CHAPTER 31 Intrathecal Therapies and Totally Implantable Drug Delivery Systems
INTRODUCTION
The discovery of opioid receptors in 19711 and their subsequent isolation in nerve tissue in 19732 in brain tissue3 and the spinal cord were foundational to the idea of using intraspinal opioids for pain control. In 1976, intrathecal opioid infusion was first reported in animals.4,5 Subsequently, intrathecal and epidural morphine was used in humans in 1979.6,7 Both modes of intraspinal delivery were effective in effectively controlling chronic nonmalignant and cancer pain.8 The discovery of a pain processing and modulating role for gamma-aminobutyic acid (GABA) receptors, adrenergic receptors, glutamate receptors, and calcium channels, amongst others, at both spinal and supraspinal levels, expanded intrathecal therapy to include non-opioid agents for intrathecal analgesic application.
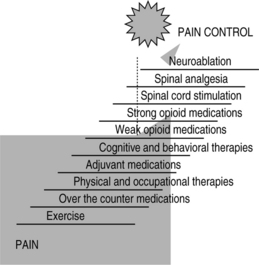
The treatment of chronic nonmalignant pain, like the treatment of cancer pain, requires that the treating physician understands the mechanism underlying the patient’s pain, the psychological and behavioral factors operant in perpetuating that pain, and the appropriate treating modalities to manage the diagnostic specific pain syndrome.9 It is important to know that there are no syndrome-specific therapies and there are many therapies to choose from for the management of chronic pain conditions. Because there are many therapies to treat chronic pain, from noninvasive to more invasive therapies, caregivers should choose therapies using a logical approach or algorithm to those choices. Fig. 31.1 is an example of a pain treatment continuum or algorithm of care that we use in our practice for patients with nonmalignant pain. This continuum of care starts with exercise at the bottom of the ladder moving up, by order of invasiveness and cost, towards more invasive therapies including implantable technologies and neuroablative therapies. This continuum of care should be placed in context by the reader and should not be considered guidelines to care. It is important to emphasize that spinal analgesia is a costly and an invasive therapy and therefore should be applied only when less invasive and less costly therapies have failed, including sequential systemic opioids trials.
INDICATIONS FOR INTRATHECAL THERAPY
Initially, intrathecal therapy was only used for patients with cancer-related pain syndromes, but over the years the indications for intrathecal therapy have expanded.10 Newer indications include neuropathic pain syndromes,11 failed back surgery syndrome (FBSS),12,13 complex regional pain syndromes,14 and head and neck pain.15 Intrathecal therapy is invasive and expensive treatment for chronic pain and should only be applied when certain criteria are applicable. These inclusion criteria for intrathecal therapy include:
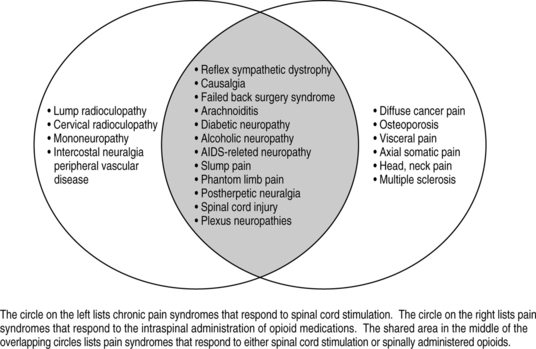
Likewise, when choosing implantable technologies for pain control such as spinal cord stimulation (SCS) or intrathecal therapy with opioids, it is equally important to know if the patient’s pain is nociceptive, neuropathic, or mixed nociceptive/neuropathic. Neuropathic pain is amenable to spinal cord stimulation for pain, while nociceptive pain is not. Intrathecal opioid therapy is useful for nociceptive pain, but minimally useful for patients with primary neuropathic pain. Intrathecal therapy with non-opioids such as local anesthetics, alpha-adrenergic agents or voltage sensitive, N-type calcium channel-blocking agents have been shown to be effective with neuropathic pain syndromes. Because intrathecal therapies are moderately efficacious for neuropathic pain syndromes when using admixtures of opioids and non-opioids there exists no clear-cut boundaries when choosing between SCS or intrathecal analgesic therapies for neuropathic pain syndromes. Fig. 31.2 is a Venn diagram that presents clear-cut diagnoses which respond to intrathecal opioids alone and those that respond to spinal cord stimulation alone. The overlapping gray area represents diagnoses that will respond to both therapies.
TRIALS FOR INTRATHECAL THERAPY
When a patient meets all criteria for intrathecal analgesia delivery and that patient has failed conservative therapies as outlined in Figure 31.1, that patient should undergo a trial for intrathecal therapy.
If the patient does not tolerate the initial drug trialed and is in need of trial of a second agent, only the drug reservoir, the existing tubing and the first 0.22 micron filter which is proximal to the more distal filter (filter nearest the catheter exit site) is changed. Because it is known that infection rates of these systems is proportional to the ‘fiddle-factor’ in personnel handling the systems, we adopt a hands-off policy to system changes and dressing changes. We first change the dressing of the system within 48 hours of surgery and then, unless the dressing becomes wet, we only change the dressing, once every 7 days. We recommend that only physicians or nurses familiar with this system be involved in dressing changes. Biopatch® is left unchanged for 7 days unless saturated with blood.
IMPLANTABLE DRUG DELIVERY SYSTEMS
The first drug delivery system approved for the delivery of intraspinal analgesics was the Infusaid, model #400 pump, which is no longer manufactured. This pump was a nonprogrammable, fixed-rate flow pump. Today, there are other FDA approved fixed-rate pumps including the Codman, model 400 pump (Fig. 31.3) and the Medtronic, Isomed Pump (Fig. 31.4). These pumps utilize a charging fluid that remains a fluid at room temperature, but becomes a gas exerting pressure on a metal bellows that extrudes drug at a fixed rate when implanted into a patient.
Other fixed-rate pumps used outside of the USA includes the Esox®, Archimedes®, Micromedes®, and the Anschutz pump®, all of which are fixed-rate pumps. Some new fixed-rate pumps are being developed by other companies and are not approved by the FDA in the USA today. An example is the AccRx Pump being developed by Advanced Neuromodulation Systems of Plano, Texas (Fig. 31.5). Because the rate of all of these fixed-rate pumps is preset and nonprogrammable, dosing changes are made by changing concentrations of the drug.

Fig. 31.5 AccRx, a fixed-rate pump in trials, manufactured by Advanced Neuromodulation Systems, Plano, Texas.
Medtronic’s Synchromed® system is the only totally implantable and programmable pump that is approved in the USA and Europe. Other companies, including Codman (a Johnson and Johnson company), Advanced Neuromodulation Systems, and Advanced Bionics (a Boston Scientific company), are developing totally programmable intrathecal delivery systems. Rate and therefore dose of drug are externally programmable (Fig. 31.6).
Besides increasing or decreasing a continuous rate of delivery of the drug, the Synchromed® system can be programmed to deliver drug in different configurations that includes programming a single bolus of drug, a timed bolus of drug, a complex continuous delivery of drug, and a continuous delivery of drug.
INTRATHECALLY ADMINISTERED OPIOIDS
Morphine remains the gold standard for intrathecal analgesic therapy. It has an extensive history, with an ample literature for intrathecal therapy, more than any other opioid.16 It is the only opioid approved by the Food and Drug Administration (FDA) for intrathecal analgesia. Other opioids are used for intrathecal therapy when patients are intolerant to intrathecal morphine and include hydromorphone,17 meperidine,18 methadone,19 fentanyl,20 sufentanil,20 and buprenorphine.21 The latter is a partial agonist that is used intrathecally in Europe. These agents are not labeled for intrathecal therapy and are not approved by the FDA, but are used empirically, based on efficacy and safety reported in the literature.
Morphine’s analgesic efficacy is well reported. A prospective survey by Kumar et al. of 16 patients (mean follow-up, 29±12 months) found that intraspinal morphine reduced pain scores for all types of pain by an average of 57%. Surprisingly, the greatest efficacy was found in patients with neuropathic pain and mixed nociceptive/neuropathic pain when compared to nociceptive pain (75% and 61% reductions, respectively).22 A national outcomes registry for low back pain collected prospective data on 136 patients using intraspinal infusion via implanted devices, 81% of whom received morphine. After 12 months, the Oswestry Low Back Pain Disability Scale ratings improved by 47% in patients with back pain and by 31% in patients with leg pain.23
The intrathecal opioid’s time to onset and duration of action, uptake and distribution, availability to supraspinal centers, and central nervous system (CNS) side effects are all governed by the opioid’s relative (to morphine) lipophilicity and its receptor affinity.24 Morphine’s low lipid solubility, high hydrophilicity, and high receptor affinity translates clinically to slow onset of action but prolonged analgesic action. Because of this hydrophilicity of morphine, it remains in the cerebrospinal fluid (CSF) longer and therefore is available to ascend to supraspinal centers through bulk flow of the CSF. Because morphine mixes in the CSF and is carried from low spinal centers to higher spinal centers and supraspinal centers, the position of the catheter tip within the thecal sac is relatively unimportant to provide segmental analgesia. On the other hand, this hydrophilicity of morphine increases the risks for CNS supraspinal side effects such as nausea, vomiting, sedation, and respiratory depression.
