CHAPTER 45 Intraventricular Meningiomas
INTRODUCTION
Intraventricular meningiomas are relatively rare and represent 1% to 2% of all meningiomas. The first recorded case of intraventricular meningiomas is probably by Shaw in 1854.1 He identified an encapsulated fibrous tumor in the trigone of the lateral ventricle. MacDowell performed a left trigonal tumor operation in 1881.2 In 1938, Cushing and Eisenhardt first described a case of meningioma located within the third ventricle.3 Since then, there have been multiple reports of cases of intraventricular meningiomas in the literature addressing the potential problems and difficulties encountered in management of these formidable surgical lesions. To date, approximately 600 cases of intraventricular meningiomas have been reported. Of these, approximately 80% are found in the lateral ventricle, 15% in the third ventricle, and 5% in the fourth ventricle.1–48
We base our analysis on a review of our experience with 50 cases of lateral ventricular meningiomas treated in our Institute from 1990 to 2006. The discussion is focused on lateral ventricular meningiomas, and meningiomas in other ventricles are not considered. In our series, lateral ventricular meningiomas represented 2.1% of the total meningiomas seen during the period. Slow progression of these essentially benign tumors and accommodative capacity of the ventricles make these tumors unique in the sense that the symptoms are subtle and long-standing and they are significantly large at the time of diagnosis. Lateral ventricular meningiomas present a surgical challenge because of the deep location, large size, firm consistency, and the frequently observed intense vascularity. Although meningiomas have been rarely recorded in other sites of the lateral ventricular cavity, most of them are located in the trigone or have an extension toward it. The presence of bulkier choroid plexus may be the reason for higher incidence of tumors in the region of atrium of lateral ventricle. The majority of the lateral ventricular meningiomas are benign in nature and resection often leads to cure from the disease. Malignant meningioma in the lateral ventricle with cerebrospinal fluid (CSF) borne distant metastasis in the brain and spinal cord is also rarely reported.16,29,45
The exact site of origin of these tumors is unclear, but the various reviews have suggested that these tumors probably arise from the meningeal cells that are transported together with the choroid plexus as the ventricular system invaginates during embryogenesis.3,22,27 This feature is supported by the fact that the vascular supply of most of these tumors is from the choroidal vessels. Due to the relationship with choroid plexus, some authors prefer to label these meningiomas as “choroidal” meningioma.35
CLINICAL FEATURES
Like most other meningiomas, intraventricular meningiomas also have a female preponderance. Although not observed in our series, some authors have identified a relatively higher incidence of intraventricular meningiomas in children.8,31,41 Some authors have observed left side predominance,21,22 while others have noted no significant side preference. These meningiomas are sometime seen as a part of neurofibromatosis 2 (NF2) complex and also in association with multiple meningiomas. The patients may not have any significant neurologic symptoms, even when the tumor has reached a massive size. Owing to the nature of growth pattern, some of these tumors may be detected incidentally. In such cases, considering the slow nature of growth and difficulties that can be encountered during surgery, a conservative observation may be a viable treatment option. High vascularity of the tumors has rarely been associated with intratumoral bleeding and acute onset of symptoms.34,36,42,46
In general, these patients usually have long-standing dull and generalized headaches as the initial presenting symptom, which increases in intensity as the tumor grows in size. Duration of headache may be as long as 10 to 15 years.38 With tumor growth, the additional symptoms of raised intracranial pressure in the form of vomiting and visual symptoms related to papilledema become prominent. Although not seen in this series, episodic severe headaches simulating symptoms generally related to colloid cysts have been observed in cases with small sized lateral ventricular meningiomas.21 Visual field defects are relatively frequent. In our series, 5 of the 14 patients with preoperative visual field defect had almost complete homonymous hemianopia. The exact cause of the visual field defect before surgery is unclear. A vascular steal from the posterior cerebral artery supply zone by the “vascular” tumor could be a probable cause of this symptom. Direct stretching and compression of the optic radiation projection fibers is another possible cause. Considering that the visual field defect improved in eight patients (57%) after surgery, involvement of fibers of optic radiation by pressure could be an important factor. Memory disturbances, neuropsychological deficits and personality changes are relatively late and were seen in 12% cases.13 Decrease in scholastic performance due to progressive cognitive changes secondary to chronically raised intracranial pressure is a frequent form of presentation in children. Verbal and visual memory is more frequently affected. The short-term or recent memory is relatively spared.
