Intussusception
Intussusception is the most frequent cause of bowel obstruction in infants and toddlers. It is an acquired invagination of the proximal bowel (intussusceptum) into the distal bowel (intussuscipiens). It was first described in 1674 by Paul Barbette of Amsterdam, defined by Treves in 1899, and operated on successfully in 1873 by John Hutchinson.1,2
Pathophysiology
Primary Intussusception
The vast majority of cases do not have a lead point and are classified as primary or idiopathic intussusceptions. The cause is generally attributed to hypertrophied Peyer patches within the bowel wall.3 Intussusception occurs frequently in the wake of an upper respiratory tract infection or an episode of gastroenteritis, providing an etiology for the hypertrophied lymphoid tissue. Adenoviruses in children older than age two, and to a lesser extent rotaviruses, have been implicated in up to 50% of cases.4,5 Other contributing evidence that viruses may play a role in intussusception includes the rise in cases during seasonal respiratory viral illnesses and the increased risk associated with previous rotavirus immunization.6 The newest immunization formulas available in the USA, RotaTeq® and Rotarix®, have not been associated with intussusception in both pre- and post-marketing studies.6–10
Secondary Intussusception
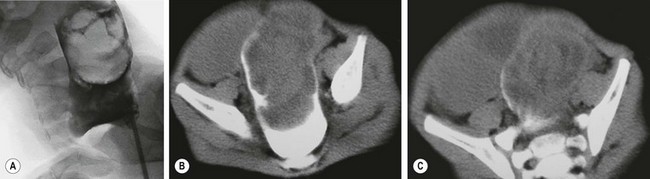
An intussusception may have an identifiable lesion that serves as a lead point, drawing the proximal bowel into the distal bowel by peristaltic activity. The incidence of a lead point varies from 1.5% to 12% and the presence of a lead point increases in proportion with age.11,12 The most common lead point is a Meckel diverticulum followed by polyps and duplications. Other benign lead points include the appendix, hemangiomas, carcinoid tumors, foreign bodies, ectopic pancreas or gastric mucosa, hamartomas from Peutz–Jeghers syndrome (Fig. 38-1), and lipomas. Malignant causes, although rare, increase in incidence with age and include lymphomas and small bowel tumors.13 Systemic diseases, including Henoch–Schönlein purpura and cystic fibrosis, have been associated with intussusception. Other rare diseases related to intussusception are celiac disease and Clostridium difficile colitis.14
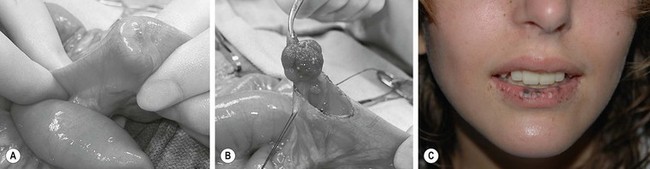
FIGURE 38-1 (A) Operative view of the outside of the jejunum shows a palpable mass as the lead point of a reduced intussusception. (B) A hamartomatous polyp is characteristic of Peutz–Jeghers syndrome. (C) Mucocutaneous macular lesions are seen in this patient with Peutz–Jeghers syndrome. Note extension of the pigmentation beyond the vermilion border.
Incidence
Idiopathic intussusception can occur at any age. Most patients are well-nourished, healthy infants, and approximately two-thirds are boys. The highest incidence occurs in infants between ages 4 and 9 months,15 and it is also the most common cause of small bowel obstruction in this age group.16 Intussusception is uncommon below 3 months and after 3 years of age. The condition has been described in premature infants where it has been postulated as the cause of small bowel atresia in some cases.17
Clinical Presentation
The classic presentation is an infant or a young child with intermittent, crampy abdominal pain associated with ‘currant jelly’ stools and a palpable mass on physical examination, although this triad is seen in less than a fourth of children.18 The abdominal pain is sudden and the child may stiffen and pull the legs up to the abdomen. The pain can also be associated with hyperextension, writhing, breath holding and vomiting. The attack often ceases as suddenly as it started. Between attacks, the child may appear comfortable but eventually will become lethargic. Small or normal bowel movements will stop as the obstruction progresses and becomes associated with bilious emesis and increasing abdominal distention. Stools may be blood tinged as impending ischemia causes mucosal sloughing and compression of mucous glands leading to evacuation of dark, red mucoid clots or ‘currant jelly’ stools. This is often a late sign as are laboratory derangements. A pitfall is to wait for the currant jelly stool, leukocytosis, and electrolyte abnormalities that are often hallmarks of ischemic bowel.
