Chapter 16 Intraoperative Irradiation
History
The use of IORT was first employed almost 100 years ago.1 The contemporary approach to IORT was initiated in the 1960s by Abe and colleagues in Japan. These investigators advocated resection (where possible) with large single-dose radiation therapy (25 to 40 Gy using cobalt-60).2 By the mid-to-late 1970s, many institutions in the United States (e.g., Howard University, Massachusetts General Hospital, the Mayo Clinic, and the National Cancer Institute [NCI]) had adopted IORT as a treatment approach using linear accelerator–based electron treatment in the operating room. Presently there are about 90 centers in at least 16 countries worldwide with active IORT programs.
Biology
When EBRT is fractionated there is a preferential therapeutic advantage for normal tissues relative to tumor as defined by the “4Rs” of classical radiobiology (normal tissue repair, tumor reoxygenation, tumor redistribution, and normal tissue repopulation). With a single large fraction of radiation therapy, these advantages are lost. In addition, large doses per fraction may result in increased risk of late effects. There is evidence that small vessel injury caused by large doses per fraction may contribute to late effects, and ischemic complications are dose dependent.3 Furthermore, tumor response to single and fractionated radiation therapy depends on the percentage of hypoxic cells within a tumor. This differential sensitivity between hypoxic and well-oxygenated cells increases with increasing dose.
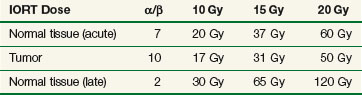
Using alpha/beta (α/β) calculations, biologically equivalent doses to a fractionated EBRT course using 2 Gy per fraction for varying IORT doses have been estimated (Table 16-1). There are disadvantages from a late effects standpoint with IORT, but many of these disadvantages are mitigated by exclusion of nontarget tissues from the radiation field by direct inspection, mobilization, and shielding.4 When combined with EBRT and resection, IORT doses of 10 to 20 Gy provide local control for most solid tumors, especially in the setting of microscopic residual tumor tissue. When combined with EBRT and surgery there is little reason to exceed IORT doses of 10 to 20 Gy. Late normal tissue complications are often the limiting sequelae of IORT administration, and careful planning and administration with techniques designed to reduce the dose to nontarget tissues are of paramount importance.
Local Control: An Important Endpoint
Pancreatic Cancer
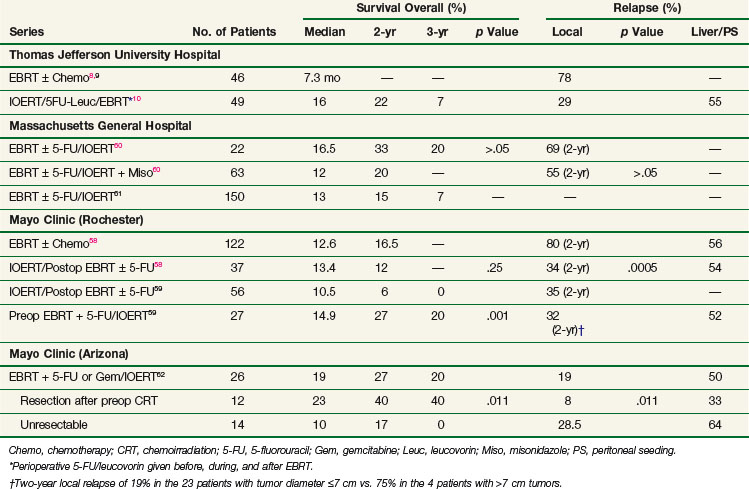
EBRT with 5-FU–based chemotherapy employed in the treatment of unresectable pancreatic cancer results in a doubling of median survival compared with surgical bypass/stenting alone (3 to 6 months vs. 9 to 13 months) and an increase in 2-year survival from 0% to 5% to 10% to 20%.5 Unfortunately, these techniques result in poor local control rates. Series from the Mayo Clinic, Thomas Jefferson University Hospital, and other institutions report local failure rates exceeding 70% to 80%6,7,8,9,10 (Table 16-2).
Retroperitoneal Sarcoma
When surgery is used as the primary treatment modality for retroperitoneal sarcomas, local failure has been reported to range from 40% to 90%. Despite the addition of EBRT to surgery, local failure rates are 40% to 80%. This is in contrast to extremity sarcomas in which local control rates approach 90%. Because of the limited tolerance of surrounding normal tissue (small intestine, stomach, liver, kidney, spinal cord), EBRT doses are limited. A randomized NCI trial evaluating IORT in retroperitoneal sarcomas demonstrated that patients receiving IORT + EBRT experienced significantly improved local control and less small bowel toxicity versus patients treated with EBRT alone (80% in-field relapse; see later discussion).11
Colon and Rectal Cancer
In patients with locally advanced (T4) or locally recurrent colon and rectal cancer, local control is difficult to achieve, despite multimodality therapy. Studies from Princess Margaret Hospital and the Mayo Clinic report local failure rates of 90% or greater in evaluable patients treated with EBRT with or without systemic therapy.12,13 In radiation-naive patients, the optimal approach in patients with locally advanced disease is preoperative EBRT combined with 5-FU–based chemotherapy followed by resection. Despite this, local recurrence occurs in 30% to 70% of patients.14
Cervical Cancer
For patients with cervical cancer, para-aortic nodal metastases are common. Despite the presence of these “distant” metastases, 15% to 20% of patients are cured by radical radiotherapy techniques, employing EBRT doses of 55 to 60 Gy. However, high complication rates have been reported with these doses and techniques.15,16 As in rectal cancer, patients with recurrent cervical cancer in the pelvis or para-aortic region have a poor long-term prognosis with 5-year overall survival (OS) rates ranging from 5% to 30%. These patients have often been previously irradiated, and re-treatment with meaningful doses of EBRT is usually not feasible given normal tissue tolerance. When patients have para-aortic or locally recurrent disease, administration of IORT is a feasible method to escalate dose and enhance local control.
Local Control: Radiation Dose, Complications, Shrinking-Field Techniques, Distant Metastases
Influence of Dose
In both animal and human models the probability of local control of a tumor by radiation is proportional to the total dose administered. The dose of radiation to control a tumor locally depends on several factors, including tumor type, clonogen number, and tumor microenvironment. Thus a given radiation dose may be able to control a small tumor with high probability and acceptable morbidity but that same dose may be insufficient against disease of larger volume. Clinical experience has generated a body of data correlating local control by tumor type and radiation dose. In vivo data for a variety of irradiated human tumors of varying sizes and types are summarized in Figure 16-1.
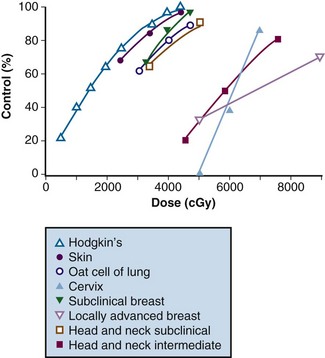
Figure 16-1 Local control versus dose of irradiation.
From Gunderson LL, Tepper JE, Biggs DJ, et al: Intraoperative ± external beam irradiation. Curr Probl Cancer 7:8, 1983.
