CHAPTER 14 Intraoperative Neurophysiologic Monitoring of the Spine
Intraoperative Monitoring of the Spinal Cord
Somatosensory-evoked potential (SEP) monitoring has been used for many years to monitor spinal function intraoperatively during various surgeries involving the spine, such as corrective surgery for scoliosis or other congenital deformities and removal of intraspinal tumors or arteriovenous malformations. SEP monitoring has been shown to reduce the incidence of neurologic damage in large studies of experienced monitoring teams.1 SEPs monitor only sensory transmission through the dorsal column pathways, however; SEPs do not provide a direct measure of motor function. In addition, the dorsal columns receive their blood supply from the posterior spinal arteries, whereas the anterior spinal arteries supply the motor pathways. Ischemic damage to the spinal cord from the anterior spinal artery may be undetectable with SEP monitoring.2,3
MEPs are a more direct technique that evaluates the motor pathway. Monitoring has been previously performed by relying on stimulation of the spinal cord directly.4 Spinal cord stimulation can be done with the use of epidural electrodes inserted after a laminectomy has been performed or by percutaneous intraspinous needle electrodes. The epidural electrodes are invasive and often require placement by a skilled anesthesiologist. Percutaneous intraspinous needles are difficult to place accurately and may not achieve adequate or consistent stimulation of the spinal cord. In addition, there is the question of whether MEPs generated through spinal cord stimulation arise solely from propagation through the motor pathway or if multiple pathways are involved in their generation.5,6 There are reports of MEP monitoring in which spinal cord stimulation resulted in no significant intraoperative changes despite postoperative neurologic motor deficits (so-called false-negative result).7 It is now believed that motor cortex stimulation with transcranial electrical stimulation provides a more reliable method for monitoring of the motor pathways. This technique is now routinely used in spinal cord monitoring together with SEPs.
Somatosensory-Evoked Potential Monitoring
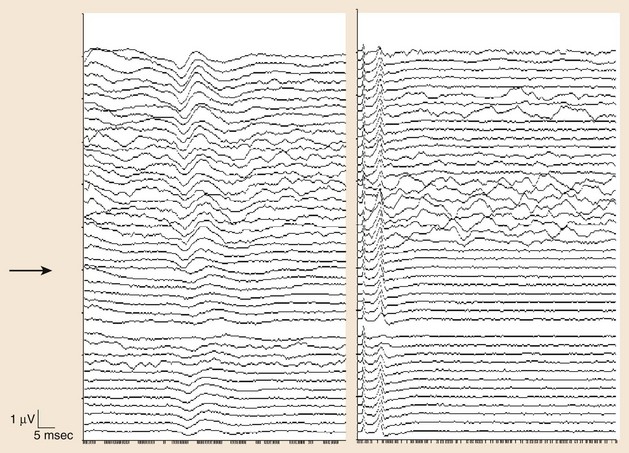
The use of SEPs in intraoperative monitoring of complex spine surgeries began in the early 1970s.8 Although SEP monitoring evaluates primarily the integrity of the posterior columns, it is often used to give an overall assessment of the spinal cord based on the assumption that many intraoperative mechanisms of injury would affect the spinal cord diffusely. An example of such an injury is spine distraction during scoliosis surgery. In addition, ischemic injury may initially result in a more diffuse dysfunction of the spinal cord that could be detected by SEPs (Fig. 14–1). SEP responses are thought to pass through large fiber somatosensory pathways of the dorsal column and possibly through the anterior spinothalamic tract, and this may be another reason why anterior spinal artery ischemia could be detected using this technique.