Physical Examination
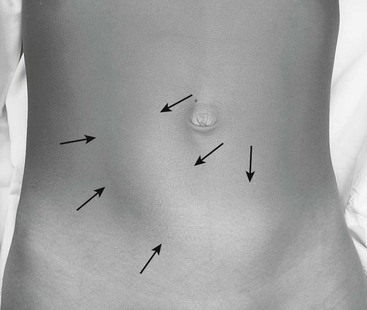
The child’s vital signs are usually normal early in the disease course. During painless intervals, the child may appear comfortable and the physical examination may be unremarkable. However, the cramping episodes usually occur every 15 to 30 minutes and re-examination may prove difficult. There may be audible peristaltic rushes, and a sausage-shaped or curved mass might be palpable anywhere in the abdomen or even visualized if the child is relatively thin (Fig. 38-2). The right lower abdominal quadrant can appear flat or empty (Dance sign) as the intussuscepted mass is drawn cephalad. On rectal examination, bloodstained mucus or blood may be encountered as a later sign. If the obstructive process worsens and bowel ischemia occurs, dehydration, fever, tachycardia, and hypotension can develop in quick succession as a result of bacteremia and bowel necrosis.
Diagnosis
Abdominal Radiography
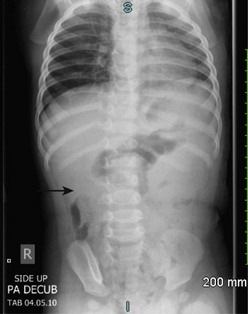
In half of cases, the diagnosis of intussusception can be suspected on plain flat and upright abdominal radiographs (Fig. 38-3). Suggestive radiographic abnormalities include an abdominal mass, abnormal distribution of gas and fecal contents, sparse large bowel gas, and air-fluid levels in the presence of bowel obstruction. However, plain films have limited value in confirming the diagnosis and are not used as the sole diagnostic test. They are best utilized as a screening tool when one of the abnormal findings listed above is found.19
Ultrasonography
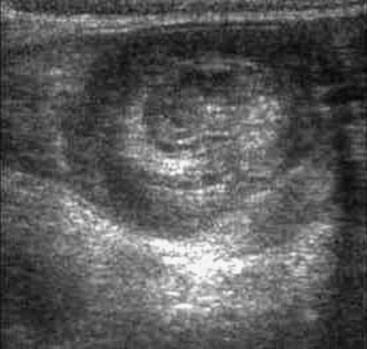
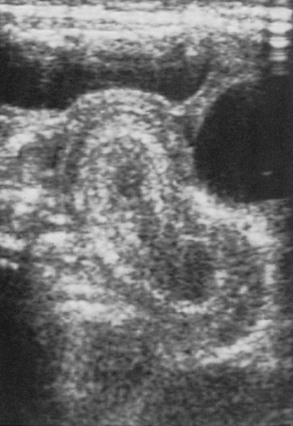
The use of abdominal ultrasound (US) for the evaluation of intussusception was first described in 1977.20 Since then, most institutions have adopted it as a screening tool because of the lack of radiation exposure, ability to identify pathologic lead points, and decreased cost.21,22 The characteristic finding on ultrasound has been referred to as a ‘target’ or ‘doughnut’ lesion (Fig. 38-4), which consists of alternating rings of low and high echogenicity representing the bowel wall and mesenteric fat within the intussusceptum in a transverse plane. The ‘pseudokidney’ sign is seen on longitudinal section (Fig. 38-5). This pattern is secondary to the edematous walls of the intussusceptum within the intussuscipiens. Ultrasonography can also guide the therapeutic reduction of an intussusception.21 Equivocal findings using this modality should mandate a conventional contrast or air enema.23
Computed Tomography and Magnetic Resonance Imaging
Neither computed tomography (CT)24 nor magnetic resonance imaging (MRI) are routinely used in the evaluation of a patient with intussusception, although either may confirm this diagnosis and/or pathologic causes for intussusception, such as a malignancy (i.e., lymphoma). The characteristic CT finding is a ‘target’ or ‘doughnut’ sign (Fig. 38-6). Transient small bowel intussusceptions that are discovered on CT or MRI are usually not clinically significant.21 Radiographic or operative treatment should be based on clinical findings in symptomatic patients.25 Laparoscopy is an excellent means to evaluate these patients if surgical intervention is needed.
