CHAPTER 1 Introduction to endoscopy
1.1 Anatomy of an endoscope
Key Points
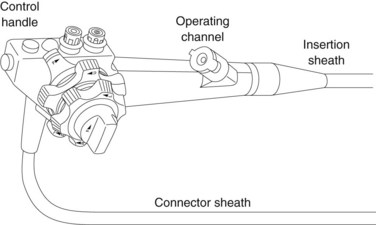
1 The control handle
The control handle (Fig. 1) combines the following elements:
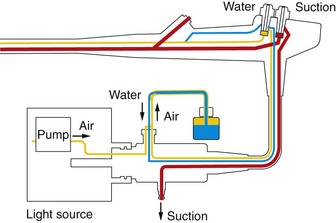
1.1 Suction and insufflation/cleaning valves
1.1.1 Suction
The cylinder (Fig. 2), which is attached to the aspirator at its base, communicates with the operating channel, to which it is joined by a circular channel.
1.1.2 Insufflation–cleaning
The cylinder (Fig. 2) integrates separate inlets and outlets for air and water. The inlet–outlet group that is closest to the base is the insufflation channel. The second group, which is higher up, is the lens cleaning channel.
This mechanism is either fully activated or deactivated, i.e. there is no intermediate position.
![]() Clinical Tips
Clinical Tips
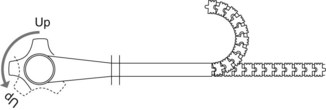
1.2 Bending section controls
The two flexing controls (Fig. 3) (large wheel for up/down, small wheel for left/right) are coaxial and each has a separate brake.
When the drum is rotated using the control wheel, it rolls up the cable, which is attached to the tip of a series of articulated steel rings that are attached to each other (like vertebrae) and can bunch up in such way as to provoke the deformation shown in Figure 3.
There are generally either four or six control buttons housed on top of the control handle.
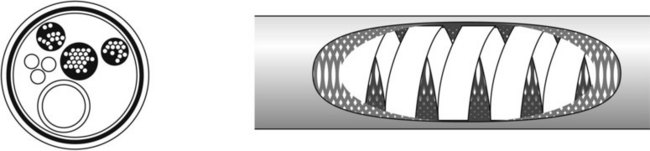
2 Main insertion tube
The insertion sheath, which is mounted on the handle, combines the following elements:
Internally, the sheath (Fig. 4) contains a spiral metal element covered with a metal plait that provides the external synthetic resin coating with support. The characteristics of the spiral determine the texture of the sheath, depending on the thickness of the metal element, and the extent to which the wound elements tend to form joints with each other. The type of metal used is also a key factor. Stainless steel is used for the upper segment, whereas bronze is used for longer colonoscopes. Alternatively, to obtain better rotational torque, two parallel coaxial spiral elements are integrated and move in opposite directions in such a way that they oppose each other.
3 Bending section
The bending section (Fig. 3), which is the continuation of the main sheath, bears a strong resemblance to an alligator’s spine. The bending section is composed of a series of circular rings that are reciprocally articulated at a 90° angle. Each ring combines hinge plates that guide the bending section cables and keep them in place. The cables, which are soldered to the distal chain, pass over the hinge plates, and when they reach the main sheath, they enter the insertion tube. The rings must be non-contiguous, so that when a cable is pulled, causing the rings to bunch up toward the sheath, they cannot pivot on their axes and abut each other. When two cables are pulled concurrently, the flexing occurs at the bisector of the angle formed by the cables. Multidirectionality is obtained by the force of these crossed impulses.
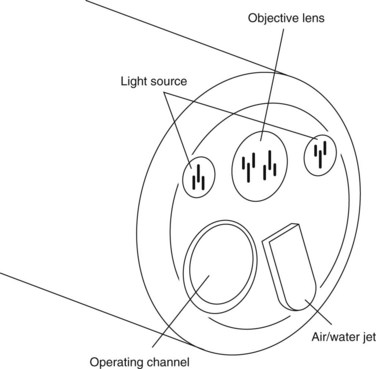
4 The optical head
The optical head (Fig. 5), which is an endoscope’s most distal component, provides either forward or side-viewing optics, depending on its intended destination and use.
Conclusion
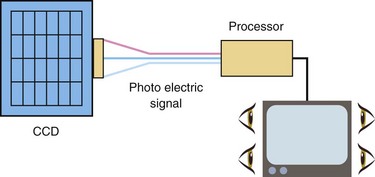
Videoendoscopes have supplanted fiberscopes. Fiberoptic image conductors have been replaced by miniature charge coupled devices (CCDs) that are increasingly sophisticated and that allow the operator to see far more detail than would be possible with the naked eye, while still employing the same types of operator controls and channels without compromising robustness or reliability (Fig. 6).
1.2 Electronic videoendoscopy
Key Points
Introduction
Fiberoptic endoscopes have a number of serious drawbacks:
The first electronic videoendoscope came on the market around 1986 (Fig. 2).
1 Electronic videoendoscopes
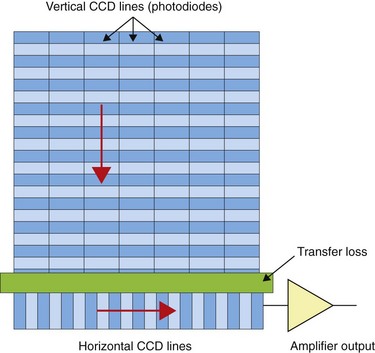
The electronic videoendoscope works like a digital camera. Its distal end combines a coupled charge device (CCD; Fig. 3). This technology allows better image transmission and storage, leading to improved diagnosis and therapy. It has a coupled charge device and a color system.
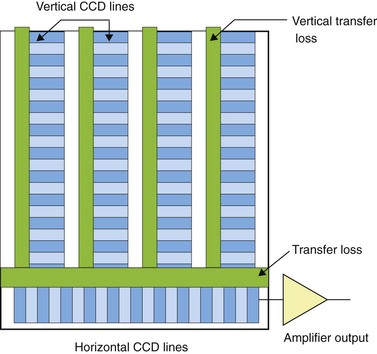
1.4 Full frame read-out CCD (Fig. 6)
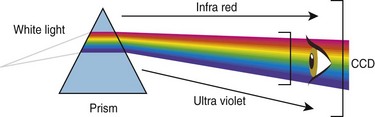
Color is not a physical reality. It is an impression generated in our brain, via our eyes, by luminous radiation from objects. In 1676, Isaac Newton showed that sunlight can be broken down into the colors of the spectrum by passing light through a prism (Fig. 7).