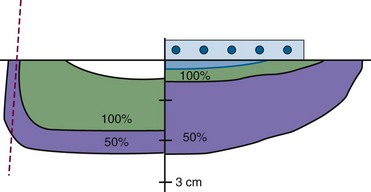
The studies of Fletcher examined local control probability as a function of radiation dose for patients undergoing treatment for breast carcinoma and squamous cell carcinoma of the upper aerodigestive tract (see web-only Table 16-1 on the Expert Consult website![]() ). For breast cancer patients, control of subclinical disease was 60% to 70% with 30 to 35 Gy, 85% with 40 Gy, and 95% with 45 to 50 Gy. For larger/palpable tumors, EBRT doses of 46 Gy, 59 Gy, and 76 to 90 Gy result in a local control probability of 20%, 35% to 50%, and 70% to 80%, respectively.17,18,19,20 These data suggest that marked improvements in local control can be achieved by escalating radiation doses.
). For breast cancer patients, control of subclinical disease was 60% to 70% with 30 to 35 Gy, 85% with 40 Gy, and 95% with 45 to 50 Gy. For larger/palpable tumors, EBRT doses of 46 Gy, 59 Gy, and 76 to 90 Gy result in a local control probability of 20%, 35% to 50%, and 70% to 80%, respectively.17,18,19,20 These data suggest that marked improvements in local control can be achieved by escalating radiation doses.
WEB-ONLY TABLE 16-1 Tumor Control Probability Correlated with Irradiation Dose and Volume of Cancer
| Dose (Gy) | Tumor Control Probability |
|---|---|
| Squamous Cell Carcinoma: Upper Aerodigestive Tract | |
| 50* | >90% subclinical |
| ~60% T1 lesions of nasopharynx | |
| ~50% 1- to 3-cm neck nodes | |
| 60* | ~90% T1 lesions of pharynx and larynx |
| ~50% T3 and T4 lesions of tonsillar fossa | |
| ~90% 1- to 3-cm neck nodes | |
| ~70% 3- to 5-cm neck nodes | |
| 70* | ~90% T2 lesions of tonsillar fossa and supraglottic larynx |
| ~80% T3 and T4 lesions of tonsillar fossa | |
| Adenocarcinoma of the Breast | |
| 50* | >90% subclinical |
| 60* | 90% clinically positive axillary nodes, 2.5-3.0 cm |
| 70* | 65% 2-3 cm primary |
| 70-80 (8-9 wk) | 30% >5 cm primary |
| 80-90 (8-10 wk) | 56% >5 cm primary |
| 80-100 (10-12 wk) | 75% 5-15 cm primary |
* 10 Gy in five fractions each week.
Modified from Fletcher GH, Shukovsky LJ: The interplay of radiocurability and tolerance in the irradiation of human cancers. J Radiol Electrol 56:383-400 1975.
Dose Versus Complications
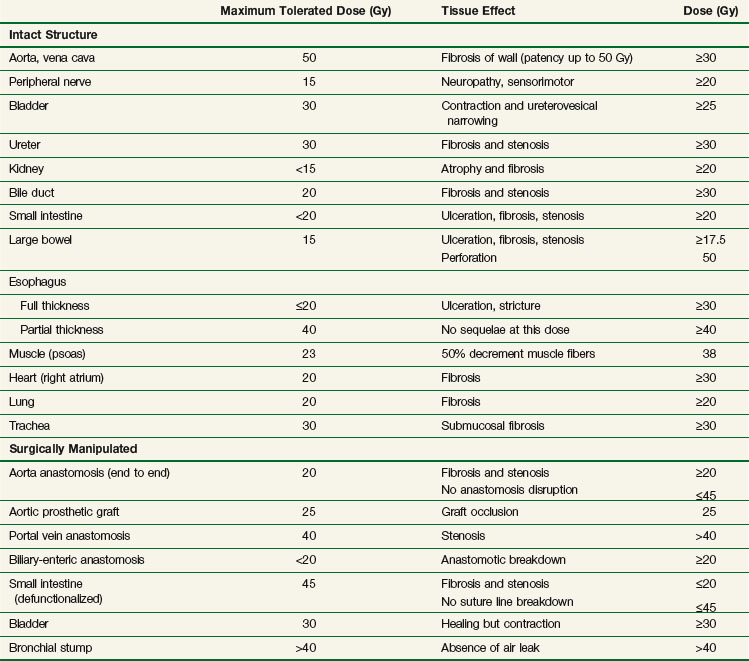
The chief limitation of EBRT to control macroscopic disease in the abdomen and pelvis is normal tissue tolerance. Normal organs such as stomach, small bowel, and kidney have tolerance levels well below the radiation doses required to eradicate most abdominal and pelvic malignancies. Exceeding these EBRT doses results in a prohibitive risk of late normal tissue damage (see web-only Table 16-2 on the Expert Consult website![]() ). Because of this, “conventional” tolerable doses of EBRT from 45 to 55 Gy using 1.8 to 2.0 Gy per fraction are not curative in most abdominal and pelvic malignancies, with resultant local persistence/local recurrence of disease common in patients treated with radiation therapy alone. This often results in tumor-related morbidity and mortality such as bowel obstruction and perforation, ureteral obstruction, and neuropathy.
). Because of this, “conventional” tolerable doses of EBRT from 45 to 55 Gy using 1.8 to 2.0 Gy per fraction are not curative in most abdominal and pelvic malignancies, with resultant local persistence/local recurrence of disease common in patients treated with radiation therapy alone. This often results in tumor-related morbidity and mortality such as bowel obstruction and perforation, ureteral obstruction, and neuropathy.
Although local control is enhanced with increasing doses of radiation, tumor dose-response curves with EBRT closely resemble normal tissue complication curves. Therefore, efforts to improve local control through escalating EBRT doses may also result in treatment-related complications (Fig. 16-2). In an R1 (microscopic residual) resection, EBRT doses of 60 Gy or higher using conventional fractionation schemes are necessary to achieve a high probability of local control. In an R2 (gross residual) resection, even higher doses are usually required. Such doses exceed normal tissue tolerance (see web-only Table 16-2 on the Expert Consult website![]() ).
).
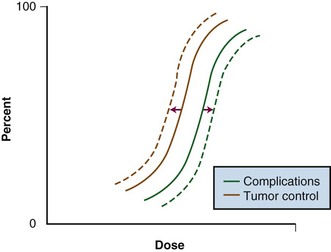
Figure 16-2 Radiation dose versus incidence of tumor control or complications.
From Gunderson LL, Tepper JE, Biggs DJ, et al: Intraoperative ± external beam irradiation. Curr Probl Cancer 7:8, 1983.
Because of the risks associated with dose escalation beyond normal tissue tolerance, an attractive alternative in patients with locally advanced malignancies is to deliver moderate doses of EBRT (i.e., at or below accepted tolerance of surrounding normal tissue). A typical course would range from 45 to 50 Gy at 1.8 to 2.0 Gy per fraction, followed by surgical exploration. After resection, IORT would be performed, avoiding or minimizing irradiation of surrounding organs by shielding or mobilization. With this approach, an increase in local control with decreased risk of normal tissue complications (relative to an EBRT-only approach) can be achieved (see Fig. 16-2: local control curve shifts to left with IORT; complication curve shifts to right with increasing EBRT doses).
Development of Distant Metastases
Preclinical data suggest that the incidence of distant metastases is related to both tumor size as well as the development of locally recurrent disease in multiple spontaneous tumor systems.21–23 In fibrosarcoma and squamous cell carcinoma cell lines in rodent models, Ramsay and associates21 reported increased rates of distant metastases in tumors measuring 6 versus 12 mm, as well as primary versus recurrent tumors. Additionally, Suit and colleagues23 showed that in mouse mammary tumors treated with single-dose irradiation, increasing rates of local failure were associated with increasing rates of distant metastases. Specifically, the incidence of metastatic disease was 31% (16 of 52) of mice with local control, 50% (9 of 18) in those with local relapse salvaged by resection, and 80% (12 of 15) in mice with local relapse in whom salvage was not attempted. Similar high rates of metastases associated with local failure have been observed in human malignancies, including cervical,24 prostate,25 head and neck,26 and breast27 cancers. These and other data suggest that metastases may arise from locally recurrent disease.