Nonoperative Management
Hydrostatic and Pneumatic Reduction
The methodology for hydrostatic reduction has not changed significantly since its first description in 1876.26 Hydrostatic reduction with barium under fluoroscopic guidance has historically been used.27 More recently, children’s hospitals have transitioned to air or water-soluble isotonic contrast because of the potential hazard of barium peritonitis in patients with intestinal perforation16,28. Successful reduction (Fig. 38-7) in uncomplicated patients is seen in about 85% of cases and ranges from 42% to 95%.29
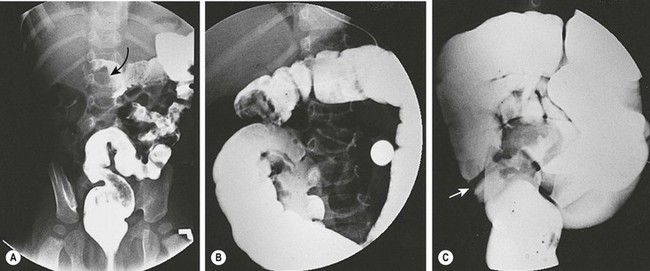
FIGURE 38-7 Fluoroscopic examination using isotonic contrast for hydrostatic reduction of intussusception. (A) Intussusception (arrow) seen in midtransverse colon. (B) Reduction has occurred to the hepatic flexure. (C) Complete reduction with reflux of contrast medium into the terminal ileum. Note the edematous ileocecal valve (arrow).
Pneumatic reduction was first described in 1897.30 It gained popularity in the late 1980s. Since then, many institutions have adopted pneumatic decompression because it is quicker, safer, less messy, and decreases the exposure time to radiation.31 The procedure is fluoroscopically monitored as air is insufflated into the rectum (Fig. 38-8). The maximum safe air pressure is 80 mmHg for younger infants and 110–120 mmHg for older infants. Potential drawbacks of pneumatic reduction include the possibility of developing tension pneumoperitoneum, and poor visualization of lead points and/or the intussusception reduction process, resulting in false-positive reductions.32–34 Rates of perforation range from 0.4–2.5% with the most recent publications citing an average rate of 0.8%.16,35
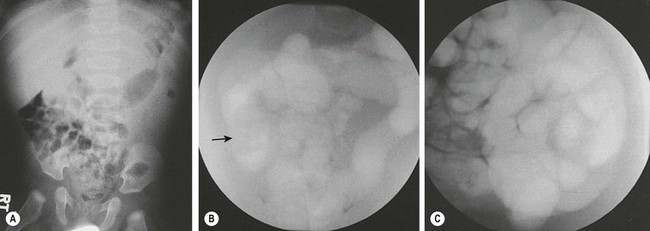
FIGURE 38-8 Plain radiography and fluoroscopic examination using air for pneumatic reduction of an intussusception. (A) Plain radiograph showing a mass effect in the right upper quadrant. (B) Pneumatic reduction to the vicinity of the cecum with the intussusception still present (arrow). (C) Complete reduction with reflux of air into multiple loops of small intestine. (Courtesy of Charles Maxfield, MD.)
Tension pneumoperitoneum is best treated with immediate cessation of the procedure and immediate release of the pneumoperitoneum using a 14, 16, or 18-gauge needle or angiocatheter above or below the umbilicus. This should be followed by immediate operative exploration.36
For unsuccessful reduction, several studies have shown improved reduction rates using a second attempt after waiting between 30 minutes to 24 hours after the initial attempt.28 In some instances, this is done in the operating room prior to laparoscopy or in conjunction with laparoscopic reduction.37
Operative Management
Open Approach
An operation is needed when nonoperative reduction is unsuccessful or incomplete, for signs of peritonitis, the presence of a lead point, or radiographic evidence of pneumoperitoneum. Preoperative preparation includes administration of broad-spectrum antibiotics, intravenous fluid resuscitation, insertion of a urinary catheter, and placement of a nasogastric tube for gastric decompression. Most commonly, the cecum and terminal ileum are involved, and can be delivered through the traditional right lower abdominal incision (Fig. 38-9). It is important to evaluate the extent of the intussusceptum before delivering it as it can extend into the rectosigmoid region in severe cases which usually requires extension of the incision.
