CHAPTER 11 Intravascular Approaches to the Treatment of Varicose Veins
Radiofrequency and Lasers
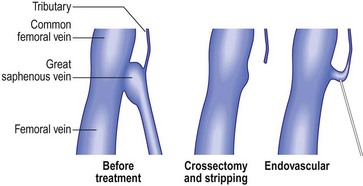
The first attempt at minimizing the extent of surgery for varicose vein disease was the application of ligation alone. It was thought that the mere ligation of the saphenofemoral junction (SFJ) without disturbance of the great saphenous vein (GSV) with invasive techniques such as stripping the entire GSV to the ankle would be effective. Unfortunately, this minimal surgical treatment was demonstrated to result in a high degree of recurrence (upward of 50% at 3 to 5 years) even when the ligation was accompanied by sclerotherapy or ambulatory phlebectomy of distal varicose veins.1–5 When these recurrences where evaluated, treatment failure was secondary to reanastomosis through hemodynamically significant perforator or anastomotic veins extending from the knee to the groin, which remained in place after ligation alone.6 Since ligation alone failed to provide acceptable degrees of improvement in abnormal venous hemodynamics, it was recommended that more invasive complete removal of the GSV from the SFJ to the knee after ligating the SFJ be performed. However, stripping typically required general anesthesia, with patients usually taking a week or more to get back to normal activities. So it appeared that, due to lack of effectiveness, the first attempts at reducing the extent of surgery by ligation alone failed to gain acceptance. Ironically, the continued necessity for stripping probably spurred the development of endovenous techniques as many patients would shudder at the thought of stripping. The race was on to develop a minimally invasive alternative using intravascular laser and RF devices to thermocoagulate endothelial cells and vein walls, producing specific destruction of the targeted vessel without the necessity of stripping or ligation.
Radiofrequency Closure
The first theories of endovenous ablation were based on the belief that specifically directing relatively omnidirectional RF energy into vein walls to cause their destruction was potentially safer, easier to engineer and more controllable than other mechanisms for doing so. Initial designs involved a mechanism by which RF current heated tissue by resistive (or ohmic) heating of a narrow rim (less than 1 mm) of tissue in direct contact with an electrode. Deeper tissue planes could be slowly heated by conduction from the small-volume region of heating, although heat was typically dissipated by conduction into surrounding normothermic tissue.7 By carefully regulating the degree of heating with microprocessor control, subtle gradations of either controlled collagen contraction or total thermocoagulation of the vein wall could be achieved.
The initial design was such that when the RF catheter was pulled back, a feedback-controlled loop regulated by readings from a thermocouple enabled the operator to heat a section of vein wall to a specified preset temperature. This was chosen for its relative safety since the temperature increase remained localized around the active electrode. This necessitated the maintenance of close, stable contact between the active electrode and the vessel wall without coagulum formation. It was believed by strictly limiting the temperature to 85°C, boiling, vaporization, and carbonization of the tissues could be avoided.8 It was also believed that heating the endothelial wall to 85°C resulted in heating the vein media to no more than 65°C, the minimal temperature at which collagen contracts.
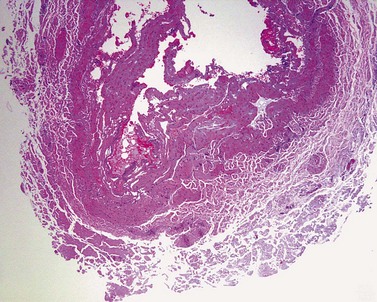
Ex-vivo studies by Reich-Schupke et al9 investigated histological changes following radiofrequency ablation at various powers and application times. When low power (5 W) and an application time up to 400 ohms was applied, histological changes were not uniform. Necrosis was limited to the endothelium in the majority of vein segments and rarely reached the media. This would most likely not result in complete vein shrinkage and occlusion. At 20 W and an application time up to an impedance of 400 ohms, histological changes included widespread necrosis of the initima and media and collagen bundle coagulation. The authors concluded that with increased power and application time, there was a more homogenous and extensive heating of the vein wall, which was thought to lead to a more successful outcome.
Vessel wall ablation using electrode-mediated RF is a self-limiting process. As coagulation of tissue occurs, there is a marked decrease in impedance that limits heat generation.10 Alternatively, if a clot builds up on the electrodes, blood is heated instead of tissue and there is a marked rise in impedance (resistance to RF). The RF generator can be programmed to rapidly shut down when impedance rises, thus assuring minimal heating of blood but efficient heating of the vein wall. The problem is that the electrodes must be manually debrided of coagulum, which requires the removal of the catheter, cleaning by the operator and then reinsertion – which is problematic during tumescent anesthesia.
The initial experience, dating back to 1998, demonstrated an efficacy equal to or better than that of ligation and stripping, with few, if any, adverse sequelae.11–23 Early experience directly comparing RF Closure with ligation and stripping procedures, even with RF performed under general anesthesia, noted equal efficacy with less pain, shorter ‘sick leave’, and faster return to normal activities.18
When performed by us, the procedure was entirely under local tumescent anesthesia, with over 90% of patients resuming normal activities 1 to 2 days postoperatively. Its main drawbacks were the high cost of single-use catheters and the necessity to withdraw the catheter manually at a speed of 2 to 3 cm per minute and frequent cleaning of coagulum on the electrodes, which made the procedure tedious at times. To speed up the procedure, Goldman12 recommended that only the most proximal 20 cm of the GSV be treated with RF and the remaining varicose GSV be treated with ambulatory phlebectomy, but this technique has not found wide acceptance. Goldman believes that the addition of ambulatory phlebectomy minimizes the possibility of recurrence from distal perforators. Proebstle et al24 found that up to 30% of tributary veins do not resolve with laser ablation of the GSV alone, thereby necessitating removal with ambulatory phlebectomy.
However, treatment of the GSV or its tributaries below the knee may not be entirely necessary, as others have shown that ligation and stripping procedures from the groin to the knee add little to the procedure’s efficacy.15,25 In addition, others have also demonstrated equal effectiveness with less than 2 years follow-up when only the proximal 30 to 40 cm of the GSV is treated without treating distal varicose tributaries.13–15,17,26
Weiss and Weiss17 evaluated patients treated with a percutaneous approach allowing access of the Closure catheter to treat the proximal GSV. Patients (mean age, 47.2 ± 12.6 years; 76% female) had symptomatic saphenous reflux with a saphenous vein diameter of 2 to 12 mm (mean, 7.4 mm). Most of the veins treated were above-knee great saphenous (73%), some entire great saphenous (21%), with the remaining including below-knee great saphenous, small saphenous, and accessory saphenous. Adjunctive procedures performed at the time of treatment were phlebectomy on more distal branches in 61% and high ligation in 21%, but the adjunctive procedures did not affect outcome.
Vein occlusion at 1 week was documented by duplex ultrasound in 300 out of 308 legs, or a success rate of 97%. Occlusion persisted at 6 weeks in 95% and at 6 months in 92%. In this report, if the saphenous vein was closed at 6 months it was noted by duplex ultrasound to remain closed to 12 months and beyond. Subsequent follow-up for up to a decade by duplex ultrasound indicates that any vein noted to have been eliminated at 12 months by RF will never recur. Typically when the GSV is treated there is closure or elimination of major tributaries at the SFJ except for the superficial or superior epigastric vein, which, intentionally not treated, continues to empty superiorly into the common femoral vein. We believe that there is a high margin of safety by maintaining flow through this tributary. The high flow rate appears to diminish the possibility of extension of any thrombus (in the unlikely event that this would occur) from the GSV and has the additional benefit of allowing normal venous flow from the lower abdominal wall into its proper drainage into the common femoral vein. By leaving the superior epigastric vein intact, thrombus in the GSV following this procedure has not been observed.13
Long-term efficacy with the RF ablation has been documented by Merchant et al27 investigating 1222 limbs (great saphenous, small saphenous, and accessory saphenous veins). Occlusion rates (evaluated via duplex ultrasound) of 96.8%, 89.2%, 87.1%, 88.2%, 83.5%, 84.9%, and 87.2% were found at 1 week, 6 months, 1 year, 2 years, 3 years, 4 years, and 5 years, respectively. Body mass index greater than 25 was associated with an increased incidence of nonocclusion, groin reflux, and recanalization. A pullback speed above 3 cm/minute at 85°C was more likely to result in nonocclusion and recanalization. In the study by Vasquez et al,28 factors associated with improved occlusion rates included increasing age, female sex, and volumes greater than 250 ml of tumescent anesthesia. The authors theorized that increased failure rates associated with male sex and younger age are secondary to variations in collagen and inflammation in these populations.