Intrathecal morphine delivery is associated with side effects. Both elevated concentrations of morphine-3-glucuronide, a metabolite of morphine in plasma, and an elevated plasma or CSF morphine-3-glucuronide over morphine-6-glucuronide ratio may play a pathogenic role in the development of the cutaneous dysesthesias, hyperalgesia, and allodynia and/or myoclonus seen with the long-term delivery of high-dose intrathecal morphine.25 It seems that morphine-3-glucuronide might exert this neuroexcitatory effect by indirect activation of the N-methyl-D-aspartate (NMDA) receptor, an inotropic glutamate receptor.26 Some patients might not tolerate morphine, but may tolerate hydromorphone, a relatively hydrophilic (to morphine) but more lipophilic agent than morphine. In some patients, supraspinal effects such as nausea and vomiting might be severe. In such cases, more lipophilic compounds which do not mix in the CSF such as fentanyl, sufentanil, or methadone might be chosen.
Intrathecal morphine delivery is known to be associated with hypothalamic–pituitary dysfunction. In a study conducted in Belgium, Abs et al.27 examined hypothalamic–pituitary axis function in 93 patients with non-cancer pain. Seventy-three patients received intrathecal morphine with a mean dose of 4.8 mg/day for a mean duration of 26.6 months. A 20-patient comparison group had comparable pain syndromes but was not treated with intrathecal morphine. A majority of patients of both genders in the intrathecal morphine group developed hypogonadotropic hypogonadism. Fifteen percent also developed central hypocortisolism and/or growth hormone deficiency, compared to none in the control group. Further, decreased libido occurred in 96% of men and 69% of women who received intrathecal opioids, compared with 10% and 20% of men and women, respectively, in the controlled group. Hormone replacement therapy ameliorated decreased libido in 10 of 14 males and 7 of 12 premenopausal females. The authors suggested further investigations to determine the need for systematic endocrine evaluations and replacement therapy in patients treated with long-term intrathecal morphine.
Hydromorphone is a mu agonist that is 8–10 times more lipid soluble28,29 and 5–10 times more potent than morphine. It is a hydrogenated ketone of morphine. Although it is not FDA approved, it is increasingly being used for intrathecal therapy.30,31 Hydromorphone is stable when contained in an infusion system (SynchroMed) and held at 37°C for 4 months.32 Because of its higher lipophilicity than morphine, hydromorphone has a quicker onset of action, a shorter duration of action and, for the same reason, fewer supraspinal side effects because of less rostral spread.
Because of the incomplete cross-tolerance among opioids, switching from morphine to hydromorphone is a consideration when morphine use is associated with too many side effects or poor analgesia. The initial dose of hydromorphone should be 50% of its equianalgesic dose to morphine (equianalgesia is 1:5–10) and should be titrated higher until analgesia is achieved.
Hydromorphone is not only successful in delivering intraspinal analgesia33 but also has a lower incidence of side effects than morphine when given epidurally. In the retrospective study comparing intrathecal morphine to hydromorphone by Anderson et al.,30 37 patients received intrathecal hydromorphone after either inadequate analgesia or unmanageable side effects occurred during treatment with intrathecal morphine sulfate alone. Pain scores improved during the 10 months’ mean follow-up after changing to hydromorphone. Drowsiness and nausea lessened after patients were switched from morphine to hydromorphone and leg edema subsided temporarily, but eventually reoccurred after extended hydromorphone exposure
A known complication of intrathecal therapies is the development of intrathecal granuloma, an intradural but extramedullary mass associated with either high dose or high concentration of intrathecally delivered agents. This complication of intrathecal analgesia will be discussed later in this chapter. In contrast to morphine, hydromorphone exposure intrathecally was not associated with the development of catheter tip granuloma in a sheep model when daily doses, equianalgesic to doses of morphine that produced granulomas, were given.34
However, hydromorphone when used in humans is known to be associated with the development of tip granuloma. In an initial report, the formation of inflammatory masses at the catheter tip was reported in 9 patients who received hydromorphone, either alone or mixed with other drugs.35 A subsequent review of the same report and database and an additional six case reports identified was published with the number of new cases reported in the literature to be 15.36 It still is unclear whether these 15 reported cases of granuloma were due to the intrathecal hydromorphone delivered or due to other agents since all patients had, at one time or another, other agents, either alone or in combination with hydromorphone, in their pumps.
The neuroexcitatory side effects of opioids, e.g., hyperalgesia, are not reported to be more frequent with hydromorphone than morphine, although hydromorphone-3-glucuronide is a more potent neuroexcitant than morphine-3-glucuronide37 which could be responsible for these symptoms.
Meperidine is the only member of the opioid family that has clinically important local anesthetic activity in doses used for analgesia. Because of this quality of meperidine, it is the only opioid in current use that is active as a sole agent for spinal anesthesia (not analgesia). Surgical procedures to the lower limbs, inguinal area, perineum or c-section have been performed using spinal meperidine alone.38,39 Meperidine has been used for labor analgesia and found not to increase c-section deliveries.40 A 0.5 mg/kg intrathecal dose of meperidine produces anesthesia for up to 6 hours or longer.38 A dose of 1 mg/kg or a total dose of 100 mg has been associated with respiratory depression, bradycardia, and hypotension.18,41
There is limited literature on the use of continuous intrathecal meperidine via implantable infusion pumps. In a case report by Harvey et al.42 a woman with chronic low back who failed other medical interventional treatment modalities achieved significant pain relief using a continuous intrathecal meperidine infusion. Another case report also showed similar success.43 According to Chrubasik et al., the dose of meperidine should be 25–30 times higher than morphine to maintain equianalgesia.44 A dose of up to 60 mg per day appears to be safe.45
Methadone. In a survey by Hassenbusch and Portenoy, 2–4% of respondents cited use of this opioid.46 Methadone is more lipid soluble than morphine, hydromorphone, and meperidine, and therefore does not produce noticeable migration within CSF to supraspinal centers. Methadone, based on this lipophilicity, should have fewer supraspinal side effects than morphine when given intrathecally.
The L-isomer of methadone has mu agonist activity and D-isomers have noncompetitive antagonism of NMDA receptors.47 In vitro studies suggest that methadone induces desensitization of the delta opioid receptor by uncoupling the receptor from its underlying G-protein. This delta opioid activity is critical for the development of morphine-induced tolerance and dependence. Animal studies showed that D-methadone reduces the development of morphine tolerance and NMDA-induced hyperalgesia by virtue of its NMDA receptor antagonist activity.48
Studies of the prolonged use of intrathecal methadone for cancer and non-cancer pain showed overall effectiveness between 37.5% and 80% for those populations studied based on greater than 50% reduction49–51 in pain or pain reduction combined with improved scores on quality of life questionnaire.19 These studies involved both cancer and non-cancer patients. Methadone was administered at total daily dosages of 5–60 mg and the duration of treatment ranged from 3 days to 37 months.
Fentanyl is an opioid analgesic which preferentially binds to mu receptors. It is highly lipophilic and has a fast onset of action in the order of 5 minutes with a peak analgesic effect of approximately 20 minutes, when given epidurally. Due to lipophilicity, its effect is largely segmental, although continuous intrathecal infusion is associated with receptor saturation and mixing in the CSF. It is equipotent when given epidurally or intravenously.52
As fentanyl is 75–100 times more potent than morphine sulfate, lower doses are needed to produce similar analgesia. Thus, considerations for side effects must be made, especially since fentanyl side effects have been noted to be more severe than those of morphine sulfate. Cephalad spread of fentanyl which could lead to respiratory depression does occur, but less so than morphine. Although it is uncertain whether fentanyl’s exact location of action is systemic or spinal, the systemic mode of action appears to be favored by some.53 While scientific data on epidural and intrathecal fentanyl are evolving, there does appear to be a role for fentanyl in treating both acute and chronic pain processes.
Two retrospective studies on intrathecal fentanyl have been reviewed. One study of 122 patients examined the complications associated with implantable drug delivery systems. This study included two patients with the combination of fentanyl and bupivacaine54 and neither of these patients experienced serious adverse events. In another study, eight patients out of a total of 29 patients with intrathecal therapy received intrathecal fentanyl, 10.5–115 mcg/day for a mean duration of 31 months.55 The authors reported a 68% reduction in pain and an overall satisfaction of 3.25 on a scale of 1 (poor) to 4 (excellent) in all eight patients.
Due to being extremely lipid soluble, it largely diffuses into the central neural tissue when administered intrathecally, leaving less bulk drug available for rostral movement, and thus fewer central side effects such as nausea, vomiting, itching, urinary retention, and respiratory depression. Because of this lipid solubility, after intrathecal administration, sufentanil concentrations in the CSF decrease more quickly when compared to morphine. The mean residence time in the CSF, i.e., the time required to eliminate 63.2% of the drug, is approximately 0.9 hours after injection of 15 mcg sufentanil56 while that of morphine (0.05 mg/kg) is 2.3 hours.57
The higher affinity of sufentanil to mu opioid receptors when compared to morphine gives sufentanil a potential benefit in delaying the development of tolerance to the drug. It has been postulated that agents with high efficacy and receptor reserve, i.e. sufentanil, should produce less tolerance than agents of lower efficacy and lower receptor reserve, i.e. morphine.58 Rats develop, over time, less tolerance to intrathecal sufentanil when compared to morphine. Also, sufentanil requires the occupancy of fewer mu receptors to produce antinociception than does morphine.