1.5 Color system selection
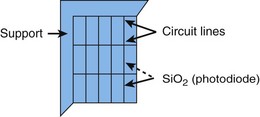
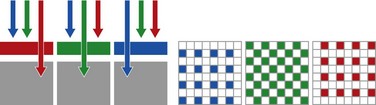
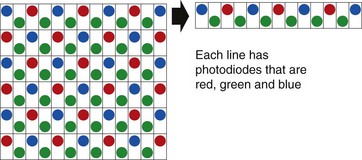
1.5.2 CCD colors: a mosaic system
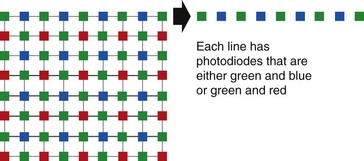
A CCD combines a mosaic filter that is transparent to one of the primary colors (red, green, or blue) or their complements (cyan, magenta, or yellow). Each photodiode captures a color and then reconstitutes a pixel using four photodiodes. Inasmuch as cyan is composed of blue and green, and yellow is composed of red and green, the color green has more potential luminance, i.e. red and blue have only 25% as much luminance as green (Fig. 8).
2 Electronics and the endoscope
2.1 Resolution
Box 1 Advantages of electronic videoendoscopes
Endoscopic imaging is making major advances thanks to the migration to digital technology.
Conclusion
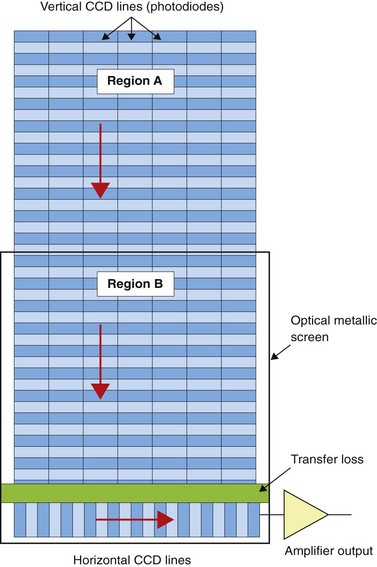
New high resolution CCDs are 45% smaller than classic CCDs and integrate octagonal photodiodes that theoretically allow storage of more information in the same surface area. With this type of CCD, 1.7 times more photodiodes can be obtained relative to the previous generation of CCDs (Figs 9, 10).
Houcke P, Canard J-M, Chirol P-L, et al. Evaluation technique des vidéo-endoscopes couramment utilisés en gastro-entérologie [Technical evaluation of gastroenterological videoendoscopes]. Hépatogastroenterology. 1997;6:495-504.
Machida H, Sano Y, Hamamoto Y, et al. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy. 2004;36(12):1094-1098.
Pelletier M. La technique du zoom ou magnification. [Zoom/magnification techniques] CREGG, Paris, 27 September 2003. Acta Endoscop. 2003;33(Suppl 3):446.
1.3 Endoscopic accessories
Key Points
1 Tissue grasping and acquisition
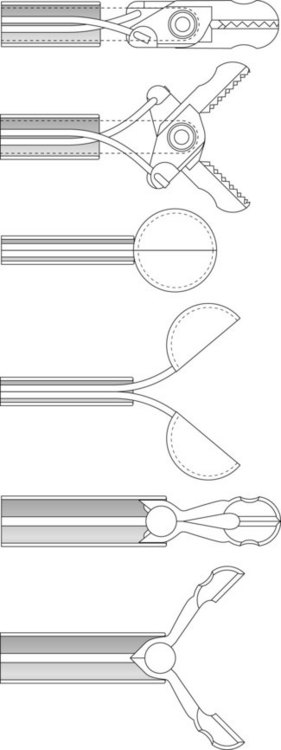
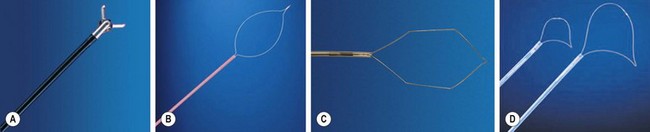
Gastrointestinal biopsies pose a major challenge for the endoscopist. They are undertaken using 5 mm-long forceps with spoon-shaped jaws (Fig. 1), which employ one of the following mechanical principles:
2 Injection
Injections are performed: (a) with the aid of a basic catheter for fluoroscopic opacification (prior to insertion of a gastrointestinal stent); (b) with the aid of a spray catheter for chromoendoscopy, or (c) via disposable needles for tattooing, hemostasis, variceal sclerotherapy, chromoendoscopy (see Ch. 2.4), EMR, submucosal dissection, and antireflux treatment (Fig. 2).
3 Clips and ligation devices
Hemostasis can be performed using clips and ligation systems. Reusable and disposable clipping systems are available and their use is described in Chapter 7.8. Clips can also be used for marking the site of a lesion or for closing small perforations that occur during polypectomy, EMR or ESD. Disposable ligation systems are available with 6–10 elastic bands for esophageal variceal ligation and to assist EMR (Ch. 7.8). They are also used for hemostasis of bleeding Dieulafoy lesions, and for Mallory–Weiss tears (Fig. 3).
4 Dilatation
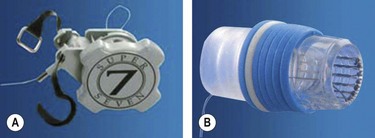
Dilatation (see Ch. 7.1) is performed using progressively larger bougie dilators over a rigid or semi-rigid metal guidewire or else using disposable balloons (Fig. 4A). Balloon diameters and length vary. The balloons are passed through the operating channel and dilatation is performed hydrostatically (except in cases of achalasia) under visual and/or fluoroscopic control.
5 Coagulation
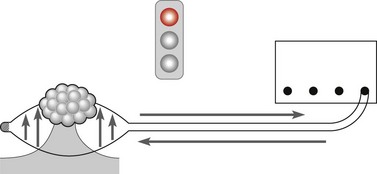
Coagulation (see Ch. 1.4) can be monopolar, bipolar (Fig. 4B), or multipolar, and can be performed using dedicated probes. Coagulation is useful for tumor debulking and for hemostasis. In monopolar coagulation, a high-frequency electric current is applied to the tissue, requiring a patient grounding pad (25–40 watt (W) pulses for 7–10 s). This method is risky as the muscle layer may be coagulated and delayed perforation may occur. Argon plasma coagulation (APC) is less risky, and is also more appealing by virtue of its cost-effectiveness and multifunctionality (60 W, 0.8–1.5 l/mn). The advantage of bipolar coagulation, which uses three electrodes, is that the electric current is conducted back to the electrosurgical generator (useful in the presence of a pacemaker).
Bipolar probes contain a lateral spiral filament at their distal end (10–20 W, 3–4 pulses lasting 10–14 s each). The contact must be tangential as the distal tip of the probe is perforated and has no conductor, thus allowing for cleaning. Some probes are equipped with a distal injection needle. Diathermic heater probes (Fig. 5A), which are used in some countries, comprise an internal thermocouple that generates a constant temperature of 250°C at the distal end (which has an anti-adhesive coating). This system also houses three 1-cm cleaning channels above the active distal portion (8-s 20–30 joule pulses).