Patient Selection and Evaluation
Sequencing and Dose: EBRT, IORT
Sequencing with Surgery
The role of preoperative versus postoperative therapy has been evaluated in rectal cancer. A large German randomized trial demonstrated that patients undergoing neoadjuvant irradiation and chemotherapy experienced significantly improved local control and less toxicity than patients receiving postoperative irradiation and chemotherapy.30
Radiation Dose and Technique
The biologic effectiveness of single-dose IORT in early responding tissues (tumor) relative to an equivalent total dose of fractionated EBRT has been estimated to be 1.5 to 2.5 times the IORT dose delivered4,31,32 (see Table 16-1). Therefore the effective tumor doses (when “normalized” to fractionated EBRT doses) of IORT treatment added to 45 to 50 Gy given by EBRT are as follows: 10-Gy IORT dose, 60 to 80 Gy; 15-Gy IORT dose, 75 to 87.5 Gy; and 20 Gy IORT dose, 85 to 100 Gy. These figures are not intended to be exact but represent educated estimates.
A discussion of the technical aspects of IORT administration is beyond the scope of this chapter. In brief, the implementation of IORT-based treatment is a multidisciplinary effort including one or multiple surgeons, radiation oncologist, anesthesiologist, operating room nurse, radiation physicist/dosimetrist, and therapist. In its broadest sense, IORT may be administered with either electrons (IOERT) or high-dose-rate photon afterloading techniques (HDR-IORT). Each method has potential advantages and disadvantages (see later and web-only Tables 16-6 and 16-7![]() ).
).
Dose-Limiting Structures and Tolerance
The development of normal tissue late effects increases with increasing radiation dose as well as dose delivered per fraction. Therefore, the incidence of late normal tissue effects in patients receiving IORT plus EBRT would be higher than those receiving EBRT alone. However, in this context the severe morbidity and mortality associated with locally recurrent tumor is often overlooked. As an example, when EBRT alone is used as the primary treatment modality for locally advanced rectal cancer, more than 90% of patients experience local persistence or local recurrence of disease with associated symptomatology. These symptoms include severe pelvic pain and neuropathy, which are difficult to manage clinically; the vast majority of patients experience disease-related death within 2 to 3 years. An argument can be made that the tumor-related morbidity/mortality approaches 100% in these patients.33
IORT tolerance for intact or surgically manipulated organs or structures in animals (primarily canines) is seen in Table 16-3. Much of this information has been derived from studies from the NCI34,35,36–38,39 and Colorado State University.40–42
Ureter
Clinical studies of the effect of IOERT on the ureters of human cancer patients have been undertaken at the Mayo Clinic.43 Doses of 10 Gy administered intraoperatively resulted in a 50% incidence of ureteral obstruction, increasing to 70% with doses from 15 to 25 Gy. This high complication rate relative to canine models may be due to age-related factors, surgical manipulation, EBRT, and/or tumor bed effects.
In an update of this experience, Mayo Clinic investigators reported on 146 patients with locally advanced malignancies receiving IORT to one or both ureters with doses between 7.5 and 30 Gy.44 They reported the risk of obstruction after IOERT is significant and increases with time and IORT dose. However, obstruction risk for ureters not receiving IOERT was also high, which suggests an underlying risk of ureteral injury from other causes (EBRT, surgical manipulation of ureters). (A more detailed discussion of ureteral tolerance with IORT can be found on the Expert Consult website![]() .)
.)
Peripheral Nerve
Peripheral nerve tissue is the principal dose-limiting normal tissue for IORT in the pelvis and retroperitoneum. Data regarding peripheral nerve tolerance and neuropathy comes from canine models as well as clinical analyses from patients treated intraoperatively.* Peripheral nerve tissue is often situated adjacent to or directly involved by tumor in the abdomen and pelvis. Because of this, the relative surgical “immobility” of the peripheral nerve, and the inability to shield the nerve from the IORT field, nerve tissue will often receive full-dose EBRT and IORT.
The mechanism of neuropathy after IORT is poorly understood. Peripheral nerve tolerance depends on the volume of nerve irradiated and total dose delivered. In animal models, histomorphologic findings after IORT have demonstrated decreased central nerve fiber density, particularly in large nerve fibers receiving more than 20 Gy. Electron microscopy analysis has demonstrated increased microtubule density and neurofilament accumulation within axons without associated myelin changes, suggesting possible hypoxic injury related to vascular changes.54
A Spanish study evaluated 45 patients with primary or locally recurrent extremity soft tissue sarcoma undergoing resection with IOERT (10 to 20 Gy).55 Nine patients received IOERT alone secondary to prior EBRT or patient refusal. Five patients developed neurotoxicity at a median of 13 months; 4 of the 5 showed objective weakness and/or sensory loss. Most patients developing neuropathy received IOERT doses greater than 15 Gy.
An analysis from the Mayo Clinic evaluated peripheral nerve tolerance in 51 patients undergoing IOERT for primary or recurrent pelvic malignancies.43 Patients received EBRT (median dose 50.4 Gy), maximal resection where possible, and IOERT boost from 10 to 25 Gy using 9- to 18-MeV electrons. Sixteen (32%) of patients experienced grade I to III peripheral neuropathy as manifested by pelvic/extremity pain, leg weakness, numbness, or tingling. Pain was severe (grade III) in 3 of 51 patients (6%) (see web-only Table 16-3 on the Expert Consult website![]() ).
).
WEB-ONLY TABLE 16-3 Mayo Clinic Series: Clinical Peripheral Neuropathy Characteristics with Pelvic IOERT
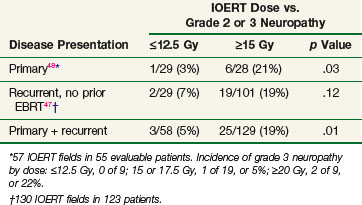
In a follow-up study, Mayo Clinic investigators evaluated 178 patients with locally advanced colorectal cancer receiving IOERT. This study suggested a relationship between increasing doses of IOERT and the incidence of clinically significant neuropathy (Table 16-4). In primary and locally recurrent colorectal patients the incidence of severe (grade III) neuropathy was approximately 5% and the incidence of any neuropathy was approximately one third. This is consistent with canine studies, suggesting that increasing IOERT doses are related to the incidence of clinical and electrophysiologic neuropathy.47,48
A more recent Mayo Clinic analysis of 607 patients with locally recurrent colorectal cancer receiving IORT reported an incidence of grade 1 to 3 neuropathy of 15% (grade 1, 5%; grade 2, 7%, grade 3, 3%). A dose-related increase in grade 2 and 3 neuropathy was seen in patients receiving 15 Gy or more versus 12.5 Gy or less.50
Results for Selected Diseases
A summary of IORT results and a discussion of future possibilities in selected disease site (pancreas, breast, colorectal, gynecologic cancers, retroperitoneal/pelvic sarcomas) are now presented. For a more detailed discussion of results of treatment for selected diseases the reader is referred to the Expert Consult website ![]() and to dedicated chapters on each site in an IORT text.56,57
and to dedicated chapters on each site in an IORT text.56,57
Pancreatic Cancer
Because of poor local control results achieved with conventional EBRT and chemotherapy, the use of IORT in the setting of pancreatic cancer is rational. The combination of EBRT plus IOERT in the setting of locally advanced/unresectable pancreatic adenocarcinoma has resulted in improvement in local control in separate series from Massachusetts General Hospital, the Mayo Clinic, and Thomas Jefferson University Hospital* (see Table 16-2). However, most patients ultimately develop liver metastases, peritoneal seeding, or both.