In a survey performed by Hassenbusch and Portenoy,46 nearly 20% of pain clinicians have used either fentanyl or sufentanil in intrathecal drug delivery systems; however, there are no clinical data of long-term use of intrathecal sufentanil in humans. It is known that in humans a 10 mcg bolus of intrathecal sufentanil produces analgesia within 5 minutes with a duration of about 19 minutes.59
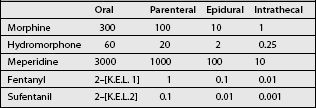
Because of its high lipophilicity, this agent is used when more hydrophilic drugs produce excessive supraspinal side effects. When converting morphine to sufentanil in an implanted pump, we, based on the potency ratio of sufentanil to morphine, arbitrarily use a dose conversion of 1 mcg of sufentanil to 1000 mcg (that is 1 mg of morphine) as shown in Table 31.1
Animal toxicology studies of intrathecal sufentanil have shown no detectable pathological effects on spinal cord histology. However, in a study performed in sheep, which have relatively smaller intrathecal spaces, a rather large dose of 7.5 mcg/kg of sufentanil did produce demonstrable neurotoxic changes. These authors did demonstrate a low-grade inflammatory response to the intrathecal catheter itself and concluded that the response was thought to represent a foreign body response.60
INTRATHECALLY ADMINISTERED NON-OPIOIDS (ADJUVANTS)
Bupivacaine. Local anesthetics are the most common adjuvant agents used with opioids for intrathecal therapy. By the year 2000, more then 20% of patients with intrathecal therapy for pain control had local anesthetics in their implantable pumps. According to this survey of implanting physicians, 60% of the responding physicians used morphine–bupivacaine combinations.46
Bupivacaine HCl has been shown to be stable and compatible with the Medtronic SynchroMed implantable pump, maintaining 96% of its initial concentration with chronic exposure to the system after 12 weeks at 37°C.61 Further, Trissel et al.62 in a stability study, showed that combinations of morphine sulfate, 5 mg/mL plus bupivacaine, 2.5 mg/mL, or morphine sulfate, 50 mg/mL plus bupivacaine, 25 mg/mL, packaged in 20 mL aliquots in plastic syringes and maintained at 4°C or 23°C for 60 days or −20°C or 37°C for 2 days remained stable. In other words, the morphine remained stable at, or greater than, 97% and the potency of bupivacaine remained greater than 95%, after the study. Frozen samples exhibited microparticulates upon thawing, suggesting that freezing of morphine–bupivacaine combinations should be avoided.
Bupivacaine is the intrathecal local anesthetic most widely used in humans. Other local anesthetics including tetracaine and lidocaine have been tried but their use has been abandoned due to their relative known toxicity.63 Recently, ropivacaine has been tried in humans;64 however, it offers little advantage over the less expensive bupivacaine.
Human postmortem studies and the long-term usage of bupivacaine do suggest clinical safety of the long-term infusion of low doses of intrathecal bupivacaine.65 There are, however, reports in the anesthesia literature of permanent neurological sequelae, specifically the cauda equina syndrome, when some local anesthetics, specifically 5% lidocaine, are used for spinal anesthesia, particularly when delivered through small-bore intrathecal catheters.66 It is unclear, as reported by the authors, whether this complication was drug related, catheter related, technique related, or a combination of these.
The positive analgesic response with the use of local anesthetic–opioid combinations intraspinally, for the treatment of cancer and non-cancer-related pain, has been previously reported.67,68 Deer et al., in a retrospective study of 109 patients, 25 with cancer pain and 84 with nonmalignant pain, studied the effect of the addition of bupivacaine intrathecally to patients who did not receive analgesia with their intrathecal opioids alone (morphine, mean dose of 8 mg/day, or hydromorphone, mean dose 1.5 mg/day).69 All patients who had a poor response to the opioid alone, who were switched to opioid–bupivacaine combinations, reported significantly lower pain scores and experienced a mean opioid dose reduction of 23. In a nonrandomized, noncontrolled prospective study, performed by van Dongen et al.,70 it was found that in 5 of 20 cancer patients with inadequate analgesia with morphine alone, the addition of bupivacaine (5–21.6 mg) improved pain relief. However, in the study, the doses of morphine were relatively low (1.2–7.2 mg/day). Recently, Mironer et al., in a randomized, double-blind, multiple-phase, crossover trial, comparing intrathecal morphine or hydromorphone alone to morphine and or hydromorphone with the addition of bupivacaine, found a statistically significant improvement in quality of life scores, but no change in pain scores, in the group with the addition of bupivacaine, when compared to the group with opioids alone.71 Lundborg et al. reported on their clinical experience using intrathecal bupivacaine infusion for three patients with complex regional pain syndrome.72 These 3 patients received bupivacaine at an average dose of 54 mg/day, maximum of 90 mg/day, and were followed for an average of 374 days during their infusion for an average of 1900 days. Although there was a moderate decrease in pain, there was no improvement in allodynia, edema, or trophic changes.
Clonidine is not approved for clinical use in the USA; however, its use is widespread and safety has been established. Because of this, and because of a significant literature on its use, this agent for intrathecal use should not be considered experimental. Some authors have found that the combination of clonidine with an opioid works synergistically and thus the combination provides better analgesia at lower doses than if the drugs were given alone.73,74
Daily intrathecal dosages in humans have ranged from 3 mcg/kg to 60 mcg/kg. Studies in different animals and humans have demonstrated no neuropathology after intraspinal clonidine.75 The main side effect of clonidine, when given intrathecally, is hypotension. Humans may also experience decreases in heart rate but no metabolic changes. Other side effects of intrathecal clonidine include dry mouth, drowsiness, dizziness, and constipation. Sudden withdrawal of clonidine can precipitate agitation and rebound hypertension.76
Some authors believe that clonidine may be useful in facilitating a ‘spinal opioid holiday’1 in patients who have become tolerant to high doses of opioids.77 Clonidine may also be effective in neuropathic pain78 and sympathetically mediated pain syndromes.79
Clonidine has been used safely in numerous patients and is stable with various agents. It is stable in a titanium reservoir infusion pump (SynchroMed, Medtronic, Inc., Minneapolis, Minn., USA) in concentrations of 0.05 mg/mL and 1.84 mg/mL, in combination with morphine sulfate at a concentration of 2 mg/mL and 20 mg/mL at 37°C for 90 days and 54 weeks, respectively.80 When an admixture of morphine sulfate, bupivacaine hydrochloride, and clonidine hydrochloride was incubated in SynchroMed-EL pumps (Medtronic, Minneapolis, Minnesota, USA) at 37°C for 90 days or stored in glass vials at 4°C and at 37°C, as controls, the concentrations remained at greater than 96% of the original concentrations.81 A color change from colorless to light yellow between days 30 and 60 did not affect the solution’s stability. No particulate matter and no clinically significant changes in osmolality were observed during this 90-day study period.
Hassenbusch et al. reported a 20-month prospective phase I/II cohort study of 31 patients (6 with cancer pain, 25 with nonmalignant pain) who received intrathecal clonidine alone at dosages between 144 and 1200 mcg (mean 872 mcg).82 Twenty-two patients achieved greater than 50% pain and symptom reduction without intolerable side effects. At 6 months, 77.3% (17/22) achieved continued good pain relief, and 59% of the total group were considered long-term successes (mean follow-up 16.7 months). Clonidine dosages did not change significantly over time in this group. Poor long-term outcomes were related to inadequate pain relief (n=4), intolerable side effects such as hypotension (n=2), impotence, lethargy, and malaise (n=1 each).
A retrospective survey of Ackerman et al. observed that only 2 of 10 patients treated with clonidine alone (doses 75–950 mcg/day) had good pain relief after 7–11 months of intrathecal therapy.83 The addition of clonidine (75–950 mcg/day) to either morphine 0.15–15 mg/day (5 patients) or hydromorphone 200–800 mcg/day (10 patients) similarly yielded very limited effect; only 3 of these patients achieved long-term pain relief (7–11 months). Although the results from this study and that of Hassenbusch et al.82 appear to contradict each other, it should be noted that Ackerman and colleagues’ report was retrospective in nature and their group was not homogeneous, since many patients received either many drugs over time and/or more than one drug at a time during the therapy period.
Siddall et al., in a randomized, prospective study, compared the efficacy of intrathecal administration of saline, morphine (0.2–1 mg), clonidine (50–100 μg), and the combination of clonidine and morphine.84 In phase I of the study, each patient received saline, clonidine, and morphine in a random sequence. One dose per day of each drug was titrated over 3 days until the occurrence of either analgesia (defined as a >50% reduction from baseline pain score) or side effects. During phase II of the study, each patient received a combination consisting of 50% of the final dosage of morphine combined with 50% of the final dosage of clonidine. The authors compared the proportion of those patients who had a positive response at any time during the assessment. Five of 15 patients responded positively to saline, 3 of 15 responded to the largest dosage of clonidine alone, 5 of 15 responded to the largest dosage of morphine alone, and 7 of 15 to the combination of one-half the largest dosage of clonidine plus one-half the largest dosage of morphine. These data suggest that morphine and clonidine is a worthwhile combination to achieve improvement in analgesia.