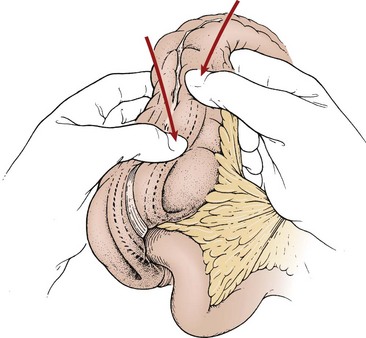
FIGURE 38-9 A right lower quadrant muscle-splitting incision allows delivery of the intussusception through the incision. Gentle and continuous massage from distal to proximal usually results in reduction of the intussusception.
Once the leading edge of the intussusceptum is identified, it is gently manipulated back toward its normal position in the terminal ileum. Excessive force or pulling is avoided to prevent injury or perforation of the bowel. Inability to manually reduce the intussusception, the finding of ischemic bowel, or identification of a lead point requires resection and bowel anastomosis or diversion, depending on the condition of the bowel and child. Ileopexy has been described in patients with recurrent intussusception after operative reduction.38 However, in a series of 278 patients, this technique was not shown to reduce re-intussusception rates when compared to operative reduction and resection of the affected area.39
Laparoscopic Approach
Initially, the use of laparoscopy in the operative management of intussusception was strictly diagnostic, or was used in cases with equivocal radiographic studies or in patients with suspected lead points, and was associated with conversion rates in up to 70% of cases.40 More recently, there has been increased success with laparoscopic reduction with some studies showing conversion rates as low as 5.4%41 but more in the range of 12–40%.37,42–44
Where laparoscopy fits into a surgeon’s therapeutic algorithm is a topic frequently discussed. It would be beneficial to identify any preoperative risk factors. No study to date has specifically addressed this topic although some have noted an increased conversion rate associated with lead points. Recently, a retrospective analysis of 65 cases found that in patients unable to be reduced laparoscopically, 33% had a lead point that necessitated conversion to open (Fig. 38-10).45 Contraindications to laparoscopy include peritonitis, hemodynamic instability, and severe bowel distension that precludes adequate visualization.41
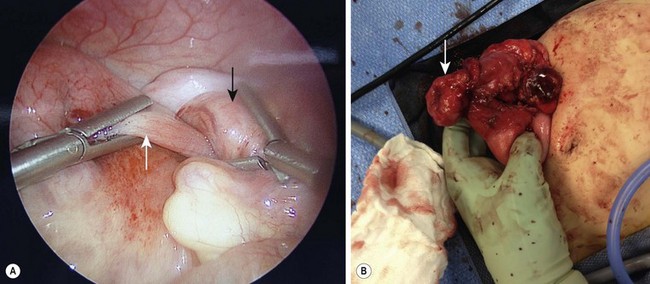
FIGURE 38-10 (A) This laparoscopic photograph shows an incompletely reduced intussusception with the intussusceptum (white arrow) telescoping into the intussuscipiens (black arrow). (B) A pathologic lead point due to a Burkitt lymphoma was found requiring conversion to open.
The majority of minimally invasive approaches describe the use of three abdominal ports: one in the infraumbilical region with two other ports along the left side of the abdomen. Laparoscopic reduction is accomplished by applying gentle pressure distal to the intussusceptum using atraumatic graspers. Although counterintuitive to the conventional open method, traction is usually required proximal to the intussuscipiens to complete the reduction (Figs. 38-11). Appendectomy is not routinely performed with laparoscopic reduction and up to the surgeon’s discretion. Careful inspection of the bowel is performed to evaluate for any signs of ischemia, necrosis, or perforation. A criticism of laparoscopic reduction is the loss of tactile sense that can lead to missed pathology. If resection is required, this can often be accomplished by exteriorizing the bowel by enlarging the periumbilical incision. If this is not possible, the operation should be converted to a laparotomy.