The incidence of seizure is relatively uncommon in patients with lateral ventricular meningiomas. Focal motor or generalized seizures as presenting symptom have been reported and were seen in three cases (6%). Sensory seizures occurred in five patients (10%). Postoperative seizures are reported in 29% to 70% patients operated by transcortical approaches. In our series, 11 (22%) patients had postoperative seizures. Parietal and temporal lobe signs have been recorded in the literature and were seen in 8% of our cases. Preoperative localizing signs such as hemiparesis, aphasia, agnosia, and alexia are rarely present even in large lateral ventricular meningiomas.17
INVESTIGATIONS
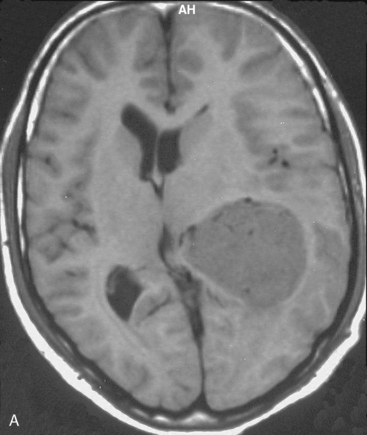
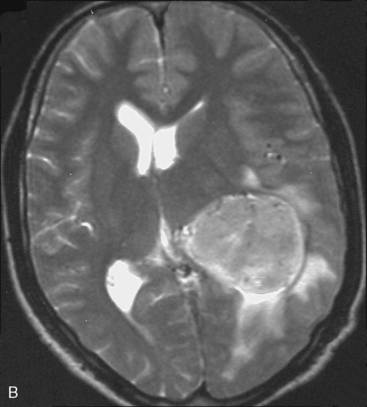
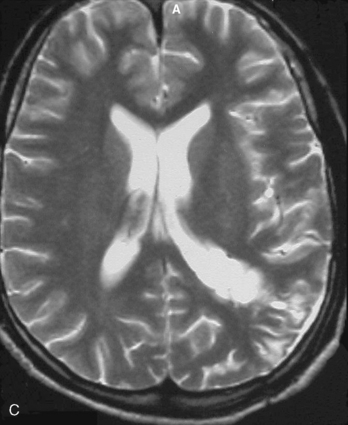
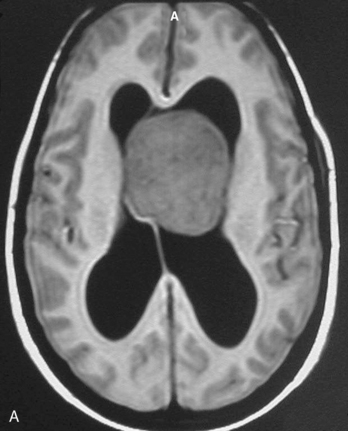
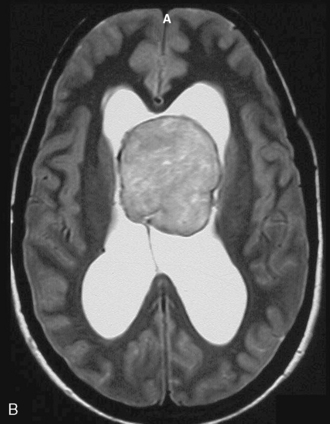
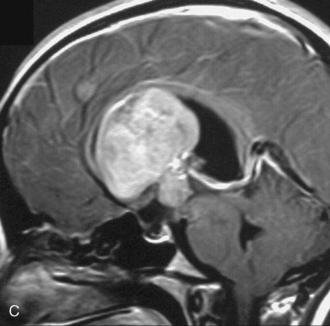
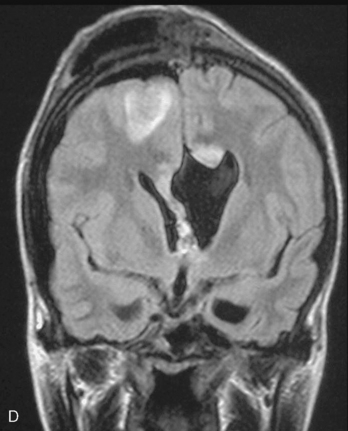
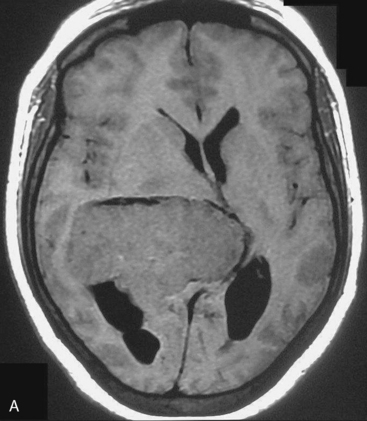
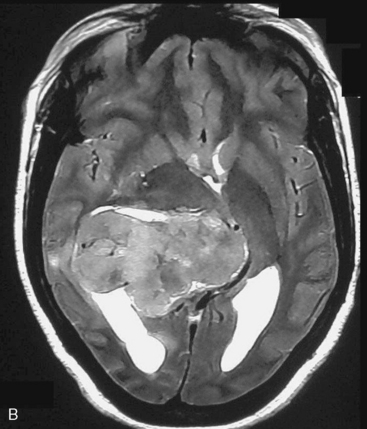
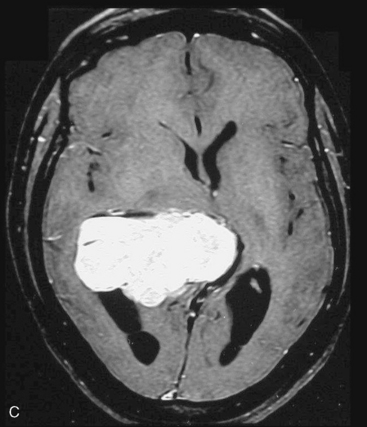
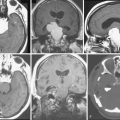
Magnetic resonance imaging scan (MRI) is an important tool for diagnosing lateral ventricular meningiomas.39,40 It differentiates intraventricular lesions from the paraventricular tumors. It helps in defining the extent of the tumor, its physical nature, and its vascularity and thereby assists the surgeon in planning the surgical approach. The most common site for the lateral ventricular meningiomas is the trigone of the lateral ventricle and irrespective of its size they are attached to the choroid plexus in the region of the trigone. Asymmetric hydrocephalus with ipsilateral dilatation of the temporal and occipital horns is also common and frequently diagnostic of intraventricular location of the lesion and meningioma. The MRI, in general, shows the lesion as iso- to slightly hypointense on T1-weighted images and iso- to mildly hyperintense on T2-weighted images. There is usually homogeneous enhancement on contrast administration. The advent of MRI has limited the role of angiography in lateral ventricular meningiomas. The MR spectroscopy and blood volume-time intensity maps may assist in the preoperative diagnosis. A high alanine-to-creatinine ratio has been reported as specific MR spectroscopic findings for meningiomas. Functional MRI may be useful in preoperative cortical mapping, so as to identify the sensorimotor and language cortices to plan an optimal surgical approach and select the safest area in which to make a cortical incision.
