Intrauterine Development and Comparative Respiratory Anatomy
I General Developmental Periods
A Fertilization period (weeks 1 through 3)
1. The sperm fertilizes the egg.
2. Blood vessels first appear.
3. Heart tubes form that will develop into the heart.
4. Blood cells form from endothelial cells within the yolk sac.
II Respiratory System Development
1. By the fourth week, brachial arches form and develop the maxillary (upper) and mandibular (lower) jaw.
2. The brachial arches also form the pharynx, mouth, oropharyngeal airway, and laryngeal cartilages.
3. The tongue develops within weeks 4 to 7.
4. The palate starts to develop in the fifth week and is complete by the 17th week of gestation.
5. A cleft lip may develop as a result of the lip not completely forming and extending into the nostril. A cleft palate occurs from malformation of the palate and may be unilateral or bilateral.
6. The nasal cavity with nasal concha develops when the oronasal membrane ruptures, allowing the oral and nasal cavities to develop. This occurs during approximately the seventh week.
7. Nasal sinuses develop during the latter part of fetal development, with further development of the ethmoid, maxillary, frontal, and sphenoidal sinuses continuing into puberty.
1. An epithelial groove will give rise to the larynx, trachea, bronchi, pulmonary epithelium, and assorted glands.
2. The tracheoesophageal septum divides into the esophagus and laryngotracheal tube.
3. The first lung bud develops from the laryngotracheal tube by 24 to 26 days of fertilization.
4. The laryngotracheal tube, along with the surrounding tissue, develops into the larynx, trachea, bronchi, and lungs.
5. Visceral and parietal pleura develop from the lung buds (bronchopulmonary buds).
6. By 5 weeks two lung buds develop.
7. The phrenic nerve innervates the diaphragm within the fourth week, and the diaphragm is completely formed by the seventh week.
8. By the 10th week, true and false vocal cords are formed.
9. Further growth of the lung buds develop into secondary buds, two on the right and one on the left.
10. This branching continues, with 24 orders of branches present at 16 weeks.
11. By 25 weeks airways have changed from glandular to tubular and increase in length and diameter.
1. Embryonic period (fertilization to 5 weeks)
a. The laryngotracheal groove forms.
b. The lung bud first appears.
c. The lung bud divides into left and right mainstem bronchi.
d. Branching of the airways begins.
e. Pulmonary arteries invade lung tissue, following the airways and dividing as the airway divides.
f. Pulmonary veins originate independently from the lung parenchyma and return to the left atrium, completing the pulmonary circuit.
2. Pseudoglandular period (5 to 13 weeks)
a. The conducting airways develop and are complete up to and including the terminal bronchiole.
b. Mucous glands and goblet cells appear.
c. Bronchi and bronchioles are lined with cuboidal epithelium.
d. Diaphragm begins to develop.
e. Muscle fibers, elastic tissue, and early cartilage formation can be seen along the tracheobronchial tree.
3. Canalicular period (13 to 24 weeks)
a. Enlargement of the conducting airways continues, with proliferation of pulmonary blood vessels.
b. Gas exchange units develop from respiratory bronchioles.
d. Meconium is present at 16 weeks.
e. Breathing movements can be detected between 18 and 20 weeks.
f. Elastic tissue develops beginning at 20 weeks.
g. Airway changes from glandular to tubular and increases in length and diameter.
h. Type I and II alveolar pneumocytes develop, with synthesis and production of surfactant starting by weeks 22 to 24.
4. Terminal sac period (24 weeks to birth)
a. Primitive alveoli develop from alveolar ducts.
b. Further development of the pulmonary vasculature occurs, as does lymphatic proliferation.
c. The fetus weighs approximately 1000 g at 26 to 28 weeks.
d. The fetal lungs represent 2% to 3% of the total body weight. This percentage decreases as the weight of the fetus increases toward the end of gestation.
e. The air sacs change from a cuboidal cellular configuration to a squamous epithelium, allowing greater diffusion of gases.
f. As the lung matures, the number of alveoli increases, and the thickness of the alveoli wall decreases.
g. At birth the number of alveoli ranges from 24 to 75 million.
h. The number of alveoli continues to increase until there are approximately 300 to 600 million alveoli in adulthood.
i. The size of the lung increases from approximately 1 to 2 m2 at 32 weeks’ gestation to the adult size of 70 m2.