In a prospective study, Uhle et al. reported on 10 patients with neuropathic pain syndromes who received clonidine at an average of 44 mcg/day in combination with morphine sulfate or buprenorphine.85 All patients had a 70–100% reduction in pain after the addition of clonidine to either morphine or buprenorphine. Four of eight patients with non-neuropathic pain syndrome also appeared to benefit from the addition of clonidine. The most frequent adverse effects noted in this study were hypotension, fatigue, dry mouth, and impaired bowel (N=10, 4, 3, 1 patients, respectively).
Tizanidine is an alpha2 agonist used clinically as a muscle relaxant. It appears to have a potential role as an intrathecal analgesic drug. In a dog model, Kroin et al.86 reported that tizanidine and clonidine at dosages of 3.0–18.0 mg/day yielded equivalent analgesia using a thermal withdrawal test, but that clonidine, when compared to tizanidine, was associated with greater toxicity (hypotension, bradycardia, and bradyarrhythmias). A further toxicity study of 3–6 mg/day in dogs was performed and these authors found no significant side effects or differences in spinal cord histopathology between the 3 mg/day and 6 mg/day groups.
Kawamata et al.87 studied the effects of clonidine and tizanidine on a rat model of neuropathic pain. Sprague-Dawley rats were chronically implanted with lumbar intrathecal catheters, and the sciatic nerve was loosely ligated. Twenty-one to 28 days after surgery, the rats received intrathecal clonidine (0.3, 1.0, and 3.0 μg) and tizanidine (1.0, 2.0, and 5.0 μg), and the antihyperalgesic effects of thermal and mechanical stimuli were examined. In addition, changes in blood pressure and heart rate, sedation level, and other side effects after intrathecal administration of drugs were studied. The authors found that the administration of 3.0 micrograms of intrathecal clonidine or 5 micrograms of tizanidine significantly reversed both thermal and mechanical hyperalgesia. The administration of 3.0 micrograms of intrathecal clonidine, but not 5.0 micrograms of tizanidine, significantly decreased mean blood pressure and heart rate and produced urinary voiding. A greater sedative effect was produced by the clonidine when compared to the tizanidine. The authors concluded that the antihyperalgesic dose of intrathecal clonidine and the antinociceptive doses produced several side effects. Intrathecal tizanidine at the dose that reversed hyperalgesia would be preferable for neuropathic pain management because of absence of hypotension and bradycardia and lower incidence of sedation.
Ziconotide is an omega-conopeptide, a synthetic form of the cone snail peptide, varpi-conotoxin MVIIA. Ziconotide is a neuron-specific, N-type voltage-gated calcium channel blocking agent with an analgesic and neuroprotective effect.88 Spinally administered ziconotide blocks neurotransmitter release from primary nociceptive afferents to prevent pain signal propagation to the brain. It has an advantage over intrathecal morphine in that it is a non-opioid and tolerance does not developed after its prolonged use. Although it has been used extensively in humans, ziconotide is approved by the FDA for clinical intrathecal use.
Ziconotide is indicated for both nociceptive and neuropathic type of pain. It has been shown to be effective not only for chronic pain but also for acute postoperative pain. Atanassoff et al.89 performed a randomized, double-blind pilot study in patients undergoing elective total abdominal hysterectomy, radical prostatectomy, or total hip replacement. After intrathecal injection of local anesthetic and before surgical incision, a continuous intrathecal infusion of either placebo or 1 of 2 doses of ziconotide (0.7 mcg/hour or 7 mcg/hour) was started and continued for 48–72 hours postoperatively. Thirty patients received the study drug and 26 of them were evaluable for efficacy. It was found that the mean daily patient-controlled analgesia (PCA) morphine equivalent consumption was less in patients receiving ziconotide than in placebo-treated patients. The visual analogue scale of pain intensity (VASPI) scores during the first 8 hours postoperatively were remarkably lower in ziconotide-treated than in placebo-treated patients. In 4 of 6 patients receiving the high dose of ziconotide (7 mcg/hour), adverse events such as dizziness, blurred vision, nystagmus, and sedation led to discontinuation of the study drug infusion. After ziconotide discontinuation, these symptoms resolved.
In a double-blind, placebo-controlled, short-term trial of ziconotide in 257 patients with non-cancer pain by Presley et al.,90 31% of the ziconotide-treated patients reported significantly lower pain scores, compared to 6% of the placebo-treated patients. Moderate to complete pain relief was achieved in 43% of patients in the ziconotide arm versus 18% in the placebo arm. Patients who received ziconotide also decreased their systemic opioid intake and reported improved quality of life compared to patients who received placebo.
Staats et al., in a double-blind, placebo-controlled, randomized trial,91 studied 111 patients (ages between 24 to 85 years) with cancer or AIDS pain with VASPI score of 50 mm or greater. Patients were randomly assigned in a 2 to 1 ratio to receive ziconotide or placebo treatment. Intrathecal ziconotide was titrated over 5–6 days followed by a 5-day maintenance phase for responders and crossover of nonresponders to the opposite treatment group. Pain relief was moderate to complete in 53% of patients in the ziconotide group compared to 17.5% in the placebo group. Five of 111 (4.5%) patients receiving ziconotide achieved complete pain relief.
In spite of being a promising analgesic, ziconotide, like all analgesics, is not free of side effects, and side effects increase with a rapid titration of the agent and decrease with reduction in dose.91 Adverse events associated with intrathecal ziconotide have included vestibular disorders such as nystagmus, abnormal gait, nausea/vomiting and dizziness, urinary retention, blurred vision, diplopia, memory impairment, and orthostatic hypotension.90–92 Apparently, these side effects do decrease over time. In the study by Penn and Paice93 involving the use of intrathecal ziconotide in three patients with both neuropathic and nociceptive pain mechanisms, in dosages between 0.2 mcg/hour and 5.3 mcg/hour, significant pain relief was achieved. Side effects seen included nausea, diarrhea, nystagmus, dysmetria, sedation, confusion, and hallucinations. With intrathecal ziconotide doses of 0.9 mcg/hour or greater, much more serious side effects occurred including disorientation, agitation, and in two of three patients, unresponsiveness, which only resolved at some point after intrathecal ziconotide was discontinued.
Midazolam has been used as a sole drug for intrathecal therapy for the management of postoperative pain.94 In animal studies, it has been shown that the antinociceptive analgesic effect of intrathecal midazolam can be reversed by naloxone; more specifically, this analgesic effect of intrathecal midazolam was blocked by delta-selective antagonists in rats.95 Midazolam has been shown to have a synergistic analgesic effect when given with bupivacaine intrathecally, and in animals this synergistic analgesic effect was demonstrated, giving midazolam with clonidine and with NMDA and AMPA receptors antagonists in rats.96,97
Because of conflicting reports of different toxicities in differing animal models, the use of midazolam, although used emperically by some in humans, continues to be controversial. Toxicity has not been associated with the intrathecal infusion of midazolam in studies on rats,96 cats,98 pigs and sheep;99 however, there has been, in contrast to these studies in rats, cats, pigs and sheep, evidenced toxicity with the intrathecal infusion of midazolam in rabbits.100 For a detailed review of preclinical safety issues with midazolam see the excellent review by Yaksh and Allen.101
In a prospective study by Rainov et al., 26 patients received a combination of various intrathecal agents as follows: four patients received midazolam 0.4 mg/day plus morphine sulfate 0.5 mg/day, clonidine 0.03 mg/day, and bupivacaine 1.0 mg/day; four patients received morphine/bupivacaine/midazolam; and two patients received morphine/midazolam.102 Overall, 19/26 patients (73%) achieved good to excellent pain relief, 6/26 patients (23%) achieved sufficient pain relief, and one patient reported poor pain relief. No information was provided regarding outcomes according to drug combination received on-study.
Baclofen is a GABA-beta agonist that has been used to treat spasticity since 1984. Intrathecal baclofen is FDA approved for the treatment of spasticity caused by upper motor neuron disease; however, questions remain as to whether baclofen is an analgesic when given intrathecally. Baclofen is stable alone or in combinations with clonidine when placed into intrathecal pump systems.103
Other non-opioid agents used intrathecally
Other agents shown to be effective for the relief of pain when given intrathecally include neostigmine,104,105 adenosine,106,107 and octreotide.108,109
Neostigmine is an acetylcholinesterase (Ach) inhibitor which increases the availability of acetylcholine at the neuromuscular and the dorsal horn level. Since muscarinic receptor2 agonists produce antinociception in rats,110 and because muscarinic receptors have been detected at the level of the substantia gelatinosa and to a lesser extent in laminae III and V of the dorsal gray matter of the spinal cord, it was felt that neostigmine would be an effective analgesic if given intraspinally in humans. Neostigmine does produce analgesia in humans; however, its use is associated with an extremely high incidence of nausea and vomiting.105
Endogenous adenosine may be involved in the mediation of the spinal antinociception induced by descending adrenergic fibers originating from the locus ceruleus. This antinociceptive effect is blocked by intrathecal aminophylline (an adenosine receptor antagonist).111 Kekesi et al.106 reported that adenosine has little antinociceptive efficacy during continuous intrathecal administration, but appears to potentiate the effect of endomorphin-1. Eisenach et al.107 compared intrathecal adenosine with intravenous adenosine for chronic neuropathic pain in seven patients with hyperalgesia and allodynia. In intrathecal group, spontaneous pain was not relieved; however, evoked dysesthesias such as allodynia and mechanical hyperalgesia were markedly reduced. Five of seven patients developed back pain after the induction of adenosine.