It is important preoperatively to differentiate these benign intraventricular meningiomas from more frequently encountered malignant tumors in the region and in the ventricle. The differential diagnosis of lateral ventricular meningioma includes choroid plexus papilloma, intraventricular ependymomas, central neurocytomas, and subependymomas.27 Each of these tumors has a characteristic nature of clinical presentation and radiologic features. The presence of a large sized and well-defined lobulated trigone centered tumor in a physically healthy and neurologically stable patient favors a preoperative diagnosis of a meningioma. The duration of the presenting symptoms is also relatively longer in intraventricular meningiomas when compared to other malignant intraventricular tumors. Intense and homogeneous tumor enhancement is more frequent in intraventricular meningiomas. The presence of intratumoral necrotic and cystic changes is relatively uncommon in intraventricular meningiomas and was seen in nine (18%) cases. As in other brain tumors, we observed that longer the duration of clinical symptoms the firmer was the consistency of the tumor. Calcification within the tumor was seen in 14% cases. Moderate to severe tumor surrounding parenchymal edema was seen in 64% of patients. Enlargement of the contralateral ventricle, probably suggestive of the chronic nature of the disease process, was seen in all our cases with medium to large sized tumors. Dilatation of the temporal and occipital horns, probably a result of focal obstruction to the CSF pathway, suggests intraventricular location of the tumor.
Other than the fact that there is no bone related or origin site changes, CT scan shows all typical features of meningiomas.49 The tumors are hypodense or slightly hyperdense tumor and have intense contrast enhancement. Calcification may be seen. Dense and large calcifications within the tumor may even be visible on plain skull radiographs.
ANGIOGRAPHY
The blood supply to lateral ventricular meningioma is from both anterior and posterior choroidal arteries. They also can receive supply from lenticulostriate and thalamoperforating vessels. The study may provide useful information on the location of the feeding arteries in relationship to the internal dome of the tumor. The carotid angiogram shows enlargement, tortuosity, and displacement of the anterior choroidal artery. Venous drainage is through the vein of Galen, internal cerebral vein, or the basal vein of Rosenthal. Large veins frequently course over the dome of the tumor before joining the main venous channel. In the venous phase, the internal cerebral veins appear stretched and displaced inferiorly and to the opposite side. The vein of Galen may be elongated and straightened. Vertebral angiogram may show enlargement and anterior displacement of the lateral posterior choroidal artery with a reversal of its curve, and marked anterior convexity.23
SURGICAL STRATEGY
Surgery on intraventricular meningiomas challenges the surgical skills and philosophical understanding of a neurosurgeon. Surgical experience and thorough knowledge of anatomy are crucial in excising these lesions.50,51 While a successful surgical resection is compatible with normal life, any error in planning the approach or executing the surgery can lead to disabling neurologic deficits or even death. The anatomy of the trigone, the arterial and venous patterns, and the relationship with visual pathways and hemispheric dominance needs to be evaluated, before attempts toward surgery. When planned, surgery has to be directed toward radical or total tumor resection, as incomplete or partial tumor resection can lead to tumor bleeding related issues. The issues that are relevant while planning surgery for intraventricular meningiomas include assessment of the size of the tumor, its consistency, its vascularity, location of the feeding blood vessels, and the direction of the growth. The basic principles that determine the success of the surgery include early access to the blood supply, minimal retraction of the brain for adequate exposure while removal of large masses, and understanding of the function of the surrounding anatomic structures. An interhemispheric approach and exposure of the medial part of the tumor to control the feeding vessels early in the operation appears to be a reasonable surgical option.28,52 However, the large sizes of the tumor and the presence of a relatively tense brain leading to difficulty in retraction of the brain led to the employment of transcortical approach in most of our cases. The selection of the cortical incision depends on the site of projection of the tumor and the safest cortical area in the proximity. Exact understanding of the well-described function of the cortical area in question is mandatory while planning the surgical approach, as severe speech and cognitive deficits can mar the surgical outcome. Electrophysiological monitoring and mapping have been identified to be useful in evaluating the best site for cortical incision. Intraoperative tractography of motor and optic tracts using neuronavigation is increasingly being used as a useful technological help. Damage to the visual tracts can be avoided by a three-dimensional understanding of the white matter tracts of the brain by cadaveric study of “fibers” of the brain by fiber dissection techniques. The optic radiation covers the entire lateral aspect of the temporal horn and atrium as it extends to the occipital horn. Anterior, inferior, and posteroinferior routes to the atrium would avoid the optic radiations.
