Chapter 1 Introduction to Allergy
History of Allergy
The concept of allergy is relatively recent in the history of clinical medicine. Until the late 19th century allergy was not considered to be a medical discipline. In fact, the concept of hypersensitivity reactions to generally innocuous substances present in the environment had not yet been developed. Seasonal catarrh or “hay fever” was seen as toxic reactions to various plant products, and therapies were developed to neutralize these “toxins.”
Despite a strong clinical interest in allergy and an active system of clinical practice, the causes of allergy were not well understood until the 1960s. During that period of time Johannson and Ishizaka independently isolated immunoglobulin E from the serum of allergy patients and demonstrated its primary role in clinical allergy. This development demonstrated the molecular basis of allergy and allowed both a rapid progression of the science related to allergy practice as well as the growth of standardized in vitro serum tests for specific IgE. These developments continued to foster a growth in clinical allergy practice, and led to the rapid influx of clinical and scientific research in the field of allergy-related immunology. These interests continue to the present day.
Prevalence of Allergic Diseases
In general, studies have suggested that allergic diseases, both respiratory and nonrespiratory, are steadily increasing in prevalence. This rise has been seen around the globe, not only in the west but also throughout the developing world. It has been estimated that 25% to 30% of the population in the western world are affected by allergic illnesses on a yearly basis, with a somewhat lower prevalence in the developing world. By a variety of indicators, the prevalence of allergic diseases such as rhinitis and asthma continue to grow steadily.1
Allergic diseases occur throughout the lifespan, but often have their origins in childhood. Infants and young children are often sensitized to foods and other macromolecules absorbed through the gut, and develop a variety of hypersensitization symptoms such as colic and eczema. These sensitivities can be enhanced by maternal food allergies, and these allergies have been demonstrated to be transmitted placentally. Among these atopic children, exposure to aeroallergens over the first few years of life often causes additional sensitization, resulting in the development of upper respiratory allergy and the condition known as allergic rhinitis. Allergic rhinitis also has a very strong genetic predisposition, with up to two-thirds of children with both parents suffering from allergic rhinitis also demonstrating symptoms of this disease. Concurrent with the development of allergic rhinitis is a rise in IgE levels in children. As will be discussed later in this chapter, IgE is the immunoglobulin involved in the immune response, and levels of IgE become significant in allergic children after the age of 2 years. Allergic rhinitis is quite common in childhood, with studies suggesting that up to 40% of children may be diagnosed by their physician with allergic rhinitis by the age of 6 years.
This progression of allergic disease from food-mediated colic and eczema, to allergic rhinitis, and finally to asthma has been referred to as the allergic march, implying that allergic disease will continue to progress in a steady manner from early childhood into adulthood.2 It is unclear at this time whether early aggressive intervention can prevent or blunt this progression of disease. It is clear, however, that this allergic march is common in many children with atopic disease.
Burden of Allergic Diseases
For example, allergic rhinitis has been demonstrated to affect a daytime function in both children and adults.3,4 These studies suggest that over 90% of children and adults have noted disruption in their abilities to work productively in the workplace or in the school when their rhinitis is symptomatic. Nearly one in four of these adults and children have missed work or school due to their symptoms. These findings demonstrate that allergic rhinitis is not only bothersome in terms of its adverse symptoms, it will impact the ability of adults and children to perform the general activities of their daily living, such as work and school attendance. Similar findings have been reported in a number of studies.