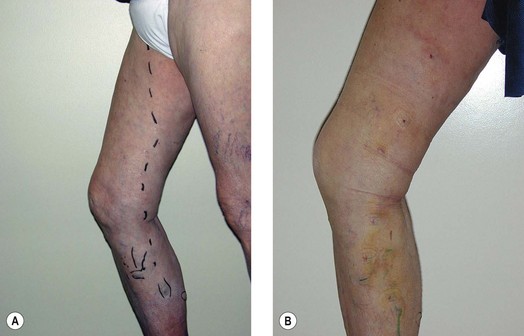
In a study by Goldman and Amiry,16 closure of the GSV with endoluminal RF thermal heating in combination with ambulatory phlebectomy was equally as effective as closure of the GSV as described above. The first 47 sequential, nonrandomized patients having an incompetent GSV from an incompetent SFJ and painful varicosities in 50 legs were treated with the VNUS Closure procedure. The varicose veins were marked with the patient standing and again with the patient lying down in the operative position with a venoscope (LLC, Lafayette, La.), as previously described (Fig. 11.1).12,29,30 After appropriate marking, the area surrounding the GSV and distal tributaries to be treated was infiltrated with 0.1% lidocaine tumescent anesthesia. The amount of tumescent fluid averaged 800 mL with a lidocaine dose of 8 mg/kg. The GSV was then accessed through a 2- to 3-mm incision in the medial midthigh, usually 20 cm inferior to the SFJ. The proximal portion of the GSV was then treated with VNUS Closure and the distal portion, including all varicose tributaries, was removed with a standard ambulatory phlebectomy technique.
Three separate papers detail a similar cohort of patients treated in multicenter studies encompassing from 16 to 31 clinics, 210 to 324 patients, and 6 to 12 month follow-up.13,14,31 The vein occlusion rate at 1 year examination was 91.6% from nine centers and 81.9% from fourteen centers. Forty-nine patients were followed at 2 years with duplex scans and showed an 89.8% closure rate. There was a 3% incidence of paresthesia which was decreased to 1.6% when treatment was confined to the thigh. Two limbs (0.8%) developed scarring from skin burns and three patients developed a deep vein thrombosis (DVT) with one embolism. The reason for the increase in adverse effects appears to be the use of general anesthesia without tumescent anesthesia by a majority of the surgeons.
Sybrandy and Wittens32 from Rotterdam reported one year follow-up of 26 patients treated with VNUS Closure. They reported five patients with postoperative paresthesia of the saphenous nerve and one with a cutaneous burn, for an overall complication rate of 23%. One patient (3.8%) had total recurrence of the GSV. One patient (3.8%) could not be treated due to a technical failure. Eight patients (30.8%) had closure of the GSV, but with persistent reflux of the SFJ. Thirteen patients (50%) had closure of both the GSV and SFJ. Overall, 88% of patients had a totally occluded GSV.
Another report describes two episodes of DVT in 29 patients treated with the RF Closure.33 Here, the surgeons treated the patient with a groin incision and passage of the catheter from the groin downward. The authors do not report the type of anesthesia used or the length of vein treated. It is presumed that patients were not ambulatory and were treated under general anesthesia.
In our experience using tumescent anesthesia in awake patients, two patients have developed focal numbness 4 cm in diameter on the lower medial leg. These resolved within 6 months. Since adopting the principles outlined above of tumescent anesthesia and moving the catheter rapidly from any points of sharp pain, no paresthesias have been noted. No skin injury or thrombus has been observed in any of our patients. Unfortunately, with both endoluminal RF and laser procedures, if patients are not ambulatory after the procedure and/or if tumescent anesthesia is not given, complications in the form of DVT, PE, or angiogenesis have been reported.34–36 Tumescent anesthesia or the placement of large volumes of dilute anesthesia in a perivascular position serves several purposes:
Contrary to the report by Hingorani et al34 we have never seen DVT in any of our patients treated with intravascular laser or RF. We believe that the reason for our lack of adverse sequelae is the use of tumescent anesthesia in awake patients with immediate ambulation and avoidance of occlusion of the superior epigastric vein. While we realize treating patients without general anesthesia is not standard practice for general or vascular surgeons,37 some vascular surgeons who perform tumescent anesthesia on awake patients with immediate ambulation have reported similar results with virtually no DVT. A DVT was noted in a female patient treated using tumescent anesthesia while awake but she weighed more than 350 pounds and did not ambulate after the endoluminal RF procedure.38,39
Salles-Cunha et al35 reported on the development of angiogenesis and fibrotic tissue along the course of the GSV treated with RF Closure. Contrary to this report, our experience with tumescent anesthesia utilized is a complete lack of detection of small vessel networks (angiogenesis) by duplex ultrasound. We believe that the reason for our lack in detecting small vessel networks is not from a lack of trying to see them, but from the minimization of inflammation that occurs with tumescent anesthesia placed in the perivascular space during either RF or laser endothelial ablation.40
ClosureFAST
In 2006, VNUS introduced the ClosureFAST catheter. This new device promised increased time efficiency and ablation of incompetent veins of any size. The 7F ClosureFAST catheter allows 7 cm segments of vein to be uniformly heated for 20 seconds at 120°C. The temperature is maintained by a radiofrequency generator through a feedback loop, and vein segments are treated serially41 with continuous pullback not needed.42 While treatment with the Closure system was limited to veins of less than 12 mm, no diameter restrictions are indicated with the ClosureFAST catheter.41,42 The manufacturer recommends the initial and most proximal 7 cm of great saphenous vein to be treated with two consecutive cycles, while the remaining vein segments may be treated with a single cycle. Each disposable catheter is US$795 (as of 11/2009). Proebstle et al41 treated, 252 GSVs with ClosureFAST and either adjuvant ambulatory plebectomy (in 71.6%) or foam sclerotherapy (in 13.9%). Mean treatment time (spanning the time between catheter insertion and removal) was 16.4 ± 8.2 minutes and 6.7 ± 1.7 treatment cycles. The linear endovenous energy density was 116.2 ± 11.6 J/cm for the initial 7 cm of GSV, and 68.2 ± 17.5 J/cm for the subsequent 7 cm. Patients were followed at 3 days, 3 weeks, 3 months, and 6 months post procedure. All patients had successful occlusion of their GSV. Via life-table analysis, occlusion rates were 99.6%. Seventy percent of patients experienced no postprocedural pain. No deep vein thrombosis or skin burns were seen. Side effects were infrequent with 3.2% paresthesias, 0.8% phlebitis, 1.6% hematomas, 2% hyperpigmentation, and ecchymoses in 6.4%. Mean patient down time was 1.0 ± 1.9 days. Finally, 99% of treated patients would recommend the ClosureFAST system to their friends.
Calcagno et al43 investigated the relationship of size to efficacy in 338 great and small saphenous veins following ClosureFAST treatment. Initial occlusion rates, evaluated between postoperative days 2 to 5, were not significant (94% in veins ≤ 12 mm and 96% in those > 12 mm). At 6 months, complete occlusion rates in veins ≤ 12 mm or > 12 mm were similar (98% and 100%, respectively). Interestingly, veins partially occluded in the immediate postoperative period developed complete occlusion at 6 months follow-up. Diameter did not affect the outcome for successful treatment of incompetent saphenous veins with ClosureFAST.
The Recovery Study by Almeida et al,44 compared 87 GSVs treated with either ClosureFAST or 980-nm diode endovenous laser. This small, short term follow-up study of only 1 month, demonstrated increased incidence of eccyhmoses, pain, phlebitis, and tenderness in the 980-nm laser group during the initial postoperative 2 weeks. These increased side effects were attributed to microperforations caused by the 980-nm diode. While quality of life and venous severity scores were more favorable in the initial 2 weeks in the ClosureFAST group, no difference was seen at 1 month follow-up. No comparisons of efficacy were provided in this short-term study.