Generators of Somatosensory-Evoked Potential Responses
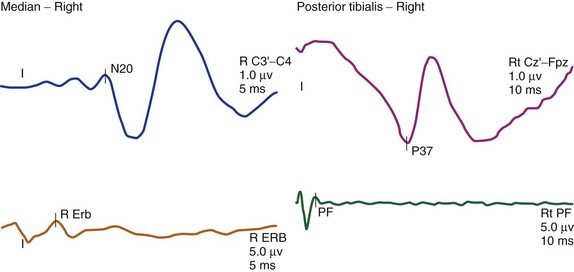
The cortical response for the lower extremity is called the P37 potential. The generator of this response arises from the primary somatosensory cortex of the leg, which is located in the mesial parietal cortex. The cortical response for the upper extremity, which is generated from the primary somatosensory cortex of the hand, is called the N20 potential (Fig. 14–2). Two important characteristics of these waveforms are (1) amplitude, which is recorded in microvolts and determined by either a baseline-to-peak or a peak-to-trough measure of the waveform, and (2) latency, which is recorded in milliseconds and is the time interval from the stimulus to the occurrence of the potential. An amplitude change from the initial baseline measure to a decrease of more than 50% is often considered a significant change in SEP amplitudes.9
Significant latency changes in SEP monitoring consist of a 10% prolongation beyond the baseline latency value (Table 14–1).10 Although these deviations from the baseline measures are thought to be significant, they should be interpreted with caution, taking into account various factors, including the evolution of the changes (e.g., a trend toward worsening is an ominous sign) and other intraoperative factors such as length of the surgery, type of anesthetic agent, and temperature effects. Also, significant latency and amplitude changes can occur in isolation. It is quite common to see a significant amplitude change without any associated latency changes. The most significant change is a complete loss of the cortical potential.
TABLE 14–1 Significant Changes in Different Monitoring Modalities
| Type of Study | Significant Changes | Highly Significant Changes |
|---|---|---|
| Somatosensory-evoked potentials | Amplitude <50%; latency >10% | Complete loss of amplitude |
| Motor-evoked potentials | Increase threshold voltage >50-100 V | Complete loss of amplitude |
| Pedicle screw stimulation | Current intensity <7-10 mA |
Another measurement made in posterior tibial or peroneal nerve SEP monitoring is the popliteal fossa potential. This is a nerve action potential that is recorded as the impulses pass within the popliteal fossa in the peripheral nervous system. The reason for this measurement is to ensure that an adequate stimulus has been applied. If there is an absence of the popliteal fossa response in addition to an absence of the leg cortical (P37) response, it suggests that the changes seen are not a result of a lesion at the level of the spinal cord. In this case, the change may be technical (e.g., the stimulating needles may have dislodged) or may be seen when the leg is ischemic (e.g., with femoral artery catheterization during thoracoabdominal aneurysm surgery or with direct compression of the peripheral nerve) (Fig. 14–3).
The other posterior tibial stimulation SEP response that can be monitored (besides the P37 response) is the P31/N34 complex, often termed the subcortical response because the generator for this response is at the level of medulla and midbrain. The benefit in recording these responses is that they are relatively more resistant to the effects of anesthesia compared with the cortical P37 response (see Fig. 14–3). The same is true for the subcortical potentials from median nerve stimulation (P14/N18) potential. In pediatric cases, the subcortical potentials may also be better formed and more easily monitored than cortical responses. Some of this effect may be the result of the variation of myelination in the younger age groups and the more significant effects of anesthetics on these patients. These differences from the adult morphology can persist into the early teenage years. Other factors affecting the responses include core body temperature changes. The core body temperature commonly decreases more than 1° C. The cooling affects the limbs more than the core body temperature, which can result in slowing of conduction.
Motor-Evoked Potential Monitoring
Noninvasive stimulation of the brain using TCES was first reported in 1980.11 Single-pulse TCES was subsequently used in monitoring motor pathways.12–17 Because of effects of general anesthesia, single-pulse stimulation was found to be less effective in reliably recording MEPs.18–22 With the introduction of the multipulse technique for motor pathway monitoring, reliable and robust MEP recording can now be obtained in most patients using some specific general anesthesia protocols.23–27 The use of the multipulse technique requires that neuromuscular blockade not be used during this part of the monitoring. Occasionally, the use of partial neuromuscular blockade may still allow for TCES monitoring.28 This method reportedly achieves more reliable stimulation of the motor cortex intraoperatively and is more resilient to the effects of general anesthesia.