Investigators at Mayo Clinic Rochester reported on 27 patients with locally advanced pancreatic cancer who received IOERT after preoperative EBRT plus chemotherapy. Local control was achieved in 78% of patients. Median survival was 14.9 months and 2-, and 5-year survival rates were 27% and 7%, respectively. This compared favorably to similarly matched patients treated with IOERT followed by EBRT (median survival, 10.5 months; 2-year OS 6%, p = .001 in favor of preoperative therapy).59
In a series from the Massachusetts General Hospital (MGH), Willett and colleagues61 described 150 patients with locally advanced pancreatic cancer who received IOERT, EBRT, and 5-FU–based chemotherapy. One-, 2-, and 5-year actuarial survival rates were 54%, 15%, and 4%, respectively. Median survival for the entire cohort was 13 months. Five patients survived beyond 5 years. Long-term survivors were treated by smaller applicator sizes, reflecting smaller tumor size.
Investigators from Mayo Clinic Arizona, have used only the sequence of preoperative chemoirradiation followed by restaging and surgical exploration with resection/IOERT, as indicated, for select patients with borderline resectable or unresectable pancreas cancer.62 A series of 26 patients with no prior treatment received IOERT after preoperative chemoirradiation; resection was performed in 12 of 26 patients before IOERT (R0 or R1, 9 patients; R2, 3 patients). Median survival for the total group was 19 months, with OS rates of 27% at 2 years and 20% at 3 years (see Table 16-2). Survival outcomes appeared to be improved in patients with resection after preoperative chemoirradiation versus those without resection (median 23 vs. 10 months; 2-year OS 40% vs. 17%; 3-year OS 40% vs. 0%; p = .011, log-rank). Liver or peritoneal relapse was documented in 13 of 26 patients (50%).
In a series from Okinawa, Japan, 210 patients underwent gross total pancreatic tumor resection plus IORT, with and without EBRT.63 The median IORT dose was 25 Gy. Local failure was seen in 15% of patients with a 2-year actuarial local control of 84%. Median survival was 19.1 months with a 2-year survival rate of 42%. Patients treated with IORT and chemotherapy had significantly more favorable OS compared with IORT alone.
A multi-institution European pooled analysis evaluated 270 patients, 91.5% of whom underwent resection. Most received either preoperative or postoperative EBRT.64 Five-year local control was 23% with a median survival of 19 months and a 5-year survival rate of 18%. A significantly greater control and survival was seen in patients receiving preoperative versus postoperative EBRT.
Breast Cancer
Intraoperative Irradiation Alone
Randomized trials have demonstrated equivalent disease-free survival (DFS) and OS in selected breast cancer patients undergoing either mastectomy or breast-conserving surgery followed by EBRT.65 Local recurrences frequently occur at or adjacent to the original tumor bed after breast-conserving surgery. There is increasing interest in the use of IORT as a supplement or alternative to EBRT in selected cases in both Europe and the United States.*
A phase II French study investigated the role of 21-Gy IOERT alone (linac) in patients older than age 65 years with T1N0 invasive ductal carcinoma (RADELEC trial).66 At a median follow-up of 30 months, only two patients developed breast recurrence requiring salvage mastectomy.
The Italian ELIOT trial (initiated in 2000) is a randomized phase III trial comparing 21-Gy IOERT versus standard EBRT whole-breast/boost therapy.73 IOERT is delivered with a mobile linear accelerator (the Novac7 [Hitesys, Aprilia, Italy]; see web-only Fig. 16-2 on the Expert Consult website![]() ) while shielding the thoracic wall with a lead plate. Veronesi and associates68 reported on 237 patients with primary tumors 2 cm or smaller undergoing wide excision with either sentinel lymph node biopsy and/or axillary lymph node dissection. Patients received IOERT using 3- to 9-MeV electrons with doses of 17 to 21 Gy (>90% received 21 Gy as the prescribed dose). At a median follow-up of 19 months, the rate of post-treatment complications was low, with 1.7% developing breast fibrosis. A follow-up report of 574 patients revealed 3 patients with local recurrence and 3 additional patients with ipsilateral recurrence in other quadrants at median follow-up of 20 months.
) while shielding the thoracic wall with a lead plate. Veronesi and associates68 reported on 237 patients with primary tumors 2 cm or smaller undergoing wide excision with either sentinel lymph node biopsy and/or axillary lymph node dissection. Patients received IOERT using 3- to 9-MeV electrons with doses of 17 to 21 Gy (>90% received 21 Gy as the prescribed dose). At a median follow-up of 19 months, the rate of post-treatment complications was low, with 1.7% developing breast fibrosis. A follow-up report of 574 patients revealed 3 patients with local recurrence and 3 additional patients with ipsilateral recurrence in other quadrants at median follow-up of 20 months.
A preliminary report from the Italian collaborative breast IOERT group described a multicenter trial comparing IOERT alone after lumpectomy to conventional-fraction EBRT in tumors less than 3 cm with negative margins. IORT patients received a single 21-Gy fraction using 6- to 9-MeV electrons. Of 314 evaluable patients, no local recurrence was detected in either treatment group at a median follow-up of 31 months although a significant difference in the incidence of grade 1 to 2 late toxicity was seen in favor of the IORT group (3% vs. 63%).74
A systematic review of seven published IORT-alone series in early breast cancer suggested that short-term local control, DFS, and OS were similar to those of reported EBRT series. Local control rates ranged from 0% to 29% with IORT. However, many of these small, single-institution studies had short follow-up (median of 2 years).75
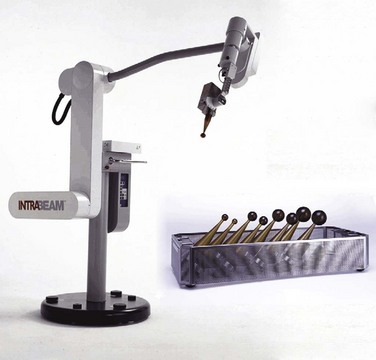
An approach of targeted IORT using the INTRABEAM system (TARGIT) (Carl Zeiss Meditec, Jena, Germany) implements a 50-kv x-ray generator mounted on a flexible floor stand with a set of spherical applicators ranging from 1.5 to 5 cm in diameter (see web-only Figure 16-1 on the Expert Consult website![]() ). Radiotherapy is delivered to the tumor bed by placing the applicator within the tumor cavity and conforming the adjacent breast tissues around the applicator before treatment. A potential disadvantage of this approach is that low-energy x-rays could potentially lead to underdosing of residual tumor cells removed from the tumor cavity. In an international phase III TARGIT trial,76 patients were randomized to IORT alone or a typical course of EBRT. If patients who had undergone resection were at high risk of local recurrence in other quadrants (e.g., extensive intraductal component, extensive lymphovascular space invasion, nodal metastases), then EBRT could be delivered postoperatively. This trial has completed accrual, and final results are pending. A preliminary report showed a recurrence rate of less than 2%, but median follow-up was only 25 months. Because late local relapse can occur, longer follow-up is needed.