Long-term studies are necessary to assess prolonged efficacy of the ClosureFAST system. Radiofrequency and 1320-nm Nd:YAG laser both stimulate collagen contraction, have negligible development of thrombi, and show decreased incidence of side effects, owing to lack of perforations of the vein wall.45 We feel a randomized, blinded trial comparing the efficacy and safety of these two technologies is justified.
Endoluminal Laser
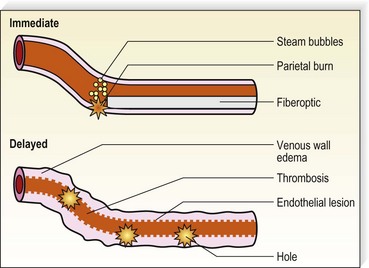
With only a slight delay following the development of RF endovenous ablation, lasers were applied to this application. Various lasers have been demonstrated to effectively close axial veins through thermal damage to endothelium with subsequent thrombosis and resorption of the damaged vein. Endoluminal laser closure has lower disposable costs since fiber optics are less expensive than more complex and more engineered RF fibers. Prior to the development of ClosureFAST, lasers were much quicker to perform, with the speed of pullback typically 10 to 20 cm/minute for 810–980-nm lasers and 6 cm/minute for the 1320-nm laser. By increasing the energy of the 1320 nm laser from 6 W to 10–12 W, the pullback rate can be increased to 2 mm/second, which doubles the speed of this endoluminal laser. Studies comparing the safety and efficacy of the higher energy/faster pullback 1320 nm laser are presently underway at the time of writing this chapter (11/2009). Endovenous laser treatment (EVLT) allows delivery of laser energy directly into the blood vessel lumen in order to produce endothelial and vein wall damage with subsequent fibrosis (Fig. 11.2). It is presumed that destruction of the GSV with laser is a function of thermal damage to the endothelium and/or vessel wall. The presumed target for lasers with wavelengths of 810, 940, 980, and 1064 nm is intravascular red blood cell absorption of laser energy. However, thermal damage with resorption of the GSV has also been seen in veins believed to be emptied of blood, although it is virtually impossible to completely eliminate hemoglobin as a chromophore by maneuvers such as leg elevation. While direct thermal effects on the vein wall probably occur, absorption by blood usually plays a role.
Some authors advocate emptying the vein of blood via manual compression, leg elevation, and tumescent anesthesia, immediately before the procedure.46,47 The presence of blood has several drawbacks including: decreased transmission of laser energy to the vein wall, potential of complete laser energy absorption by blood resulting in thrombosis and recanalization, and melting of the laser tip via carbonization. However, the presence of blood leads to steam bubble production, which may contribute as a secondary mechanism to EVLT efficacy.46,48
The extent of thermal injury to tissue is strongly dependent on the amount and duration of heat to which the tissue is exposed. Linear endovenous energy density (LEED) is defined as the total joules delivered divided by total centimeters of treated vein. While some authors recommend a LEED above 70 J/cm to reduce the incidence of recanalization and recurrence,49 others have shown no statistical difference in failure rates based on LEED.50 In addition, since each laser wavelength has a unique effect on the endothelial cells, water content of the blood and/or red blood cells, the total energy of one laser wavelength may not have the same efficacy as another wavelength and cannot be casually compared. Moritz and Henriques51 investigated the time–temperature response for tissue exposed to up to 70°C. They found that skin can withstand temperature rises for very short exposure times and that the response appears to be logarithmic as the exposure times become shorter. For example, an increase in body temperature to 58°C will produce cell destruction if the exposure is longer than 10 seconds. Tissues, however, can withstand temperatures up to 70°C if the duration of exposure is less than 1 second. Thus, any tissue injury from brief exposure to temperatures less than 50°C would be expected to be reversible.
One in vitro study model has predicted that thermal gas production by laser heating of blood in a 6-mm tube results in 6 mm of thermal damage.26,52 These authors used 810-, 940-, and 980-nm diode lasers with multiple 15-J, 1-second pulses to treat the GSV. A median of 80 pulses (range, 22–116) were applied along the treated vein every 5 to 7 mm. Histologic examination of excised veins demonstrated thermal damage along the entire treated vein with evidence of perforations at the point of laser application described as ‘explosive-like’ photo-disruption of the vein wall. This produced the homogeneous thrombotic occlusion of the vessel. This effect occurred only with blood-filled veins, not with saline-filled veins, attesting to the absorption of laser energy by hemoglobin (Hb) and HbO2 at these wavelengths. Since a 940-nm laser beam can only penetrate 0.3 mm in blood,53 the formation of steam bubbles may contribute to the mechanism of action. Multiple in vitro and animal models were performed to delineate the mechanism of action for vein closure. A consecutive series of events occur in endovenous ablation:
Carbonization seen histologically on the vein wall indicates a direct contact with the laser fiber. This interaction leads to fibrosis and is believed by some to be the primary mechanism of vein wall closure. Thrombus formation results from steam bubble production at the laser tip48,54 and is thought to contribute to vein closure. However, the volume of steam produced in EVLT is not enough to result in significant collagen damage. Of note in the experiments of Disselhoff et al48 is that continuous mode resulted in more carbonization, steam bubble formation, and higher and more persistent endovenous temperatures than was found with an intermittent (pulsed) mode. Interestingly, in a study by Der Kinderen55 no histological differences were seen between veins treated with continuous or intermittent modes.
Another possibility for the mechanism of action of EVLT is similar to that of RF closure – collagen contraction. Collagen has been noted to contract at about 50°C, while necrosis occurs at between 70°C and 100°C.56 Whether collagen contraction, thermal damage, or a combination of the two effects is responsible for destruction and resorption of the GSV is unknown and remains controversial.
A metanalysis by Van den Bos and colleagues57 compared occlusion rates following EVLT (all wavelengths included), RF ablation, ultrasound guide foam sclerotherapy (UGFS), and high ligation with stripping from 64 studies and 12,320 limbs. At 3 years, success rates were 94.5% for laser ablation, 84.2% for RF, 77.4% for UGFS, and 77.8% for high ligation with stripping. After 5 years, treatment success was seen in 95.4%, 79.9%, 73.5%, and 75.7% for laser ablation, RF ablation, UGFS, and high ligation and stripping, respectively. The authors found efficacy for high ligation and stripping, RF ablation, and UGFS were equal, but EVLT was more effective than the other three regimens. The incidence of deep venous thromboses were less than 1% in both the radiofrequency and laser ablation and less than 2% in conventional surgery. Risk factors for the development of deep venous thrombosis post EVLT include: general or epidural anesthesia, presence of coagulation disorder, or incorrect placement of the laser fiber tip.58
810-nm Diode laser
Initial reports have shown this technique with an 810-nm diode laser to have excellent short-term efficacy in the treatment of the incompetent GSV, with 96% or higher occlusion at 9 months and less than a 2% incidence of transient paresthesia.59–65 Two year success rates ranged from 93% to100%.49,63,65 Although most patients experience some degree of postoperative ecchymosis and discomfort, major side effects are rare. The lack of significant heating of perivenous tissues probably explains the low complication rate found and argues well for the continued lack of significant complications.
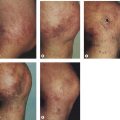
Our patients treated with EVLT with an 810-nm diode laser have shown an increase in post-treatment purpura and tenderness versus RF. Most of our patients do not return to complete functional normality for 2 to 3 days, as opposed to the 1 day ‘down-time’ with RF Closure of the GSV. Since the anesthetic and access techniques for the two procedures are identical, we believe that nonspecific perivascular thermal damage is the probable cause for this increased tenderness. In addition, recent studies suggest that pulsed 810-nm diode laser treatment with its increased risk for perforation of the vein, as opposed to continuous treatment which does not have intermittent vein perforations, may be responsible for the increase in symptoms with EVLT versus RF treatment (Fig. 11.3).
As mentioned above, deep venous thromboses occur in less than 1% of patients following endovenous laser ablation.57 Kabnick66 classified the development of ‘endovenous heat-induced thrombosus (EHIT) at the superficial-deep venous junction’ as follows:
Class 1 – thrombus in close proximity to the junction.