A The lung begins secreting fluid by the 70th day of gestation.
B This fluid is composed of a combination of sodium, potassium, chloride, bicarbonate, and a small percentage of protein in water.
C The presence of lung fluid assists lung growth and the development of the functional residual capacity (FRC).
D Fetal breathing helps secrete lung fluid and mixes lung fluid with amniotic fluid.
E Because of this process, amniotic fluid can be analyzed to determine lung maturation (see Section V, Amniotic Fluid).
F Amniocentesis is the procedure in which amniotic fluid is removed from the uterus for examination.
A Surfactant is synthesized and secreted by type II alveolar pneumocytes.
B Surfactant first appears between 22 and 24 weeks’ gestation.
C Surfactant reduces surface tension, maintaining alveolar stability and preventing atelectasis.
D Protein makes up 10% to 20% of the surfactant, and 80% to 90% of the protein is phospholipids. A small percentage of cholesterol is also present.
E Two important phospholipids, lecithin and sphingomyelin, are present in surfactant.
F Sphingomyelin is present early in gestation and remains constant from 18 weeks to approximately 34 weeks before decreasing in concentration.
G Lecithin, the major phospholipid of adult surfactant, abruptly increases between 32 and 34 weeks’ gestation.
H The increased concentration of lecithin denotes lung maturation. The increase of lecithin in surfactant reduces the incidence of respiratory distress syndrome (RDS).
I Without appropriate surfactant production, newborns will have reduced lung compliance, decreased FRC, increased work of breathing, and greater oxygen consumption.
J Inadequate surfactant levels can occur in a newborn as a result of:
A Amniotic fluid is composed of amniotic cells, maternal blood, and fetal urine.
B There is approximately 30 ml of amniotic fluid at 10 weeks, which increases to 1 L by term.
C The fetus swallows amniotic fluid, which is absorbed by the gastrointestinal tract. Every 3 hours, the placenta exchanges amniotic fluid.
D Amniotic fluid protects the fetus and acts as a cushion surrounding the fetus. It also allows growth and development, movement, and maintenance of a thermoneutral environment.
E Amniocentesis can determine sex, lung maturity, biochemical abnormalities, and chromosomal defects.
F Lung maturity is determined by the concentration of lecithin and sphingomyelin.
G The ratio of lecithin to sphingomyelin (L/S) determines the incidence of RDS.
H An L/S ratio of ≥2.0 indicates a mature lung and low incidence of RDS.
I An L/S ratio of 1.0 to 1.5 indicates a transitional lung with a moderate incidence of RDS.
J An L/S ratio of <1.0 indicates a high incidence of RDS.
K Another test to determine lung maturity is the shake test.
1. A mixture of amniotic fluid, saline, and alcohol is placed in a test tube, shaken for 15 minutes, and then allowed to stand.
2. A complete ring of bubbles around the tube indicates appropriate fetal production of surfactant.
3. These results can be compared with an L/S ratio of >2.0.
4. Absence of bubbles indicates that surfactant maturity is incomplete.
A Maternal health and individual physiology, pregnancy complications, and maternal behaviors affect the health and development of the fetus.
B Any condition that leads to interference with placental blood flow or the transfer of oxygen to the fetus can cause adverse outcomes.
C Table 25-1 list maternal conditions and related neonatal outcomes.