). Radiotherapy is delivered to the tumor bed by placing the applicator within the tumor cavity and conforming the adjacent breast tissues around the applicator before treatment. A potential disadvantage of this approach is that low-energy x-rays could potentially lead to underdosing of residual tumor cells removed from the tumor cavity. In an international phase III TARGIT trial,76 patients were randomized to IORT alone or a typical course of EBRT. If patients who had undergone resection were at high risk of local recurrence in other quadrants (e.g., extensive intraductal component, extensive lymphovascular space invasion, nodal metastases), then EBRT could be delivered postoperatively. This trial has completed accrual, and final results are pending. A preliminary report showed a recurrence rate of less than 2%, but median follow-up was only 25 months. Because late local relapse can occur, longer follow-up is needed.
Intraoperative plus External Beam Irradiation
The addition of boost treatment after lumpectomy and conventional EBRT has been shown to reduce local recurrence rates by 50% in all age groups versus EBRT alone. Potential advantages of an IORT boost versus EBRT boost are as follows: more precise delivery of irradiation to the tumor bed, skin sparing with avoidance of associated late cosmetic sequelae, and a smaller boost area with a more homogeneous dose distribution.
In the Medical College of Ohio and Centre Regional de Lutte Contre le Cancer (France) combined series,70 72 patients with early-stage breast cancer underwent lumpectomy with axillary lymph node dissection followed by 10- to 15-Gy IOERT using 6- to 20-MeV electrons. Patients later received EBRT doses of 45 to 50 Gy at 1.8 to 2.0 Gy per fraction. No significant complications were observed, and cosmetic results were described as excellent. Eight of 72 patients developed minor palpable fibrosis at the lumpectomy site. At a minimum 2-year follow-up in all patients, no patients had experienced local relapse.
At the University of Salzburg, IOERT combined with EBRT was used in 351 consecutive patients from October 1998 to April 2002 and results were reported in the initial 170 patients treated through December 2000.71 Local control results were compared with those from patients treated with EBRT alone. At the time of publication, the 3-year local control with an IOERT versus EBRT boost was 100% versus approximately 97%.
Investigators at the University of Heidelberg reported a preliminary experience in 155 breast cancer patients using a 20-Gy orthovoltage IORT boost followed by EBRT in women with T1 to T2 breast cancers. The 5-year local relapse-free survival was 98.5% at a median follow-up of 34 months. Grade 3 fibrosis of the tumor bed was found in 5% of patients at 3 years.72
A European pooled analysis by Sedlmayer and colleagues77,78 evaluated 1200 patients with limited breast cancer resection plus IOERT (median dose, 9.7 Gy; range, 5 to 17 Gy) followed by whole-breast EBRT using standard fractionation. Patients undergoing immediate secondary mastectomy due to extensive margin involvement were excluded. At median follow-up of 59.6 months, local tumor control was 99.3%. The authors concluded an IOERT boost during breast-conservation therapy results in optimal dose delivery and outstanding local control rates.
Early results of the TARGIT trial67 using IORT as boost therapy reported 183 patients treated with the INTRABEAM system of 5 or 7.5 Gy followed by EBRT. At median follow-up of 16 months, actuarial 2-year local control was 99% with serious complications (including fistula, wound dehiscence, and ulceration) occurring in 11% of patients.
Retroperitoneal and Pelvic Soft Tissue Sarcomas
National Cancer Institute Randomized Phase III Trial
The NCI conducted a randomized phase III trial in patients undergoing surgical resection of primary retroperitoneal sarcoma. All patients underwent gross total resection, although most had microscopically involved margins. Patients were randomized to receive 20 Gy IOERT followed by 35 to 40 Gy of EBRT postoperatively versus postoperative EBRT alone to a dose of 50 to 55 Gy. Patients receiving IOERT were treated with concurrent misonidazole given 15 to 30 minutes before treatment. EBRT treatments were delivered over 4 to 5 weeks at 1.5 to 1.8 Gy per fraction in both study arms; however, patients receiving only EBRT received an additional 15 Gy using similar fractionation by reduced fields. The incidence of in-field locoregional recurrence was significantly lower among patients receiving IOERT compared with patients receiving only EBRT (3/15 vs. 16/20, p <.001). Patients receiving IOERT experienced fewer episodes of radiation enteritis than patients receiving only EBRT (2/15 vs. 10/20, p <.05); however, radiation-related peripheral neuropathy was more frequent in patients receiving IOERT (9/15 vs. 1/20, p <.01).11
Mayo Clinic Experience
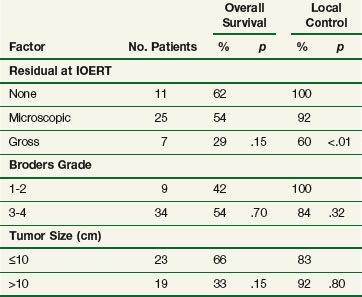
Investigators at the Mayo Clinic (Rochester)79 reported on 87 patients with primary or recurrent retroperitoneal or intrapelvic sarcomas receiving IOERT as a component of treatment. Seventy-seven patients received EBRT (53 preoperatively, 12 postoperatively, 12 both) to a median dose of 48 Gy, usually through a shrinking-field technique. Fifteen patients (17%) had gross residual disease after resection, 56 (64%) had microscopic residual disease, and 16 (18%) had either negative margins or no residual disease. Median IOERT dose was 15 Gy (range, 9 to 30 Gy). Five-year OS was 47%. Patients with tumors larger than 10 cm experienced a significantly worse survival compared with smaller lesions (5-year OS 28% vs. 60%, p = .01) and patients with gross residual disease after resection experienced a worse 5-year survival versus patients whose gross residual disease was totally resected (37% vs. 52%, p = .08). Five-year local control rates in patients with gross, microscopic, and no residual tumor were 41%, 60%, and 100%, respectively. Factors influencing 5-year local control are shown in Table 16-5 for patients with primary sarcomas, and in web-only Table 16-4 on the Expert Consult website![]() ). Patients with R0 or R1 resection experienced significantly improved local control (web-only Table 16-5 on the Expert Consult website shows factors influencing survival after treatment
). Patients with R0 or R1 resection experienced significantly improved local control (web-only Table 16-5 on the Expert Consult website shows factors influencing survival after treatment![]() ). Patients with tumors greater than 10 cm were significantly less likely to be long-term survivors.
). Patients with tumors greater than 10 cm were significantly less likely to be long-term survivors.
WEB-ONLY TABLE 16-4 Mayo Clinic IOERT Analysis: Factors Influencing 5-Year Local Control in Resected Retroperitoneal Sarcomas
WEB-ONLY TABLE 16-5 Mayo Clinic Analysis: Factors Influencing Overall Survival at 5 Years in Resected Retroperitoneal Sarcomas with IOERT
A further update of the Mayo Clinic experience reported on 226 IORT patients (52% primary) treated from 1981 to 2008.80 Most (63%) had high-grade tumors, and 36 (16%) had received prior EBRT. In previously unirradiated patients, EBRT was delivered preoperatively in 70% of patients, postoperatively in 10% and both in 10%. R0 and R1 resection was achieved in 39% and 50% of patients, respectively. Median IORT dose was 12.5 Gy. Five-year OS was 50% (R0 resection 52%, R1 55%, R2 28%). For the entire population, 5-year local failure was 29% (R0 18%, R1 31%, R2 61%), with distant metastases developing in 42% of patients. Central failure in the IORT field occurred in only 10% of patients. The authors concluded that (1) patients with retroperitoneal sarcoma undergoing gross total resection experienced improved local control relative to subtotal resection when treated with combined modality treatment including IORT, (2) improved outcomes were seen in patients with primary versus recurrent disease, and (3) the high rates of distant relapse suggest more effective systemic therapy is needed for patients with high-grade disease.
