Chapter 9 Intravascular ultrasound in coronary artery disease
 The observation of the three-layered appearance by intravascular ultrasound occurs due to the acoustic impedance between adjacent structures.
The observation of the three-layered appearance by intravascular ultrasound occurs due to the acoustic impedance between adjacent structures. The earliest accumulations of atherosclerotic plaque consist of crescentic intimal thickening of an intermediate echointensity.
The earliest accumulations of atherosclerotic plaque consist of crescentic intimal thickening of an intermediate echointensity. With a greater accumulation of plaque, there is a greater complexity to the plaque which may be differentiated by broad ultrasound criteria relating to echoreflectivity.
With a greater accumulation of plaque, there is a greater complexity to the plaque which may be differentiated by broad ultrasound criteria relating to echoreflectivity. Intravascular ultrasound provides accurate online quantitative information regarding lumen size and residual plaque load before and after interventional procedures.
Intravascular ultrasound provides accurate online quantitative information regarding lumen size and residual plaque load before and after interventional procedures. Intravascular ultrasound has confirmed that with increasing plaque accumulation, there is a remodelling process whereby the vessel initially expands to accommodate the plaque load up to around 40% of vessel area.
Intravascular ultrasound has confirmed that with increasing plaque accumulation, there is a remodelling process whereby the vessel initially expands to accommodate the plaque load up to around 40% of vessel area. During balloon angioplasty, localised calcium has a direct role in promoting dissection by increasing shear stress within the plaque at the junction between tissue types with differing elastic properties.
During balloon angioplasty, localised calcium has a direct role in promoting dissection by increasing shear stress within the plaque at the junction between tissue types with differing elastic properties. A strong argument for the use of IVUS in balloon angioplasty is that it may allow a ‘stent-like’ result to be achieved by optimising the acute lumen gain from balloon dilatation alone.
A strong argument for the use of IVUS in balloon angioplasty is that it may allow a ‘stent-like’ result to be achieved by optimising the acute lumen gain from balloon dilatation alone. In the MUSIC trial of additional IVUS guidance in optimal stent deployment, criteria for stent expansion were defined and even with only 80% of patients achieving these criteria, a six-month angiographic restenosis rate of 8.3% was reported.
In the MUSIC trial of additional IVUS guidance in optimal stent deployment, criteria for stent expansion were defined and even with only 80% of patients achieving these criteria, a six-month angiographic restenosis rate of 8.3% was reported.INTRODUCTION
The development of intravascular ultrasound (IVUS) has been a major development in the invasive imaging of coronary arteries. Clinical studies in intravascular ultrasound began in 1989 with the development of catheters initially in the 5–6 French gauge (F) range with the most recent catheter size miniaturised to 2.6 F.1 Intravascular ultrasound has permitted not only a greater understanding of plaque morphology and its response to interventional procedures but has provided accurate online quantitative information regarding lumen size and residual plaque load, an important predictor of restenosis. The presence of disease not only at the site of focal stenosis but also in reference segments believed by angiography to be free of disease has modified interventional practice significantly. With continued improvement in image quality through increasing ultrasound frequency from 30 MHz through 40 MHz currently available and ultimately 50 MHz, the morphology of plaque and the offline ability to characterise plaque will provide additional information in the management of atherosclerotic disease. It is likely that continued technical developments will enhance and define the role of intravascular ultrasound in coronary interventional practice.
BASIC INTERPRETATION
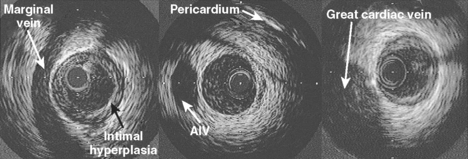
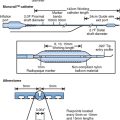
An appreciation of the coronary anatomy and its relationship to structures around it is important to the accurate interpretation of intravascular ultrasound images. These spatial relations are best appreciated at the time of slow catheter pull-back from distal to proximal vessel done using an automated pull-back device. Advances in image quality and improved tissue penetration has allowed the use of epivascular structures in addition to side branches as reference points for tomographic and axial orientation (Fig. 9.1).
The media consists of multiple layers of smooth muscle cells arranged helically and circumferentially around the lumen of the artery, woven through a matrix of elastic fibers and collagen. The coronary arteries are less elastic than other similar sized arteries and thus resemble a transition towards more muscular peripheral arteries. The normal medial thickness ranges from 125–350 *gmm (mean 200 *gmm) although in the presence of plaque, the medial thickness may be considerably thinner, approximately 100 *gmm,2 or completely involuted and replaced by plaque in severe disease. The external elastic lamina encircles the medial layer. It is composed of elastin but is thinner and more fenestrated than the internal lamina, and is not more than 20 *gmm in thickness.