In addition, in patients with allergic rhinitis, the presence of the disease impacts on other aspects of function. Children with allergic rhinitis learn less effectively than those without the disease.5 Adults and children with allergic rhinitis have difficulty falling asleep and staying asleep.6 Furthermore, many of the older treatments for allergic rhinitis, particularly the first-generation antihistamines such as diphenhydramine, further adversely affect quality of life and function through their sedating and anticholinergic side effects.7
While the effects of upper airway allergy on function and quality of life can be significant, the adverse effects of asthma are even more dramatic. Asthma is a disease that is often poorly treated, due to a variety of factors including poverty, delay in diagnosis, inappropriate treatment, and patient nonadherence. It affects sleep, learning, daytime function, and has a significant negative impact on quality of life. Asthma deaths continue to number around 180000 annually on a worldwide basis, with delay in diagnosis and inadequate treatment being primary driving factors.8
Comorbidities of Allergic Diseases
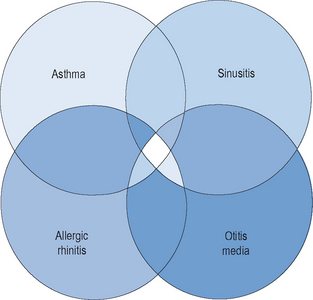
The majority of allergic diseases affect the upper and lower respiratory tracts. Respiratory illnesses that have a very direct allergic pathogenesis include allergic rhinitis and asthma. Other respiratory illnesses such as otitis media and acute and chronic rhinosinusitis have significant elements of allergy, at least in the expression of their symptoms, and perhaps in their pathogenesis as well (Figure 1.1). Among patients with allergic rhinitis, both adults and children, there is a greater prevalence of these other allergic illnesses than in the nonallergic population. In addition, among patients with allergic rhinitis, there is a higher likelihood of the development of rhinosinusitis, asthma, and otitis media than in patients who do not have allergic rhinitis. For that reason, the presence of allergy must be considered in any individual who presents with chronic respiratory symptoms, either upper or lower.
Over the past decade, an awareness of the close interrelationship between upper and lower airway inflammatory diseases has been appreciated. Due to similarities in epithelial cells and membranes, inflammatory mediators, and pathophysiological mechanisms, the entire airway has been conceptualized as a unified system. It has been observed that diseases that affect one portion of this airway system will often affect other respiratory sites as well. This observation has led to a model described as the “unified airway model,” also known as the model of “one airway, one disease.”9,10 Allergic rhinitis and asthma, therefore, are considered diseases along a pathophysiological spectrum, whose mechanisms exert similar influences in discrete portions of a unified airway system. This model has been useful conceptually, in explaining many observations of concurrent inflammation in both the upper and lower airway.
In 2000, the World Health Organization brought together an international panel of experts to examine the association between upper and lower airway inflammatory diseases. This panel issued a consensus document known as the ARIA document (Allergic Rhinitis and its Impact on Asthma). In the ARIA guidelines, the close association between allergic rhinitis and asthma was detailed, with specific recommendations for treatment of these coexisting diseases. The document stated “The upper and lower airways may be considered as a unique entity influenced by a common, evolving inflammatory process, which may be sustained and amplified by interconnected mechanisms.” In addition, it went on to argue that “When considering a diagnosis of rhinitis or asthma, an evaluation of both the upper and lower airways should be made.”11
Basic Immunology
The term “immune” is derived from the Latin word immunitas that refers to the specific exemption that was granted to Roman senators in their state duties. This concept is carried forward into today’s legal system, where witnesses can be given immunity from prosecution for cooperating with a criminal investigation. In health and disease, the immune system is designed to provide this same exemption or protection through preventing or limiting the effects of the disease on the organism. The immune system has a number of properties, which are detailed in Box 1.1.
▪ Types of Immunity
Adaptive immunity refers to the body’s ability to respond specifically to foreign invaders based upon the programmed, direct response of the coordinated immune system to the specific foreign factor. This specific immune response involves a series of events that directs antigen-specific mechanisms toward the foreign invaders. Cellular components of the immune system, primarily B and T lymphocytes, under direction of soluble mediators such as cytokines, will respond to foreign antigens and mount a specific response directed at limiting injury and eliminating these foreign antigens.
Antigens are generally protein molecules that are a discrete component of the foreign material to which the individual is exposed. They have specific three-dimensional configurations known as epitopes that allow them to be recognized by antibodies and to trigger the initiation of the immune response. These epitopes bind to antibodies in a specific manner, similar to that of a key within a lock. The binding of antigen and antibody initiates a cascade of events that begins the specific response. A series of mechanisms allows this immune response to be maximized, yet regulated and specifically directed toward the type and magnitude of the response (Box 1.2).