Massachusetts General Hospital Experience
Investigators from MGH described 37 patients with primary or recurrent retroperitoneal sarcoma who underwent EBRT (median dose, 45 Gy) and resection. Twenty patients received IOERT (10 to 20 Gy). In 29 (78%) patients receiving gross total resection and IOERT, 5-year survival and local control rates were 74% and 83%, respectively. In comparison, 13 patients undergoing gross total resection without IOERT had 5-year survival and local control rates of 30% and 61%, respectively. Four patients receiving IOERT experienced significant complications, including neuropathy, hydronephrosis, and fistula formation.81
A subsequent report from the same institution described 103 patients with primary retroperitoneal sarcoma, 62 of who underwent gross total resection. In this group, patients with high-grade disease and/or involved margins received EBRT with or without IOERT, particularly where a localized area of close margin of residual tumor was identified. This study again demonstrated a trend for IORT to further improve survival versus use of EBRT alone with a significant increase time to both local and distant relapse. In completely resected patients, a trend toward improved survival with IOERT was seen compared with EBRT alone (5-year OS 77% vs. 45%, p = .13).82
European Pooled Analysis
A European pooled analysis described 122 curatively approached patients (81 with recurrent disease) with retroperitoneal sarcoma undergoing maximum resection plus IOERT (median dose, 15 Gy). Most received adjuvant EBRT. Five-year OS, DFS, local control, and freedom from metastatic disease rates were 64%, 28%, 40%, and 50%, respectively. Five-year local control within the IOERT field was 72%. In patients receiving IOERT, EBRT, and R0 resection, 5- and 10-year OS were 80% and 5- and 10-year local control rates were 100%. Only 5% of patients experienced an IOERT in-field relapse after R0 resection, with 23% after R1 resection and 75% after R2 resection. Late complications of grade 2 or higher were seen in 21% of patients. The authors concluded that in selected patients IOERT resulted in excellent local control and survival with accepted morbidity.83
Conclusions and Future Possibilities
IORT combined with EBRT and resection offers an effective means of improving local control in patients with primary and recurrent retroperitoneal sarcomas, as demonstrated in a randomized trial from the NCI as well as multiple U.S. and European single-institution studies.* A randomized NCI trial showed an 80% tumor bed relapse rate with adjuvant EBRT alone, likely owing to an inability to deliver effective EBRT doses given normal tissue constraints. Because these results are similar to reports of resection alone, the use of adjuvant EBRT without IORT after marginal resection could be questioned. A more practical approach would be to administer preoperative EBRT after confirmation of diagnosis by thin-needle biopsy. This would be followed by resection at an institution with IORT capabilities.
Even with improved local control rates, locoregional and distant failure remain common modes of failure, emphasizing the need for improved therapies. Pilot studies have evaluated the role of concomitant chemoirradiation with preoperative EBRT, IORT, and maintenance chemotherapy for resectable moderate- and high-grade retroperitoneal and pelvic sarcomas.87 An Italian Sarcoma Group phase II study is evaluating preoperative EBRT with high-dose continuous infusion ifosfamide, using IOERT or postoperative EBRT for radiation dose escalation after maximal resection.
Gynecologic Cancers
Mayo Clinic Experience
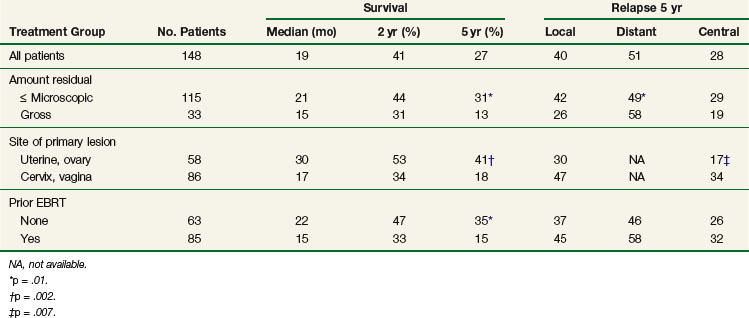
A Mayo Clinic analysis described 148 patients with primary (23 patients) or recurrent (125 patients) gynecologic malignancies treated with IOERT-containing regimens.88–90 Preoperative or postoperative EBRT was delivered in 113 patients; 85 (57%) had received prior EBRT. The 5-year OS for all patients was 27%, and the 5-year local failure rate was 40%89,90 (Table 16-6). On subset analysis, 115 patients with R0 or R1 resection had improved 5-year OS compared with patients with R2 resection (31% vs. 13%, p = .01); patients with uterine or ovarian primary origin had better survival than those with cervical or vaginal primary tumors (5-year OS 41% vs. 18%, p = .002); patients with no prior EBRT had better 5-year OS than those with prior EBRT (35% vs. 15%, p = .01). The rate of distant metastases at 5 years was 49% (R0 resection 63%, R1/R2 41%; p = .04). Fewer metastases were seen in patients treated with MVAC chemotherapy (methotrexate, vinblastine, doxorubicin [Adriamycin], cisplatin) in a prior analysis.88
Pamplona Experience
Spanish investigators reported on 67 patients with primary (31 patients) or recurrent (36 patients) cervical cancer. Previously unirradiated patients were generally treated with preoperative EBRT to 45 Gy at 1.8 Gy per fraction with concurrent cisplatin and 5-FU. Previously irradiated patients underwent immediate resection or neoadjuvant chemotherapy if their disease was unresectable. A median IOERT dose of 12 Gy was delivered for primary disease, and 15 Gy was used for recurrent disease after resection. Ten-year local control rate (within the IOERT field) was 69% for the entire group; local control rates for primary and recurrent disease were 93% and 46%, respectively. Factors adversely influencing local control included involved parametrial margins, gross residual disease, and pelvic lymph node involvement. Patients with two or more risk factors had a 10-year local control rate of 0%. Ten-year survival for the entire group was 35% (primary disease 58%, recurrent disease 14%). Patients with gross residual, microscopic residual, and no residual disease had 10-year survival rates of 0%, 9%, and 45%, respectively. Eight of 67 (12%) patients experienced chronic pain; motor neuropathy was noted in 1 patient (3%). All failures within the IOERT field occurred concomitant with relapse in the pelvis and/or distant metastases.91
Xian Experience
A study from Xian Jiaotong University in China was reported on 160 patients with stage IIb carcinoma of the cervix.92 All received IOERT during hysterectomy and selected lymphadenectomy. Ninety-eight patients were treated contemporaneously with standard therapy without surgery. IOERT-treated patients received 20 Gy of EBRT at 2 Gy per fraction followed by two treatments with iridium-192, receiving 12 to 16 Gy to point A, followed by surgery and IOERT using 12-MeV electrons with doses of 18 to 20 Gy. Patients received standard therapy of 50 Gy of EBRT at 2 Gy per fraction followed by seven iridium-192 treatments, delivering a dose of 35 Gy to point A. In these latter patients the central field was shielded after the EBRT dose reached 20 Gy. The 5-year OS, DFS, and local control rates in the IOERT patients were 89%, 86%, and 96%, respectively, versus 68%, 56%, and 59% in the standard therapy group (p <.05). The IOERT patients had fewer complications.