The appearance of the three-layered appearance by intravascular ultrasound occurs due to the acoustic impedance between adjacent structures. For example, the lumen and intima are usually well-delineated due to the large acoustic impedance between fluid and tissue. The three-layered appearance of the vessel wall is dependent on the intima being of sufficient size to be identified with the resolution of the current generation of ultrasound transducers and in the presence of a sufficient acoustic interface between media and adventitia.3 At a frequency of 30 MHz, the threshold of intimal thickening required to resolve a definite intimal layer is approximately 160 *gmm. Previous work has shown that there is a progressive increase in the thickness of the intimal layer with increasing age.4 In an autopsy study done to evaluate the relationship between ultrasound images and tissue histology in 16 intact hearts from subjects ranging in age from 13 to 55 years with no history of coronary artery disease, segments with a three-layered appearance had a significantly greater intimal thickness (243 *b+ 105 *gmm) than non-layered segments (112 *b+ 55 *gmm) with a threshold between the two of 178 *gmm.4 As this threshold is crossed in males over the age of 30 years, it is apparent that histologically normal arteries will only have a two-layered appearance in the rare patients younger than this undergoing ultrasound examination.
The media appears as a thin middle layer by intravascular ultrasound and is often referred to as the sonolucent zone as it is less echodense than the intima or adventitia due to a lesser collagen content. Intravascular ultrasound imaging was performed in vitro on 6 histologically normal and 104 minimally diseased arteries in patients aged 13 to 83 years to test the hypothesis that normal coronary arteries produce a three-layer image that corresponds to the histologic layers of intima, media and adventitia.5 The results showed a very strong correlation between area of the echolucent ultrasound layer with the media and the inner echogenic layer with intimal area. In addition, a three-layered appearance was consistently seen when the internal elastic membrane was present with or without intimal hyperplasia. If the internal elastic membrane was absent, a three-layer appearance was still seen if the collagen content of the media was low. However, a two-layer appearance was observed when there was absence of the internal elastic membrane as well as a high collagen content of the media.5 In addition, the relative composition of the intimal layer also determined the ability to discriminate the three vessel wall layers. Thus, over a given coronary artery segment, the three-layered appearance may alternate with a two-layered appearance due to the relative content of elastin and collagen. However, for the purposes of quantitation, the acoustic impedance between the combination of adventitia and external elastic lamina with the intima permits accurate measurement of plaque and vessel area. In the left main stem and at the proximal part of the right coronary artery, the three-layered appearance may be lost due to the increase in elastin content in transition from the highly elastic aortic root.
ATHEROSCLEROTIC DISEASE
Intravascular ultrasound is the current imaging technology of choice for studying the morphology of atherosclerotic plaque in vivo and continues to have an important diagnostic role (Table 9.1). Early studies used a large 8 F catheter to correlate ultrasound appearances with histological findings in arteries collected at the time of autopsy.6 The arteries studied were a combination of elastic, transitional (musculo-elastic) and muscular types with respect to media/adventitia appearance. The results confirmed that a highly accurate measurement of luminal area was achieved comparing ultrasound to direct measurement of perfused isolated arteries. A distinct interface between media and adventitia was obtained only where there was a significant difference in the acoustic qualities of the two layers (namely, loose collagen in the adventitia of elastic arteries or where there was a minimal smooth muscle cell component in the adventitia of transitional/muscular arteries). The interface between plaque and media was only apparent where there was a dense internal elastic lamina or a significant amount of necrotic material in the plaque.
| Detection of angiographically silent disease |
Estimation of functional stenosis severity
The earliest accumulations of atherosclerotic plaque consist of crescentic intimal thickening of an intermediate echointensity. A common site for initial and increased plaque accumulation occurs at branch points and bifurcations due to the shear stress effect of blood flow. Transplant vasculopathy is a good model for the early development of coronary artery disease as these patients undergo ultrasound studies at angiographic follow-up early after transplantation. In one study, intravascular ultrasound was used to study epicardial arteries in 25 recently transplanted hearts from young donors (mean age 28 years).7 In this unique study group, all donors aged under 25 years had a homogeneous non-layered vessel wall. Another group of donors of mean age 32 years manifested a three-layered appearance. In five hearts, significant eccentric intimal thickening >500 *gmm was shown in donors with risk factors for coronary disease, implying early coronary disease in the presence of angiographically normal arteries. Subsequent work by the same group in a larger group of transplant recipients over a period of long-term follow-up has shown that all 60 hearts had variable degree of concentric intimal thickening after one year, 42 of whom had normal coronary arteries at angiography.8
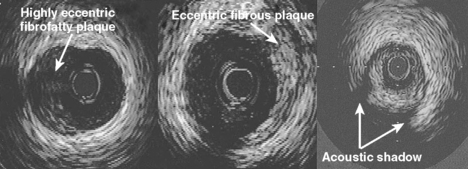
With a greater accumulation of plaque, there is a greater complexity to the plaque which may be differentiated by broad ultrasound criteria. A fibrous plaque has an echodensity intermediate between less echodense media or lipid and more echodense calcification. Thus by comparing the brightness of the tissue in question to that of the adventitia, a relative grading of the plaque may be obtained. Such fibrous plaques with similar brightness to adventitia may then be described as hard or soft (with respect to the grey scale) depending on the presence or absence of shadowing behind the plaque (Fig. 9.2). Fatty plaques are significantly more echolucent and when large may be appreciated as lipid pools. However, because shadowing in relation to a fibrous plaque may be misinterpreted as a lipid collection, there is a tendency to broadly classify plaques containing lipid as fibrofatty in nature.