TABLE 25-1
Maternal Condition and Neonatal Outcomes
| Maternal Condition | Fetal or Neonatal Outcome |
| Previous pregnancy complication | Same outcome as previous fetus |
| Diabetes mellitus | LGA, congenital malformations, RDS, hypoglycemia |
| Pregnancy-induced hypertension | Prematurity, SGA (pre-eclampsia) |
| Maternal age <17 years | Low birth weight, prematurity |
| Maternal age >35 years | Prematurity, chromosomal defects |
| Placenta previa | Prematurity, bleeding, SGA |
| Placenta abruptio | Fetal asphyxia, bleeding |
| Alcohol consumption | SGA, CNS dysfunction, mental retardation, facial dysmorphology |
| Smoking | SGA, prematurity, mental retardation, SIDS |
| Drug use | Placental abruption, IUGR, prematurity, CNS abnormalities, withdrawal disorders |
From Wilkins RL, Stoller JK, Scanlan CL: Egan’s Fundamentals of Respiratory Care, ed 8, St. Louis, 2003, Mosby.
A Major activities of the placenta include metabolism, transfer of nutrients and wastes, and endocrine secretion.
B Provides exchange of oxygen, carbon dioxide, and metabolic nutrients between fetal and maternal blood supplies.
C Blood supplies of the mother and fetus are in close proximity to one another but are not in actual contact.
D Fetal blood enters the placenta by way of two umbilical arteries and leaves by one umbilical vein.
E During birth contractions of the uterus slow blood flow to the placenta, and gas exchange may be reduced.
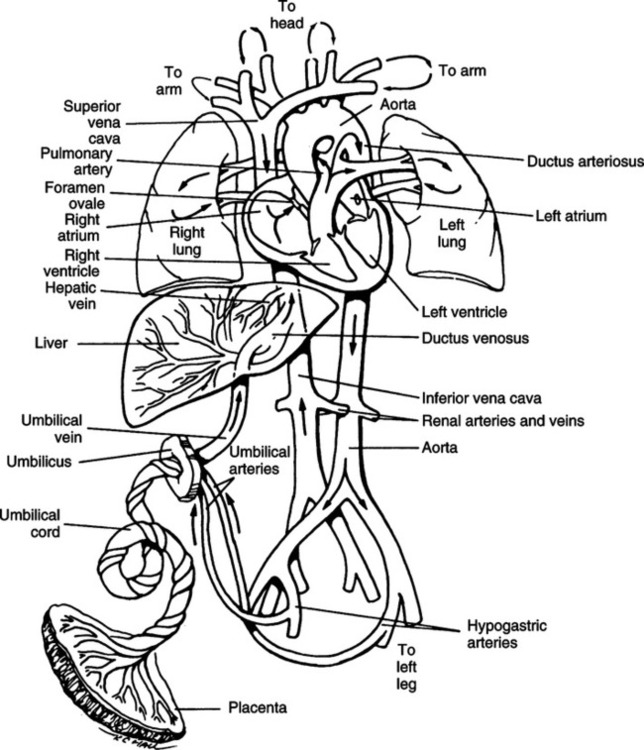
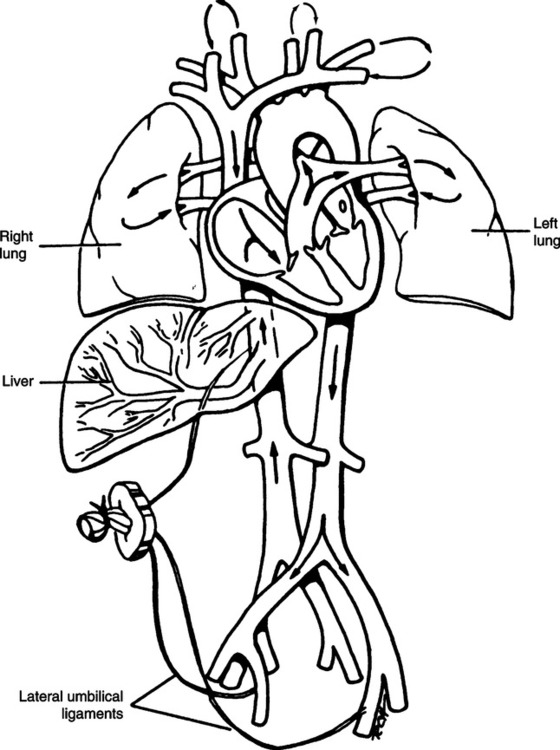
VIII Fetal Circulation (Figure 25-1)
A After oxygenated blood leaves the placenta, a portion of the blood enters the portal sinus to perfuse the kidney. The remainder enters the ductus venosus, bypassing the liver and entering the inferior vena cava.