6 Tissue resection
Sectioning (see Ch. 7.11) occurs using 200–500 volts HF current that generates an electric arc between the diathermy snare and the tissue. The latest generation of electrosurgical generators allows automatic stabilization of fluctuations in potential and intensity. The heat generated at the points where the electric arc comes into contact with the tissue is so high that the tissue is immediately vaporized. Following this, as the snare moves across the tissue, electric arcs are generated continuously wherever the gap between the tissue and snare is small enough, thus producing the resection.
It is useful to have a range of snares (Figs. 5B,C,D): monofilament; braided; small size (10 mm); large size (20–30 mm); asymmetric for esophageal EMR; and barbed for large colonic EMRs. Transparent caps with an edge groove (into which the loop inserts) are also essential.
7 Gastrointestinal stents
A range of self-expanding metal stents (SEMS) (see Ch. 7.2) are available for use in the esophagus, stomach, duodenum, biliary tree, and colon. Most of today’s gastrointestinal prostheses are made of hardened steel (articulated components that are 2.5 cm in diameter and that do not become shorter on expansion), nitinol or Elgiloy (mesh or webbing composed of one or more wires, the length of which decreases by 30% as the prosthesis expands). The stents are straight, and may or may not have funnel-shaped or ‘dog-bone’ tips. Anchorage, extraction and antireflux valve systems are also available for these devices and some may be completely or partially membrane-covered to minimize tumor ingrowth.
American Society for Gastrointestinal Endoscopy Committee. Technology Status Evaluation Report. Endoscopic hemostatic devices. Gastrointest Endosc. 2009;69(6):987-996.
American Society for Gastrointestinal Endoscopy Committee. Technology Status Evaluation Report. Guidewires for use in GI endoscopy. Gastrointest Endosc. 2007;65(4):571-576.
American Society for Gastrointestinal Endoscopy Committee. Technology Status Evaluation Report. Gastrointest Endosc. 2007;65:741-749.
1.4 Electrosurgical generators: procedures and precautions
Key Points
1 Electrophysical basis of electrosurgery
2 Problems associated with older electrosurgical generators
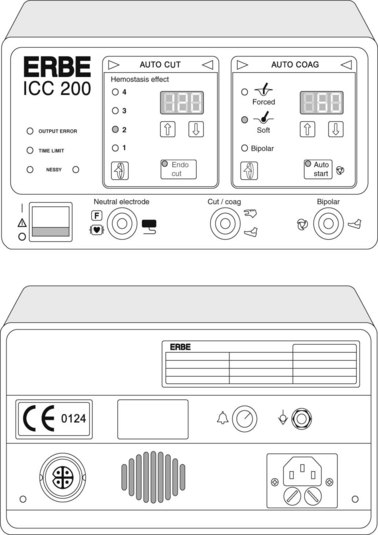
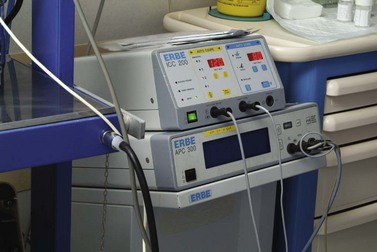
The power created by older electrosurgical generators is constant and does not vary with tissue and cutting surface impedance. Cutting speed and electric arc intensity are the only variable parameters with these devices. In today’s generators, electric-arc intensity is constant and controlled. Cutting speed is preadjusted in endoscopic diathermy mode without the need for any action on the part of the operator. The electrosurgical generator’s output power is regulated automatically in accordance with the contact surface. The most common unit is the ERBE ICC 200 (Fig. 3), recently replaced by the VIO 200 or 300 series.
2.2 Sectioning may inadvertently result in tissue coagulation
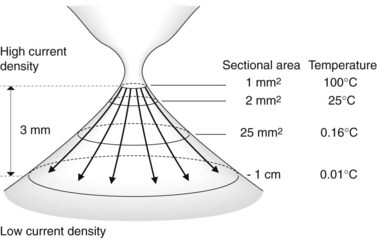
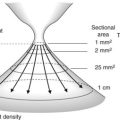
This can occur if too little current is applied to the target contact surface (Fig. 4). Sectioning a 1 mm2 contact surface requires a high level of current density. This same current applied to a 1 cm2 surface will be unduly low and will induce coagulation. New electrosurgical generators avoid this problem by automatically adjusting the instrument’s output current to the characteristics of the tissue being sectioned, within the limits of the maximum-current setting, which must be high enough to allow sectioning, as otherwise the tissue will be coagulated.
3 Principles of endoscopic diathermy (electrosurgery)
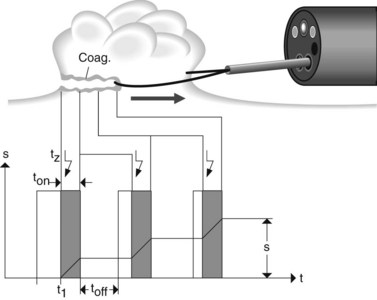
In endoscopic diathermy, all of the electrical settings that are applied to the section and its characteristics are automatically controlled and adjusted so as to achieve optimal cutting performance throughout the process (Fig. 5). The electrical arc’s voltage and intensity between the tissue and cutting wire are measured, analyzed and stabilized by an onboard microprocessor. Endoscopic diathermy is a fractionated process that is carried out via the following stages:
All of these parameters are regulated automatically via the device’s power.
3.2 What to do if the resection does not start immediately
There are two possible scenarios in this case:
6 Alarm systems
These alarm systems allow for detection of:
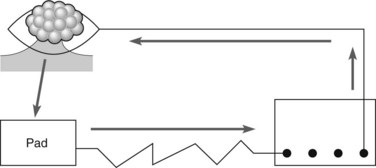
They ensure that endoscopic electrosurgery is safe for the patient and operator. The grounding pad monitoring system (NESSY) guarantees that no skin burn will occur under the neutral pad, providing that double-face pads are used (Fig. 8).
7 Statutory requirements of endoscopy rooms
The endoscopy room must meet the following statutory requirements:
9 Practical tips for endoscopic electrosurgery
9.1 General tips
9.2 Standard settings for an ERBE ICC 200 electrosurgical generator
The endoscopic diathermy (‘endo-cut’) function can only be obtained using the yellow pedal, in which case cutting and coagulation will alternate automatically (Fig. 9).