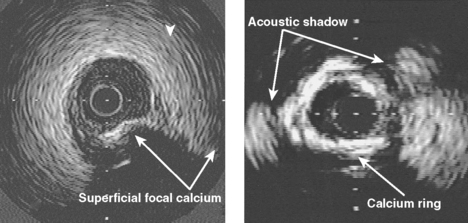
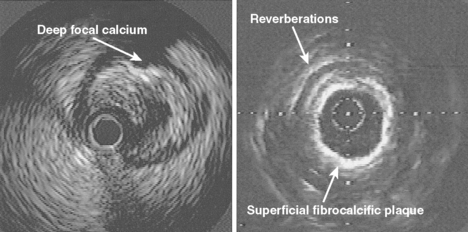
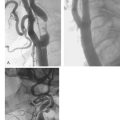
Calcification is commonly seen by intravascular ultrasound as a bright echo with shadowing behind often associated with reverberation artifact in the area of the shadow due to oscillation of the ultrasound beam between calcium and transducer (Fig. 9.3). Calcium may be seen in relatively small plaque accumulations indicating the age of the plaque or the site of previous plaque rupture and repair. In one series of patients undergoing balloon angioplasty, 82% of arterial segments exhibited small areas of calcium which were visible in only 8% of angiograms at the lesion site or in 155 of more proximal segments by fluoroscopy.9 In the GUIDE (Guidance by Ultrasound Imaging for Decision Endpoints) trial (Phase I), 70% of target lesions had areas of calcium by ultrasound, compared to 40% of angiograms.10 Calcification may be graded from absent (0) to severe (3+) by the extent of the arc subtended by a fibrocalcific matrix.11 In general, at least 180 degrees of calcium is required to achieve a mass of calcium identifiable by angiography (74% identification by fluoroscopy) increasing to 86% of cases identified if more than two quadrants or calcium length *vm 6 mm is present.12
By definition, plaque calcification results in shadowing of deeper structures, obscuring evaluation of underlying arterial wall components. Furthermore, shadowing may occur without any obvious calcification as the calcium may be out of plane and not visualised unless hit by the ultrasound beam in a perpendicular fashion. The calcium may be distributed in the plaque in several ways: as a deep deposit in an arc at the intima-media border, in a superficial rim at the luminal surface, or as a concretion within a fibrous plaque (Fig. 9.4). In one study of 110 patients, superficial calcium was present in 50%, deep calcium in 15% and both in 35%.12 On occasion, the fibrous cap may be intensely echoreflective with shadowing extending to and around the periphery of the artery suggesting a more uniform distribution of calcium throughout the wall. Dense fibrotic plaques may be sufficiently underpenetrated by the ultrasound beam to cause significant shadowing, and such plaques are usually referred to as fibrocalcific.
The presence of thrombus is often more difficult to establish as it is frequently mistaken for soft plaque.13 Thrombus often appears as a scintillating mass with a lobular edge and classically moves in an undulating manner separate from the movement of the artery. Incomplete microchannels are sometimes identified but many intra-coronary thrombi may be very difficult to differentiate as they may be relatively small and not very separate from the arterial wall. Luminal blood is characterised by a continually mobile speckled pattern. In contrast to conventional echocardiography, the gain of the intravascular ultrasound console should be set to allow its identification separate from possible thrombus. Where the flow of blood is significantly impaired, the backscatter may be sufficiently intense to mimic thrombus or soft plaque. It may be differentiated from the latter by injection of saline converting the lumen briefly to greater echolucency. Advanced signal analysis of the radiofrequency pattern may help to provide a rational and objective criteria to differentiate thrombus from fibrofatty plaque.14
Intravascular ultrasound has confirmed the observations first made by Glagov et al. that with increasing plaque accumulation, there is a remodelling process whereby the vessel expands to accommodate the plaque load.15 The extent of remodelling in a given artery may be highly variable with segments of positive remodelling, no change, and negative remodelling (shrinkage). Even in angiographically normal segments, the average plaque burden in the normal reference segment may comprise 40% of the total vessel cross-sectional area. The inability of angiography to detect this occult disease is due to the presence of positive arterial remodelling and the diffuse nature of disease throughout the entire vessel length in many patients. It has been suggested that discrete coronary artery lesions only become apparent angiographically when the accumulation of plaque above a threshold of 40% of vessel area, for example, overcomes the ability of the vessel to expand any further.16–18
CORONARY INTERVENTION
Intravascular ultrasound has provided an invaluable insight into the characteristics of atherosclerotic plaque with an accurate online means for measuring the dimensions of the index artery prior to and after intervention. Furthermore, the reduction in the diameter of ultrasound catheters (to less than 1 mm in diameter) has allowed an assessment to be made prior to intervention as well as describing vessel morphology after the procedure. Additionally, it may provide additional prognostic information regarding the likelihood of acute and subacute vessel closure and longer-term restenosis. In the context of stenting, an improved clinical outcome may be achieved with ultrasound guidance (Table 9.2).