SURGICAL APPROACH
Cushing discussed a temporoparietal approach.3 The middle temporal gyrus approach was described by Olivocrona.53 Cramer was probably the first to use the more popular posterior parieto-occipital incision to approach the meningiomas located in the atrium.17 Yasargil recommended a parieto-occipital interhemispheric approach for a variety of intraventricular lesions. The incision was in the parasplenial-precuneal area.53 Kempe and Blaylock suggested a posterior transcallosal approach to resect tumors in the ventricular trigone of the dominant hemisphere.30
Transcortical Approach
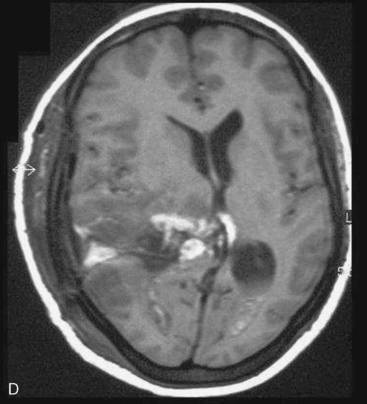
Depending on the location of the tumor, the direction of its spread, and its minimum distance from the surface, the safest part of the cortex in the vicinity is selected for the approach. The medium and large sized meningiomas within the trigone and body of the lateral ventricle are approached by the posterior parietal-superior parietal lobule or superior parieto-occipital approach. The parieto-occipital approach is along the orientation parallel to the optic radiation over the cerebral convexity and is most suitable to avoid damage to the optic radiation. Moreover, this is often the thinnest region overlying the tumor positioned in the trigone (Figs. 45-1, 45-2, and 45-3). The incision extends from postcentral fissure to the parieto-occipital fissure approximately 3 cm from the falx. The incision lies medial to the majority of the visual fibers and parallel to their projections. The visual field deficits, which remain a significant problem, are attributed to damage caused by manipulation of the tumor at the border of the lateral ventricular ependyma. In our series, five patients operated via this approach had visual field defects. Postoperative hemispheric motor deficits will depend on the extent of cerebral cortical manipulation. It is sometimes surprising to see that even extensive cortical manipulation may lead to no significant cortical dysfunction.
Cortical damage of the dominant hemisphere may result in alexia, agraphia, acalculia, and ideomotor apraxia.37 Gerstmann’s syndrome is also believed to result from lesions in this area. Lesions in this region of the nondominant hemisphere may impair memory for visual information, hemineglect, and constructional apraxia. A homonymous visual field deficit is frequently encountered because the visual projection fibers that run parallel to the lateral aspect of the ventricle are transected.
Interhemispheric Approach
Interhemispheric approach is more suitable for tumors located in the body of the lateral ventricle or those having a significant extension toward it (see Fig. 45-2). Small sized trigonal tumors where the retraction of the brain off the falx is relatively safe are suitable for interhemispheric approach. Even large trigonal tumors having a more medial extension are frequently suitable. The advantage of interhemispheric approach is that the feeding blood vessels can be addressed early in the operation. On the other hand, the disadvantage is that the exposure sometime becomes limited. Moreover, prolong or excessive retraction of the medial occipital cortex can affect the optic radiation fibers.
Distal Sylvan Approach
Such an approach has been discussed for approach for removal of small lesions in the atrium. After the exposure, the sylvian fissure is widely opened.43 The transverse gyrus of Heschl is the landmark for orientation of the region and for atrium of the lateral ventricle. The medial end of the transverse gyrus of Heschl corresponds to the posterior end of the insular cortex. The longitudinal axis corresponds to the access to the atrium through the posterior end of the insular cortex. The disadvantage of the approach is that it is suitable only for significantly small lesions. The wide opening of the Sylvian fissure in the presence of a tumor exposes the possibility of perforator injury. On the dominant side, optic radiations may be injured as they are in the path. Insular cortex related to hearing sensation may be affected.