B The oxygen saturation of the blood (Sao2) coming from the placenta is approximately 80%. The Pao2 is 27 to 29 mm Hg.
C Blood coming from the inferior vena cava has perfused the lower body tissues and has reduced Sao2 and Pao2. Thus as the oxygenated blood from the ductus venosus enters the inferior vena cava and mixes, the Sao2 decreases to approximately 67%.
D The blood enters the right atrium from the inferior vena cava, where it mixes with blood returning from the upper part of the body and head. This further reduces the saturation to approximately 62%.
E The blood flow entering the right atrium is divided into two streams, with the larger stream entering the left atrium by way of the foramen ovale.
F The foramen ovale is an opening of the interatrial septum between the right and left atria.
G This opening remains patent because of the increase in blood pressure in the right side of the heart relative to that of the left.
H The blood enters the left atrium and then mixes with a small amount of deoxygenated blood returning from the lungs by way of the pulmonary veins. This blood enters the left ventricle and is pumped out the aorta.
I A portion of this blood is directed to the head and upper extremities. This flow of blood has a higher oxygen content and Sao2 than the flow that is pumped out of the right ventricle.
J The second stream of blood in the right atrium is pumped to the right ventricle and out the pulmonary artery. This blood has mixed with blood coming from the superior vena cava.
K Approximately 10% of the cardiac output from the right side of the heart enters the pulmonary arteries and the lung. The lungs need little blood at this time because gas exchange occurs within the placenta.
L The pulmonary arteries are constricted as a result of the low Pao2 and lung fluid compressing the vessels. The pulmonary vascular resistance generally is high, and the peripheral vascular resistance generally is low.
M A large portion of the blood volume from the right side of the heart (approximately 90% of blood entering the right heart) enters the arch of the aorta by way of the ductus arteriosus. This ductus connects the pulmonary arteries and aorta and creates a right-to-left shunt. The saturation of this blood is approximately 50%.
N Blood flow moves out of the heart through the ductus arteriosus to the arch of the aorta, descending aorta, and thoracic aorta. Here blood is directed to the kidney, gut, and lower part of the body.
O A major portion of the blood (approximately 50% of the cardiac output) enters the placenta and is oxygenated.
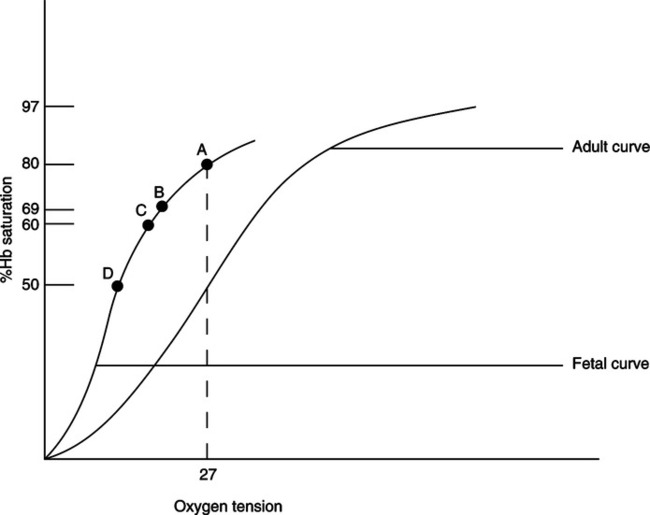
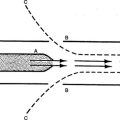
IX Transfer of Oxygen From Maternal to Fetal Blood (Figure 25-2)
A Maternal blood enters the placenta through the spiral arteries. The Pao2 of the maternal blood is approximately 100 mm Hg.