| PRE INTERVENTION |
Pre-intervention imaging
With the development of catheters as small as 2.6 F, intravascular ultrasound may be used to assess lesions prior to intervention and direct appropriate therapy. In general, there are two major determinants of device selection – plaque load or burden, and the extent and severity of calcification. With an extensive plaque load, a debulking strategy has become common in interventional practice. Directional coronary atherectomy has been used to debulk lesions although it is only generally successful when the lesion is free of superficial calcium. In the latter case, high speed rotational atherectomy may be used to ablate superficial calcium in lesions with a large plaque load, either as a stand alone procedure or in preparation for further treatment.19
In one study of pre-intervention imaging, 313 target lesions underwent intravascular ultrasound resulting in a change of therapy in 40% of cases.20 This comprised 6% of patients who underwent therapy where none had been planned due to a significant disparity in the assessment of lesion severity between ultrasound and angiography; 7% had revascularisation deferred for the same reason, and a further 13% had a change in revascularisation strategy or selection including referral for bypass surgery in 1% due to demonstration of unsuspected significant unprotected left main involvement.20
Accurate balloon sizing is central to achieving the largest acute lumen gain after balloon angioplasty and with ultrasound, it is possible to demonstrate arterial remodelling and vessel expansion adjacent to diseased segments and balloon size accordingly. A strong argument for the use of intravascular ultrasound (IVUS) in balloon angioplasty is that it may allow a ‘stent-like’ result to be achieved by optimising the acute lumen gain from balloon dilatation alone. To investigate the safety of such an approach in selected cases, the CLOUT (Clinical Outcomes with Ultrasound Trial) pilot trial has been reported.21 This is a multicentre investigation of the use of IVUS in balloon ‘over-sizing’ to achieve an improved acute outcome. The authors hypothesised that adaptive remodelling at the lesion site and in adjacent segments would accommodate this approach. Angiographically guided balloon angioplasty was performed in 104 lesions in 102 patients (types B1 and B2 lesions). IVUS was then performed to measure the proximal and distal reference segments, and if remodelling was present, a larger balloon size was calculated from whichever of the proximal or distal reference segments was smallest from the formula: balloon size = (mean lumen diameter + mean vessel diameter)/2. Quarter-size balloons were then used to achieve this with increased balloon sizes ranging from 0.25 to 1.25 mm. The mean reference segment plaque area was 51 *b+ 15%. A total of 73% of patients had balloon up-sizing, increasing the balloon:artery ratio from 1.12 *b+ 0.15 to 1.30 *b+ 0.17. As a result, minimum lumen diameter increased from 1.95 *b+ 0.49 to 2.21 *b+ 0.47 mm (percent diameter stenosis decreased from 28 *b+ 15% to 18 *b+ 14%). Despite this more aggressive balloon sizing strategy, the angiographic dissection rate was unchanged (37% vs 40%) with no increase in major complication rate (1.9%). Whether or not this more aggressive approach impacts on clinical outcome is awaited.
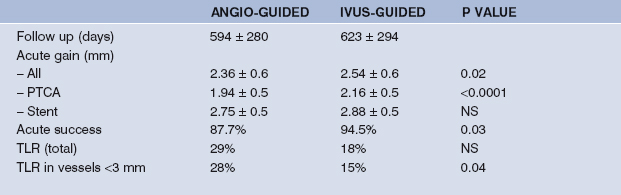
One study performed to see whether IVUS guidance of balloon angioplasty leads to better outcome is the SIPS (Strategy of IVUS-guided PTCA and Stenting) trial.22 Patients enrolled in the study were randomised to either IVUS-guidance using the ORACLE FOCUS™ balloon – a combined balloon catheter and ultrasound transducer – or angiographic guidance of standard practice of balloon dilatation. The aim of the study was to achieve >65% reference minimal lumen area. A total of 355 lesions in 269 patients were enrolled with a 50% stent rate in both arms of the study. IVUS-guided angioplasty resulted in an increased MLD with a reduction in percent diameter stenosis (Table 9.3). A similar acute gain was also seen in patients receiving stents with IVUS-guidance, perhaps reflecting smaller vessels in the IVUS-guided stent group. Procedural success was higher in the IVUS-guided group. No significant difference in duration, contrast use, maximum inflation pressure or equipment used was seen although there was a trend towards a lower number of balloon catheters used in the IVUS-guided arm, 1.22 *b+ 0.94 vs 1.39 *b+ 1.03. With respect to acute outcome, there were no deaths and no difference in myocardial infarction (MI), but there was an increase in target vessel revascularisation in the standard angioplasty group, 4.6% vs 0.8%, resulting in a major adverse cardiac event rate (MACE) of 6.1% vs 0.8% (p < 0.05), compared with the IVUS-guided group. Of interest, those undergoing IVUS-guided PTCA had the lowest target segment revascularisation over the follow-up period of almost two years. This reflected the fact that stenting was conditional and many patients received a stent because of a complication at the time of intervention. Furthermore, with IVUS-guidance, larger balloons were used due to the recognition of remodelling in diseased segments, accounting for stenting in smaller vessels in this group. Once stented, no difference was seen with respect to major events however. The long-term clinical results of this trial are awaited.