SURGICAL TECHNIQUE
In general, the histopathologic features of intraventricular meningiomas are no different from meningiomas elsewhere in the brain.8
POSTOPERATIVE MANAGEMENT
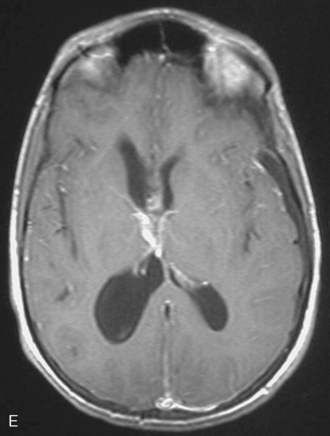
Patients with preoperative ventricular enlargement or large tumors are at risk for postoperative subdural hematoma and hygroma formation. Tanaka reported subdural fluid collections in 40% of patients after removal of intraventricular tumors, and symptomatic collections required surgical drainage in 11% of cases.54 In our series, although 36% of patients were noted to have subdural hygroma surgical intervention was not needed in any of these patients. The problem of hydrocephalus in intraventricular meningiomas is very closely associated with respect to clinical signs and symptoms and postoperative management. In our opinion, shunting for hydrocephalus should be avoided and may be counterproductive.
[1] Shaw A. Fibrous tumor in the lateral ventricle of the brain, bony deposits in the arachnoid membrane of the right hemisphere. Trans Path Soc Lond. 1853–1854;5:18-21.
[2] MacDowell TW. Large calcareous tumor involving chiefly the innerand middle portions of the left temporosphenoidal lobe and pressingupon the left crus and optic thalamus. Edinburgh Med J 1088;1881:1826.
[3] Cushing H., Eisenhardt L. Meningiomas, Their Classification, Regional Behavior, Life History and Surgical End Results. Springfield, IL: Charles C Thomas, 1938.
[4] Abbott K.H., Courville C.B. Intraventricular meningiomas: Review of the literature and report of two cases. Bull Los Angeles Neurol Soc. 1942;7:12-28.
[5] Arseni C., Ionescu S., Maretsis M. Meningiomas of the lateral ventricle. Psychiatr Neurol Neurochir. 1968;71:319-336.
[6] Bhatoe H.S., Singh P., Dutta V. Intraventricular meningiomas: a clinicopathological study and review of the literature. Neurosurg Focus. 2006;20(3):E9.
[7] Bret P., Gharbi S., Cohadon F., Remond J. Meningioma of the lateral ventricle. 3 recent cases [in French]. Neurochirurgie. 1989;35:5-12.
[8] Byard R.W., Bourne A.J., Clark B., Hanieh A. Clinicopathological and radiological features of two cases of intraventricular meningiomas in childhood. Pediatr Neurosci. 1989;15(5):260-264.
[9] Caner H., Acikgoz B., Ozgen T., Colak A., Onol B. Meningiomas of the lateral ventricle. Report on six cases. Neurosurg Rev. 1992;15:303-306.
[10] Castillo R.G., Getse A.W. Meningioma of the third ventricle. Surg Neurol. 1985;24(5):525-528.
[11] Cedzich C., Schramm J., Bingham K. Intraventricular tumors of the ventricles I-III. 1993;54:179-185.
[12] Ceylan S., Iibay K., Kuzeyli K., Kalelioglu M., Aktruk F., Ozoran Y. Intraventricular meningiomas of the fourth ventricle. Clin Neurol Neurosurg. 1992;94(2):181-184.
[13] Chaskis C., Buisseret T., Michotte A., D’Haens J. Meningioma of the fourth ventricle presenting with intermittent behaviour disorders: a case report and review of literature. J Clin Neurosci. 2001;8(4A):59-62.