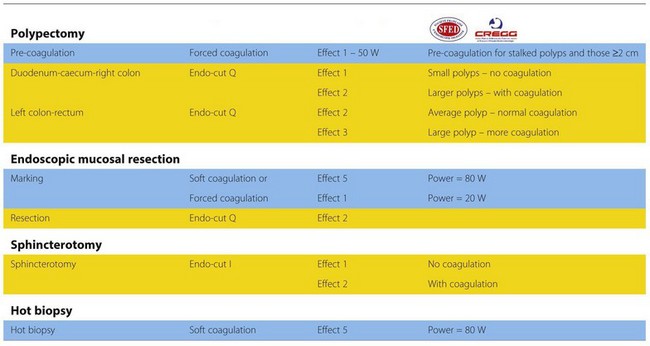
9.3 Polypectomy
9.5 Endoscopic mucosal resection (EMR)
There are small but significant differences with the newest range of ERBE electrosurgical generators (VIO 200 or 300) (Fig. 10). Two different endo-cut functions (Q and I) exist: ‘endo-cut Q’ is designed for polypectomy and functions much as described above with slow cutting and more coagulation. The standard setting for a polypectomy is 120 W, effect level 3 (the effect level can be reduced to 2 if less coagulation is desired). There are, however, many adjustable settings and general recommendations are outlined in Tables 1 and 2. ‘Endo-cut I’ is designed for sphincterotomy with a different cutting-coagulation cycle, designed for quick cutting and less coagulation, although it can be adjusted.
Table 1 Manufacturer’s recommended settings for ERBE VIO series electrosurgical generators
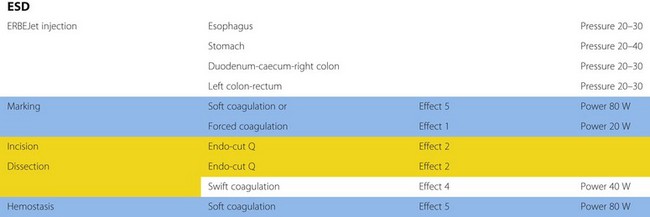
Table 2 Manufacturer’s recommended settings for ERBE VIO series electrosurgical generators when performing endoscopic submucosal dissection (ESD)
ASGE Technology Committee. Technology status evaluation report. Electrosurgical generators. Gastrointest Endosc. 2003;58:656-660.
ASGE Technology Committee. Technology status evaluation report. The argon plasma coagulator. Gastrointest Endosc. 2002;55:807-810.
Canard JM, Ponchon T, Napoléon B, et al. Bistouris électriques: principes et précautions d’utilisation [Electrosurgical generators: usage principles and precautions] SFED. www.sfed.org, September 2003.
Canard JM, Védrenne B. Clinical application of argon plasma coagulation in gastrointestinal endoscopy: has the time come to replace the laser? Endoscopy. 2001;33(4):353-357.
Farin G, Grund KE. Technology of argon plasma coagulation with particular regard to endoscopic applications. End Surg. 1994;2:71-77.
Maier M, Kohler B, Benz C, et al. A new HF current electrosurgical generator with integrated self modifying system (endo-cut mode) for endoscopic sphincterotomy: a prospective randomized trial. Gastrointest Endosc. 1995;41:308.
Rey JF, Beilenhoff U, Neumann CS, Dumonceau JM. European Society of Gastrointestinal Endoscopy (ESGE) guideline: the use of electrosurgical units. Endoscopy. 2010;42(9):764-771.
1.5 Organizational structure of an endoscopy unit
Key Points
Introduction
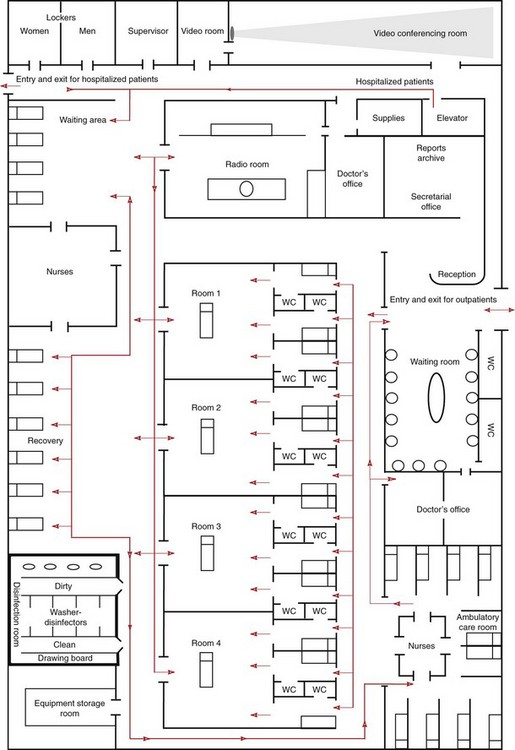
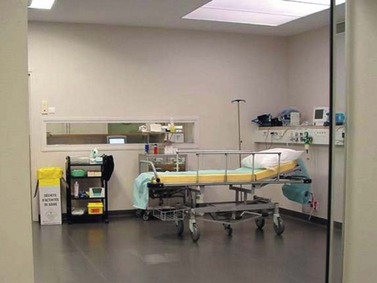
Endoscopic centers (Fig. 1) should be equipped with facilities for the whole gamut of diagnostic and therapeutic endoscopy for the lower and upper GI tract, and should also be equipped so that non-GI tract endoscopy can also be performed – particularly bronchoscopy, ENT, and urological endoscopy.
1 Rooms
1.1 Endoscopy rooms
1.1.1 Characteristics of an endoscopy room
1.1.2 Endoscopy room equipment
1.2 Postoperative monitoring room and recovery room
All endoscopy units that administer general anesthesia or IV sedation should have a postoperative monitoring room where each patient’s vital signs are monitored by a nurse (cardiac activity, pulse, blood pressure, and oxygen saturation). When the patient fully awakens they should be taken to the recovery room, where they remain until the doctor in charge authorizes their discharge. In some countries, nurse-led discharge policies exist, allowing them to discharge patients who meet key safety criteria, e.g. mobile, pain-free, normal observations. The postoperative monitoring room must comply with national safety standards (Fig. 3).
1.3 Cleaning room
The disinfecting room (Fig. 4) should be a separate, self-enclosed space that communicates with each endoscopy room via a technical window, or preferably a door that opens automatically. This room is used to clean and disinfect endoscopes. Medical supplies should not be sterilized in the disinfecting room. This should be done in a central sterilization facility that is used for the operating rooms, if the endoscopy unit is part of a hospital. Cleaning and disinfecting rooms for medical equipment must comply with legal regulations pertaining to design, methodology, and personnel training.
1.3.1 Additional work surfaces should be provided
1.4 Endoscope storage room
The endoscope storage room (Fig. 5) must be separate from the disinfecting room and should contain vented cabinets to store endoscopes, endoscope accessories, and small implements. Endoscopes should be stored vertically. The utility of cabinets with high efficiency particulate air filtration (HEPA) systems, and their location, have yet to be clearly established but they may allow clean endoscopes to be stored for up to 72 hours without the need for reprocessing prior to use.
3 Medical and paramedical personnel
3.3 Medical secretaries
All secretaries should have the same credentials and skill sets and should be interchangeable.