Octreotide (Sandostatin), a synthetic octapeptide derivative of somatostatin, has been found to be beneficial in the treatment of chronic pain, although the mechanisms underlying its therapeutic effect are not completely understood. Somatostatin is distributed in the substantia gelatinosa. It has been shown to have analgesic effect without adverse effects if given intrathecally, but the high cost, more than US$20 000.00 per year, prevents its widespread use.113 In a recent double-blinded study, octreotide was found to exert primarily an antihyperalgesic rather than analgesic effect on visceral pain perception.112
SELECTION OF DRUGS FOR LONG-TERM INTRATHECAL INFUSION
In year 2000, an expert panel of physicians was convened to review what was known about common practices of European and American physicians using intrathecal agents and to review what was known about the drugs that were being used by these physicians up to 1999. This Polyanalgesic Consensus Conference 2000 published their review of common and experimental intrathecal agents and proposed guidelines for the appropriate use of these agents.112 Most recently a second expert panel, the Polyanalgesic Consensus Conference 2003, convened to review newer information since 1999 and to modify the 2000 guidelines. The purposes of this expert consensus panel were to: review the medical literature since 1999 pertaining to intraspinal agents; update the algorithm for intraspinal drug selection; propose guidelines for optimizing drug concentration and dosing during therapy; develop consensus regarding the evidence required to support use of a drug for long-term intrathecal infusion; and clarify existing regulations and guidelines pertaining to the use of compounded drugs for intrathecal administration.113 Regarding guidelines for intrathecal therapy, the panel suggests six lines of approach (Fig. 31.7).
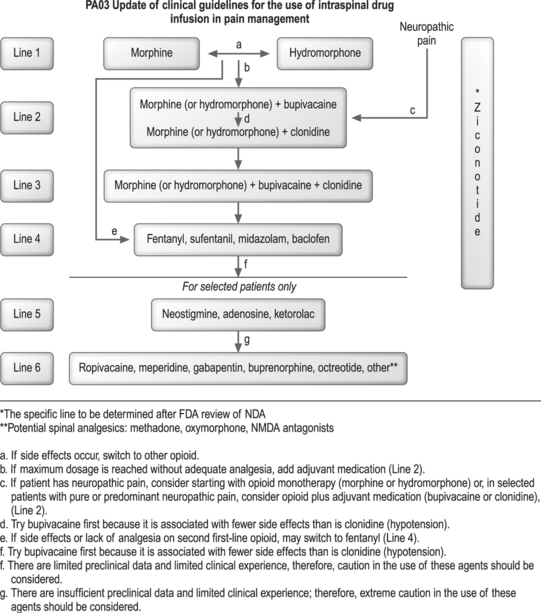
Fig. 31.7 Amended guidelines of the Polyanalgesic Consensus Conference, 2003.
(Used with permission of the authors.)
According to the recommendations of this expert panel, first-line therapy, a Line-1 approach, includes morphine, the only opioid approved by the FDA for long-term intrathecal administration, and hydromorphone, which is supported by an extensive medical literature28–34 and clinical experience. In order to avoid the risk of the development of catheter tip granuloma, the panel recommends an upper limit for drug dosage and concentration as shown in Table 31.2
Table 31.2 Recommended intrathecal dosages and concentrations
| Drug | Dosage (mg/day) | Concentration (mg/mL) |
|---|---|---|
| Morphine | 15 | 30 |
| Hydromorphone | 10 | 30 |
| Bupivacaine | 30 | 38 |
| Clonidine | 1.0 | 2.0 |
Recommended intrathecal dosages and concentrations106 to prevent the formation of intrathecal tip granuloma. These recommendations represent general recommendations of the expert consensus panel and are dependent upon the specific patient and the clinical experience of the physician; thus, maximum dosage and/or concentration may vary from these. Re volorem num voloreet ut vullamc ortisim zzriliscilit prate
If side effects come before efficacy, or if efficacy is not established at the highest recommended dose with either morphine or hydromorphone (as shown in Table 31.2), the panel recommends switching to the alternate drug on Line-1 or moving to Line-2 therapy. The latter approach, moving to Line-2 therapy, is appropriate if the pain is neuropathic in nature. Some of the experts on the panel endorsed the idea of omitting Line-1 completely and moving to Line-2 if the patient has severe neuropathic pain that had not responded to systemically administered opioids.
Line-4 therapies include the lipophilic opioids, fentanyl and sufentanil, the GABA-alpha agonist, midazolam, and the GABA-beta agonist, baclofen. The strategy here, basically, is to switch opioid treatment from morphine or hydromorphone to fentanyl or sufentanil. Baclofen is approved for a long-term infusion for spasticity. Data, however, do support its use as an analgesic agent for intrathecal therapy. Although the data for their safety and efficacy are limited, intrathecal fentanyl and sufentanil have been used in clinical practice.20,53 As stated before, the high lipophilicity of these agents minimizes their diffusion to rostral pain centers. Therefore, the supraspinal side effects induced by these agents are less than those induced by the more hydrophilic agents such as morphine or hydromorphone. In the United States, midazolam is not available commercially as a preservative-free compound for intrathecal therapy.101
COMPOUNDING OF DRUGS FOR INTRATHECAL DELIVERY
Because many physicians use off-labeled analgesic agents for intrathecal delivery, and because many of these agents are not manufactured in concentrations necessary for intrathecal use, many physicians rely on the compounding of these off-labeled analgesics for their practices. The Polyanalgesic Consensus Conference 2003113 felt it necessary to guide this part of the physician’s practice [compounding] to assure that patients would not be hurt by poor practice. According to the American Society of Health System Pharmacists (ASHSP),114 compounding is defined as the process of mixing of ingredients to prepare a medication for patient’s use, including dilution, admixture, repackaging, reconstitution, and other manipulations of sterile products.
The United States Pharmacopoeia (USP)115 and the ASHSP have issued standards for compounding sterile products. These standards also apply to the compounding of solutions for intrathecal drug delivery. According to these standards, all sterile compounded and preservative free preparations, administered via an intrathecal delivery system, are classified as, at least, level 2 (medium risk), and many are classified as level 3 (high risk) preparations.114,115
The Polyanalgesic Consensus Conference 2003 suggests a set of considerations that need to be respected when preparing com pounded formulations for intraspinal delivery.113 The following is a list of does and don’ts when compounding intrathecal agents:
THE SIDE EFFECTS OF INTRASPINAL ANALGESIC THERAPY
Gastrointestinal complications
Constipation. The incidence of constipation from intrathecal delivery of opioids is less than when the opioids are given systemically.116 The management of intrathecal opioid induced constipation is the same as the management of systemic opioid induced constipation using stool softeners and gentle laxatives. If this approach does not resolve the problem, one should consider switching to another opioid or, if possible, lowering the dose of the opioids.
Nausea and vomiting. Opioids are thought to induce nausea and vomiting by a direct action on the chemoreceptor trigger zone (CTZ), an area of the hindbrain, which is outside the blood–brain barrier. This is supported by evidence showing that ablation of the CTZ prevents the induction of vomiting by opioids.117 The mechanism of action of opioids in emesis is, however, complicated. Biphasic dose–response curves have been reported and, in certain circumstances, opioids can have antiemetic actions.118 Vomiting as a side effect of intrathecal administration of opioids is infrequent; however, should it happen, antiemetic therapy usually will resolve the problem. If nausea and vomiting persist, the clinician should use an agent that has less spread to supraspinal centers when given intrathecally. Agents that have less spread to supraspinal centers are agents of greater lipophilicity, including methadone, fentanyl, and sufentanil.
Urinary retention is an early common side effect of intrathecally administered opioids in males, especially elderly males, and is infrequent in females. Urinary retention is most common when therapy is first initiated, but fortunately resolves spontaneously with continued therapy. Bethanechol (Urecholine) is the agent of choice for urinary retention.116 It is indicated for the treatment of acute postoperative and postpartum nonobstructive (functional) urinary retention and for neurogenic atony of the urinary bladder with retention. Dosage must be individualized, depending on the type and severity of the condition to be treated. The usual adult oral dose ranges from 10 to 50 mg three or four times a day. The effects of the drug appear in 30–90 minutes and persist for approximately 1 hour. If necessary, the effects of the drug can be abolished promptly with atropine. Should urinary retention persist, in spite of adequate pharmacologic therapy, decreasing the dose of intrathecal medication would be the next step, and if that does not work, perhaps changing agents from a hydrophilic agent to a lipophilic agent would be appropriate.
Pruritus a fairly common side effect. The incidence of this opioid side effect is higher in opioid-naive patients than in those patients who are opioid tolerant. The use of antihistamines may ameliorate the problem; however, using opioid antagonists or partial antagonists, in combination with opioids in the pump, has been suggested as a possible solution.45
Central nervous system side effects
Sexual and endocrine dysfunction. The prevalence of these side effects is quite high in patients who are receiving intrathecal opioid therapy. In a study of 73 patients, the majority had hypogonadotropic hypogonadism, 13% developed central hypocorticism, and 17% growth hormone deficiency. These abnormalities affected the sexual function in this group of patients.27
Leg and pedal edema. These side effects are not infrequent in patients receiving intrathecal opioid therapy. In a study by Aldrete and da Silva et al.,119 five out of 23 patients (21.7%) who received intrathecal opiates for longer than 24 months developed pedal or leg edema. However, the symptoms improved by the discontinuation or reduction of the dose; the most effective treatment was elevation of the legs and dose reduction. The use of diuretics with elastic stockings can be helpful in overcoming this side effect. In our experience, the incidence of peripheral edema is greater with the use of hydrophilic agents such as morphine or hydromorphone than with lipophilic agents such as fentanyl, sufentanil, and methadone. Consequently, if peripheral edema develops and does not respond to diuresis, we switch hydrophilic agents to lipophilic agents.