Immune System Components
The immune system is composed of both cellular and molecular components that are involved in various regulatory and effector functions. The molecular components of the immune system include specific agents, such as antibodies and antigens, and nonspecific agents, such as cytokines. These two components work in a coordinated manner in controlling and facilitating the immune response.
▪ Cellular Components of the Immune System
All of the cells of the immune system are derived from pluripotent stem cells in the bone marrow. During hematopoiesis, these cells will develop along two cells lines: lymphoid and myeloid. The lymphoid lineage will differentiate into three types of discrete immune cells: B lymphocytes, T lymphocytes, and NK (natural killer) cells. These cells are involved in all aspects of the immune response. The myeloid lineage, in contrast, will differentiate into all the other blood cells, including erythrocytes, platelets, neutrophils, eosinophils, basophils, monocytes, and mast cells (Box 1.3). With the exception of the erythrocytes and platelets, the remaining cells are referred to as leukocytes.
Lymphocytes
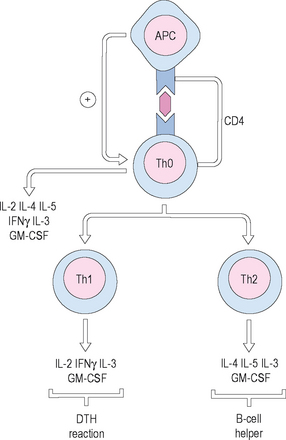
T-helper cells are further divided into two populations: T-helper-1 (Th1) cells and T-helper-2 (Th2) cells. Th1 cells respond primarily to invasion by microbial pathogens, and elaborate specific Th1 cytokines, including interleukin (IL)-2, IL-3, and interferon (IFN)-gamma. They mediate cytotoxicity and local inflammatory responses. Th2 cells, by contrast, are more involved in the humoral response, and are the primary T cell population involved in allergy and atopy. Th2 cells elaborate different cytokines, referred to as Th2 cytokines, including IL-4, IL-5, IL-6, IL-10, and IL-13. These cytokines are involved in shifting the immune response toward the Th2 orientation, and in shifting the production of antibody toward immunoglobulin (Ig) E, the antibody primarily responsible for the allergic response (Figure 1.2).
Mononuclear cells
Antigen-presenting cells (APCs) are specialized macrophages that are able to recognize foreign substances as antigen and process that antigen by enzymatic degradation. They are found in the skin, lymph nodes, and spleen. The APCs then process the antigen, placing relevant fragments onto their surfaces in conjunction with major histocompatibility complexes (MHC) I and II. They then present these complexes to T-helper cells through interaction with the T-cell receptors (TCRs). This interaction then activates the T cell, which can then proceed with initiation of the immune response through interactions with B cells and elaboration of cytokines.
▪ Molecular Components of the Immune System
A variety of soluble mediators are involved in the triggering and control of the immune reaction. These mediators include the classes of antibodies and cytokines, both of which are protein molecules that serve discrete functions within the immune system. The effects of these soluble mediators are responsible for the portion of the immune reaction known as humoral immunity.
Immunoglobulins
Immunoglobulins are glycoproteins that are produced by B cells in response to antigenic stimulation. They are the primary immune effectors and are responsible for specificity of the immune response. Five classes of immunoglobulins have been described, and these are classified as IgG, IgM, IgA, IgD, and IgE (Figure 1.3). The basic structure of the immunoglobulin molecule is similar among these five classes, and consists of four protein chains linked by disulfide bonds in the configuration of a “Y.” The base of this Y-shaped molecule is referred to as the Fc portion, which is responsible for binding to cell surfaces, and the arms of the molecule, referred to as the Fab portion, are freely exposed to the microenvironment to allow binding to specific antigens and activation of cellular immune mechanisms. Antibodies are produced by activated B cells after they are transformed into plasma cells. These plasma cells are programmed to produce one specific type of immunoglobulin, and are efficient in secreting large amounts of these antibodies rapidly.