The method of MEP monitoring has been revolutionized by the use of multipulse TCES. Previous methods for MEP recording used a variety of stimulation and recording techniques. Spinally elicited neurogenic responses were used in the past and were putatively stated to be a result of activation of the motor pathways in the spinal cord. More recent evidence has suggested that these spinally elicited neurogenic responses are generated through activation of the sensory pathways and retrograde activation of alpha motor neurons. In a collision experiment using stimulation of the spinal cord followed by stimulation of the posterior tibial nerve at various interstimulus intervals, the neurogenic responses were abolished, suggesting that the potentials were colliding in the spinal cord.6
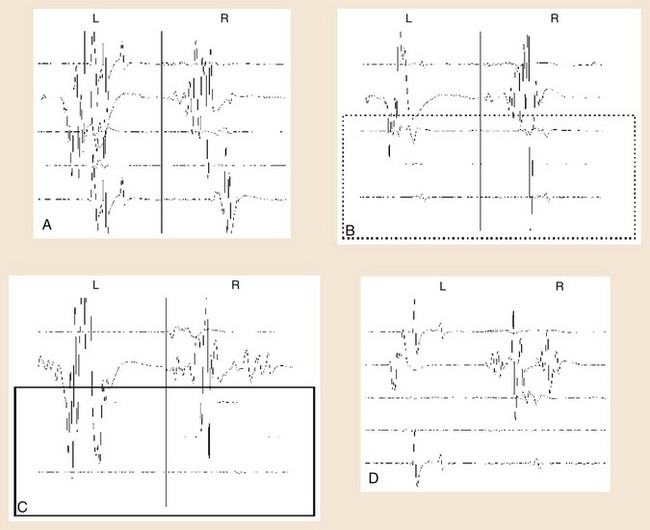
At the beginning of TCES-MEP monitoring, threshold voltages for each side of the body and MEP amplitudes are calculated.23 The motor cortex on the side of the brain receiving the anodal stimulus is typically the first region to activate at the lowest stimulus threshold. The initial current used is typically 100 V, with a train of stimuli delivered to the cortex. After the stimulation, an MEP response is monitored in the muscles contralateral to the side receiving the anodal stimulus. If no response is seen, the voltage is increased typically by 50-V increments, and the process is repeated until an MEP response is seen in all the muscles contralateral to the anodal stimulus. This voltage is called the threshold voltage for that side.
Determining significant changes during the course of surgery typically is most reliable if there is an absolute loss of myogenic responses to stimulation (Fig. 14–4). Some authors have also suggested that reduction of MEP amplitude to 25% of baseline amplitude values is predictive of motor pathway impairments.29 Significant changes can also be determined by the change of voltage required to obtain MEPs of greater than 50 V beyond baseline thresholds used in obtaining MEPs at the beginning of monitoring (see Table 14–1).30
Clinical Use of Intraoperative Monitoring
SEPs have become a useful modality in monitoring scoliosis surgery and have been shown to reduce the risk of neurologic deficit. The occurrence of definite neurologic deficits in the presence of unchanged SEP recordings has been estimated to be approximately 0.063%.31 Intraoperative neurophysiology can play a neuroprotective role (through the detection of early changes) and an educational role during surgery.32 Surgeons who use intraoperative neurophysiologic monitoring regularly can learn which surgical techniques and maneuvers have a risk for producing a neurologic deficit and avoid those methods.