The authors concluded that the use of IOERT in the intermediate-stage cervical cancer provides excellent tumor control and survival while allowing a reduction of EBRT and intracavitary components of radiation therapy with resultant improved quality of life and lower cost.92
Stanford Experience
Stanford investigators evaluated 36 patients with gynecologic malignancies treated with orthovoltage IORT to 44 sites.93 The primary site included cervix (47%), endometrium (31%), and vulvar (14%) malignancies. Most (72%) patients had failed prior radiation therapy, and 89% had recurrent disease. Mean IORT dose was 11.5 Gy, accompanied by mean EBRT dose of 44 Gy (EBRT in 53% of patients); approximately 25% received adjuvant chemotherapy. Five-year locoregional control, distant metastases–free survival, and disease-specific survival were 44%, 51%, and 47%, respectively. For cervical cancer patients these values were 45%, 60%, and 46%, respectively. The authors concluded that IORT after surgery seems to confer long-term local control in carefully selected gynecologic patients.
Colorectal Cancer: Primary and Recurrent Disease
Primary Locally Advanced Cancers
In an MGH series, 64 patients with locally advanced primary rectal cancer underwent preoperative irradiation (with or without 5-FU) followed by resection and IOERT. Patients undergoing margin negative resection had a 5-year actuarial local control and disease-specific survival of 91% and 63%, respectively. Patients with microscopically involved margins experienced 5-year local control and disease-specific survival rates of 65% and 47%, respectively, and in patients with gross disease the rates were 57% and 14%, respectively94 (Table 16-7).
TABLE 16-7 Primary Rectal (Massachusetts General) or Colorectal (Mayo Clinic) IOERT Series: Disease Control and Survival by Degree of Resection
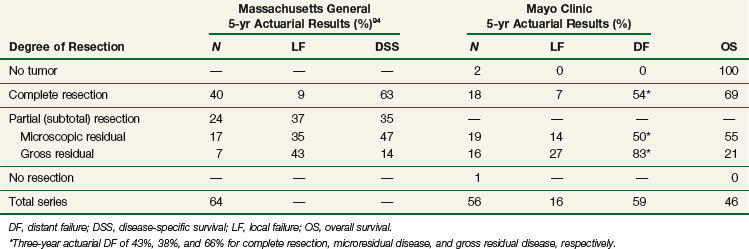
A report from Mayo Clinic Rochester48 described 56 patients with primary locally advanced colorectal cancer undergoing EBRT (45 to 55 Gy, usually with concurrent 5-FU administration) followed by resection and IOERT (10 to 20 Gy). Five-year OS for all patients was 46%. Patients with R0 or R1 resection had an improved OS relative to patients with R2 resection (5-year OS 59% vs. 21%, p = .0005). Failure within the IOERT field occurred in 4 of 16 patients (25%) with R2 resection versus 2 of 39 (5%) with R0 or R1 resection (p = .01) (see Table 16-7).
An update from Mayo Clinic50 analyzed 146 patients with locally unresectable primary colorectal cancers who received IOERT in addition to preoperative or postoperative combined modality therapy. Median survival was 44 months with a 5-year OS of 52%. Three-year local and distant relapse rates were 10% and 43%, respectively. Patients receiving preoperative combined modality therapy appeared to have a survival advantage versus those receiving postoperative therapy (median survival 76 vs. 26 months, 5-year OS 55% vs. 38%, p = .02).
Madrid investigators describe 558 patients with T3 to T4 rectal cancer, 281 of whom received preoperative combined modality therapy plus IOERT and 277 who received postoperative chemoradiotherapy without IOERT. Patients receiving preoperative therapy plus IORT versus postoperative therapy alone (despite higher stage disease at presentations) had a significant improvement in pelvic control (92% vs. 84%, p = .03), DFS (65% vs. 56%, p = .05), and OS (68% vs. 58%, p = .016).51
A European pooled analysis of 651 IOERT-treated patients from four major centers showed that 5-year OS was 67% with 5-year local control of 88% in patients with locally advanced rectal cancer.53 Positive circumferential margins were a strong predictor for both OS and local relapse, and the addition of preoperative chemoirradiation appeared to improve 5-year OS (70% vs. 64%, p <.05).52 An update of the European pooled analysis in 605 patients demonstrated that chemoirradiation led to more downstaging and complete remissions compared with radiotherapy alone. Local recurrence, distant metastasis, and OS rates were 12%, 29%, and 67%, respectively. Risk factors for local recurrence included lack of downstaging with preoperative therapy, lymph node involvement, margin involvement, and lack of postoperative chemotherapy.
Locally Recurrent Colorectal Cancer
Patients developing local recurrence after curative resection of primary colon or rectal cancer are treated with palliative intent at most institutions. Local recurrence from rectosigmoid cancer often causes pelvic pain owing to nerve involvement in the presacral space or pelvic sidewall. The likelihood of margin negative resection is low. For patients undergoing surgery alone for pelvic recurrence from rectal cancer, the reported 5-year survival rate is 0%.95
When IOERT is combined with EBRT ± chemotherapy and surgical salvage, 5-year OS in the range of 20% can occur.47,95,96,97,98 In an analysis from the Massachusetts General Hospital96 of 41 patients with locally recurrent rectosigmoid cancer undergoing IOERT, patients with gross residual disease experienced 5-year local control and DFS rates of 21% and 7%, respectively, versus 47% and 21% with clear or microscopically positive margins. Eindhoven investigators99 described a series of 147 patients with locally recurrent colorectal cancer receiving IOERT. Median OS was 28 months with 5-year OS, DFS, metastases-free survival, and local control of 32%, 34%, 50%, and 54% respectively. R0 resection was associated with improved disease outcomes. Patients treated with IOERT alone had worse outcomes versus those who were re-irradiated or treated with full-dose preoperative EBRT.
A Mayo Clinic (Rochester) report95 described the outcome of 106 patients undergoing palliative resection of locally recurrent, nonmetastatic rectal cancer. Forty-two patients received IOERT as a component of treatment (most 15 to 20 Gy) and 41 had EBRT (most ≥45 Gy). Patients with R2 resection had a significantly worse outcome versus those with R1 resection (5-year OS 9% vs. 33%, p = .03). IOERT versus no-IOERT patients had 5-year OS of 19% versus 7% (p = .0006).
An updated Mayo Clinic analysis100 described 175 patients with locally recurrent colorectal cancer (123 with no prior EBRT, 52 with prior EBRT) undergoing IOERT. Five-year OS in previously unirradiated patients was 20% versus 12% in previously irradiated patients. Three-year local control rate in previously unirradiated patients was 75% versus 51% in those previously irradiated. Three-year distant metastases rates were 64% and 71%, respectively.
An even more recent Mayo Clinic analysis50 described 607 patients with recurrent colorectal cancer who received IOERT as a component of treatment. Five-year OS was 30%. In patients undergoing R0 resection, 5-year OS was 46%. Recurrence within a previously irradiated field was associated with an increased risk of local relapse (3-year local relapse 39% vs. 20%, p <.001) but not survival. On multivariate analysis, complete resection, no prior chemotherapy, and treatment after 1996 were associated with improved survival. Three-year local and distant relapse rates were 27% and 55%, respectively.
Future Possibilities
Based on the just-presented and other data it appears improved local control and survival may be achieved when IORT is combined with preoperative chemoirradiation for locally advanced or locally recurrent colorectal cancer. Many patients will develop distant metastases, and relapse within the IORT and EBRT fields is common if gross total resection is not obtained. Based on the proven survival benefit of concurrent 5-FU–based regimens with EBRT in colorectal and other gastrointestinal malignancies, 5-FU–based chemotherapy should be administered concurrently with EBRT. Although adjuvant 5-FU with leucovorin has previously been shown to improve survival in patients with advanced-stage colorectal cancer, the addition of newer therapies (oxaliplatin, capecitabine, irinotecan, bevacizumab) has demonstrated further survival benefit in patients with stage IV disease. These agents are being evaluated as adjuvant therapy in patients with stage II and III disease (see Chapter 48). Given the high rate of subsequent distant metastases in locally advanced and recurrent colorectal cancer patients, significant improvement in long-term survival will likely be achieved through improvements in systemic agents.
