.
55 Safian RD, Niazi KA, Strzelecki M, et al. Detailed angiographic analysis of high-speed mechanical rotational atherectomy in human coronary arteries. Circulation. 1993;88(3):961-968.
56 Tran T, Brown M, Lasala J. An evidence-based approach to the use of rotational and directional coronary atherectomy in the era of drug-eluting stents: when does it make sense? Catheter Cardiovasc Interv. 2008;72(5):650-662.
57 Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503-1516.
58 Cannon CP, Weintraub WS, Demopoulos LA, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344(25):1879-1887.
59 Wallentin L, Lagerqvist B, Husted S, Kontny F, Stahle E, Swahn E. Outcome at 1 year after an invasive compared with a non-invasive strategy in unstable coronary-artery disease: the FRISC II invasive randomised trial. FRISC II Investigators. Fast Revascularisation during Instability in Coronary artery disease. Lancet. 2000;356(9223):9-16.
60 Lagerqvist B, Husted S, Kontny F, Stahle E, Swahn E, Wallentin L. 5-year outcomes in the FRISC-II randomised trial of an invasive versus a non-invasive strategy in non-ST-elevation acute coronary syndrome: a follow-up study. Lancet. 2006;368(9540):998-1004.
61 Andersen HR, Nielsen TT, Rasmussen K, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349(8):733-742.
62 Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. 2006;295(21):2511-2515.
63 Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341(9):625-634.
64 Ellis SG, da Silva ER, Heyndrickx G, et al. Randomized comparison of rescue angioplasty with conservative management of patients with early failure of thrombolysis for acute anterior myocardial infarction. Circulation. 1994;90(5):2280-2284.
65 Bowers TR, O’Neill WW, Grines C, Pica MC, Safian RD, Goldstein JA. Effect of reperfusion on biventricular function and survival after right ventricular infarction. N Engl J Med. 1998;338(14):933-940.
66 Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961-972.
67 Inoue K, Owaki T, Nakamura T, Kitamura F, Miyamoto N. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg. 1984;87(3):394-402.
68 Cohen DJ, Kuntz RE, Gordon SP, et al. Predictors of long-term outcome after percutaneous balloon mitral valvuloplasty. N Engl J Med. 1992;327(19):1329-1335.
69 Kuntz RE, Tosteson AN, Berman AD, et al. Predictors of event-free survival after balloon aortic valvuloplasty. N Engl J Med. 1991;325(1):17-23.
70 Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet. 1986;1(8472):63-67.
71 Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol. 2004;43(4):698-703.