Post intervention imaging
Balloon angioplasty
Initial in vitro validation studies were done to correlate the appearance of diseased arteries at ultrasound post balloon dilatation with histology.23 The consistent finding following balloon dilatation was tearing of the plaque with separation of the ends of the tear and an increase in lumen cross-sectional area, with some stretching of the less diseased wall. In this study, the lumen area by ultrasound and at section were similar (R = 0.88).23 Dissection of the plaque (separation of intima from media) was a common finding which appeared as an increase in the sonolucent area corresponding to media. One feature described was the presence of arterial flaps with protrusion of plaque into the lumen, less frequently seen in vivo due to blood flow.
The presence of calcium in a coronary lesion determines the response of the artery to balloon dilatation. In one study of patients after balloon angioplasty of peripheral and coronary vessels, intralesional calcium and the relative size of dissection for each lesion was determined.24 A total of 76% of patients had significant dissection/plaque fracture after angioplasty. In 71% of these patients, significant localised calcium deposits were identified within the plaque, and the vast majority of dissections (87%) were adjacent to the calcific portion of the wall. Comparing the relative size of dissections with respect to the neoluminal area, dissections in calcified areas were larger than those in lesions without calcium, 28% vs 11%, respectively. It was concluded that localised calcium had a direct role in promoting dissection by increasing shear stress within the plaque at the junction between tissue types with differing elastic properties.25 Given these findings, it may be that deep calcium deposits protect against deep medial injury despite a large plaque fracture by deflecting the pressure axially. Further characterisation of lesion morphology with newer ultrasound catheters is required to address these issues in a clinical context.
An important use for intravascular ultrasound in interventional procedures is that it can accurately measure lumen and vessel area both at the lesion site and at the reference segment allowing the operator to size angioplasty balloons more exactly. In a key study of 223 coronary vessels treated by a Palmaz–Schatz stent, directional atherectomy or laser balloon angioplasty, 83% of patients underwent follow-up angiography six months after treatment.25 The traditional dichotomous definition of restenosis (*vm 50% diameter stenosis) was used along with a cumulative graphical method. Although the restenosis rates were 19%, 31% and 50% for stents, directional atherectomy, and laser balloon angioplasty respectively, the late lumen loss was equivalent across the groups. This indicated that the major procedural determinant on restenosis was the acute lumen gain (2.6, 2.2, and 2.0 mm in the three groups) leading to the ‘Bigger is Better’ hypothesis.25
Stent deployment
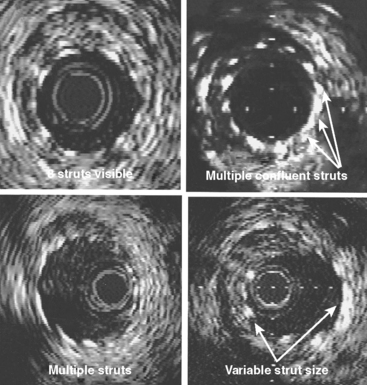
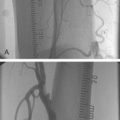
The MUSIC (Multicentre Ultrasound guidance of Stents In Coronaries) trial was designed to examine the additional value of IVUS in determining optimal stent deployment with Aspirin alone in vessels >3.0 mm (Fig. 9.5). Using the initial criteria of complete apposition (symmetry >0.7) and complete expansion (minimum stent area [MSA] >90% average of proximal/distal segments or MSA >100% of lowest reference segment or MSA >90% proximal segment, and subsequently revised to 80%, 90%, 80% respectively if MSA >9 mm2), a subacute thrombosis rate of 1.3% was reported at follow-up of mean 198 days.26 The need for bypass surgery was 0.6% and repeat angioplasty was 4.5%. Of interest, even using the revised IVUS criteria, only 80% of patients achieved this. A six-month angiographic restenosis rate of 8.3% was reported (9.3% if the stent thrombosis cases were included) which is a remarkable result suggesting a major clinical benefit form IVUS-guided stent deployment and this data largely led to the design of the OPTICUS trial.