5 Quality assurance of endoscopy
This mechanism applies in France to institutions that are recognized as health centers within the framework of the institution’s accreditation procedures. In other countries, quality metrics are employed for continuous service improvement. The database maintained by the French health public health organization known as Haute Autorité de Santé (HAS), now allows for hospital department self-assessment, which promotes the following: improvements in patient management; reduced risk of nosocomial infection, optimization of department management practices (in terms of human resources, finances, logistics, and information systems); evaluation of professional practices. In the UK, all endoscopy units complete an online self-assessment tool every 6 months called the Global Rating Scale (www.grs.nhs.uk). This assesses multiple aspects of quality, including procedural quality indicators, patient experience, the endoscopy workforce and the quality of training. It provides a benchmark for endoscopy services and highlights areas for improvement.
Conclusion
Box 1 The main requirements for the organizational structure of an endoscopy unit
1.6 Gastrointestinal endoscopy training
Key Points
1 Principles of certification and re-certification
General principles have been developed by societies which document the minimum requirements for certification or re-certification of an individual. These are listed in Box 1. A minimum number of procedures are still required for general endoscopy (Table 1). A key change in assessing the adequacy of an individual’s training is that competence is not solely based on completing a minimum number of procedures, but should also involve direct observation and successful completion of objective criteria. Several societies now require a formal summative assessment prior to certification. Guidelines have also been developed to certify non-physicians performing endoscopy in countries where there are too few endoscopists.
Table 1 Guidelines for minimum numbers of general endoscopy procedures required for certification
| Procedure | Number of supervised procedures | |
|---|---|---|
| USA | France | |
| EGD | 130 | 300 |
| Flexible sigmoidoscopy/proctoscopy | 30 | 100 |
| Colonoscopy | 140 | 100 |
| Esophageal dilation | 20 | 10 |
| PEG | 15 | Not stated |
| Esophageal stent placement | 10 | 10 |
| Pneumatic dilation for achalasia | 5 | Not Stated |
| Tumor ablation | 20 | 10 |
| Advanced endoscopy | Not applicable | 100a |
| Non-variceal hemostasis | 25 (including 10 active bleeders) | 30 |
| Variceal hemostasis | 20 (including 5 active bleeders) | |
| Polypectomy | 30 | 50 |
| Abdominal ultrasound | Not applicable | 300 |
| pH and manometry | See Box 3 | 50 |
a This includes laser, APC, dilation, and stent insertion.
Advanced endoscopic training is usually obtained and additional training and competence assessed and credentialed separately (Box 2). Ideally, a formal training program should be completed. Animal models and short courses are useful adjuncts but cannot replace formal training.
Box 2 ASGE guidelines for EUS certification
The endoscopist should demonstrate the following:
The above requirements are in addition to competence in general endoscopy and the general certification requirements detailed in Box 1.
Box 3 ASGE training requirements for capsule endoscopy
For re-certification, physicians must document evidence of ongoing training and an adequate case load to maintain endoscopic skills. In addition to adequate numbers of procedures, the success, ability to perform therapeutic interventions and complication rate should be assessed (Table 2). Continuing medical education and continuous quality improvement are also key components of re-certification.
Table 2 Recommended numbers of interventional procedures
| Procedure | Number of supervised procedures | |
|---|---|---|
| USA | France | |
| EUS | 75 | 150 |
| Mucosal Lesions | ||
| Submucosal abnormalities | 40 | |
| Pancreaticobiliary | 75 | |
| EUS-guided FNA: Nonpancreatic Pancreatic |
25 25 |
|
| ERCP | 200 With at least an 80% cannulation rate |
150 |
| Laparoscopy | 25 | 50 |
The ASGE recommend a minimum of 125 supervised cases for competence of mucosal and submucosal abnormalities, with a minimum of 150 supervised cases, of which 75 are pancreaticobiliary and 50 EUS-FNA for comprehensive competence.
2 Endoscopic training using models
2.4 Erlangen active simulator for interventional endoscopy (EASIE)
EASIE uses resected stomachs of adult pigs which have been washed, and frozen (Figs 1–4). The stomachs are fastened to a board at six points, with the distal end of the esophagus attached to a tube that simulates the gastrointestinal tract. ERCP training can also be performed, in which case the stomach-duodenum-liver is preserved to allow for access to the bile ducts via the papilla. Current EASIE models include: hemostasis; polypectomy; APC; dilation; stent placement and ERCP. Endotrainer uses the same models, with some improvements.
Aabakken L, Adamsen S, Kruse A, et al. Performance of colonoscopy simulator: experience from a hands-on endoscopy course. Endoscopy. 2000;32:911-913.
American Association for the Study of Liver Diseases, the American College of Gastroenterology, American Gastroenterological Association Institute, American Society for Gastrointestinal Endoscopy. The gastroenterology core curriculum. Third edition. Gastroenterology. 2007;132:2012-2018.
ASGE. Guidelines for certification and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001;54:811-814.
ASGE. Guidelines for certification and granting privileges for gastrointestinal endoscopy. Gastrointest Endosc. 1998;48:679-682.
ASGE. Guidelines for training in endoscopic ultrasound. Gastrointest Endosc. 1999;49:829-833.
Bar-Meir S. A new endoscopic simulator. Endoscopy. 2000;32:898-900.
British Society of Gastroenterology. Non-medical endoscopist. A report of the working party of the British Society of Gastroenterology. www.bsg.org.uk.
Eisen GM, Baron TH, Dominitz JA, et al. Methods of granting hospital privileges to perform gastrointestinal endoscopy. Gastrointest Endosc. 2002;55:780-783.
Faigel DO, Baron TH, Adler DG, et al. ASGE. Guidelines for certification and granting privileges for capsule endoscopy. Gastrointest Endosc. 2005;61:503-505.
Greff M, Mignon M. Europe and initial and continuing medical education in hepato-gastroenterology. Gastroenterol Clin Biol. 1996;20(2):13-15.
Hochberger J, Maiss J, Hahn EG. The use of simulators for training in GI endoscopy. Endoscopy. 2002;34:727-729.
Hochberger J, Maiss J, Magdeburg B, et al. Training simulators and education in gastrointestinal endoscopy: current status and perspectives in 2001. Endoscopy. 2001;33:541-549.
Ikenberry SO, Anderson MA, Banerjee S, et al. ASGE. Endoscopy by nonphysicians. Gastrointest Endosc. 2009;69(4):767-770.
2004 JAG: Guidelines for the training, appraisal and assessment of trainees in gastrointestinal endoscopy. www.thejag.org.uk, 2004.
Neumann M, Hochberger J, Felzmann T, et al. Part 1. The Erlanger endotrainer. Endoscopy. 2001;33:887-890.
1.7 Endoscopy nurses
Key Points
1 Sphere of responsibility
Figure 2 An endoscopist, an anaesthesiologist and two endoscopic nurses during a therapeutic colonoscopy.
1.2 Technical knowledge
An endoscopy nurse must have the following technical attributes:
1.4 Infection control
Endoscopy nurses are also in charge of unit environmental hygiene and endoscopy disinfection processes (see Ch. 1.10).