TCES-evoked MEP monitoring during spinal surgery is a safe and reliable method of monitoring corticospinal tract activity and is indispensable for high-risk surgeries.33 There is no evidence that TCES has resulted in the development of epilepsy or brain damage. Some other risks are associated with TCES monitoring, including tongue or lip laceration and rarely mandibular fractures. The use of a soft bite-block may prevent these injuries. Relative contraindications to TCES include epilepsy, cortical lesions, convexity skull deficits, increased intracranial pressure, cardiac disease, intracranial electrodes or shunts, cardiac pacemakers, or other implantable biomedical devices.34
One study that looked at the reproducibility of various methods that could be monitored during scoliosis surgery found that MEPs could be obtained in 80% of patients and SEPs could be obtained in 93% of patients.35 In spinal surgery, MEPs obtained from upper and lower extremities were consistently recorded in 22 patients with multipulse stimulation using trains of three to six pulses separated by 2 msec, with responses measuring more than 100 µV in all but 1 patient. These responses persisted with nitrous oxide concentration of up to 74%. One patient had loss of responses from one lower limb in which increased weakness was noted for a few days after surgery; in 3 patients, there was an increase in weakness or spasticity without any accompanying intraoperative MEP changes.36 In another study,37 MEPs during TCES were reproducibly recorded during spinal surgery in 40 patients with partial neuromuscular blockade. In two patients, there were some significant changes in the motor potentials that correlated with postoperative neurologic deficits. No postoperative neurologic deficits were observed in nine patients in whom MEP amplitudes decreased to less than 20% of baseline values.37
TCES-induced MEPs have been used to monitor cases of intramedullary spinal cord tumor resection. In 32 consecutive patients, MEPs were elicited in 19 patients before myelotomy, and 3 of these patients had MEP amplitude decrease less than 50% from baseline; all of these patients had postoperative neurologic deficits.38
In a review of 160 patients undergoing scoliosis surgery, a combination of SEP and transcranial MEP monitoring was successfully recorded in 81% of the patients, with changes seen in 5% of monitored cases that were reversible after taking appropriate surgical corrective measures. None of these patients had new postoperative deficits or worsening of preexisting deficits. This combination of techniques was considered safe, reliable, and accurate and obviated the need for the “wake-up” test.39
Use of TCES-evoked MEPs has been easily accomplished with an anesthetic combination of narcotic drip accompanied by nitrous oxide. The use of isoflurane in addition to this combination resulted in a tendency for deterioration of MEP amplitude responses.40 MEPs elicited by TCES are more feasible with total intravenous anesthesia compared with balanced anesthesia using nitrous oxide, isoflurane, and fentanyl. Some of the suppressant effects of balanced anesthesia can be overcome with higher stimulation intensities and repetitive stimulation.41
Pedicle Screw Stimulation
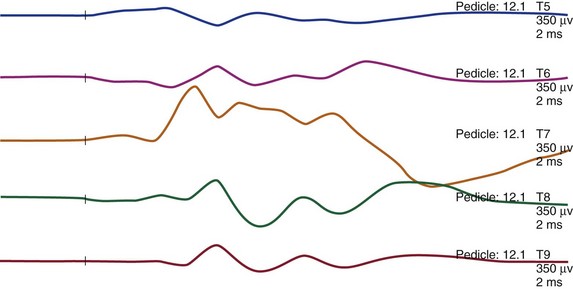
Intraoperative assessment during pedicle screw insertion can be used to minimize the risk of nerve root trauma from a misdirected screw. The integrity of pedicle screw placement can be assessed by directly stimulating the screw and by simultaneously recording myogenic responses from the appropriate myotomes. Using a direct monopolar nerve stimulator, with serial increments of the level of current intensity from 1 to 20 mA, triggered EMG recordings can be performed (Fig. 14–5). Absence of a myogenic response up to 10 mA is thought to indicate an intact pedicle. The presence of a pedicle breach is suspected by a stimulation-induced myogenic response at less than 7 to 10 mA (see Table 14–1).7
Summary
Pearls
Pitfalls
Key Points
1 Nuwer MR, Dawson EG, Carlson LG, et al. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: Results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6-11.
2 Toleikis JR, Skelly JP, Carlvin AO, et al. Spinally elicited peripheral nerve responses are sensory rather than motor. Clin Neurophysiol. 2000;111:736-742.
3 Calancie B, Harris W, Broton JG, et al. “Threshold-level” multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: Description of method and comparison to somatosensory evoked potential monitoring. J Neurosurg. 1998;88:457-470.