Class 2 – thrombus extending past the junction occluding less than 50% of the vein.
Class 3 – thrombus extending past the junction occluding more than 50% of the vein.
Class 4 – completely occluded deep venous thrombosis.
Isolated cases of pulmonary emboli have been reported in 810-nm, 940-nm, 980-nm, and 1320-nm endovenous lasers.58,63,66–70 Seromas and hematomas occur infrequently.68,71,72 Other rare events include the development of arteriovenous fistulas,73 which can form from thromboses via inflammation and neovascularization.74 The area most at risk for arteriovenous fistula development is the popliteal fossa, as the SSV lies in close proximity to the superficial sural artery.58 Tumescent anesthesia was not utilized in this patient. The authors proposed the use of tumescent anesthesia to aid in the separation of the veins from nearby arteries with a resultant decreased incidence of arteriovenous fistulas.73 Septic thromboplebitis from Staphlococcus aureus has been described after EVLT; in that case, the patient made a full recovery following extensive debridement and intravenous antibiotics.75 Retained guidewires have been described post EVLT, resulting in dyspnea and chest pain. Guidewires more than twice the length of the sheath are recommended to decrease the chance of this complication. Finally, an endovenous catheter sheath fragment was found in a patient’s heart septum, resulting in arrythmias.58 If the laser fiber tip fails to project outside of the protective sheath that is used to guide the optical fiber, the fiber can melt the plastic sheath and release a fragment into the systemic circulation with devastating consequences.58,76
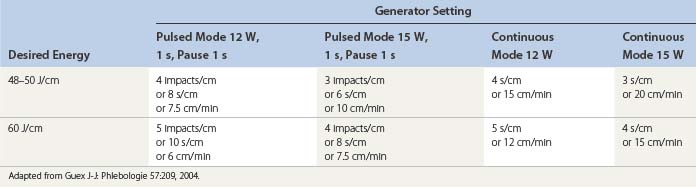
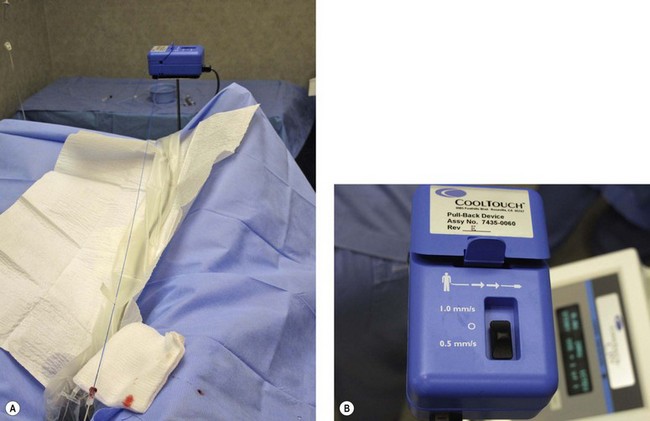
Continuous pullback speed of a fiberoptic is faster than electrode-based RF Closure pullback and may be simplified by the technique described by Guex77 using a cutaneous centimetric scale and an external electronic metronome when it is not included in the laser generator software. Power and speed are easily calculated from Table 11.1.26,78,79 Corcos79 has developed a manual technique of pulling the fiber back and forth within the vein in an attempt to limit vein wall perforation. Even with his expert technique in judging by feel when the vein has been sufficiently damaged to stop the back and forth movement, he still has found perforation in 2 of 24 treated veins and full-thickness injury in 22 of 24 veins. In our experience, trying to vary the fluence and treating with a continuous laser pullback versus pulsed pullback has not resulted in an elimination of vein perforation.56
Although most studies report 1 to 2 years of follow-up, we have been seeing patients back in follow-up now 4 years after the procedure with recurrent or new GSV. Sadick et al80 reported a 4-year follow-up evaluation of 94 limbs treated with the 810-nm diode laser (continuous mode, 14 W, 1–2 mm/second pullback) combined with ambulatory phlebectomy. The overall recurrence rates was 4.3%, with the majority of recurrences within the first 6 months. Our impression is that our patients undergoing 810-nm diode laser treatment with continuous pullback at 12 W, including ambulatory phlebectomy of distal veins, have a far higher recurrence rate than those treated with RF Closure at similar follow-up times. Our estimate is a 20% recurrence rate with 810-nm diode laser versus a 10% recurrence rate with RF Closure.
940-nm Diode laser
A longer wavelength such as 940-nm has been hypothesized to penetrate deeper into the vein wall with resulting increased efficacy. This is a false assumption since penetration is not due to length of the wave in nanometers but is a result the intensity of absorption by water and can be predicted based on water absorption data. A report of 280 patients with 350 treated limbs with 18-month follow-up demonstrated complete closure in 96%.81 Twenty vein segments were examined histologically. When veins were treated with 1 second duration pulses at 12 J, perforations were not present. When the fluence was increased to 15 J with 1.2- and 1.3-second pulses, microperforations did occur but were said to be self-sealing. The author suggests that his use of tumescent anesthesia as well as the above-mentioned laser parameters are responsible for the lack of significant perforations and enhanced efficacy.
An additional study of 109 treated GSV followed for 12 months with duplex scanning demonstrated a 10% recurrence rate.24 Another 5% exhibited incomplete proximal recanalization over 12 months. A 3 to 12 month evaluation of 33 patients who had an incompetent small saphenous vein showed no recanalization.82 An analysis of the 10% of nonoccluded/closed veins suggested that low laser fluences were the primary reason for recanalization.83 Proebstle et al84 investigated the 940-nm diode laser (continuous mode) at two settings – 15 W (5 mm/second pullback) and 30 W (3–4 mm/second) – in 263 great saphenous veins. At 12 months, occlusion rates were 82.7% and 97% in the 15 W and 30 W groups, respectively. No difference in side effects was found between the two cohorts.
980-nm Diode laser
A 980-nm laser has also been used to treat/close the GSV.68,85–87 Complete closure without adverse effects was seen in the 20 and 15 patients with 1- and 3-month follow-up, respectively. The authors experience with the 980-nm laser is similar to their experience with the 810- and 940-nm lasers. They speculate that the 980-nm wavelength would allow increased penetration into the vein wall with better results. However, no major differences between 810-, 940- and 980-nm lasers were found in an in vitro study.52
A comparison study in 60 limbs randomized to receive either 810-nm or 980-nm endoluminal laser treatment of the GSV revealed minimal differences between the two lasers immediately after the procedure and at 12-months follow-up.88 A short-term success rate of 97% was shown in a multicenter study of 1703 limbs treated with the 980-nm diode laser.68 A 97.1% 4-year occlusion rate was reported in 511 GSVs (continuous mode, pullback 3 mm/second, 10 W).87
1064-nm Nd:YAG laser
Three published studies have evaluated a 1064-nm Nd:YAG endoluminal laser.89–91 In one study,89 the lateral saphenous goat vein was used. Occlusion was more likely when fluence exceeded 84 J/cm2. More importantly, treated vessels were not perforated even with a fluence of 224 J/cm2. A diffusing fiber was also used to obtain circumferential damage.
The paper by Chang and Chua90 reported a clinical study using an endoluminal 1064-nm Nd:YAG laser in the treatment of incompetent GSVs in 151 men and women with 252 treated limbs. Unfortunately, the surgeons also ligated the SFJ, which did not allow for a determination of the efficacy of SFJ ablation. Spinal anesthesia was used. Laser power was set at 10 or 15 W, delivered with a pulse duration of 10 seconds, with manual retraction of the laser fiber at a rate of 10 seconds/cm. Skin overlying the treated vein was cooled with cold water. Unfortunately, this treatment resulted in superficial burns in 4.8% of patients, paresthesia in 36.5%, superficial phlebitis in 1.6%, and localized hematomas in 0.8%. This wavelength has not gained wide acceptance due to the high complication rate. This can be predicted by relatively poor absorption by water leading to greater penetration and non-specific heating.