In OPTICUS (OPTimal Intra-Coronary Ultrasound in Stenting), a multicentre, randomised trial of 550 patients with angiographic follow-up has been recently reported.27 The primary endpoints were defined as binary restenosis and angiographic MLD at six months follow-up. The secondary endpoints were 6- and 12-month clinical follow-up and the economics of ultrasound-guided stenting. Again, patients were randomised to angiography- or IVUS-guided stent deployment of either Palmaz–Schatz or NIR stents (thus giving OPTICUS the angiography arm for comparison that MUSIC didn’t have). Lesions were more challenging than described in the Benestent or Stress trials with 62% AHA/ACC type B2 and 15% type C lesions. The MUSIC expansion criteria were achieved in 64% of patients. Initial reports from the study have confirmed a reduction in the need for repeat in-hospital angioplasty although at follow-up despite an improved minimal luminal diameter (MLD) post-stent (and an impressive increase in angiography-guided post-stent MLD), clinical and angiographic outcome was no different comparing both groups.27
To evaluate objectively whether ultrasound-guided stent deployment results in an additional clinical benefit over angiography alone, the CRUISE (Can Routine Ultrasound Influence Stent Expansion?) substudy of STARS (STent Anti-thrombotic Regimen Study) has now been reported.28 Patients undergoing ultrasound-guided stent deployment in nine centres were compared with seven centres in which stenting was guided by angiography alone followed by blinded IVUS assessment (IVUS-documentary). A total of 499 patients were followed up from an initial 525 patients with larger balloon sizing (3.88 *b+ 0.51 vs 3.69 *b+ 0.59 mm, p <0.001) and greater dilatation pressure (18.0 *b+ 2.6 vs 16.6 *b+ 3.0 atmospheres, p <0.001) used in the IVUS-guided and IVUS-documentary groups, respectively. A total of 36% of patients in the IVUS-guided group had a change in deployment strategy based on the ultrasound images. This was associated with superior stent expansion (MSA) in the IVUS-guided group, 7.78 *b+ 1.72 mm2 vs 7.06 *b+ 2.13 mm2 (p < 0.001). At nine-month follow-up, a 44% reduction in the clinical end-point of target vessel revascularisation (TVR) was demonstrated (8.5% vs 15.3%, p < 0.05) (Table 9.4).28 This was an encouraging clinical outcome although the apparent benefits of IVUS guidance could have been accentuated by other differences in treatment between the different centres, given the operators’ individual discretion regarding optimisation of final stent deployment. Also, a clinical restenosis rate (TVR) rather than angiographic restenosis rate was selected as a primary endpoint in CRUISE and yet a significantly greater number of multivessel disease patients were in the IVUS-documentary group (44%) compared with the IVUS-guided group (27%), although this was not an independent predictor of TVR by multivariate analysis.28
The AVID (Angiography Versus Intravascular ultrasounD) trial is a multicentre comparison of IVUS-guided stenting with angiography-guided stenting, similar in concept to the CRUISE study. The initial results were reported in 1999.29 A total of 759 patients undergoing elective single or multiple stent placement in native vessels >2.5 mm or saphenous vein grafts in 24 centres were included. Patients were randomised after optimal angiography-guided stent deployment (<10% residual stenosis). In the angiography-guided group, documentary IVUS was performed (with un-blinding only if significant dissection was noted [2.6%]). In the IVUS-guided group, larger balloons or additional stents were required in 43% of cases to achieve the criteria of full stent apposition and MSA >90% of the distal reference area. As a result, the final MSA was greater in the IVUS-guided group (7.54 *b+ 2.86 mm2 vs 6.94 *b+ 2.46 mm2, p< 0.01). After 12 months follow-up, the primary clinical endpoint of target lesion revascularisation (TLR) was 8.4% in the IVUS-guided group versus 12.4% in the angiography-guided group (p = 0.08). When protocol violations such as the inclusion of vessels smaller than 2.5 mm were excluded, the difference achieved statistical significance, 4.9% vs 10.8% (p = 0.02). The benefit of IVUS-guidance was particularly evident in three subgroups – saphenous vein grafts (TLR 5.7% vs 20.4%, p = 0.05), vessels with a diameter stenosis greater than 70% (TLR 3.5% vs 14.9%, p = 0.003), and vessels with a distal reference diameter less than 3.25 mm (TLR 7.9% vs 14.6%, p = 0.04).
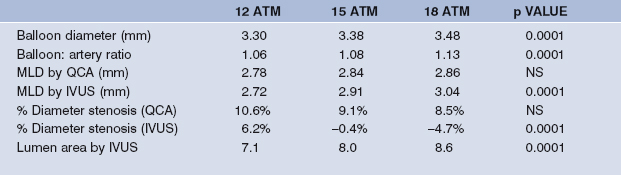
Although the availability of recent generation balloon expandable stents has diminished the practice of high pressure (>14 atmospheres) dilatation, the OSTI (Optimal StenT Implantation) trial was completed in North America. This trial was designed to study the relationship between balloon pressure and stent deployment by IVUS. To date, 89 Palmaz–Schatz stents have been implanted in 79 lesions in 76 patients, post-dilating at 12, 15 and 18 atmospheres.30 Palmaz–Schatz stent dimensions increase with increasing post-dilatation pressure which was described by IVUS but not quantitative angiography. Of interest, routinely used criteria for stent expansion, e.g. MUSIC criteria (37% at 12 bar, 61% at 15 bar, 74% at 18 bar) may still not be met despite 18 atmospheres pressure and a balloon: artery ration >1.1 (Table 9.5). In the second OSTI study, focal balloons achieved maximal stent expansion within 0.2 mm of the external elastic lamina resulting in a clinical restenosis rate (target lesion revascularisation) of 8.3%.31
High-speed rotational atherectomy
Rotational atherectomy uses a rotating diamond-coated burr to abrade atherosclerotic plaque at speeds as high as 180 000 rpm. The technique works through the method of differential cutting whereby the burr selectively abrades harder tissue and is deflected away from normal vessel wall thus removing superficial calcium and dense fibrous plaque through micro-embolisation, pushing softer plaque away from the cutting path of the device. The appearance of vessels undergoing rotational atherectomy as a debulking strategy has been described.32 In this study, 28 patients (22 calcified plaques, a third of which were circumferential) underwent ultrasound imaging after the procedure (with 71% having adjunct balloon angioplasty). Following rotablation, a distinct, circular intima-lumen interface was achieved with the lumen size 20% larger than the largest burr used. Deviations from a circular geometry occurred only in areas of soft plaque or superficial tissue disruption of calcified plaque. This study confirmed that there was no significant damage to the media and no dissections were caused by the procedure. A residual plaque load of 54% was reported indicating that athero-ablation using a burr strategy of 70–80% of the reference vessel lumen by angiography still resulted in a significant residual plaque even with adjunctive balloon dilatation in the majority of patients.32
A subsequent study of sequential ultrasound imaging before and after rotational atherectomy did confirm dissection planes in 26% of cases following rotablation increasing to 76% of cases after adjunct balloon dilatation with spread of the dissection plane to areas not only with the calcified plaque but also adjacent to the plaque, in part contributing to the expansion of the lumen area.33 There was a reduction in plaque area from 15.7 *b+ 4.1 to 13.0 *b+ 4.7 mm2 after initial rotablation, but still a residual percent cross-sectional narrowing of 74% indicating the need for adjunct balloon angioplasty to achieve an adequate final lumen area. In this study, the arc of calcium decreased significantly with full thickness calcium removal in some patients. Even without a measurable decrease in calcium arc, significant calcium ablation still occurred as evidenced by an increase in lumen area, uncovering of deeper calcium deposits, and the uncovering of deeper adventitial structures not seen pre-rotational atherectomy. In everyday practice, pre-interventional ultrasound scanning can direct athero-ablation appropriately. Superficial calcium not apparent by angiography is amenable to successful rotablation if the calcium arc is >180 degrees and extends to more than half of the lesion length. This preliminary debulking may then be supplemented by balloon angioplasty or intra-coronary stenting.