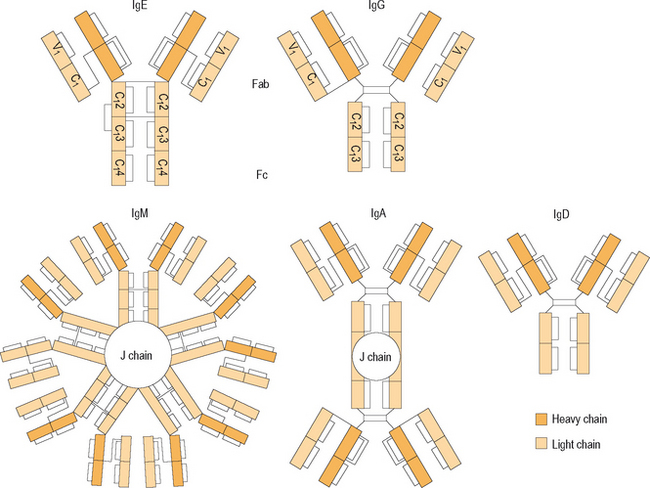
Figure 1.3 Domain structure of different antibody classes.
(Reproduced with permission from Holgate ST. Allergy, 2nd edn, p 245, figure 16.4. Published by Mosby–Elsevier Inc. ©2001.)
Cytokines
Cytokines are small regulatory polypeptides that are responsible for communication among cells of the immune system. They are potent messengers that are produced by many types of nucleated cells, including lymphocytes, macrophages, epithelial cells, endothelial cells, and fibroblasts. The cytokines that are produced specifically by lymphocytes have also been referred to as lymphokines, and those that act specifically between white blood cells are known as interleukins. Other classes of cytokines include interferon, tumor necrosis factor (TNF), and colony stimulating factor (CSF).
Of major interest in the immune response is the class of cytokines known as interleukins. These chemical mediators serve to direct and control the immune response through intercellular communication. They act specifically at IL receptors on cells and have specific functions in the immune response. For example, IL-4 is responsible for isotype switching, signaling plasma cells to shift their production of immunoglobulin from IgG to IgE. IL-5 is responsible for growth and proliferation of B cells and for eosinophil maturation and chemotaxis. A more complete list of interleukins and their functions is found in Box 1.4.
▪ Stages of the Immune Response
On initial exposure to this antigen, however, a specific response is also generated that lags behind the nonspecific response. This phase is referred to as the primary immune response. In this phase the newly recognized antigen is processed within specialized macrophages, the APCs, and the antigenic portion of those molecules is presented along with MHC molecules to T-helper cells. These T cells work through direct interaction with B lymphocytes, and signal the B cells to begin production of specific classes of immunoglobulin with discrete specificity for the antigen that was just presented. The B cells then produce antibody. This primary response again is somewhat delayed and modest in magnitude.
Types of Immune Hypersensitivity Reactions
While the immune response is usually appropriate and targeted to the level and type of challenge, it is possible for that response to become inappropriate, or for an immune reaction, once initiated, to continue without adequate cessation. In addition, agents that should ordinarily not be recognized as harmful antigens can sometimes be mistaken by the immune system as harmful and can initiate an inappropriate immune response. In those cases, this immune response can be injurious to the host and can result in significant morbidity and even mortality. These poorly controlled or overexuberant responses of the immune system are referred to as hypersensitivity reactions. These hypersensitivity reactions have often been classified into four specific types of responses, although other authors have argued that several more responses may be present. These four classes of hypersensitivity responses, described by Gell and Coombs, are presented in Table 1.1 and will be discussed below.