1320-nm Nd:YAG laser
In an attempt to heat vein walls more directly by heating water in the vein walls (and to reduce the risks of superheating of hemoglobin and subsequent thrombus formation), Goldman and Weiss helped to develop a 1320-nm endoluminal laser. At this wavelength, tissue water is the target and the presence or absence of red blood cells within the vessels has relatively little effect. An important element was the inclusion of a mechanical catheter drawback system and a radial delivery of energy to provide more uniform and predictable heating of the vessel. Studies in porcine GSV demonstrated full-thickness thermal damage at 5 W with the 1320-nm laser and at 20 W with the 1064-nm laser.91 In a mathematical model comparing the 1320-nm and 980-nm lasers, the 1320-nm laser had increased venous wall absorption and produced vein wall damage at a lower energy.92
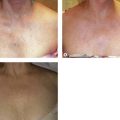
Clinical studies have demonstrated close to 100% efficacy without evidence of vessel perforation with use of the 1320-nm Nd:YAG intravascular laser in 24 patients with 6- to 12-month follow-up (Fig. 11.4).93 We investigated the 1320-nm laser in 64 patients at 5 to 6 W with pullback of 1 mm/second. Follow-up extended up to 5 years, with a mean follow-up of 25.3 months. A 7.8% failure rate was found.94 Clinical results as well as postoperative adverse sequelae were identical to those seen with VNUS Closure treatment (Fig. 11.5).
Weiss et al95 have compared 36 GSVs treated with the 810-nm diode laser to 42 GSVs treated with the 1320-nm Nd:YAG laser and 174 GSVs treated with RF closure. They found a 95% success rate with both RF and 1320-nm endoluminal treatment, without adverse effects. In contrast, the 810-nm diode laser achieved an 86% success rate, with 52% of patients experiencing significant pain interfering with walking for 2 to 3 days and 99% with significant bruising covering 75% or greater of the treated area. Proebstle et al96 evaluated 282 limbs treated with 1320-nm Nd:YAG (continuous mode, pullback 1 mm/second, 8 W), 940-nm diode (continuous mode, pullback 4–5 mm/second, 15 W), and 940-nm diode (continuous mode, pullback 3 mm/second, 30 W). Success rates at 3 months were 97%, 90.3%, and 100%, respectively. No statistical differences in phlebitis or paresthesias were noted between the three groups, but the 1320-nm group had significantly less pain. The 1320-nm laser resulted in lower incidences of ecchymoses than the 940-nm at 30 W.
1470-nm Diode laser
With the concept of targeting water without targeting hemoglobin, additional wavelengths targeting tissue water are in the process of being introduced for endovenous ablation. Successful implementation of the 1320-nm laser by us resulted in decreased adverse events and high efficacy rates, and so it was predictable that other available wavelengths that avoid hemoglobin but heat water would be tried. Most recently a 1470-nm diode laser was tested, as this wavelength has a high affinity for water. Since this wavelength is rapidly absorbed by the water content of blood and utilizes a fiber tip with a spacer instead of the traditional bare tip fiber, there is a significant compression of the vein is required along with copious tumescent anesthesia for fiber and vein wall contact.97 In a study by Pannier,98 117 limbs were treated with the 1470-nm diode (continuous mode, 15 W) and a LEED of either greater than or less than 100 J/cm. Both groups maintained 100% occlusion at 1-year follow-up. All patients were prophylactically treated with 7 days of low molecular weight heparin. No deep vein thrombosis or pulmonary emboli occurred. Paresthesias arose in 9.3%, with an increased incidence noted in the patients treated with a LEED of over 100 J/cm. As the two groups displayed similar efficacy, the authors recommend an LEED of less than 100 J/cm be utilized to decrease the risk of side effects. One hundred and six GSVs were randomized to receive either 1470-nm or 980-nm, both at 15 W with adjuvant ambulatory phlebectomy. Significantly less pain, ecchymoses, induration, transient paresthesias, and time to return to daily activities were seen in the 1470-nm laser cohort.99
1500-nm Diode laser
The 1500-nm diode laser is another recent advancement in endovenous laser ablation technology. Similar to the 1470-nm diode laser, the 1500-nm diode targets water in the vein wall. Compared to the 980-nm laser, the 1500-nm laser showed more uniform destruction of the vein wall in an animal model.100 Vuylsteke et al101 treated 158 GSVs with a 1500-nm diode laser (6 W above the knee and 5 W below the knee, using continuous mode and a pullback rate of 1 mm/second). The average LEED was 53.4%. An occlusion rate of 93.3% was found at 6 months follow-up. Ecchymosis was mild in 31.6% of patients, moderate to severe in 19%, and undetectable in 49.4% of patients. Moderate pain was detected in 1% of patients and no paresthesias were encountered. The authors suspect the decreased incidence of the adverse events was due to lack of perforation in the vein wall. A further comparison was made with their previous study investigating the 980-nm diode (10 W) laser occlusion and adverse event rates. Though the efficacy rates were equivalent, side effects such as ecchymoses, induration, discomfort, and paresthesias were statistically less with the 1500-nm diode.
Endovenous laser treatment of the small saphenous vein
Varicose veins are associated with an incompetent SSV in one-fifth of patients. The SSV course lies in close proximity to the sural nerve. As a result, increased incidences of paresthesias have been noted following EVLT of the SSV. Endovenous laser ablation using the 980-nm diode (accessed by micropuncture technique, continuous mode, 10–14 W, pullback 3–5 mm/second) for SSV was investigated by Gibson et al. Ninety-four percent of patients had at least one adjuvant treatment at the time of endovenous laser ablation: foam sclerotherapy, microphlebectomy, ligation of perforators, or EVLT of the GSV. Of the 210 small saphenous veins treated via tumescent anesthesia, 100% were occluded at 1 week. Three months post-treatment, 96% of SSV remained occluded. Nonocclusive deep venous thromboses extending into the popliteal vein were found in 5.7% of patients at 1 week postoperative. Numbness developed in 1.6%.102
A study by Park et al103 explored the 980-nm diode laser (assessed percutaneously, pulsed mode, 12–15 W, 2 mm/second pullback) in treating 390 incompetent SSVs. All patients were treated using 70 to 220 mL of tumescent anesthesia. One-third of patients had concomitant EVLT of their GSV. High closure rates of the SSV were reported: 99.7% at 1 week postoperative and 99.4% at 1 year. The majority of patients experienced ecchymoses and skin tightness, which resolved in 2 weeks. Other side effects were infrequent: 2.3% phlebitis and 2% paresthesias. No skin burns or deep vein thrombosis developed.
The 810-nm diode laser treated 41 SSVs (continuous mode, 8–10 W, pullback 2–3 mm/second) and 135 GSVs (continuous mode, 12–14 W, pullback 1.5–2 mm/second) under tumescent anesthesia in a paper by Jung et al.104 Spinal anesthesia was added if phlebectomy was simultaneously performed. Recanalization was found in 7.3% of the 41 SSVs at the 3 month postoperative follow-up. One case of foot drop was reported following endovenous laser ablation of the SSV and ambulatory phlebectomy. Following surgery, intramuscular hemorrhage resulted in muscular edema and pressure on compression of the peroneal nerve. The patient recovered following 2 months of physical therapy.
Endovenous laser therapy for stasis ulcers
Viarengo et al105 investigated the 980-nm diode laser with postoperative compression versus compression alone in the treatment of stasis ulcers. Compression consisted of elastic support hose, elastic bandages, or Unna boots. All 52 patients suffered from ulceration for over 1 year prior to study enrollment. After 3 months of treatment, ulcerations had improved in 62.9% of the EVLT with compression compared to 12% in the compression group. At 12-months follow-up, ulceration healing was 81.5% and 24% in the EVLT with compression and compression alone groups, respectively. A 44.4% recurrence rate was noted with compression alone. No ulcerations recurred in those treated with the 980-nm diode laser with compression. No infections arose in either cohort. In the paper by Fernandez et al,106 102 (6.54%) of their patients possessed preoperative ulcers. All ulceration showed healing in a mean of 5.2 weeks post 810-nm diode endovenous laser ablation; however, three cases experienced reopening of their ulcerations.