Tolerance is a phenomenon in which exposure to a drug results in the diminution of effect, either desired or undesired, or the need for a higher dose to maintain the effect, desired or undesired. Tolerance can be considered an adaptation process which may be traced back to cellular and molecular levels. Receptor mechanisms of tolerance include downregulation and upregulation of numbers of receptors. Also, there is evidence that the higher the intrinsic activity of the opioids at only one receptor site, fewer receptors are needed in order to induce a potent analgesic effect; therefore, the incidence of tolerance is less. Drugs with high intrinsic activity include sufentanil and fentanyl.120 NMDA receptor activation through protein kinase, phospholipase C translocation, and activation of nitric oxide synthetase also contributes to the formation of tolerance.120,121 If titrating the dose up does not resolve the problem, changing into a new opioid or giving the patient a ‘holiday’ from opioid intrathecal therapy should be considered.
Complications
Surgical complications
CSF leakage
Leakage of CSF around the catheter as it enters the dural sac is a frequent occurrence after intrathecal catheter placement. Fortunately, CSF leakage is a self-limiting occurrence. CSF leakage usually resolves within a week after catheter implantation. However, should the problem persist, resulting in either a postural headache or leakage of CSF from the skin suture site which might require an epidural blood patch as a treatment option of PDPH, care should be taken when this procedure is performed. This autologous blood patch is prefereably done one level below the entrance level of the catheter to avoid catheter damage and should be performed under fluoroscopy to prevent catheter damage. If CSF leakage persists with formation of a subcutane ous CSF collection (hygroma), aspiration of the CSF collection must be avoided to prevent development of an infection. Aspiration does not cure the problem of the leak and fistula between the thecal sac and subcutaneous collection. Instead, applying an abdominal binder that increases pressure in the pocket might decrease CSF drainage and may help the fistula to close spontaneously. If that fails, surgical intervention might be indicated to close the fistula.
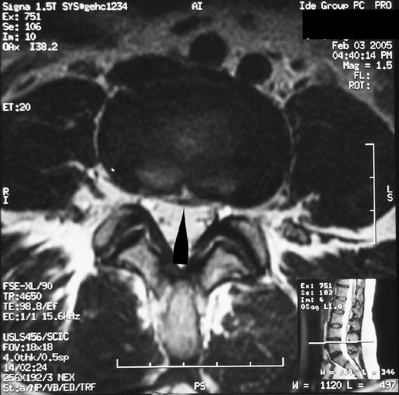
Catheter tip granuloma
In 1991, North et al. first reported a granulomatous region associated with the intrathecal catheter tip causing mass effect and neurologic dysfunction.122 In a literature review on the subject by Coffey and Burchiel, in November of 2000, 41 cases, 16 from the literature and 25 reported to Medtronic, Inc. and to the FDA, were identified.35 The mean duration of therapy in these cases was 24.5 months. Most cases were located in the thoracic region. Intrathecal drugs involved included morphine or hydromorphone, either alone or mixed with other drugs in 39 out of the 41 cases. Thirty patients underwent surgery to relieve spinal cord or cauda equina compression. Eleven of the patients were nonambulatory and one died due to pulmonary embolism. Microscopically, the masses were composed of chronic inflammatory cells with variable degrees of granuloma formation. In these cases, there was a region of central necrosis resembling an abscess that contained no polymorphonuclear leukocytes. Although tissue stains for microorganisms were uniformly negative, intraoperative cultures were positive in three cases, thought to be a secondary process.
Yaksh et al., observing masses in humans and in two species of animals, suggested a probable relationship existing between the mass formation and opioid dose and concentration.123 Some authors have suggested placing catheter tips below the conus medullaris to avoid catastrophic complications caused by granulomatous mass formation over the spinal cord. However, in a case report by Fernandez et al.,124 a patient placed on hydromorphone, 110 mg per day with a concentration of 400 mg/mL, developed a catheter tip granuloma in the sacral region. In this case, the catheter was placed caudally into the lumbosacral region. This patient presented with saddle anesthesia and bowel/bladder incompetence, and after surgery, the patient was left permanently disabled.
1 Goldstein A, Lowney LI, Pal PK. Stereospecific and nonspecific interactions of the morphine congenitor levorphanol in subcellular fractions of mouse brain. Proc Natl Acad Sci USA. 1971;68:1742-1747.
2 Pert C, Synder S. Opiate receptor: demonstration in nerve tissue. Science. 1973;48:1011-1014.
3 Kuhar MH, Pert CB, Synder SH. Regional distribution and opiate receptor binding in monkey and human brain. Nature. 1973;245:447-450.
4 Lamotte C, Pert CB, Synder SH. Opiate receptor binding primate spinal: distribution and change after dorsal root section. Brain Res. 1976;11:407-412.
5 Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357-1358.
6 Wang JK, Naus LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149-151.
7 Behar M, Olshwang D, Magora F, et al. Epidural morphine in treatment of pain. Lancet. 1979;I:527-528.
8 Ventafrida V, Figliuzzi M, Tamburini M, et al. Clinical observation on analgesia elicited by intrathecal morphine in cancer patients. Bonica JJ, Ventafrida V, editors. Advances in pain research and therapy, vol. 2.. Raven, New York, 1979;559-565.
9 Krames ES. Intraspinal analgesia for nonmalignant pain. In: Steve D. Waldman. Interventional pain management, 2nd edn. 2001:609–620.
10 Krames ES. Intraspinal opioid therapy for chronic nonmalignant pain: current practice and clinical guidelines. J Pain Symptom Manage. 1996;11:333-352.
11 Winkelmuller M, Winkelmuller W. Long-term effect of continuous intrathecal opioid treatment in chronic pain of nonmalignant etiology. J Neurosurg. 1996;85:458-467.
12 Hassenbusch SJ, Stanton-Hicks MD, Soukup J, et al. Sufentanil citrate and morphine/bupivacaine as alternative agents in chronic epidural infusion for intractable non-cancer pain. Neurosurgery. 1991;29:76-82.
13 Schuchard M, Krames ES, Lanning RM. Intraspinal analgesia for nonmalignant pain: a retrospective analysis for efficacy, safety and feasibility in 50 patients. Neuromodulation. 1998;1:46-56.
14 Borolat G, Schwartzman RJ, Aries L. Chronic intrathecal morphine infusion for intractable pain in reflex sympathetic dystrophy. In: Proceedings of the 8th meeting of the European Society for Steriotatic and Functional Neurosurgery. Budapest, Hungary, 1981:81.
15 Nitescu P, Joberg M, Applegren L, et al. Complications of intrathecal opioids and bupivacaine in the treatment of refractory cancer pain. Clin J Pain. 1995;11:45-62.
16 Krames E. Best practice and research. Clin Anesthesiol. 2002;4:619-649.
17 Macres S, Richeimer S. Successful treatment of erythromelalgia with intrathecal hydromorphone and clonidine. Clin J Pain. 2000;16:310-313.
18 Ong B, Segstro R. Resperatory depression associated with meperidine spinal anesthesia. Can J Anesth. 1994;41:725-727.
19 Mironer YE, Tollison CD. Methadone in the intrathecal treatment of chronic nonmalignant pain resistant to other neuroaxial agents. The first experience. Neuromodulation. 2001;4:25-31.
20 Meininger D, Byhahn C, Kessler P. Intrathecal fentanyl, sufentanil, or placebo combined with hyperbaric mepivacaine 2% for parturients undergoing elective cesarean delivery. Anesth Analg. 2003;96:852-858.
21 Shah FR, Halbe AR, Panshal ID. Good child CS, improvement of postoperative pain relief by the addition of midazolam to an intrathecal injection of buprenorphine and bupivacaine. Eur J Anesthesiol. 2003;20:904-910.
22 Kumar K, Kelly M, Pirlot T. Continuous intrathecal morphine treatment for chronic pain of nonmalignant etiology: long-term benefits and efficacy. Surg Neurol. 2001;55:79-86. discussion 86–88
23 Deer T, Chapple I, Classen A, et al. Intrathecal drug delivery for treatment of chronic low back pain: report from the National Outcomes Registry for Low Back Pain. Pain Med. 2004;5(1):6-13.
24 Cousins MJ, Cherry DA, Gourlay GK. Acute and chronic pains use of spinal opioids. In: Cousins MJ, Bridenbaugh P, editors. Neural blockade in clinical anesthesia and management of pain. 2nd edn. Philadelphia: JB Lippincott; 1988:955-1029.
25 SJogren P, Thunedborg LP, Christrup L, et al. Is development of hyperalgesia, allodynia and myoclonus related to morphine metabolism during long-term administrating: six case histories. Acta Anaesthesiol Scanda. 1988;42:1070-1075.
26 Hemstapat K, Monteith GR, Smith D, et al. Morphine-3-glucoronide’s neuroexcitatory effects are medicated via indirect activation of N-methyl-D-aspartic acid receptors: mechanistic studies in embryonic cultured hippocampal neurons anesthesia, and analgesia. Anesth Analg. 2003;97(2):494-505.