[14] Chen H.J., Lui C.C., Chen L. Meningioma in the anterior III ventricle in a child. Childs Nerv Syst. 1986;2(3):160-161.
[15] Couillard P., Karml M.Z., Abdelkader A.M. Microsurgical removal of an intraventricular meningiomas with ultrasound guidance, and balloon dilatation of operative corridors: case report and technical note. Surg Neurol. 1996;45(2):155-160.
[16] Couldwell W.T., Frankhauser H., de Tribolet N. Osseous metastases from a benign intraventricular meningiomas. Case report. Acta Neurochir (Wein). 1992;117(3–4):195-199.
[17] Cramer F. The intraventricular meningiomas: a note on the neurologic determinants governing the surgical approach. Arch Neurol. 1960;3:98.
[18] Criscuolo G.R., Symon L. Intraventricular meningiomas. A review of 10 cases of the National Hospital, Queen Square (1974–1985) with reference to the literature. Acta Neurochir (Wein). 1986;83:83-91.
[19] Delandsheer J.M. Les meningiomas du ventricule lateral. Neurochirurgie. 1965;11:4-83.
[20] de la Torre E., Alexander E.Jr, Davis C.H.Jr, Crandell D.L. Tumors of the lateral ventricles of the brain: report of eight cases, and suggestions for clinical management. J Neurosurg. 1963;20:461-470.
[21] Fornari M., Savoiardo M., Morello G., Solero C.L. Meningiomas of the lateral ventricles. Neuroradiological and surgical considerations in 18 cases. J Neurosurg. 1981;54:64-74.
[22] Guidetti B., Delfini R., Gagliardi F.M., Vagnozzi R. Meningiomas of the lateral ventricles. Clinical, neuroradiologic, and surgical considerations in 19 cases. Surg Neurol. 1985;24:364-370.
[23] Huang Y.S., Araki C. Angiographic confirmation of lateral ventricle meningiomas. A report of five cases. J Neurosurg. 1854;11:337-352.
[24] Hussein S. Operative management of the trigono-atrial lesion. Zentralbl Neurochir. 1997;58(4):177-182.
[25] Jun C.L., Nutik S.L. Surgical approaches to intraventricular meningiomas of the trigone. Neurosurgery. 1985;16:416-420.
[26] Innichinski Z., Kopczynski S. Meningioma within the lateral ventricle. Neurochirurgia (Stuttg). 1970;13:124-130.
[27] Jelinek J., Smirniotopoulos J.G., Parisi J.E., Kanzer M. Lateral ventricular neoplasms of the brain: differential diagnosis based on clinical, CT, and MR findings. AJR Am J Roentgenol. 1990;155:365-372.
[28] Jun C.L., Nutik S.L. Surgical approaches to intraventricular meningiomas of the trigone. Neurosurgery. 1985;16:416-420.
[29] Kamiya K., Inagawa T., Nagasako R. Malignant intraventricular meningioma with spinal metastasis through the cerebrospinal fluid. Surg Neurol. 1989;32:213-218.
[30] Kempe L.G., Blaylock R. Lateral-trigonal intraventricular tumors. A new operative approach. Acta Neurochir (Wien). 1976;35:233-242.
[31] Kloc W., Imielinski B.L., Wasilewski W., Stempniewicz M., Jende P., Kawaccki Z. Meningiomas of the lateral ventricles of the brain in children. Childs Nerv Syst. 1998;14(8):350-358.
[32] Kobayashi S., Okazaki H., MacCarty C.S. Intraventricular meningiomas. Mayo Clin Proc. 1971;46:735-741.
[33] Koumtchev Y.N., Dimitrov Z.I., Gozmanov G.R., Peshev Z.B. Meningioma of the lateral cerebral ventricle. A case report. Folia Med (Plovdiv). 2002;44:93-96.
[34] Lang I., Jackson A., Strang F.A. Intraventricular hemorrhage caused by intraventricular meningioma. CT appearance. AJNR Am J Neuroradiol. 1995;16:1378-1381.