Technique for endoluminal laser ablation using a standard sharp fiberoptic
Varicose veins are marked with the patient standing and again with the patient lying down in the operative position with a Venoscope, as previously described.29,30 After appropriate marking, the area surrounding the GSV and distal tributaries to be treated is infiltrated with lidocaine 0.1% tumescent anesthesia. The amount of tumescent fluid averages 800 mL with a lidocaine dose of approximately 8 mg/kg. An alternative is semitumescent anesthesia with a cocktail of 20 mL lidocaine with epinephrine + 20 mL normal saline + 10 mL sodium bicarbonate, injected in the saphenous fascia under ultrasound guidance (Fig. 11.6). The GSV is then accessed either under duplex guidance according to Seldinger technique at any level, or through a 2- to 3-mm incision (Fig. 11.7).
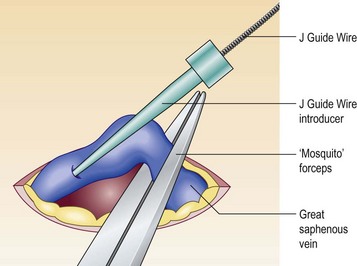
Figure 11.7 Introduction of J Guide Wire through phlebotomy when echo-guided access is not possible.
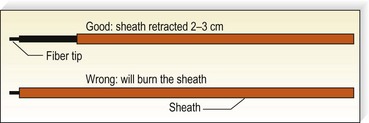
A 500 to 600-µm laser fiber is inserted into the vein within a protective sheath so that only the distal 2 to 3 mm of laser fiber exits from the sheath (Fig. 11.8). A helium–neon (He:Ne) aiming beam that is continuously illuminated when the laser is on insures that the laser fiber is outside of the sheath. If the laser fiber retracts within the sheath, thermal destruction of the sheath occurs.
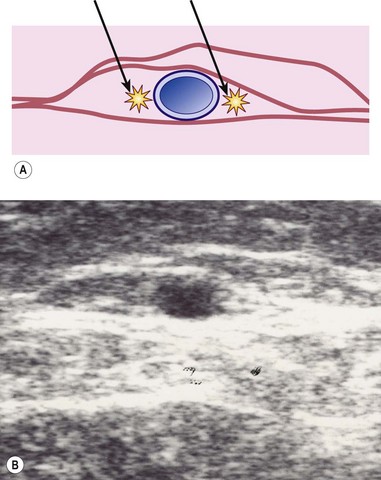
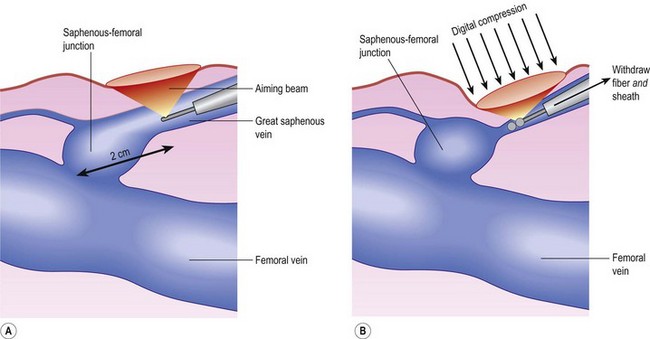
Correct placement of the laser fiber tip 2 cm distal to the SFJ (Fig. 11.9) is confirmed through catheter length measurement, duplex examination, and viewing the He:Ne aiming beam through the skin (Fig. 11.10). The laser is in a continuous firing mode at a manufacturer recommended energy setting, which is wavelength dependent, with either slow mechanical withdrawal at a rate to approximate 1 mm/second or, in the case of the 1320-nm system, a continuous pullback mechanism set for 1 mm/second (Fig. 11.11). This technique minimizes pain and maintains efficacy of treatment. The treated leg is then wrapped in gauze to absorb the tumescent fluid with an overlying compression bandage. The patient returns the next day, when the bandage is changed to a 30- to 40-mmHg graduated compression stocking. The compression stocking is then worn for 7 days continuously and then while the patient is ambulatory for another 7 days.
1 Jakobsen BH. The value of different forms of treatment for varicose veins. Br J Surg. 1979;66:182.
2 Munn SR, Morton JB, Macbeth W, et al. To strip or not to strip the long saphenous vein? A varicose veins trial. Br J Surg. 1981;68:426.
3 McMullin GM, Coleridge Smith PD, Scurr JH. Objective assessment of high ligation without stripping the long saphenous vein. Br J Surg. 1991;78:1139.
4 Rutherford RB, Sawyer JD, Jones DN. The fate of residual saphenous vein after partial removal or ligation. J Vasc Surg. 1990;12:426.
5 Sarin S, Scurr JH, Coleridge Smith PD. Assessment of stripping the long saphenous vein in the treatment of primary varicose veins. Br J Surg. 1992;79:889.
6 Van Rij AM, Jiang P, Solomon C, et al. Recurrence after varicose vein surgery: a prospective long-term clinical study with duplex ultrasound scanning and air plethysmography. J Vasc Surg. 2003;38:935.
7 Haines DE. The biophysics of radiofrequency catheter ablation in the heart: the importance of temperature monitoring. Pacing Clin Electrophysiol. 1993;16(3 Pt 2):586.
8 Haines DE, Verow AF. Observations on electrode-tissue interface temperature and effect on electrical impedance during radiofrequency ablation of ventricular myocardium. Circulation. 1990;82:1034.
9 Reich-Schupke S, Mumme A, Stucker M. Histopathological findings in the treatment of varicose veins after bipolar radiofrequency-induced thermotherapy – results of an ex-vivo experiment. Derm Surg. 2010. [in press]
10 Lavergne T, Sebag C, Ollitrault J, et al. Radiofrequency ablation: physical bases and principles. Arch Mal Coeur Vaiss. 1996;89(Spec No 1):57.
11 Weiss RA, Goldman MP. Controlled radiofrequency-mediated endovenous shrinkage and occlusion. In: Goldman MP, Weiss RA, Bergan JJ, editors. Varicose veins and telangiectasias: diagnosis and treatment. 2nd ed. St. Louis: Quality Medical Publishing; 1999:217-224.
12 Goldman MP. Closure of the greater saphenous vein with endoluminal radiofrequency thermal heating of the vein wall in combination with ambulatory phlebectomy: preliminary 6-month follow-up. Dermatol Surg. 2000;26:452.
13 Chandler JG, Pichot O, Sessa C, et al. Treatment of primary venous insufficiency by endovenous saphenous vein obliteration. Vasc Surg. 2000;34:201.
14 Manfrini S, Gasbarro V, Danielsson G, et al. Endovenous management of saphenous vein reflux. J Vasc Surg. 2000;32:330.
15 Chandler JG, Pichot O, Sessa C, et al. Defining the role of extended saphenofemoral junction ligation: a prospective comparative study. J Vasc Surg. 2000;32:941.
16 Goldman MP, Amiry S. Closure of the greater saphenous vein with endoluminal radiofrequency thermal heating of the vein wall in combination with ambulatory phlebectomy: 50 patients with more than 6-month follow-up. Dermatol Surg. 2002;28:29.
17 Weiss RA, Weiss MA. Controlled radiofrequency endovenous occlusion using a unique radiofrequency catheter under duplex guidance to eliminate saphenous varicose vein reflux: 2-year follow-up. Dermatol Surg. 2002;28:38.
18 Rautio T, Ohinmaa A, Perala J, et al. Endovenous obliteration versus conventional stripping operation in the treatment of primary varicose veins: a randomized controlled trial with comparison of the costs. J Vasc Surg. 2002;35:958.
19 Merchant RF, DePalma RG, Kabnick LS. Endovascular obliteration of saphenous reflux: a multicenter study. J Vasc Surg. 2002;35:1190.
20 Pichot O, Kabnick LS, Creton D, et al. Duplex ultrasound scan findings two years after great saphenous vein radiofrequency endovenous obliteration. J Vasc Surg. 2004;39:189.
21 Perrin M. Endoluminal treatment of lower limb varicose veins by endovenous laser and radiofrequency techniques. Phlebology. 2004;19:170.
22 Merchant RF, Pichot O, Myers KA. Four-year follow-up on endovascular radiofrequency obliteration of great saphenous reflux. Dermatol Surg. 2005;31:129.