1.5 Training
Endoscopy nurses play a number of key training roles in terms of induction and knowledge transfer for new staff members and trainees. They are also involved in appraisal, staff development and clinical research. In the UK, the national ‘GIN’ training program has been developed to train endoscopy nurses to be local facilitators who run regular nurse training courses. In the USA, the Society of Gastrointestinal Nurses and Associates (SGNA) is an important forum for nurse training in endoscopy and gastroenterology. The European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA) is similar, and runs regular training and update courses (Table 1).
| Country/Region | Society | Website |
|---|---|---|
| USA | Society of Gastrointestinal Nurses and Associates (SGNA) | www.sgna.org |
| Europe | European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA) | www.esgena.org |
| UK | British Society of Gastroenterology Endoscopy Associates Committee (BSG-EAG) | www.bsg.org.uk; www.jets.nhs.uk/GIN |
| FRANCE | Groupement des infirmier(e)s pour la formation en endoscopie (GIFE) | www.gife.fr |
European Society of Gastroenterology and Endoscopy Nurses and Associates. ESGENA Core curriculum for endoscopy nursing. www.esgena.org, 2008.
European Society of Gastroenterology and Endoscopy Nurses and Associates. European job profile for endoscopy nurses. Endoscopy. 2004;36(11):1025-1030.
Society of Gastroenterology Nurses and Associates. Standards of clinical nursing practice and role delineations. www.sgna.org, 2010.
1.8 Patient information
Key Points
Patient information
PILs should be structured and the broad headings listed in Box 1 provide a useful framework for their development.
Most national endoscopy societies have downloadable PILs for common procedures on their websites and some of these are listed below. Boxes 2 and 3 give examples of patient leaflets for colonoscopy and ERCP, which may serve as a useful guide.
Box 2 Colonoscopy Information
Your appointment details, information and consent form
Please bring this booklet with you.
An appointment for your Colonoscopy has been arranged at:
Hospital name: ___________________________________________
Date: ___________________________________________________
Telephone number: _______________________________________
Endoscopist/Consultant: ___________________________________
The endoscopic procedure
Box 3
ERCP Informationa
Your appointment details, information and consent form
Please bring this booklet with you.
An appointment for your ERCP has been arranged at:
Hospital name: ___________________________________________
Date: ___________________________________________________
Telephone number: _______________________________________
Endoscopist/Consultant: ___________________________________
Medicines and medical conditions
What are the risks and complications?
aReproduced with permission, courtesy of Dr Richard Tighe, Norfolk and Norwich Hospital, UK.
American Society for Gastrointestinal Endoscopy. www.asge.org/PatientInfoIndex.aspx?id=1022.
British Society of Gastroenterology. www.bsg.org.uk/sections/bsg-endoscopy-general/related-documents.html.
Gastroenterological Society of Australia. www.gesa.org.au/leaflets.cfm.
Societe Francaise d’Endoscopie Digestive. www.sfed.org/Generalites/Fiches-info-patients.html.
1.9 Medicolegal aspects of endoscopy
Key Points
Introduction
2 Complications
Many years ago, the commonest complications arising from endoscopy were sedation-related events, but better sedation practice has made these uncommon nowadays. Large audits suggest that both immediate and delayed complications are perhaps more common than realized, but have also highlighted the fact that many complications are preventable and that delayed recognition is associated with worse patient outcomes and a greater likelihood of litigation. Specific procedure-related complications will, however, inevitably occur from time to time, when undertaking invasive procedures, and these are discussed in detail in Chapter 8.
3 Consent for endoscopic procedures
3.1 Patient information
This is discussed in Chapter 1.8 and should include as much information as is reasonable to allow patients to make an informed decision. It must be easy to understand, and patients must have adequate time to read and contemplate the information before making their decision. It is generally not acceptable for patients to be presented with information and/or a consent form for signature immediately prior to the proposed procedure. Knowing how much information to provide to patients without causing undue concern is not easy, but a description of the procedure, alternatives, the need for sedation and potential risks is essential. All common complications (occurring with a frequency of 1% or more) should be discussed, as should rare complications, if they are potentially serious or fatal, or may lead to long-term disability (e.g. perforation, pancreatitis). The principle is that serious complications, no matter how rare, might influence a patient’s decision to proceed, and therefore needs to be discussed beforehand.
ASGE Standards of Practice Committee. Informed consent for GI endoscopy. Gastrointest Endosc. 2007;66(2):213-218.
British Society of Gastroenterology. Guidance for obtaining a valid consent for elective endoscopic procedures. A Report of the Working Party of the British Society of Gastroenterology. www.bsg.org.uk, April 2008.
Stanciu C, Novis B, Ladas S, et al. Recommendations of the ESGE workshop on Informed Consent for Digestive Endoscopy. First European Symposium on Ethics in Gastroenterology and Digestive Endoscopy, Kos, Greece, June 2003. Endoscopy. 2003;35(9):772-774.
1.10 Cleaning, disinfection, sterilization, and storage of endoscopy equipment
Key Points
1 Definitions of terms
2 Disinfection phases for immersible flexible endoscopes
Box 1 Minimizing the risk of transmission of variant CJD
2.1 Preliminary cleaning
However, before an endoscope is immersed, it must undergo a leakage test.
2.2 Initial cleaning (basin 1)
2.3 First rinsing (basin 2)
2.4 Second cleaning (basin 1)
2.5 Intermediate rinsing (basin 2)
2.6 Disinfection (basin 3)
This phase is efficient only if the cleaning before is well done.
2.7 Final rinse (basin 4)
The water quality for gastrointestinal endoscopy must meet either of the following criteria:
Purge the channels, which is the final step in this phase.
Change the final rinsing water after each endoscopy procedure.
3 Endoscope disinfection using AERs
Two types of AER are available:
The manufacture and use of AERs must comply with ISO 15883. The use of ‘pass-through’ machines is recommended in several countries (see Ch. 1.5) but this has major implications for the design and layout of endoscopy units.
Today’s AERs perform cycles composed of two cleaning phases.
4 Cleaning procedure for accessories
5 The ideal endoscopy unit
5.1 Endoscope cleaning room equipment and layout
The equipment and layout of this room (see also Ch. 1.5) must be specifically intended for the cleaning and maintenance of medical devices. It should designed in accordance with the ‘forward movement’ principle, i.e. equipment flow is one-way from dirty to clean.
The room must meet the following criteria:
5.2 Personnel
Box 3 Good practice for endoscope disinfection – 16 key elements
ASGE Standards of Practice Committee. Infection control during GI endoscopy. Gastrointest Endosc. 2008;67:781-790.
Beilenhoff U, Neumann CS, Rey JF, et al. ESGE-ESGENA guideline: Cleaning and disinfection in gastrointestinal endoscopy. Update 2008. Endoscopy. 2008;40:939-957.