27 Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85:2215-2222.
28 Shulman MS, Walkerlin G, Yamaguchi L, et al. Experience with epidural hydromorphone for post thoracotomy pain relief. Anesthes Analges. 1987;66:567-570.
29 Mahler PL, Forest W. Relative analgesic potencies of morphine and hydromorphone in postoperative pain. Anesthesia. 1975;42:602-607.
30 Anderson VC, Cooke B, Burchiel K. Intrathecal hydromorphone for chronic nonmalignant pain: a retrospective study. Pain Med. 2001;2:287-297.
31 Du Pen S, Du Pen A, Hillyer J. Intrathecal hydromorphone for intractable nonmalignant pain: A retrospective study. Pain Med. 2006;7:10-15.
32 Hildebrand KR, Elsberry DE, Anderson VC. Stability and compatibility of hydromorphone hydrochloride in an implantable infusion system. J Pain Symptom Manage. 2001;22:1042-1047.
33 Brodsky JB, Chaplay SR, Brose WG, et al. Continuous epidural hydromorphone for post phoracotomy pain relief. Ann Thorac Surg. 1990;50:888-893.
34 Johansen MJ, Satterfield WC, Baze WB, et al. Continuous intrathecal infusion of hydromorphone: Safety in the sheep model and clinical implications. Pain Med. 2004;5:14-25.
35 Coffey RJ, Burchiel K. Inflammatory mass lesions associated with intrathecal drug infusion catheters: report and observations on 41 patients. Neurosurgery. 2002;50:78-86. discussion 86–87
36 Yaksh TL, Hassenbusch S, Burchiel K, et al. Inflammatory masses associated with intrathecal drug infusion: A review of preclinical evidence and human data. Pain Med. 2002;3:300-312.
37 Wright AW, Mather LE, Smith MT. Hydromorphone-3-glucoronide is a more potent neuro-excitant than its structural analogue morphine-3-glucoronide. Life Sci. 2001;69:409-420.
38 Patel D, Janardhan Y, Meri B, et al. Comparison of intrathecal meperidine and lidocaine in endoscopic urologic procedures. Can J Anesthes. 1990;37:567-570.
39 Thi TV, Orliaguet G, Liu N, et al. A dose range study of intrathecal meperidine combined with bupivacaine. Acta Anaesthesiol Scand. 1992;36:516-518.
40 Sharma SK, McIntire DD, Wiley J, et al. Labor analgesia and cesarean delivery: an in patient meta-analysis of nulliparous women. Anesthesiology. 2004;100:142-148.
41 Cozian A, Pinand M, Lepage JY, et al. Effects of meperidine spinal anesthesia on hemodynamics, plasma catecholamines, angiotensin I, aldosterone, and histamine concentrations in elderly men. Anasthesia. 1986;64:815-819.
42 Harvey SC, O’Neil MG, et al. Continuous intrathecal meperidine via an implantable pump for chronic, nonmalignant pain. Ann Pharmacother. 1997;31(11):1306-1308.
43 Mironer YE, Grumman S. Experience with alternative solutions in intrathecal treatment of chronic nonmalignant pain. Pain Dig. 1999;9:299-302.
44 Chrubasik J, Chrubasik S, Friedrich G, et al. Long-term treatment of pain by spinal opiates: an update. Pain Clin. 1992;5:147-156.
45 Mironer YE. Neuraxial opioid therapy. In: Tollison CD, Satterthwaite JR, Tollison JW, editors. Practical pain management. 3rd edn. Philadelphia: Williams & Wilkins; 2002:135-154.
46 Hassenbusch SJ, Portenoy RK. Current practices in intraspinal therapy – a survey of clinical trends and decision making. J Pain Symptom Manage. 2000;20:S4-S11.
47 Gorman AL, Elliot KJ, Inturrisi CE. The D and L isomers of methadone bind to the noncompetitive site on the N-methyl-D-aspartade (NMDA) receptor in rat forebrain and spinal cord. Neurosci Lett. 1997;223:5-8.
48 Davis AM, Inturrisi CE. d-Methadone blocks morphine tolerance and N-methyl-D-aspartate-induced hyperalgesia. J Pharmacol Exp Ther. 1999;289:1048-1053.
49 Shir Y, Shapiras S, Shenkman Z, et al. Continuous epidural methadone treatment for cancer pain. Clin J Pain. 1991;7:339-341.
50 Mironer YE, Haasis JCIII, Chapple ET, et al. Successful use of methadone in neuropathic pain: a multicenter study by the national forum of independent pain clinicians. Pain Dig. 1999;9:191-193.
51 Mironer YE, Tollison CD. Methadone in the intrathecal treatment of chronic nonmalignant pain resistant to other neuroaxial agents: the first experience. Neuromodulation. 2001;4:25-31.
52 Reynold L, Ked Laya D. Spinal administration of opioids. Anesthesiology. 1984;61:276-310.
53 Loper KA, Ready BL, Downey M, et al. Epidural and intravenous fentanyl infusion are clinically equivalent after knee surgery. Anesth Analg. 1990;70:72-75.
54 Kamran S, Wright BD. Complications of intrathecal drug therapy. Neuromodulation. 2001;4:111-115.
55 Willis KD, Doleys DM. The effects of long-term intraspinal infusion therapy with noncancer pain patients: evaluation of patient, significant-other, and clinic staff appraisals. Neuromodulation. 1999;2:241-253.
56 Hansdottir V, Hedner T, Woestenborghs R, et al. The CSF and plasma pharmacokinetics of sufentanil after intrathecal administration. Anesthesiology. 1991;74:264-269.
57 Ionescu TI, Drost RH, Roelofs JM, et al. The pharmacokinetics of intradural morphine in major abdominal surgery. Clin Pharmacokinet. 1988;14:178-186.
58 Sosnowski M, Yaksh TL. Differential cross-tolerance between intrathecal morphine and sufentanil in the rat. Anesthesia. 1990;73:1147.
59 Camann WR, Denny RA, Holby ED, et al. A comparison of intrathecal, epidural, and intravenous sufentanil for labor analgesia. Anesthesia. 1992;77:351-353.
60 Rawal N, Nuutinen L, Raj PP, et al. Behavioral and histologic effects following intrathecal administration of butorphanol, sufentanil, and nalbuphine in sheep. Anesthesia. 1991;75:1029-1034.
61 Hildebrand KR, Elsberry DD, Deer TR. Stability, compatibility, and safety of intrathecal bupivacaine administered chronically via an implantable delivery system. Clin J Pain. 2001;17:239-244.
62 Trissel LA, Pham L. Physical and chemical stability of low and high concentrations of morphine sulfate and bupivacaine hydrochloride packaged in plastic syringes. Intl J Pharm Compounding. 2002;6:70-73.
63 Li DF, Hahar M, Cole G, et al. Neurological toxicity of the subarachnoid infusion of bupivacaine, lignocaine, or 2-chloroprocaine in the rat. Br J Anaesth. 1985;57:424-429.
64 Dahm P, Lundborg C, Janson M, et al. Comparison of 0.5% intrathecal bupivacaine with 0.5% intraspinal ropivacaine in the treatment of refractory cancer and noncancer pain conditions: Results from a prospective, crossover, double-blind, randomized study. Reg Anesth Pain Med. 2000;25:480-487.
65 SJoberg M, Karlsson PA, Nordborg C, et al. Neuropathologic findings after long-term intrathecal infusion of morphine and bupivacaine for pain treatment in cancer patients. Anesthesia. 1992;74:424-429.
66 Rigler ML, Drasner K, Krejcie TC, et al. Cauda equina syndrome after continuous spinal anesthesia. Anesth Analg. 1991;72:275-281.
67 Dupen SL, Williams AR. Management of patients receiving combined epidural morphine and bupivacaine for the treatment of cancer pain. J Pain Symp Manage. 1992;27:125-127.
68 Krames ES, Lanning RM. Intrathecal infusional analgesia for nonmalignant pain: analgesic efficacy of intrathecal opioid with or without bupivacaine. J Pain Symp Manage. 1993;8:539-548.
69 Deer TR, Caraway DL, Kim CK, et al. Clinical experience with intrathecal bupivacaine in combination with opioid for the treatment of chronic pain related to failed back surgery syndrome and metastatic cancer pain of the spine. Spine J. 2002;2:274-278.
70 van Dongen RTM, Crul BJP, von Egmond J. Intrathecal coadministration of bupivacaine diminishes morphine dose progression during long-term intrathecal infusion in cancer patients. Clin J Pain. 1999;15:166-172.
71 Mironer YE, Haasis JC, Chapple I, et al. Efficacy and safety of intrathecal opioid/bupivacaine mixture in chronic nonmalignant pain: a double-blind, randomized, crossover, multicenter study by the National Forum of Independent Pain Clinicians (NFIPC). Neuromodulation. 2002;5:208-213.
72 Lundborg C, Dahm P, Nitescu P, et al. Clinical experience using intrathecal (IT) bupivacaine infusion in three patients with complex regional pain syndrome type I (CRPS-I). Acta Anaesthesiol Scand. 1999;43:667-678.
73 Spaulding TC, Fielding S, Venafro JJ, et al. Antinociceptive activity of clonidine and its potentiation of morphine analgesia. Eur J Pharmacol. 1979;58:19-25.