4 Deletis V, Sala F. The role of intraoperative neurophysiology in the protection or documentation of surgically induced injury to the spinal cord. Ann N Y Acad Sci. 2001;939:137-144.
5 MacDonal DB. Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol. 2002;19:416-429.
1 Nuwer MR, Dawson EG, Carlson LG, et al. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: Results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6-11.
2 Lesser RP, Raudzens P, Luders H, et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol. 1986;19:22-25.
3 Zornow MH, Grafe MR, Tybor C, et al. Preservation of evoked potentials in a case of anterior spinal artery syndrome. Electroencephalogr Clin Neurophysiol. 1990;77:137-139.
4 Lueders H, Gurd A, Hahn J, et al. A new technique for intraoperative monitoring of spinal cord function: Multichannel recording of spinal cord and subcortical evoked potentials. Spine. 1982;7:110-115.
5 Su CF, Haghighi SS, Oro JJ, et al. “Backfiring” in spinal cord monitoring: High thoracic spinal cord stimulation evokes sciatic response by antidromic sensory pathway conduction, not motor tract conduction. Spine. 1992;17:504-508.
6 Toleikis JR, Skelly JP, Carlvin AO, et al. Spinally elicited peripheral nerve responses are sensory rather than motor. Clin Neurophysiol. 2000;111:736-742.
7 Minahan RE, Sepkuty JP, Lesser RP, et al. Anterior spinal cord injury with preserved neurogenic “motor” evoked potentials. Clin Neurophysiol. 2001;112:1442-1450.
8 Nash CLJr, Lorig RA, Schatzinger LA, et al. Spinal cord monitoring during operative treatment of the spine. Clin Orthop. 1977;126:100-105.
9 Jones SJ, Edgar MA, Ransford AO. Sensory nerve conduction in the human spinal cord: Epidural recordings made during scoliosis surgery. J Neurol Neurosurg Psychiatry. 1982;45:446-451.
10 Nuwer MR, Daube J, Fischer C, et al. Neuromonitoring during surgery. Report of an IFCN Committee. Electroencephalogr Clin Neurophysiol. 1993;87:263-276.
11 Merton PA, Morton HB. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285:227.
12 Agnew WF, McCreery DB. Considerations for safety in the use of extracranial stimulation for motor evoked potentials. Neurosurgery. 1987;20:143-147.
13 Amassian VE, Cracco RQ. Human cerebral cortical responses to contralateral transcranial stimulation. Neurosurgery. 1987;20:148-155.
14 Amassian VE, Stewart M, Quirk GJ, et al. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74-93.
15 Cracco RQ. Evaluation of conduction in central motor pathways: Techniques, pathophysiology, and clinical interpretation. Neurosurgery. 1987;20:199-203.
16 Cracco RQ, Amassian VE, Maccabee PJ, et al. Comparison of human transcallosal responses evoked by magnetic coil and electrical stimulation. Electroencephalogr Clin Neurophysiol. 1989;74:417-424.
17 Day BL, Rothwell JC, Thompson PD, et al. Motor cortex stimulation in intact man: II. Multiple descending volleys. Brain. 1987;110:1191-1209.
18 Jellinek D, Jewkes D, Symon L. Noninvasive intraoperative monitoring of motor evoked potentials under propofol anesthesia: Effects of spinal surgery on the amplitude and latency of motor evoked potentials. Neurosurgery. 1991;29:551-557.
19 Jellinek D, Platt M, Jewkes D, et al. Effects of nitrous oxide on motor evoked potentials recorded from skeletal muscle in patients under total anesthesia with intravenously administered propofol. Neurosurgery. 1991;29:558-562.
20 Kalkman CJ, Drummond JC, Ribberink AA. Low concentrations of isoflurane abolish motor evoked responses to transcranial electrical stimulation during nitrous oxide/opioid anesthesia in humans. Anesth Analg. 1991;73:410-415.
21 Hicks R, Burke D, Stephen J, et al. Corticospinal volleys evoked by electrical stimulation of human motor cortex after withdrawal of volatile anaesthetics. J Physiol. 1992;456:393-404.