BSG Guidelines for Decontamination of Equipment for Gastrointestinal Endoscopy: The Report of a working party of the British Society of Gastroenterology Endoscopy Committee 2008. www.bsg.org.uk/clinical-guidelines/endoscopy
Endoscopy and individuals at risk of vCJD for public health purposes. A consensus statement from the British Society of Gastroenterology Decontamination working group and the ACDP TSE working group; Endoscopy and vCJD sub-gro. http://www.dh.gov.uk/ab/ACDP/TSEguidance/index.htm.
ISO 15883-15881 Washer-disinfectors. Part 1: General requirements, terms and definitions and tests. www.iso.org.
Marchetti B, Boustière C, Chapuis C, et al. La désinfection du matériel en endoscopie digestive. Acta Endosc. 2007;37:699-704.
2006 Patient safety and reduction of risk of transmission of Creutzfeldt–Jakob disease (CJD) via interventional procedures. National Institute for Health and Clinical Excellence www.nice.org.uk/guidance/IPG196, 2006.
1.11 Gastrointestinal biopsies and histology
Introduction
The editors would like to acknowledge Brigitte Marchetti.
Low-grade dysplasia involves the following cellular changes:
High-grade dysplasia demonstrates the following changes:
A microinvasive malignancy exists if the lesion breaches the muscularis mucosae. When an endoscopic mucosal resection has been performed, the submucosa should be examined to ensure that it has not been infiltrated. Box 1 documents the criteria for careful monitoring versus surgical referral in patients in whom the muscularis mucosa has been breached.
1 Sample collection in endoscopy
Biopsy specimens must be placed in fixative immediately or mounted on slides. The specimens are numbered and patient identification details added. Formaldehyde can be used to stain any specimen, particularly those containing mucin, fat or endocrine cells. Formaldehyde does not alter cellular structures, particularly DNA, so that molecular biology techniques can be performed on the tissue if required. Box 2 shows what to document on a pathology form. Samples that are obtained following endoscopic mucosal resection or dissection should be pinned (Box 3).
2 Processing of biopsy samples
2.1 Biopsies
3 Histology of the GI tract
3.1 The esophagus
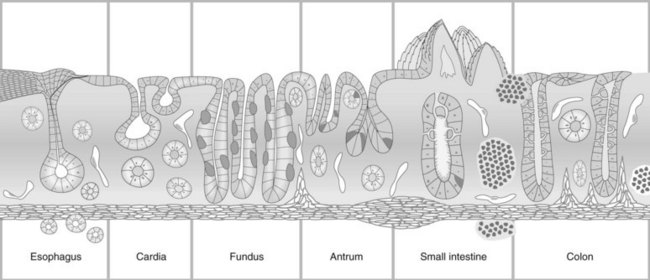
The esophagus consists of the mucosa, submucosa, and muscularis propria (Fig. 1). The mucosa contains non-keratinized stratified squamous epithelium, lamina propria, and muscularis mucosae. The submucosa contains small aciniform cells that secret mucins and connective fibers (papillas), which extend far into the mucosa. The muscularis propria consists of striated muscle in the upper third, both striated and smooth muscle in the middle, and smooth muscle in the lower third. The esophagus contains no serous membrane.
3.1.1 Squamous cell cancer of the esophagus
In microinvasive squamous cell cancer of the esophagus, the tumor extends through the basal membrane up to, but not invading, the muscularis mucosae (PT1m) or submucosa (PT1sm). Lugol’s iodine solution is used to identify and determine the extent of squamous cell cancer, as lesions with low levels of glycogen (i.e. tumor cells) do not absorb the contrast material. Other stains which are used include methylene blue, acetic acid, and indigo carmine (see Ch. 2.4).
3.1.2 Barrett’s esophagus
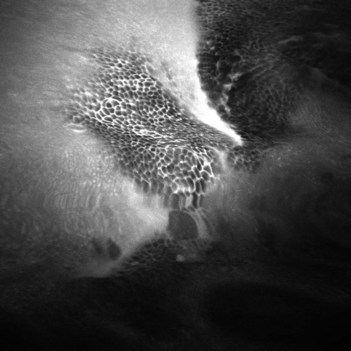
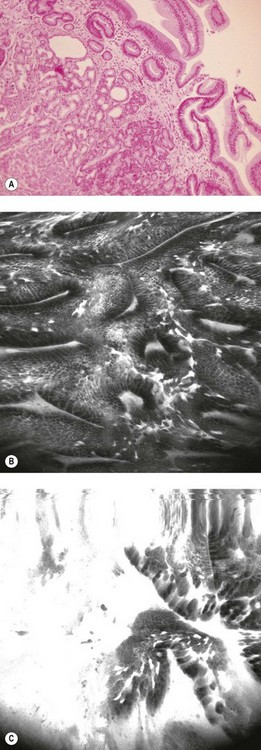
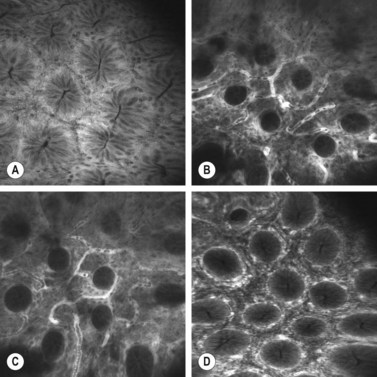
Four quadrant biopsies should be taken every 2 cm from the Barrett’s mucosa. Advanced imaging techniques (Figs 2, 3) can be used to screen for Barrett’s esophagus (see Chs 6.1 and 6.2). The gastroesophageal junction can be visualized using confocal endomicroscopy (Fig. 2), as can Barrett’s esophagus (Fig. 3B) and dysplasia (Fig. 3C), which correlate with the pathological findings (Fig. 3A). Biopsies should be submitted to pathology in separate containers to permit focusing of subsequent biopsies, should dysplasia be detected. Depending on whether high-grade, low-grade or no dysplasia is found, this determines management and endoscopic screening intervals (for screening intervals for Barrett’s esophagus, see Ch. 3).