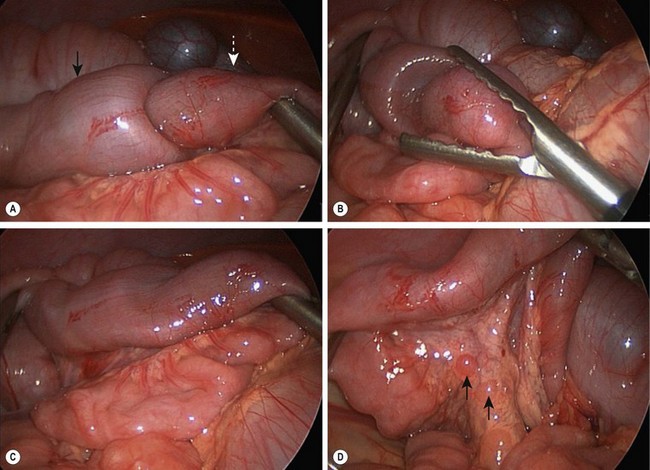
FIGURE 38-11 Laparoscopic reduction of intussusception with hypertrophied lymph nodes is depicted in these four operative photographs. (A) Intussusceptum (white arrow) is seen telescoping into the intussuscipiens (black arrow). (B) The intussusception has almost been completely reduced. (C) This intussusception has been completely reduced and the bowel appears viable. (D) Hypertrophied mesenteric lymphadenopathy (arrows) is seen. This lymphadenopathy may reflect a recent viral illness.
Recurrent Intussusception
Recurrent intussusception has been described in association with nonoperative intervention in 10–15% of cases, with about one-third occurring within 24 hours and the majority within 6 months of the initial episode.46 Recurrences are less likely to occur after operative reduction or resection.47 After laparoscopic reduction, a recurrence rate as high as 10% has been reported.37
Patients with recurrent intussusception tend to be seen earlier in their course because their parents are more aware of how to recognize the signs and symptoms. Success rates with enema reduction after one recurrence are comparable to those with the first episode and are better if the child did not previously require operative reduction. This finding has led to a nonoperative approach for initial management of recurrence in most patients as long as they are not toxic or show signs of peritonitis or hemodynamically instability.29,46 A concern in recurrent intussusception is occult malignancy. Unfortunately, the clinical findings or pattern of recurrence do not predict the presence of a malignant lead point and radiographic reduction with ultrasound is recommended to look for an occult pathology.48,49
Postoperative Intussusception
Postoperative intussusception is a rare clinical entity that has been described after ileocolic intussusception reduction and resection, retroperitoneal dissections, long intra-abdominal procedures, a Ladd procedure, or extra-abdominal operations.50,51 It accounts for 3% to 10% of cases of postoperative bowel obstruction and most often occurs in the initial 10 days following a procedure.52,53 Ileus and adhesive obstruction are more frequently encountered as a cause for intestinal obstruction in the postoperative patient. Thus, an index of suspicion is needed and ultrasound is a useful diagnostic tool.51 Most postoperative intussusceptions are ileoileal and respond to operative reduction without resection.
References
1. Barbette, P. Oeuvres Chirurgiques et Anatomiques. Geneva: Francois Miege; 1674.
2. Hutchinson, J. A successful case of abdominal section for intussusception. Proc R Med Chir Soc. 1873; 7:195–198.
3. Stringer, MD, Pablot, SM, Brereton, RJ. Paediatric intussusception. Br J Surg. 1992; 79:867–876.
4. Okimoto, S, Hyodo, S, Yamamoto, M, et al. Association of viral isolates from stool samples with intussusception in children. Int J Infect Dis. 2011; 15:e641–e645.
5. Bines, JE, Liem, NT, Justice, FA, et al. Risk factors for intussusception in infants in Vietnam and Australia: Adenovirus implicated, but not rotavirus. J Pediatr. 2006; 149:452–460.
6. Belongia, EA, Irving, SA, Shui, IM, et al. Real-time surveillance to assess risk of intussusception and other adverse events after pentavalent, bovine-derived rotavirus vaccine. Pediatr Infect Dis J. 2010; 29:1–5.