23 Stotter L, Schaaf I, Bockelbrink A. Comparative outcomes of radiofrequency endoluminal ablation, invagination stripping, and cryostripping in the treatment of great saphenous vein insufficiency. Phlebology. 2006;21:60.
24 Proebstle TM, Gul D, Lehr HA, et al. Infrequent early recanalization of greater saphenous vein after endovenous laser treatment. J Vasc Surg. 2003;38:511.
25 Blomgren L, Johansson G, Dahlberg-Akerman A, et al. Changes in superficial and perforating vein reflux after varicose vein surgery. J Vasc Surg. 2005;42:315.
26 Proebstle TM, Lehr HA, Kargl A, et al. Endovenous treatment of the greater saphenous vein with a 940-nm diode laser: thrombotic occlusion after endoluminal thermal damage by laser-generated steam bubbles. J Vasc Surg. 2002;35:729.
27 Merchant RF, Pichot O. Long-term outcomes of endovenous radiofrequency obliteration of saphenous reflux as a treatment for superficial venous insufficiency. J Vasc Surg. 2005;42:502.
28 Vasquez MA, Wang J, Mahathanaruk M, et al. The utility of the Venous Clinical Severity Score in 682 limbs treated by radiofrequency saphenous vein ablation. J Vasc Surg. 2007;45:1008.
29 Weiss RA, Goldman MP, Weiss MA. Transillumination mapping prior to ambulatory phlebectomy. Dermatol Surg. 1998;24:447.
30 Smith S, Goldman MP. Tumescent anesthesia in ambulatory phlebectomy. Dermatol Surg. 1998;24:453.
31 Kabnick LS, Merchant RF. Twelve and twenty-four month follow-up after endovascular obliteration of saphenous vein reflux – a report from the multi-center registry. J Phlebol. 2001;1:17.
32 Sybrandy JEM, Wittens CHA. Initial experience in endovenous treatment of saphenous vein reflux. J Vasc Surg. 2002;36:1207.
33 Komenaka IK, Nguyen ET. Is there an increased risk for DVT with the VNUS closure procedure? J Vasc Surg. 2002;36:1311.
34 Hingorani A, Ascher E, Markevich N, et al. Deep venous thrombosis following radiofrequency ablation (RFA) of greater saphenous vein (GSV): a word of caution. J Vasc Surg. 2004;40:500.
35 Salles-Cunha SX, Comerota AJ, Tzilinis A, et al. Ultrasound findings after radiofrequency ablation of the great saphenous vein: descriptive analysis. J Vasc Surg. 2004;40:1166.
36 US Food and Drug Administration. Manufacturer and User Facility Device Experience (MAUDE database). Access at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Search.CFM
37 Goldman MP, Weiss RA, Gradman W. Regarding ‘Deep venous thrombosis following radiofrequency ablation (RFA) of greater saphenous vein (GSV): a word of caution’. J Vasc Surg. 2005;41:737.
38 Gale AA, Dosick SM, Seiwart AJ, Comorota AJ. Regarding ‘Deep venous thrombosis following radiofrequency ablation (RFA) of greater saphenous vein (GSV): a word of caution’. J Vasc Surg. 2005;41:374.
39 Ravi R, Rodriguez-Lopez J, Ramaiah V, Diethrich EB. Regarding ‘Extension of saphenous thrombus into the femoral vein: a potential complication of new endovenous ablation techniques’. J Vasc Surg. 2005;41:182.
40 Goldman MP, Weiss RA. Regarding ‘Ultrasound findings after radiofrequency ablation of the great saphenous vein’. J Vasc Surg. 2005;41:914.
41 Proebstle TM, Vago B, Alm J, et al. Treatment of the incompetent great saphenous vein by endovenous radiofrequency powered segmental thermal ablation: first clinical experience. J Vasc Surg. 2008;47:151.
42 Gohel MS, Davies AH. Radiofrequency ablation for uncomplicated varicose veins. Phlebology. 2009;24:42.
43 Calcagno D, Rossi JA, Ha C. Effect of saphenous vein diameter on closure rate with ClosureFAST radiofrequency catheter. Vasc Endovascular Surg. 2009;43:567.
44 Almeida JI, Kaufman J, Göckeritz O, et al. Radiofrequency endovenous ClosureFAST versus laser ablation for the treatment of great saphenous reflux: a multicenter, single-blinded, randomized study (RECOVERY study). J Vasc Interv Radiol. 2009;20:752.
45 Almeida JI, Raines JK. Radiofrequency ablation and laser ablation in the treatment of varicose veins. Ann Vasc Surg. 2006;20:547.
46 Mordon S, Wassmer B, Servell P, et al. Is a vein filled with blood a good model for studying endovenous laser ablation? Lasers Surg Med. 2009;41:543.
47 Lu X, Ye K, Li W, et al. Endovenous ablation with laser for great saphenous vein insufficiency and tributary varices: a retrospective evaluation. J Vasc Surg. 2008;48:675.
48 Disselhoff BC, Rem AI, Verdaasdonk RM, et al. Endovenous laser ablation: an experimental study on the mechanism of action. Phlebology. 2008;23:69.
49 Theivacumar NS, Darwood R, Gough MJ. Neovascularisation and recurrence 2 years after varicose vein treatment for sapheno-femoral and great saphenous vein reflux: a comparison of surgery and endovenous laser ablation. Eur J Vasc Endovasc Surg. 2009;38:207.
50 Prince EA, Ahn SH, Dubel GJ, Soares GM. An investigation of the relationship between energy density and endovenous laser ablation success: does energy density matter? J Vasc Interv Radiol. 2008;19:1449.
51 Moritz AR, Henriques ECJr. Studies of thermal injury II. The relative importance of time and surface temperature in the causation of cutaneous burns. Am J Pathol. 1947;23:695.
52 Proebstle TM, Sandhoffer M, Kargel A, et al. Thermal damage on the inner vein wall during endovenous laser treatment: key role of energy absorption by intravascular blood. Dermatol Surg. 2002;28:596.
53 Roggan A, Friebel M, Dorschel K, et al. Optical properties of circulating human blood in the wavelength range 400–2500 nm. J Biomed Opt. 1999;50:523.
54 Fan CM, Rox-Anderson R. Endovenous laser ablation: mechanism of action. Phlebology. 2008;23:206.
55 Der Kinderen DJ, Disselhoff BC, Koten JW, et al. Histopathologic studies of the below-the-knee great saphenous vein after endovenous laser ablation. Dermatol Surg. 2009;35:1985.
56 Goldman MP. Endovenous laser treatment of the greater saphenous vein at 810 nm. Lasers Surg Med. 2002;31(Suppl):37.
57 Van Den Bos R, Arends L, Kockaert M, et al. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg. 2009;49:230.
58 Van Den Bos RR, Neumann M, De Roos KP, Nijsten T. Endovenous laser ablation-induced complications: review of the literature and new cases. Dermatol Surg. 2009;35:1206.
59 Min RJ, Zimmet SE, Isaacs MN, Forrestal MD. Endovenous laser treatment of the incompetent greater saphenous vein. J Vasc Interv Radiol. 2001;12:1167.
60 Navarro L, Min RJ, Bone C. Endovenous laser: a new minimally invasive method of treatment for varicose veins – preliminary observations using an 810-nm diode laser. Dermatol Surg. 2001;27:117.
61 Sadick NS, Wasser S. Combined endovascular laser with ambulatory phlebectomy for the treatment of superficial venous incompetence: a 2-year perspective. J Cosmet Laser Ther. 2004;6:44.
62 Todd K III, Fronek H, Issacs M, et al. Endovenous laser treatment of saphenous vein reflux: twelve month evaluation in 291 patients, presented at the Annual Meeting of the American College of Phlebology 2005.
63 Nwaejike N, Srodon PD, Kyriakides C. 5-years of endovenous laser ablation (EVLA) for the treatment of varicose veins – a prospective study. Int J Surg. 2009;7:347.
64 Elmore FA, Lackey D. Effectiveness of endovenous laser treatment in eliminating superficial venous reflux. Phlebology. 2008;23:21.
65 Min RJ, Khilnani N, Zimmet SE. Endovenous laser treatment of saphenous vein reflux: long-term results. J Vasc Interv Radiol. 2003;14:991.