3.2 The stomach
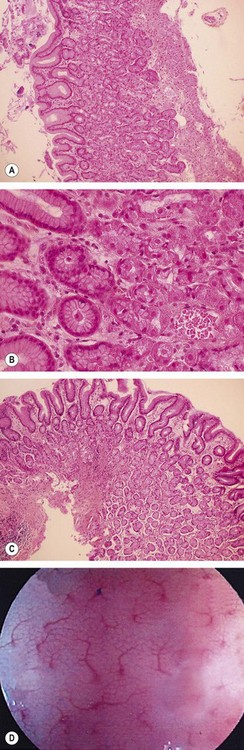
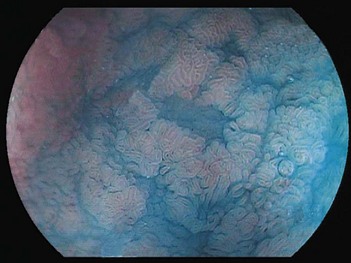
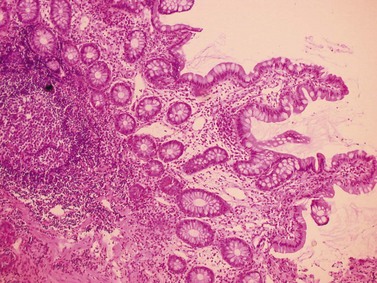
The cardia is a 5–30 mm area composed of individual or interlinked cardiac glands, which contain identical mucus secreting cells. The fundus (Fig. 4A,B) contains two regions. The first is delineated by furrows containing vessels with thick mucosa. Its crypts are round and regular and are surrounded by a vascular web (Fig. 4D), with numerous narrow, rectangular glands, which are perpendicular to the surface. The second region is composed of mucus and parietal cells. The body of the fundic gland is mainly composed of parietal cells, but also contains larger cells that are rich in pepsin or zymogens. The antrum (Fig. 4C) contains a thinner mucosa that is 200–1000 µm thick. Its epithelium is more irregular than the fundus. The antrum’s crypts are elongated and contain smaller glands that are mainly composed of mucus cells that occur around primary or secondary crypts. Cells producing gastrin and serotonin are also found here.
3.2.1 Helicobacter pylori infection
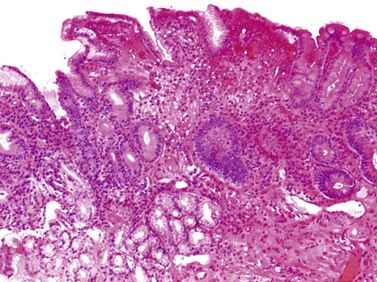
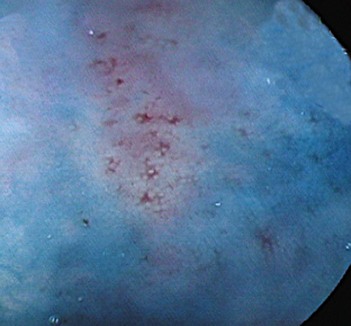
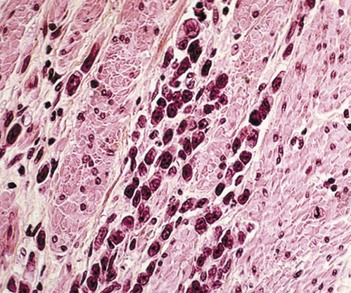
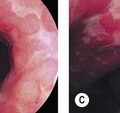
This initially provokes acute gastritis, accompanied by a polynuclear infiltration, followed by chronic gastritis accompanied by a lympho-plasmocyte infiltration (Fig. 5), which provokes lymphoid follicles. The latter occurs mainly in the antrum, but also extends to the fundus. An endoscopic examination may reveal mini- or macro-nodules that constitute submucosal lymphoid nodules. Micro-ulceration can be seen with zoom magnification (Fig. 6). Helicobacter pylori infection is initially accompanied by elevated acid secretion, which induces chronic duodenitis, which in turn is associated with gastric metaplasia and colonization by bacteria. Chronic Helicobacter pylori infection is associated with atrophy, which can be associated with reduced acid secretion and should be investigated during screening for intestinal metaplasia (Fig. 7).
3.3 Duodenum and jejunum
![]() Clinical Tip
Clinical Tip
Duodenal biopsies should be taken in the distal duodenum below the ampulla of Vater, as the villi in the first part of the duodenum are only three-quarters of the height of the villi below the papilla (Fig. 9).
3.4 Rectum, colon, and ileum
The ileal mucosa is composed of long villi that are identical to those in the jejunum; goblet cells outnumber absorbent cells by a ratio of 5 : 1 (Fig. 10).
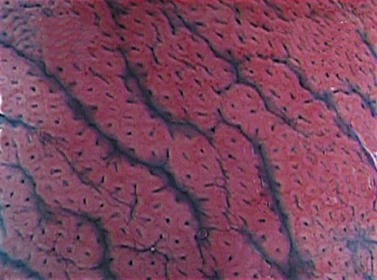
The colonic mucosa, which is mainly composed of goblet, absorbant and endocrine cells, and varies in thickness from 500–1000 µm (owing to the absence of Paneth cells) (Figs 11–13). Small numbers of intraepithelial lymphocytes (<5% of the epithelial cells) are observed in normal colic mucosa. The basal membrane is <5 µm thick, and the crypts (1700/cm2), are rectangular, and terminate with parallel intestinal glands. The goblet cells are more numerous in the upper part of the gland. Endocrine cells occur in the lower segment. The lamina propria is composed of fibroblasts, smooth mucosal fibers, macrophages, capillaries and small lymphocytes containing a few lymphoid follicles. The muscularis mucosae is 20–40 µm thick, and the submucosa is composed of loose connective tissue that is rich in arterioles and mini-veins and contains the Meissner plexus. The terminal vascularization is web-like and contains glandular crypts. Peri-cryptic micro-arches are more numerous in the caecum than in the sigmoid colon, whereas colonic mucosa thickness varies inversely.
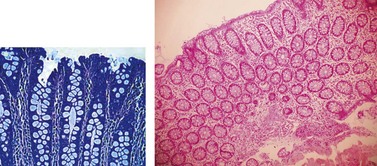
Figure 11 Normal colonic mucosa with regular glands rich in goblet cells. Two axes with perpendicular slices.
3.4.1 Benign polyps
Benign polyps include hyperplasic, inflammatory, and juvenile hamartomatous polyps.
Box 4 Hereditary polyposis syndromes
3.4.2 Neoplastic polyps
There are several types of adenoma:
Guidelines on who to screen are available in Chapter 4.
Box 5 When to biopsy colonic mucosa?
3.4.4 Inflammatory bowel disease (IBD)
Patients with long-standing IBD should undergo screening for dysplasia with biopsies taken every 10 cm in addition to targeted biopsies of any focal visible abnormalities (see Ch. 2.4 for details on chromoendoscopy; Ch. 4 for details of screening intervals, and Ch. 6.1 for details on new imaging techniques to detect dysplastic lesions).
Axon A, Lambert R, Robaszkiewisz M, et al. The second European endoscopy forum (Sintra, Portugal, 17–18 June 1999). Endoscopy. 2000;32:411-418.
Kiesslich R, Neurath FM. Endoscopic confocal imaging. Clin Gastroenterol Hepatol. 2005;3(Suppl 1):S58-S60.
Kuramoto S, Oohara T. Flat early cancers of the large intestine. Cancer. 1989;64:950-955.
Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255.
Tsukuma H, Oshima A, Narahara H, et al. Natural history of early gastric cancer: a non concurrent long term follow up study. Gut. 2000;47:618-621.