7. Shui, IM, Baggs, J, Patel, M, et al. Risk of intussusception following administration of a pentavalent rotavirus vaccine in ultrasound infants. JAMA. 2012; 307:598–604.
8. Buttery, JP, Danchin, MH, Lee, KJ, et al. Intussusception following rotavirus vaccine administration: Post-marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011; 29:3061–3066.
9. Ruiz-Palacios, GM, Perez-Schael, I, Velazquez, FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006; 354:11–22.
10. Vesikari, T, Matson, DO, Dennehy, P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006; 354:23–33.
11. Blakelock, RT, Beasley, SW. The clinical implications of non-idiopathic intussusception. Pediatr Surg Int. 1998; 14:163–167.
12. West, KW, Grosfeld, JL. Intussusception in Infants and Children. Philadelphia: WB Saunders; 1999.
13. Rampone, B, Roviello, F, Marrelli, D, et al. Late recurrence of malignant melanoma presenting as small bowel intussusception. Dig Dis Sci. 2006; 51:1047–1048.
14. Park, JH. CMH. Intussusception associated with pseudomembranous colitis [Letter to the Editor]. J Pediatr Gastroenterol Nutr. 2008; 46:470–471.
15. Huppertz, HI, Soriano-Gabarro, M, Grimprel, E, et al. Intussusception among young children in Europe. Pediatr Infect Dis J. 2006; 25(Suppl. 1):S22–S29.
16. Applegate, KE. Clinically suspected intussusception in children: Evidence-based review and self-assessment module. AJR Am J Roentgenol. 2005; 185(Suppl. 3):S175–S183.
17. Kong, FT, Liu, WY, Tang, YM, et al. Intussusception in infants younger than 3 months: A single center’s experience. World J Pediatr. 2010; 6:55–59.
18. Kaiser, AD, Applegate, KE, Ladd, AP. Current success in the treatment of intussusception in children. Surgery. 2007; 142:469–477.
19. Weihmiller, SN, Buonomo, C, Bachur, R. Risk stratification of children being evaluated for intussusception. Pediatrics. 2011; 127:e296–e303.
20. Burke, LF, Clark, E. Ileocolic intussusception–a case report. J Clin Ultrasound. 1977; 5:346–347.
21. Henrikson, S, Blane, CE, Koujok, K, et al. The effect of screening sonography on the positive rate of enemas for intussusception. Pediatr Radiol. 2003; 33:190–193.
22. Navarro, O, Daneman, A. Intussusception. Part 3: Diagnosis and management of those with an identifiable or predisposing cause and those that reduce spontaneously. Pediatr Radiol. 2004; 34:305–312.
23. Gu, L, Zhu, H, Wang, S, et al. Sonographic guidance of air enema for intussusception reduction in children. Pediatr Radiol. 2000; 30:339–342.
24. Fecteau, A, Flageole, H, Nguyen, LT, et al. Recurrent intussusception: Safe use of hydrostatic enema. J Pediatr Surg. 1996; 31:859–861.
25. Kornecki, A, Daneman, A, Navarro, O, et al. Spontaneous reduction of intussusception: Clinical spectrum, management and outcome. Pediatr Radiol. 2000; 30:58–63.
26. Hirschsprung, H. Et Tilfaelde af suakat Tarminvagination. Hospitals-Tidende. 1876; 3:321–327.
27. Ravitch, MM. Intussusception in Infants and Children. Springfield, IL; 1959.
28. Daneman, A, Navarro, O. Intussusception. Part 1: A review of diagnostic approaches. Pediatr Radiol. 2003; 33:79–85.
29. Navarro, OM, Daneman, A, Chae, A. Intussusception: the use of delayed, repeated reduction attempts and the management of intussusceptions due to pathologic lead points in pediatric patients. AJR Am J Roentgenol. 2004; 182:1169–1176.
30. Holt, LE. The Diseases of Infancy and Childhood: For the Use of Students and Practioners of Medicine. New York: Appleton; 1897.
31. Guo, JZ, Ma, XY, Zhou, QH. Results of air pressure enema reduction of intussusception: 6,396 cases in 13 years. J Pediatr Surg. 1986; 21:1201–1203.