66 Kabnick LS. Complications of endovenous thermal ablation therapies, 34th Golbal: Vascular and Endovascular Issues, Techniques and Horizons™.
67 Myers K, Fris R, Jolley D. Treatment of varicose veins by endovenous laser therapy: assessment of results by ultrasound surveillance. Med J Aust. 2006;21:199.
68 Hamel-Desnos C, Gérard JL, Desnos P. Endovenous laser procedure in a clinic room: feasibility and side effects study of 1,700 cases. Phlebology. 2009;24:125.
69 Kabnick LS. Endolaser venous system (980 nm) for the treatment of saphenous venous insufficiency: 7611 limbs. Int Angio. 2005;24(Suppl):80.
70 Barrett J. Personal Communication: Jan 15, 2007.
71 D’Othee BJ, Ghiorse D. Non-infected, non-haematic fluid collections after endovenous laser ablation of the saphenous veins: a noteworthy complication. Phlebology. 2008;23:47.
72 Vuylsteke M, Van Den Bussche D, Audenaert EA, Lissens P. Endovenous laser obliteration for the treatment of primary varicose veins. Phlebology. 2006;21:80.
73 Eidson JL3rd, Shepherd LG, Bush RL. Aneurysmal dilatation of the great saphenous vein stump after endovenous laser ablation. J Vasc Surg. 2008;48:1037.
74 Labropoulos N, Bhatti A, Leon L, Borge M, Rodriguez H, Kalman P. Neovascularization after great saphenous vein ablation. Eur J Vasc Endovasc Surg. 2006;31:219.
75 Dunst KM, Huemer GM, Wayand W, Shamiyeh A. Diffuse phlegmonous phlebitis after endovenous laser treatment of the greater saphenous vein. J Vasc Surg. 2006;43:1056.
76 Holdstock JM, Marsh P, Whiteley MS, Price BA. It is possible to cause damage to a laser fibre during delivery of tumescent anaesthesia for endovenous laser ablation (EVLA). Eur J Vasc Endovasc Surg. 2008;36:473.
77 Guex J-J. Description d’un moyen simple permettant de doser et de régulariser l’application en continu de l’énergie laser lors des traitements endo veineux. Phlebologie. 2004;57:209.
78 Weiss RA. Comparison of endovenous radiofrequency versus 810 nm diode laser occlusion of large veins in an animal model. Dermatol Surg. 2002;28:56.
79 Corcos L, Dini S, De Anna D, et al. The immediate effects of endovenous diode 808-nm laser in the greater saphenous vein: morphologic study and clinical implications. J Vasc Surg. 2005;41:1018.
80 Sadick NS, Wasser S. Combined endovascular laser plus ambulatory phlebectomy for the treatment of superficial venous incompetence: a 4-year perspective. J Cosmet Laser Ther. 2007;9:9.
81 Bush RG. Regarding ‘Endovenous treatment of the greater saphenous vein with a 940-nm diode laser: thrombolytic occlusion after endoluminal thermal damage by laser generated steam bubbles’. J Vasc Surg. 2003;36:242.
82 Proebstle TM, Gul D, Kargl A, Knop J. Endovenous laser treatment of the lesser saphenous vein with a 940-nm diode laser: early results. Dermatol Surg. 2003;29:357.
83 Proebstle TM, Krummenauer F, Gul D, Knop J. Nonocclusion and early reopening of the great saphenous vein after endovenous laser treatment is fluence dependent. Dermatol Surg. 2004;30:174.
84 Proebstle TM, Moehler T, Herdemann S. Reduced recanalization rates of the great saphenous vein after endovenous laser treatment with increased energy dosing: definition of a threshold for the endovenous fluence equivalent. J Vasc Surg. 2006;44:834.
85 Gerard JL, Desgranges P, Becquemin JP, et al. Feasibility of ambulatory endovenous laser for the treatment of greater saphenous varicose veins: one month outcome in a series of 20 outpatients. J Mal Vasc. 2002;27:222.
86 Oh C-K, Jung D-S, Jang H-S, Kwon K-S. Endovenous laser surgery of the incompetent greater saphenous vein with a 980-nm diode laser. Dermatol Surg. 2003;29:1135.
87 Desmyttère J, Grard C, Stalnikiewicz G, et al. Endovenous laser ablation (980 nm) of the small saphenous vein in a series of 147 limbs with a 3-year follow-up. Eur J Vasc Endovasc Surg. 2010;39:99.
88 Kabnick LS. Outcome of different endovenous laser wavelengths for great saphenous vein ablation. J Vasc Surg. 2006;43:88.
89 Parente EJ, Rosenblatt M. Endovenous laser treatment to promote venous occlusion. Lasers Surg Med. 2003;33:115.
90 Chang C-J, Chua J-J. Endovenous laser photocoagulation (EVLP) for varicose veins. Lasers Surg Med. 2002;31:257.
91 Goldman MP, Detwiler SP. Endovenous 1064-nm and 1320-nm Nd:YAG laser treatment of the porcine greater saphenous vein. Cos Dermatol. 2003;16:25.
92 Mordon SR, Wassmer B, Zemmouri J. Mathematical modeling of 980-nm and 1320-nm endovenous laser treatment. Lasers Surg Med. 2007;39:256.
93 Goldman MP, Mauricio M, Rao J. Intravascular 1320-nm laser closure of the great saphenous vein: a 6- to 12-month follow-up study. Dermatol Surg. 2004;30:1380.
94 Woodhall KE, Nootheti PK, Palm MD, Goldman MP. Long term follow-up study of intravascular 1320-nm laser closure of the great saphenous vein: 64 patients with more than 1-year follow-up. Am J Cosm Surg. 2009;26:236.
95 Weiss RA, Munavalli G, Bellew SG, Beasley K. Comparison of endovenous saphenous vein obliteration techniques: 810 nm versus 1320 nm versus radiofrequency. Lasers Surg Med. 2005;S17:34.
96 Proebstle TM, Moehler T, Gül D, Herdemann S. Endovenous treatment of the great saphenous vein using a 1,320 nm Nd:YAG laser causes fewer side effects than using a 940 nm diode laser. Dermatol Surg. 2005;31:1678.
97 Almeida J, Mackay E, Javier J, et al. Saphenous laser ablation at 1470 nm targets the vein wall, not blood. Vasc Endovascular Surg. 2009;43:467.
98 Pannier F, Rabe E, Maurins U. First results with a new 1470-nm diode laser for endovenous ablation of incompetent saphenous veins. Phlebology. 2009;24:26.
99 Doganci S, Demirkilic U. Comparison of two different laser wavelengths and fibers in the treatment of great saphenous vein varicosities: a prospective randomized controlled study, Submitted to Eur J Vasc Endovasc Surg 2009.
100 Vuylsteke M, Van Dorpe J, Roelens J, et al. Endovenous laser treatment: a morphological study in an animal model. Phlebology. 2009;24:166.
101 Vuylsteke ME, Vandekerckhove PJ, De Bo T, et al. Use of a new endovenous laser device: results of the 1,500 nm laser. Ann Vasc Surg. 2010;24:205.
102 Gibson KD, Ferris BL, Polissar N, et al. Endovenous laser treatment of the small [corrected] saphenous vein: efficacy and complications. J Vasc Surg. 2007;45:795.
103 Park SJ, Yim SB, Cha DW, et al. Endovenous laser treatment of the small saphenous vein with a 980-nm diode laser: early results. Dermatol Surg. 2008;34:517.
104 Jung IM, Min SI, Heo SC, et al. Combined endovenous laser treatment and ambulatory phlebectomy for the treatment of saphenous vein incompetence. Phlebology. 2008;23:172.
105 Viarengo LM, Potério-Filho J, Potério GM, et al. Endovenous laser treatment for varicose veins in patients with active ulcers: measurement of intravenous and perivenous temperatures during the procedure. Dermatol Surg. 2007;33:1234.
106 Fernández CF, Roizental M, Carvallo J. Combined endovenous laser therapy and microphlebectomy in the treatment of varicose veins: Efficacy and complications of a large single-center experience. J Vasc Surg. 2008;48:947.