One particular complementary role of IVUS and rotablation may be in the treatment of in-stent restenosis. Although several small studies exist, it is only within the last two years that a randomised controlled trial has been conceived – ARTIST (Angioplasty versus Rotablation for Treatment of Intra-STent Stenosis/Occlusion).34 This is a multicentre trial randomising patients with intra-stent restenosis or occlusion to balloon angioplasty or rotablation in restenosed or occluded stents in native vessels. Important exclusion criteria are coil stents, stents deployed distal to or at bend points >45 degrees, and stents not fully expanded by angiography or pre-procedure ultrasound. The primary endpoints of the study are acute success with quantitative angiographic assessment after the procedure (diameter stenosis <30% visually without additional stenting is defined a procedural success) and at six months follow-up. Preliminary data has reported an angiographic restenosis rate of 42% with a clinical restenosis rate of 36%.34 The acute and long-term outcome in 100 patients was recently reported and confirmed slow-flow in only 3% with only 2% sustaining an enzyme rise of >3 *bx creatine-kinase.35 With quantitative IVUS, 77% of acute gain occurred through rotablation and 23% through adjunctive balloon dilatation. At a mean of 13 months follow-up, repeat in-stent restenosis occurred in 28% with a target vessel revascularisation (TVR) of 26%.
1 Yock PG, Fitzgerald PJ, Popp RL. Intravascular ultrasound. Sci Am. 1995;2:68-77.
2 Waller BF. The eccentric coronary atherosclerotic plaque: morphologic observations and clinical relevance. Clin Cardiol. 1989;12:14.
3 Picano E, Landini L, Lattanzi F, et al. Time domain echo pattern evaluations from normal and atherosclerotic arterial walls: a study in vitro. Circulation. 1988;3:654.
4 Fitzgerald PJ, St. Goar FG, Connolly RJ, et al. Intravascular ultrasound imaging of coronary arteries. Is three layers the norm? Circulation. 1992;86:154-158.
5 Maheswaran B, Leung CY, Gutfinger DE, et al. Intravascular ultrasound appearance of normal and mildly diseased coronary arteries — correlation with histologic specimens. Am Heart J. 1995;130:976-986.
6 Nishimura RA, Edwards WD, Warnes CA, et al. Intravascular ultrasound imaging: in vitro validation and pathologic correlation. J Am Coll Cardiol. 1990;16:145-154.
7 St. Goar FG, Pinto FJ, Alderman EL, et al. Intra-coronary ultrasound in cardiac transplant recipients: in vivo evidence of ‘angiographically silent’ intimal thickening. Circulation. 1992;85:979-987.
8 St. Goar FG, Pinto FJ, Alderman EL, et al. Detection of coronary atherosclerosis in young adult hearts using intravascular ultrasound. Circulation. 1992;86:756-763.
9 Tobis JM, Mallery J, Mahon D, et al. Intravascular ultrasound imaging of human coronary arteries in vivo: analysis of tissue characterization with comparison to in vitro histological specimens. Circulation. 1991;83:319-326.
10 GUIDE trial investigators. IVUS-determined predictors of restenosis in PTCA and DCA: final report from the GUIDE trial, Phase II. J Am Coll Cardiol. 1996;27:156A. (Abstract).
11 Farb A, Virmani R, Atkinson JB, Kolodgie FD. Plaque morphology and pathologic changes in arteries from patients dying after coronary balloon angioplasty. J Am Coll Cardiol. 1990;16:1421-1429.
12 Mintz GS, Douek P, Pichard A, et al. Target lesion calcification in coronary artery disease: an intravascular ultrasound study. J Am Coll Cardiol. 1992;20:1149-1155.