22 Hicks RG, Woodforth IJ, Crawford MR, et al. Some effects of isoflurane on I waves of the motor evoked potential. Br J Anaesth. 1992;69:130-136.
23 Calancie B, Harris W, Broton JG, et al. “Threshold-level” multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: Description of method and comparison to somatosensory evoked potential monitoring. J Neurosurg. 1998;88:457-470.
24 Jones SJ, Harrison R, Koh KF, et al. Motor evoked potential monitoring during spinal surgery: Responses of distal limb muscles to transcranial cortical stimulation with pulse trains. Electroencephalogr Clin Neurophysiol. 1996;100:375-383.
25 Rodi Z, Deletis V, Morota N, et al. Motor evoked potentials during brain surgery. Pflugers Arch. 1996;431:R291-R292.
26 van Dongen EP, ter Beek HT, Schepens MA, et al. Effect of nitrous oxide on myogenic motor potentials evoked by a six pulse train of transcranial electrical stimuli: A possible monitor for aortic surgery. Br J Anaesth. 1999;82:323-328.
27 van Dongen EP, ter Beek HT, Schepens MA, et al. The influence of nitrous oxide to supplement fentanyl/low-dose propofol anesthesia on transcranial myogenic motor-evoked potentials during thoracic aortic surgery. J Cardiothorac Vasc Anesth. 1999;13:30-34.
28 van Dongen EP, ter Beek HT, Schepens MA, et al. Within-patient variability of myogenic motor-evoked potentials to multipulse transcranial electrical stimulation during two levels of partial neuromuscular blockade in aortic surgery. Anesth Analg. 1999;88:22-27.
29 Meylaerts SA, Jacobs MJ, van Iterson V, et al. Comparison of transcranial motor evoked potentials and somatosensory evoked potentials during thoracoabdominal aortic aneurysm repair. Ann Surg. 1999;230:742-749.
30 Calancie B, Harris W, Broton JG, et al. “Threshold-level” multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: Description of method and comparison to somatosensory evoked potential monitoring. J Neurosurg. 1998;88:457-470.
31 Nuwer MR, Dawson EG, Carlson LG, et al. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: Results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6-11.
32 Deletis V, Sala F. The role of intraoperative neurophysiology in the protection or documentation of surgically induced injury to the spinal cord. Ann N Y Acad Sci. 2001;939:137-144.
33 Cioni B, Meglio M, Rossi GF. Intraoperative motor evoked potentials monitoring in spinal neurosurgery. Arch Ital Biol. 1999;137:115-126.
34 MacDonal DB. Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol. 2002;19:416-429.
35 Luk KD, Hu Y, Wong YW, et al. Evaluation of various evoked potential techniques for spinal cord monitoring during scoliosis surgery. Spine. 2001;26:1772-1777.
36 Jones SJ, Harrison R, Koh KF, et al. Motor evoked potential monitoring during spinal surgery: Responses of distal limb muscles to transcranial cortical stimulation with pulse trains. Electroencephalogr Clin Neurophysiol. 1996;100:375-383.
37 Lang EW, Beutler AS, Chesnut RM, et al. Myogenic motor-evoked potential monitoring using partial neuromuscular blockade in surgery of the spine. Spine. 1996;21:1676-1686.
38 Morota N, Deletis V, Constantini S, et al. The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery. 1997;41:1327-1336.
39 Stephen JP, Sullivan MR, Hicks RG, et al. Cotrel-Dubousset instrumentation in children using simultaneous motor and somatosensory evoked potential monitoring. Spine. 1996;21:2450-2457.
40 Calancie B, Klose KJ, Baier S, et al. Isoflurane-induced attenuation of motor evoked potentials caused by electrical motor cortex stimulation during surgery. J Neurosurg. 1991;74:897-904.
41 Pechstein U, Nadstawek J, Zentner J, et al. Isoflurane plus nitrous oxide versus propofol for recording of motor evoked potentials after high frequency repetitive electrical stimulation. Electroencephalogr Clin Neurophysiol. 1998;108:175-181.