B Fetal blood enters the placenta through two umbilical arteries, which divide to form a vascular network. The Pao2 of the fetal blood entering the placenta is approximately 17 mm Hg.
C As both circulations come into proximity of one another, maternal blood releases oxygen to fetal circulation while at the same time accepts metabolic waste from the fetal circulation.
D The metabolic components alter the pH of maternal blood, shifting the oxyhemoglobin curve to the right, which reduces the affinity of hemoglobin for oxygen. This allows more oxygen to be released to fetal blood.
E The fetal oxyhemoglobin dissociation curve is shifted to the left as a result of the release of metabolic waste and the presence of fetal hemoglobin (HbF).
F Oxygen is able to combine with HbF to a greater extent than adult hemoglobin (HbA) because 2,3-diphosphoglycerate (2,3-DPG) does not affect HbF.
G One of the primary mechanisms regulating the release of oxygen from HbA is the binding of 2,3-DPG to β chains of hemoglobin. HbF has no β chains; therefore, 2,3-DPG cannot attach to it.
H As a result the oxyhemoglobin dissociation curve is shifted further to the left than that seen in the adult. Therefore, even with a low maternal Pao2, HbF is able to maintain a higher Sao2 than seen in maternal circulation. However, because the curve is shifted to the left, release of oxygen at the tissue level is impeded (see Figure 25-2).
I Maternal blood flow leaving the placenta and returning to the mother has a Pao2 of approximately 38 to 40 mm Hg.
J The fetal blood flow leaving the placenta has an umbilical artery Pao2 of approximately 29 mm Hg. The Sao2 is 80%.
K In contrast, an adult with a Pao2 of 27 mm Hg would have an Sao2 of 50%.
L At birth approximately 77% of the total hemoglobin is HbF. Within 8 to 11 months, only 1% to 2% of the total hemoglobin will be HbF.
X Transition From Fetal to Newborn Circulation (Figure 25-3)
A By the end of the normal gestational period of 38 to 40 weeks, the fetus has completely developed and is able to assume extrauterine life.
B After birth inflation of the lungs and transition of fetal circulation to newborn circulation occur.
C Vaginal birth of the fetus is initiated by contraction of the uterus.
D The fetus moves head first through the birth canal, where the chest is compressed. Intrathoracic pressures of 30 to 160 cm H2O develop, which forces lung fluid from the airways.
E Further presentation of the fetus allows passive recoil and the first introduction of air into the lungs. The first breath requires an opening pressure of 60 to 80 cm H2O to overcome the surface tension at the air-liquid interphase.
F A greater flow of blood enters the pulmonary vasculature as a result of vasodilation from the increase in Pao2 and partial removal of lung fluid. Additional lung fluid is removed by lymphatic drainage.
G For a short period a small left-to-right shunt exists as pulmonary artery pressure decreases and the ductus arteriosus remains patent.
H The ductus arteriosus constricts from the increased Pao2. This diverts more blood into the pulmonary vasculature. The ductus arteriosus remains partially open after birth but closes within 3 weeks.
I If the newborn develops hypoxia after birth, the ductus arteriosus remains open and continues to shunt blood. This reduces the pulmonary blood flow and further reduces the Pao2.
J The ductus arteriosus responds by constricting to increased Pao2 with the administration of supplemental oxygen.
K Prostaglandin synthetase inhibitors such as indomethacin (Indocin) are used to constrict the ductus arteriosus.
L During fetal development prostaglandin E1 and E2, along with the decreased Pao2, maintain the opening of the ductus arteriosus, thus ensuring that blood with a higher saturation will be routed to the brain.