74 Wilcox GL, Carlson KH, Jochim A, et al. Mutual potentiation of anti-nociceptive activity of morphine and clonidine on motor and sensory responses in rat spinal cord. Brain Res. 1987;405:84-93.
75 Hodgson P, Neal J, Pollock J, et al. The neurotoxicity of drugs given intrathecally. Anasth Analg. 1999;88:797-809.
76 Thompson’s physicians desk reference. Duraclon, Montvale, NJ, 2003;1254-1255.
77 Coombs DW, Sanders RL, LaChance D, et al. Epidural clonidine analgesia; use of clonidine, DADLE, and intraventricular morphine. Anasthesia. 1985;62:358-363.
78 Eisenach JC. Overview; first international symposium on alpha-2 adrenergic mechanisms of spinal anesthesia. Regional Anesthes. 1993;18:I-V.
79 Rauck RL, Eisenach JC, Kackson KE, et al. Epidural clonidine for refractory reflex sympathetic dystrophy. Anesthesia. 1991;75:A657.
80 Hildebrand KR, Elsberry DD, Hassenbusch SJ. Stability and compatibility of morphine–clonidine admixtures in an implantable infusion system. J Pain Symptom Manage. 2003;25:464-471.
81 Classen AM, Wimbish GH, Kupiec TC. Stability of admixture containing morphine sulfate, bupivacaine hydrochloride, and clonidine hydrochloride, in an implantable infusion system. J Pain Symptom Manage. 2004;28(6):603-611.
82 Hassenbusch SJ, Gunes S, Wachsman S, et al. Intrathecal clonidine in the treatment of intractable pain: a phase I/II study. Pain Med. 2002;3:85-91.
83 Ackerman LL, Follett KA, Rosenquist RW. Long-term outcomes during treatment of chronic pain with intrathecal clonidine or clonidine/opioid combinations. J Pain Symp Manage. 2003;26:668-677.
84 Siddall PJ, Molloy AR, Walker S, et al. The efficacy of intrathecal morphine and clonidine in the treatment of pain after spinal cord injury. Anesth Analg. 2000;91:1493-1498.
85 Uhle EI, Becker R, Gatscher S, et al. Continuous intrathecal clonidine administration for the treatment of neuropathic pain. Stereotact Funct Neurosurg. 2000;75:167-175.
86 Kroin JS, McCarthy RJ, Penn RD. Continuous intrathecal clonidine and tizanidine in conscious dogs: analgesic and hemodynamic effects. Anesth Analg. 2003;96:776-782.
87 Kawamata T, Omote K, et al. Antihyperalgesic and side effects of intrathecal clonidine and tizanidine in a rat model of neuropathic pain. Anesthesiology. 2003;98(6):1480-1483.
88 Mathur VS, et al. Neuronal N-type calcium channels: new prospect in pain therapy. Pharmaceutical News. 1999;5:25-29.
89 Atanassoff PG, Hartmannsgruber MW, et al. Ziconotide, a new N-type calcium channel blocker, administered intrathecally for acute postoperative pain. Regional Anesthes Pain Med. 2000;25:274-278.
90 Presley RW, Yearwood TL, Charapata SG, et al. Intrathecal ziconotide in the treatment of opioid-refractory neuropathic and nonmalignant pain: a controlled clinical trial. Submitted to Anesthesia and Analgesia.
91 Staats PS, Yearwood T, Charapata SG, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled clinical trial. JAMA. 2004;291:63-70.
92 Staats PS, Luthardt F, Shipley J, et al. Long-term intrathecal ziconotide therapy: a case study and discussion. Neuromodulation. 2001;4:121-126.
93 Penn RD, Paice JA. Adverse effects associated with the intrathecal administration of ziconotide. Pain. 2000;85:291-296.
94 Valentine JM, Lyons G, Bellamy MC. The effect of intrathecal midazolam on postoperative pain. Eur J Anaesthes. 1996;13:589-593.
95 Goodchild CS, Guo Z, Masgreave A, et al. Antinociception by intrathecal midazolam involves endogenous neurotransmitters acting at spinal cord delta opioid receptors. Br J Anaesth. 1996;77(6):758-763.
96 Nishiyama T, Hanaoka K. The synergistic interaction between midazolam and clonidine in spinally mediated analgesia in two different pain models in rats. Anesthes Analg. 2001;93:1025-1031.
97 Nishiyama T, Gyermek L, Lee C, et al. Synergistic analgesic effects of intrathecal midazolam and NMDA or AMPA receptor antagonists in rats. Can J Anesth. 2001;48:288-294.
98 Nishiyama T, Matsukawa T, Hanaoka K. Acute phase histopathological study of spinally administered midazolam in cats. Anesthes Analg. 1999;89:717-720.
99 Johansen MJ, Gradert TL, Satterfield WC, et al. Safety of continuous intrathecal midazolam infusion in the sheep model. Anesthes Analg. 2004;98(6):1528-1535.
100 Erdine S, Yucel A, Ozyuvaci E, et al. Neurotoxicity of midazolam in the rabbit. Pain. 1999;80:419-423.
101 Yaksh TL, Allen JE. The use of intrathecal midazolam in humans: a case study of process. Anesth Analg. 2004;98:1536-1545.
102 Rainov NG, Heidecke MD, Burkert W. Long-term intrathecal infusion of drug combinations for chronic back and leg pain. J Pain Symptom Manage. 2001;22:862-871.
103 Goodwin K, Kim N-H, Zuniga R. Stability of a baclofen and clonidine hydrochloride admixture for intrathecal administration. Hospital Pharm. 2001;26:950-954.
104 Lauretti GR, Reis MP, Prado WA, et al. Dose–response study of intrathecal morphine versus intrathecal neostigmine, their combination or placebo for postoperative analgesia in patients undergoing anterior and posterior vaginoplasty. Anesth Analg. 1996;82:1182-1187.
105 Klamt JG, Dos Reis MP, Neto JB, et al. Analgesic effect of subarachnoid neostigmine in two patients with cancer pain. Pain. 1996;66:389-391.
106 Kekesi G, Dobos L, Benedek G, et al. The antinociceptive potencies and interaction of endogenous ligands during continuous intrathecal administration: adenosine, agmatine and endorphine-1. Anesth Analg. 2004;98:420-426.
107 Eisenach JC, Rauck RL, Curry R. Intrathecal, but not intravenous adenosine reduces allodynia in patients with neuropathic pain. Pain. 2003;105:65-70.
108 Scwetz I, Naliboff B, Munakata J, et al. Antihyperalgesic effect of octreotide in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19:123-131.
109 Paice JA, Penn RD, Kroin JS. Intrathecal octreotide for relief of intractable nonmalignant pain: 5-year experience with two cases. Neurosurgery. 1996;38:203-207.
110 Yaksh TL, Dirksen R, Harty GJ. Antinociceptive effects of intrathecally injected cholinomimetic drugs in the rat and cat. Eur J Pharmacol. 1985;117:81-88.
111 Han BF, Zhang C, Qi JS, et al. ATP sensitive potassium channels and endogenous adenosine are involved in spinal antinociceptive produced by locus coeruleus stimulation. Acta Physiol Sinica. 2002;54:139-144.
112 Bennett G, Burchiel K, Buchser E, et al. Clinical guidelines for intraspinal infusion: report of an expert panel. J Pain Symptom Manage. 2000;20:S37-S43.
113 Hassenbusch S, Portenoy R, et al. Polyanalgesic Consensus Conference 2003: an update on the management of pain by intraspinal drug delivery – report of an expert panel. J Pain Symptom Manage. 2004;27(6):540-563.
114 American Society of Health System Pharmacists ASHP. Guideline on quality assurance for pharmacy-prepared sterile products. Am J Health Syst Pharmacol. 2003;60:1440-1446.
115 USP. General chapter 797: pharmaceutical compounding sterile preparations. US Phamacopeia. 2004; Jan 1.
116 Naumann C, Erdine S, et al. Drug adverse events and system complications of intrathecal opioid delivery for pain: origins, detection, manifestations and management. Neuromodulation. 1999;2:92-107.
117 Wang SC. Emetic and antiemetic drugs. In: Root WS, Hofmann FG, editors. Physiological pharmacology, II. New York: Academic Press; 1965:255-328.
118 Barnes NM, Bunce KT, Naylor RJ, et al. The actions of fentanyl to inhibit drug-induced emesis. Neuropharmacology. 1991;30:1073-1083.
119 Aldrete JA, da Silva JMC. Leg edema from intrathecal opiate infusions. Eur J Pain. 2000;4:361-365.
120 Freye E, Latasch L. Development of opioid tolerance – molecular mechanisms and clinical consequences. Anasthesiol Intensivemed Notfallmed Schmerzther. 2003;38(1):14-26.
121 Hsu MM, Wong CS. The roles of pain facilitatory systems in opioids tolerance. Acta Anaesthesiol Sin. 2000;38(3):155-166.
122 North RB, Cutchis PN, Epstein JA, et al. Spinal cord compression complicating subarachnoid infusion of morphine: case report and laboratory experience. Neurosurgery. 1991;29:778-784.
123 Yaksh TL, Hassenbusch S, Burchiel K, et al. Inflammatory masses associated with intrathecal drug infusion: a review of preclinical evidences and human data. Pain Med. 2002;3:300-312.
124 Fernandez G, Madison-Michael L, Feler CA. Catheter tip granuloma associated with sacral region intrathecal drug administration. J Neuromodulation. 2003;6:225-228.