[35] Lapras R., Deruty R. Tumours of the lateral ventricle. Symon L., et al, editors. Advances and Technical Standards in Neurosurgery, 1984. New York: Verlag
[36] Lee E.J., Choi K.H., Kang S.W., Lee I.W. Intraventricular hemorrhage caused by lateral ventricular meningioma: a case report. Korean J Radiol. 2001;2:105-107.
[37] Levin H.S., Rose J.E. Alexia without agraphia in a musician after transcallosal removal of a left intraventricular meningioma. Neurosurgery. 1979;4:168-174.
[38] Lyngdoh B.T., Giri P.J., Behari S., Banerji D., Chhabra D.K., Jain V.K. Intraventricular meningiomas: A surgical challenge. J Clin Neurosci. 2007;14:442-448.
[39] Mani R.L., Hedgcock M.W., Mass S.I., Gilmor R.L., Enzmann D.R., Eisenberg R.L. Radiographic diagnosis of meningioma of the lateral ventricle. Review of 22 cases. J Neurosurg. 1978;49:249-255.
[40] Majos C., Cucurella G., Aguilera C., Coll S., Pons L.C. Intraventricular meningiomas. MR imaging and MR spectroscopic findings in two cases. AJNR. 1999;20(5):882-885.
[41] Mircevski M., Mirceska D., Bojadziev I., Basevska R. Surgical treatment of intraventricular meningiomas in childhood. Acta Neurochir Suppl (Wein). 1985;35:89-91.
[42] Murai Y., Yoshida D., Ikeda Y., Teramoto A., Kojima T., Ikakura K. Spontaneous intraventricular hemorrhage caused by lateral ventricular meningioma—case report. Neurol Med Chir (Tokyo). 1996;36:586-589.
[43] Nagata S., Sasaki T. Lateral transsulcal approach to asymptomatic trigonal meningiomas with correlative microsurgical anatomy: Technical case report. Neurosurgery. 2005;56(ONS Suppl. 2):ONS-438.
[44] Ojemann R.G. Intraventricular meningioma. Neurosurgery. 1992;40:321-383.
[45] Peh W.C., Fan Y.W. Case report: intraventricular meningioma with cerebellopontine angle and drop metastases. Br J Radiol. 1995;68:428-430.
[46] Smith V.R., Stein P.S., MacCarty C.S. Subarachnoid hemorrhage due to lateral ventricular meningiomas. Surg Neurol. 1975;4:241-243.
[47] Wall A.E. Meningiomas within the lateral ventricle. J Neurol Neurosurg Psychiatry. 1954;17:91-103.
[48] Yoshida K., Onozuka S., Kawase T., Ikeda E. Lateral ventricular meningioma encapsulated by the dura-like membrane. Neuropathology. 2000;20:56-59.
[49] Kendall B., Reider-Grosswasser I., Valentine A. Diagnosis of masses presenting within the ventricles on computed tomography. Neuroradiol. 1983;25:11-22.
[50] Ebeling U., Reulen H.J. Neurosurgical topography of the optic radiation in the temporal lobe. Acta Neurochir (Wien). 1988;92:29-36.
[51] Kawashima M., Li Xiaoyong L., Rhoton A.L., Ulm A.J., Oka H., Fujii K. Surgical approaches to the atrium of the lateral ventricle: microsurgical anatomy. Surg Neurol. 2006;65:436-445.
[52] Yasargil M.G. Parieto-occipital interhemispheric approach. Yasargil M.G., editor. Microneurosurgery, vol. IVB. Theime, New York, 1996;322-323.
[53] Olivecrona H., Tonnis W. Handbuch de Neurochirurgie, Berlin Heidelberg, vol. 4. Springer, New York, 1974.
[54] Tanaka Y., Sugita K., Kobayashi S., Takemae T., Hegde A.S. Subdural fluid collection following transcortical approach to intra- or paraventricular tumours. Acta Neurochir (Wien). 1989;99:20-25.