M After complete presentation of the fetus, the umbilical cord is clamped and cut, which discontinues umbilical circulation and placental function.
N The umbilical arteries and vein constrict.
O The ductus venosus closes within 3 to 7 days and forms the ligamentum venosum.
P Blood flow now follows the normal circulatory pathway through the liver.
Q Left atrial pressure increases as a result of greater return of blood from the pulmonary veins. This pressure change functionally closes the foramen ovale. Anatomic closure from proliferation of fibrous and endothelial tissue occurs within a few weeks of birth. Changes in pressures in the left and right sides of the heart can reopen the foramen ovale.
R A number of factors contribute to the first and subsequent breaths of the newborn. These include:
XI Laboratory Values of the Newborn
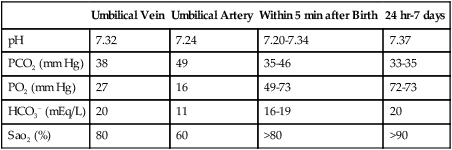
A Normal blood gas values of a healthy term newborn are listed in Table 25-2.
TABLE 25-2
Normal Term Newborn Blood Gases
| Umbilical Vein | Umbilical Artery | Within 5 min after Birth | 24 hr-7 days | |
| pH | 7.32 | 7.24 | 7.20-7.34 | 7.37 |
| PCO2 (mm Hg) | 38 | 49 | 35-46 | 33-35 |
| PO2 (mm Hg) | 27 | 16 | 49-73 | 72-73 |
| HCO3− (mEq/L) | 20 | 11 | 16-19 | 20 |
| Sao2 (%) | 80 | 60 | >80 | >90 |
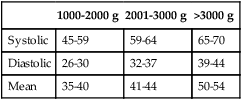
B Blood pressure during the first 12 hours of life for various-sized newborns is listed in Table 25-3.
TABLE 25-3
Blood Pressure of Various-Sized Newborns During the First 72 Hours of Life
| 1000-2000 g | 2001-3000 g | >3000 g | |
| Systolic | 45-59 | 59-64 | 65-70 |
| Diastolic | 26-30 | 32-37 | 39-44 |
| Mean | 35-40 | 41-44 | 50-54 |
C Blood volume is 80 to 90 ml/kg of birth weight.
D Blood chemistry results on the first day of life are listed in Table 25-4.
TABLE 25-4
Blood Chemistry Results of the Newborn
| Na+ | 147 mEq/L (126-159) |
| K+ | 6.5 mEq/L (5.6-8.9) |
| Cl− | 104 mEq/L (98-114) |
| Total CO2 | 20 mEq/L (18-22) |
1. Newborn: 77% of total hemoglobin
2. 6 months: 4.7% of total hemoglobin
3. 8 to 11 months: 1% to 2% of total hemoglobin
4. Newborn P50 is lower than that of the adult; however, it does approximate the adult P50 by 4 to 6 months.
G Pulmonary function values in the normal newborn
1. Respiratory rate: 30 to 60 breaths/min, mean of 40 breaths/min
2. Tidal volume: 5 to 7 ml/kg of birth weight
3. Vital capacity: 45 ml/kg of birth weight
4. FRC: 25 ml/kg of birth weight
5. Total lung capacity: 60 ml/kg of birth weight
6. Total lung compliance: 2.6 ml/cm H2O
7. Deadspace volume: 2.0 to 2.2 ml/kg of birth weight
8. Oxygen consumption: 7 ml/kg/min
XII Comparative Neonatal Respiratory Anatomy (Table 25-5)
TABLE 25-5
Comparison of Neonatal and Adult Respiratory Anatomy
| Structure | Neonate | Adult |
| Head/body size ratio | 1:4 | 1:8 |
| Tongue size | Large | Proportional |
| Laryngeal shape | Funnel-shaped | Rectangular |
| Narrowest portion of upper airway | Cricoid cartilage | Rima glottidis |
| Shape and location of epiglottis | Long/C1 | Flat, C4 |
| Level of tracheal bifurcation | T3-4 | T5 |
| Compliance of trachea | Compliant, flexible | Noncompliant |
| Angle of mainstem bronchi | 10 degrees right, 30 degrees left | 30 degrees right, 50 degrees left |
| Anteroposterior transverse diameter ratio | 1:1 | 1:2 |
| Thoracic shape | Bullet-shaped | Conical |
| Resting position of diaphragm | Higher than adult | Normal |
| Location of heart | Center of chest, midline | Lower portion of chest, left of midline |
| Body surface area/body size ratio | 9 times adult | Normal |
A Neonatal head: Very large, approximately one fourth of total body length, in contrast to the adult head, which is approximately one eighth of body height.
