Chapter 246 Intrauterine Contraceptive Device Insertion
REQUIRED EQUIPMENT
TECHNIQUE
Mirena
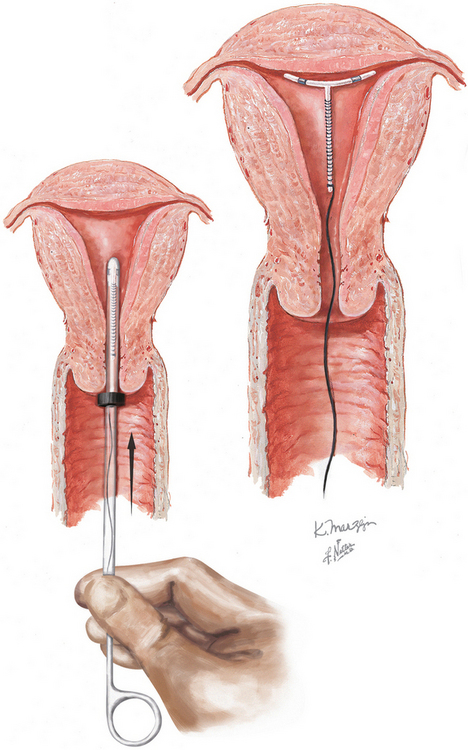
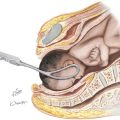
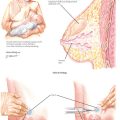
To place the IUCD in the uterine cavity, the tip of the IUCD and insertion tool are placed against the disinfected cervical os, and traction on the os is applied. Gentle pressure is exerted, advancing until the flange is approximately 1.5 to 2 cm from the cervix. This will allow sufficient room for the arms of the IUCD to expand on deployment. While this position is maintained the slider is pulled back to the raised horizontal line on the handle. This will release the arms from the inserter tube. After 30 seconds are allowed for the arms to regain their full extension, the inserter should be gently advanced until the flange meets the cervix, ensuring proper fundal placement of the device. While the inserter is held steady, the slider is pulled to its fully retracted position, releasing the IUCD. Being careful that the treads are hanging freely, the device is now removed and the threads trimmed about 3 cm from the cervix.
Oloto EJ, Bromham DR, Murty JA. Pain and discomfort perception at IUD insertion—Effect of short-duration, low-volume, intracervical application of two per cent lignocaine gel (Instillagel (TM)) —A preliminary study. Br J Fam Plann. 1997;22:177.
Walsh TL, Bernstein GS, Grimes DA, et al. Effect of prophylactic antibiotics on morbidity associated with IUD insertion: results of a pilot randomized controlled trial. IUD Study Group. Contraception. 1994;50:319.
Anteby E, Revel A, Ben-Chetrit A, et al. Intrauterine device failure: relation to its location within the uterine cavity. Obstet Gynecol. 1993;81:112.
Duenas JL, Albert A, Carrasco F. Intrauterine contraception in nulligravid vs parous women. Contraception. 1996;53:23.
Farley TM, Rosenberg MJ, Rowe PJ, et al. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339:785.
Grimes DA, Schulz KF. Prophylactic antibiotics for intrauterine device insertion: a metaanalysis of the randomized controlled trials. Contraception. 1999;60:57.
Hubacher D, Lara-Ricalde R, Taylor DJ, et al. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med. 2001;345:561.
Lethaby AE, Cooke I, Rees M. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 4, 2005. CD002126
Polis CB, Schaffer K, Blanchard K, et al. Advance provision of emergency contraception for pregnancy prevention. Cochrane Database Syst Rev. 2, 2007. CD005497
Zhou L, Xiao B. Emergency contraception with Multiload Cu-375 SL IUD: a multicenter clinical trial. Contraception. 2001;64:107.
American College of Obstetricians and Gynecologists. Intrauterine device. ACOG Practice Bulletin 59. Obstet Gynecol. 2005;105:223.
American College of Obstetricians and Gynecologists. Noncontraceptive uses of the levonorgestrel intrauterine system. ACOG Committee Opinion 337. Obstet Gynecol. 2006;107:1479.
Grimes DA. Intrauterine device and upper-genital-tract infection. Lancet. 2000;356:1013.
Mishell DRJr. Family planning. In: Katz VL, Lentz GM, Lobo RA, Gershenson DM, editors. Comprehensive Gynecology. 5th ed. Philadelphia: Mosby/Elsevier; 2007:306.
Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol. 2002;187:1699.
World Health Organization. Mechanism of action, safety and efficacy of intrauterine devices. Geneva: WHO, 1997.