| Type of reaction | Examples | |
|---|---|---|
| Type I | Immediate hypersensitivity | Anaphylaxis, allergy |
| Type II | Cytotoxic | Hemolysis |
| Type III | Immune complex | Serum sickness |
| Type IV | Delayed hypersensitivity | Contact sensitivity |
▪ Type I: Immediate Hypersensitivity
The immediate hypersensitivity response is responsible for allergic disease and anaphylaxis. It is mediated by IgE antibody, and is triggered by binding of antigen to IgE molecules on the surfaces of mast cells. When the individual has been previously sensitized to a specific antigen, that antigen promotes the development of antigen-specific IgE that is produced through mechanisms previously discussed. Those IgE molecules bind by their Fc portions to the surfaces of mast cells, and will persist indefinitely awaiting subsequent reexposure. When the person comes into contact with that specific antigen once again, the antigen will bind to adjacent IgE molecules on the mast cell surface, resulting in cross-linking of those molecules. This binding will result in influx of calcium ion into the cells, with resulting degranulation and release of mediators into the tissues and systemic circulation. The primary product that is released from these preformed granules is histamine, a vasoactive amine that binds to receptors on target cells and initiates a series of inflammatory events. This response occurs rapidly on reexposure to antigen, with symptoms often present within minutes of contact. This rapid release of histamine with brisk onset of symptoms is referred to as the early phase allergic response. It is primarily mediated by histamine, which causes end-organ effects such as vasodilatation, transudation of plasma, tissue edema, stimulation of neural endings, and smooth muscle constriction. These tissue effects result in the patient symptoms of sneezing, itching, congestion, rhinorrhea, and wheezing. In severe cases, systemic effects of histamine release will include systemic vasodilation, hypotension, shock, and even death. This profound response is referred to as anaphylaxis.
▪ Type II: Antibody-Dependent Cytotoxicity
Type II reactions are mediated by IgM and IgG antibodies that develop to antigens present intrinsically on the individual’s host cells. These antigens are recognized as foreign (non-self), and stimulate an antibody-dependent immune reaction that is directed by NK cells, leading to cell lysis and death. Host tissues that are often involved in these reactions include erythrocytes, basement membranes, and glandular epithelial elements. Complement fragments are also generated through this response, leading to activation and chemotaxis of macrophages, with damage produced at the level of the cell membrane. An example of this type II response is seen in hemolytic anemia of the newborn, mediated by Rh antibodies in the maternal circulation that can be transferred across the placenta to the newborn, resulting in hemolysis.
Summary of Immunology
At times, however, the immune system will respond to antigens that ordinarily would not stimulate an immune response, and may do so in such a way that it causes significant symptomatology and morbidity. This process underlies the allergic response and the development of atopic diseases such as allergic rhinitis, atopic dermatitis, and asthma. The allergic reaction, therefore, consists of a complex interaction of a number of different components of the immune system, resulting in the expression of various symptoms.
1 http://www.aaaai.org/media/resources/media_kit/allergy_statistics.stm. Last accessed September 28, 2006.
2 Hahn EL, Bacharier LB. The atopic march: the pattern of allergic disease development in childhood. Immunol Allergy Clin North Am. 2005;25:231-246.
3 Thompson AK, Juniper E, Meltzer EO. Quality of life in patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2000;85:338-347.
4 Gregory C, Cifaldi M, Tanner LA. Targeted intervention programs: creating a customized practice model to improve the treatment of allergic rhinitis in a managed care population. Am J Manag Care. 1999;5:485-496.
5 Marshall PS, Colon EA. Effects of allergy season on mood and cognitive function. Ann Allergy. 1993;71:251-258.
6 Juniper EF, Guyatt GH, Dolovich J. Assessment of quality of life in adolescents with allergic rhinoconjunctivitis: development and testing of a questionnaire for clinical trials. J Allergy Clin Immunol. 1994;93:413-423.
7 Vuurman EF, van Veggel LM, Uiterwijk MM, et al. Seasonal allergic rhinitis and antihistamine effects on children’s learning. Ann Allergy. 1993;71:121-126.
8 Braman SS. The global burden of asthma. Chest. 2006;130(suppl):4S-12S.
9 Grossman J. One airway, one disease. Chest. 1997;111(2 suppl):11S-16S.
10 Passalacqua G, Ciprandi G, Canonica GW. The nose–lung interaction in allergic rhinitis and asthma: united airways disease. Curr Opin Allergy Clin Immunol. 2001;1:7-13.
11 Bachert C, van Cauwenberge P, Khaltaev N, et al. Allergic rhinitis and its impact on asthma. In collaboration with the World Health Organization. Executive summary of the workshop report 7–10 December 1999, Geneva, Switzerland. Allergy. 2002;57:841-855.