1. The neonatal tongue is large in relation to the size of the oral cavity.
2. Tongue size is the primary factor forcing neonates to be obligate nose breathers. Normally only during crying will an infant actively ventilate through the mouth.
3. Because of tongue size, nasal continuous positive airway pressure (CPAP) without use of endotracheal intubation can be easily accomplished.
4. Positive end-expiratory pressure (PEEP) levels up to approximately 8 to 10 cm H2O can be used; PEEP levels above this usually cause an oral leak.
C Neonatal neck: Short and normally is creased.
1. The length is approximately 2 cm compared with 5 to 6 cm in the adult.
2. The neonatal larynx is funnel shaped, whereas the diameter of the adult larynx is more or less constant.
3. The narrowest portion of the neonate’s upper airway is the cricoid cartilage; in the adult, the rima glottidis is the narrowest point. The normal anteroposterior diameter of the neonatal glottis is approximately 7 to 9 mm, and the anteroposterior diameter of the cricoid cartilage is approximately 4 to 6 mm.
a. Endotracheal tube size must be based on the diameter of the cricoid cartilage.
b. The larynx is much higher in relation to the oral pharynx, and the opening of the larynx is more in a straight line than in the adult.
c. Therefore, an infant frequently extends the neck when in respiratory distress, whereas an adult will thrust the head forward.
1. Stiffer, relatively longer, and U- or V-shaped compared with a flatter and much more flexible epiglottis in the adult.
2. Located at the level of the first cervical vertebra; located in the adult at the fourth cervical vertebra.
1. The neonatal trachea is approximately 4 cm long compared with 10 to 13 cm in the adult.
2. The anteroposterior diameter is approximately 3.5 mm, and the lateral diameter is approximately 5 mm.
3. Normally the trachea is located to the right of the midline.
4. Bifurcation of the trachea is at the third or fourth thoracic vertebra in the neonate and at the fifth thoracic vertebra in the adult.
5. The angle of right and left mainstem bronchi widens with age. At birth the angles from the midline are 10 degrees for the right and 30 degrees for the left; in adulthood the angles are approximately 30 and 50 degrees, respectively.
6. Cartilage of the trachea may not be fully formed and often is more flexible than in the adult.
1. The neonatal thoracic cage has nearly equal anteroposterior and transverse diameters, and appearance generally is bullet shaped.
2. Range of movement is limited, and the ribs are basically fixed in a horizontal position.
3. The diaphragm is much higher than in the adult because of the relative size of the abdominal viscera. The diaphragm shows minimal movement during ventilation.
4. The heart is located in the center of the chest and slightly higher than in the adult. When external cardiac massage is performed, compression should be applied over the middle of the body of the sternum.
A The neonate’s body surface area in relation to its size is about nine times that of the adult.
B Thus maintenance of body heat is a significant problem for the neonate and is even more so for the premature infant.
C The skin of the neonate plays a much greater role in water and heat balance than that of the adult. This is a result of the large body surface area-to-weight ratio, which allows significant evaporation of water. In the neonate, 80% of body weight is water; in the adult, only 55% to 60% is water.