32. Kirks, DR. Air intussusception reduction: ‘the winds of change’. Pediatr Radiol. 1995; 25:89–91.
33. Peh, WC, Khong, PL, Chan, KL, et al. Sonographically guided hydrostatic reduction of childhood intussusception using Hartmann’s solution. AJR Am J Roentgenol. 1996; 167:1237–1241.
34. Maoate, K, Beasley, SW. Perforation during gas reduction of intussusception. Pediatr Surg Int. 1998; 14:168–170.
35. Tareen, F, Ryan, S, Avanzini, S, et al. Does the length of the history influence the outcome of pneumatic reduction of intussusception in children? Pediatr Surg Int. 2011; 27:587–589.
36. Sohoni, A, Wang, NE, Dannenberg, B. Tension pneumoperitoneum after intussusception pneumoreduction. Pediatr Emerg Care. 2007; 23:563–564.
37. Kao, C, Tseng, SH, Chen, Y. Laparoscopic reduction of intussusception in children by a single surgeon in comparison with open surgery. Minim Invasive Ther Allied Technol. 2011; 20:141–145.
38. Waldhausen, JH. Intussusception. In: Mattei P, ed. Fundamentals of Pediatric Surgery. New York: Springer, 2011.
39. Koh, CC, Sheu, JC, Wang, NL, et al. Recurrent ileocolic intussusception after different surgical procedures in children. Pediatr Surg Int. 2006; 22:725–728.
40. van der Laan, M, Bax, NM, van der Zee, DC, et al. The role of laparoscopy in the management of childhood intussusception. Surg Endosc. 2001; 15:373–376.
41. Bailey, KA, Wales, PW, Gerstle, JT. Laparoscopic versus open reduction of intussusception in children: A single-institution comparative experience. J Pediatr Surg. 2007; 42:845–848.
42. Kia, KF, Mony, VK, Drongowski, RA, et al. Laparoscopic vs open surgical approach for intussusception requiring operative intervention. J Pediatr Surg. 2005; 40:281–284.
43. Burjonrappa, SC. Laparoscopic reduction of intussusception: An evolving therapeutic option. JSLS. 2007; 11:235–237.
44. Bonnard, A, Demarche, M, Dimitriu, C, et al. Indications for laparoscopy in the management of intussusception: A multicenter retrospective study conducted by the French Study Group for Pediatric Laparoscopy (GECI). J Pediatr Surg. 2008; 43:1249–1253.
45. Hill, SJ, Langness, SM, Wulkan, ML, Laparoscopic versus Open Reduction of Intussusception in Children: Experience Over a Decade Poster presented at Southeastern Surgical Congress Feb 2012 Birmingham, AL, 2012.
46. Niramis, R, Watanatittan, S, Kruatrachue, A, et al. Management of recurrent intussusception: Nonoperative or operative reduction? J Pediatr Surg. 2010; 45:2175–2180.
47. Mirza, B. Recurrent intussusception: Management options. APSP J Case Rep. 2011; 2:9.
48. Champoux, AN, Del Beccaro, MA, Nazar-Stewart, V. Recurrent intussusception. Risks and features. Arch Pediatr Adolesc Med. 1994; 148:474–478.
49. Navarro, O, Dugougeat, F, Kornecki, A, et al. The impact of imaging in the management of intussusception owing to pathologic lead points in children. A review of 43 cases. Pediatr Radiol. 2000; 30:594–603.
50. Holcomb, GW, III., Ross, AJ, III., O’Neill, JA, Jr. Post-operative intussusception: Increasing frequency or increasing awareness? South Med J. 1991; 84:1334–1339.
51. Bai, YZ, Chen, H, Wang, WL. A special type of postoperative intussusception: Ileoileal intussusception after surgical reduction of ileocolic intussusception in infants and children. J Pediatr Surg. 2009; 44:755–758.
52. Linke, F, Eble, F, Berger, S. Postoperative intussusception in childhood. Pediatr Surg Int. 1998; 14:175–177.
53. Laje, P, Stanley, CA, Adzick, NS. Intussusception after pancreatic surgery in children: A case series. J Pediatr Surg. 2010; 45:1496–1499.