13 Siegel RJ, Ariani M, Fishbein MC, et al. Histopathologic validation of angioscopy and intravascular ultrasound. Circulation. 1991;84:109-117.
14 Metz JA, Preuss P, Komiyama N, et al. Discrimination of soft plaque and thrombus based on radiofrequency analysis of intravascular ultrasound. J Am Coll Cardiol. 1996;27(Suppl. A):200A. (Abstract).
15 Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic arteries. N Engl J Med. 1987;316:1371-1375.
16 Kakuta T, Currier JW, Haudenschild CC, Ryan TJ, Faxon DP. Differences in compensatory vessel enlargement, not intimal formation, account for restenosis after angioplasty in the hypercholesterolemic rabbit model. Circulation. 1994;89:2809-2815.
17 Post MJ, Borst C, Kuntz RE. The relative importance of arterial remodeling compared with intimal hyperplasia in lumen renarrowing after balloon angioplasty. Circulation. 1994;89:2816-2821.
18 Currier JW, Faxon DP. Restenosis after percutaneous transluminal coronary angioplasty: have we been aiming at the wrong target? J Am Coll Cardiol. 1995;25:516-520.
19 Ellis SG, Popma JJ, Buchbinder M, et al. relation of clinical presentation, stenosis morphology, and operator technique to the procedural results of rotational atherectomy-facilitated angioplasty. Circulation. 1994;89:882-892.
20 Mintz GS, Pichard AD, Kovach JA, et al. Impact of pre-intervention intravascular ultrasound imaging on transcatheter treatment strategies in coronary artery disease. Am J Cardiol. 1994;73:423-430.
21 Stone GW, Linnemeier T, St. Goar FG, Mudra H, Sheehan H, Hodgson JMcB. Improved outcome of balloon angioplasty with intra-coronary ultrasound guidance — core lab angiographic and ultrasound results from the CLOUT study. J Am Coll Cardiol. 1996;27(Suppl. A):155A. (Abstract).
22 Frey AW, Dörfer K, Lange W, Hodgson JB. The impact of vessel size on long-term outcome after intra-coronary ultrasound guidance on provisional stenting. Circulation. 1998;98(Suppl. I):1494. (Abstract).
23 Tobis JM, Mallery JA, Gessert J, et al. Intravascular ultrasound cross-sectional arterial imaging before and after balloon angioplasty in vitro. Circulation. 1989;80:873-882.
24 Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty: an observational study using intravascular ultrasound. Circulation. 1992;86:64-70.
25 Kuntz RE, Safian RD, Levine MJ, Reis GJ, Diver DJ, Baim DS. Novel approach to the analysis of restenosis after the use of three new coronary devices. J Am Coll Cardiol. 1992;19:1493-1499.
26 de Jaegere P, Mudra H, Figulla H, et alfor the MUSIC Study Investigators. Intravascular ultrasound-guided optimized stent deployment. Immediate and 6 months clinical and angiographic results from the Multicenter Ultrasound Stenting in Coronaries Study (MUSIC study). Eur Heart J. 1998;19:1214-1223.
27 Mudra H, Macaya C, Zahn R, et al. Interim analysis of the Optimization with ICUS to reduce stent restenosis. Circulation. 1998;98(Suppl. I):1908. (Abstract).
28 Fitzgerald PJ, Oshima A, Hayase M, et al. Final results of the Can Routine Ultrasound Influence Stent Expansion (CRUISE) study? Circulation. 2000;102:523-530.
29 Russo RJ, Attubato MS, Davidson CJ, DeFranco AC, Fitzgerald PJ, Iaffaldano RA, et al. Angiography versus intravascular ultrasound-directed stent placement: final results from AVID. Circulation. 1999;100(Suppl. I):I-234. (Abstract).
30 Stone GW, St. Goar F, Fitzgerald PJ, et al. The optimal stent implantation trial — final core lab angiographic and ultrasound analysis. J Am Coll Cardiol. 1997;29(Suppl. A):369A. (Abstract).
31 Stone GW, Bailey S, Roberts D, et al. Long-term results following maximal stenting using ultrasound guided focal balloon stent overexpansion — the second Optimal Stent Implantation study. Circulation. 1998;98(Suppl. I):826. (Abstract).
32 Mintz GS, Potkin BN, Keren G, et al. Intravascular ultrasound evaluation of the effect of rotational atherectomy in obstructive atherosclerotic coronary artery disease. Circulation. 1992;86:1383-1393.
33 Kovach JA, Mintz GS, Pichard AD, et al. Sequential intravascular ultrasound characterization of the mechanisms of rotational atherectomy and adjunct balloon angioplasty. J Am Coll Cardiol. 1993;22:1024-1032.
34 Radke PW, Hoffmann R, Haager PK, Janssens U, vom Dahl J, Klues HG. Predictors of recurrent restenosis after rotational atherectomy of diffuse in-stent retenosis at 6 month angiographic follow-up: a serial intravascular ultrasound study. Circulation. 1998;98(Suppl. I):3772. (Abstract).
35 Sharma SK, Duvvuri S, Dangas G, et al. Rotational atherectomy for in-stent restenosis: acute and long-term results of the first 100 cases. J Am Coll Cardiol. 1998;32:1358-1365.