Conclusions and Future Possibilities
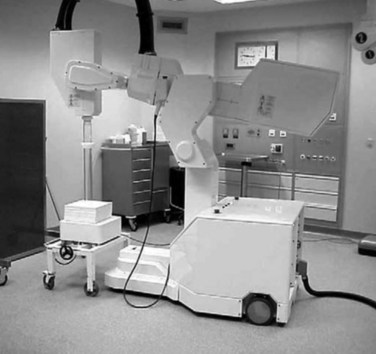
Many limitations and perceived drawbacks of IORT in past decades were due to inefficiencies associated with nondedicated facilities. Patients were often transported from the operating suite to the radiation oncology department where they were treated with nondedicated linear accelerators. These inconveniences have been overcome with dedicated IOERT, HDR-IORT, and/or orthovoltage facilities. Presently, dedicated IORT suites within or adjacent to the operating room exist at many institutions in the United States, Europe, and Far East. These facilities simplify treatment by avoiding transportation and sterility problems. A major limiting factor is the expense associated with outfitting a dedicated room (e.g., retrofitting an operating room with proper shielding, purchase of a linear accelerator dedicated for use in the operating room, construction of a separate IORT suite adjacent to the operating room). However, newer technologies have lowered these costs. Contemporary options include mobile IOERT units (Mobetron, Liac [Info & Tech, Rome, Italy], Novac) as well as mobile HDR-IORT.101 The Mobetron is a mobile, self-shielded compact linear accelerator with C-arm design that generates electron energies of 4 to 12 MeV (IntraOp Medical, Inc., Santa Clara, California, Fig. 16-3). This can be transported in and out of the operating theater. HDR-IORT units are remote afterloading devices that use an iridium-192 source (Figs. 16-4 and 16-5). In contrast to the Mobetron, HDR-IORT requires room shielding, which may be achieved by retrofitting an existing room or construction of a smaller shielded room adjacent to the operating suite. With either IORT or HDR-IORT, patients are monitored by camera during radiation administration. After completion, the HDR-IORT unit can be transported to the radiation oncology department for outpatient HDR-appropriate malignancies, including gynecologic and prostate malignancies. Mobile IOERT devices used in Europe include the Novac7 (miniature linear accelerator mounted on a robotic arm (see web-only Fig. 16-2 on the Expert Consult website![]() ) and Liac machines. The highest electron energies available with the Novac7 are 7 to 10 MeV. The INTRABEAM system for breast cancer IORT consists of a 50-kV x-ray generator mounted on a flexible floor stand and a set of spherical applicators ranging from 1.5 to 5 cm in diameter (see web-only Fig. 16-1 on the Expert Consult website
) and Liac machines. The highest electron energies available with the Novac7 are 7 to 10 MeV. The INTRABEAM system for breast cancer IORT consists of a 50-kV x-ray generator mounted on a flexible floor stand and a set of spherical applicators ranging from 1.5 to 5 cm in diameter (see web-only Fig. 16-1 on the Expert Consult website![]() ).
).

Figure 16-4 Source housing for iridium-192 source. This device allows computer-assisted treatment delivery during IORT.
Courtesy Varian Medical Systems.
Figure 16-5 Harrison-Anderson-Mick (HAM) applicator used to guide the iridium-192 source during HDR-IORT.
A detailed description of the relative advantages/disadvantages of IOERT and HDR-IORT has been discussed elsewhere and is beyond the scope of this chapter (see web-only Tables 16-6 and 16-7 on the Expert Consult website![]() ). In summary, treatment/procedure times are generally shorter with IOERT compared with HDR-IORT. Additionally, IOERT allows variation of electron energies and therefore treatment of both superficial and deeper-seated targets, whereas HDR-IORT is appropriate only for targets less than or equal to 0.5 cm in thickness (Fig. 16-6). The flexible Harrison-Anderson-Mick (HAM) applicator used in HDR-IORT may allow more conformal treatment along curved body surfaces (e.g., large pelvic sidewall fields, lateral abdominal wall, and thoracic cage), which may prove difficult with rigid IOERT applicators (see Fig. 16-5). Separate, matching fields may be required to treat larger target areas with IOERT-based applicators. A comprehensive IORT program would ideally have IOERT, HDR-IORT, as well as perioperative brachytherapy available to treat all disease sites and situations. These modalities should be viewed as complementary and not competitive.
). In summary, treatment/procedure times are generally shorter with IOERT compared with HDR-IORT. Additionally, IOERT allows variation of electron energies and therefore treatment of both superficial and deeper-seated targets, whereas HDR-IORT is appropriate only for targets less than or equal to 0.5 cm in thickness (Fig. 16-6). The flexible Harrison-Anderson-Mick (HAM) applicator used in HDR-IORT may allow more conformal treatment along curved body surfaces (e.g., large pelvic sidewall fields, lateral abdominal wall, and thoracic cage), which may prove difficult with rigid IOERT applicators (see Fig. 16-5). Separate, matching fields may be required to treat larger target areas with IOERT-based applicators. A comprehensive IORT program would ideally have IOERT, HDR-IORT, as well as perioperative brachytherapy available to treat all disease sites and situations. These modalities should be viewed as complementary and not competitive.
WEB-ONLY TABLE 16-6 Relative Advantages and Disadvantages of IOERT versus HDR-IORT Brachytherapy after Gross Total or Near-Total Resection (Maximum Tumor Thickness ≤0.5 cm)
| IOERT Potential Advantage If Technically Feasible | Potential Disadvantages of IOERT | Potential Solution to Disadvantages |
|---|---|---|
| Better dose homogeneity | Surface dose <90% with 6 ± 9 MeV | Add bolus over tumor bed to improve surface dose; use HDR-IORT |
| Faster treatment time | ||
| Less shielding required in operating room | Unable to include area at risk in single field within either abdomen or pelvis | Use abutting IOERT fields (difficult in pelvis); use HDR-IORT |
| Can treat full thickness of organ or structure at risk with relative homogeneity (e.g., aorta or vena cava, bladder side wall) | Area at risk is technically inaccessible because of location | Use HDR-IORT; surgically displace small bowel or stomach with vascularized flap (omentum, muscle) and give postoperative EBRT boost or perioperative brachytherapy |
WEB-ONLY TABLE 16-7 Potential Differences between IOERT and HDR-IORT
| IOERT | HDR-IORT | |
|---|---|---|
| Actual treatment time | 2-4 min | 5-30 min |
| Total procedure time | 30-45 min | 45-120 min |
| Treatment sites | Accessible locations | All areas where depth at risk is ≤0.5 cm from surface of applicator |
| Surface dose | Lower (75%-93%) | Higher (150-200%) |
| Dose at depth (2 cm) | Higher (70%-100%) | Lower (30%) |
| Dosimetric homogeneity (surface to depth) |
≤10% variation | ≥100% variation |
From Nag S, et al: Intraoperative irradiation with electron-beam or high-dose-rate brachytherapy. In Gunderson L, Willett C, Harrison L, Calvo F (eds): Intraoperative Irradiation. Totowa, NJ, Humana Press, 1999, pp 111-130.
Although there is a large body of data supporting the use of IORT in various malignancies, there is a relative paucity of phase III randomized trials. This is at least in part due to the limited number of IORT facilities in any given country as well as relative rarity of diseases commonly treated with IORT. Completion of phase II/III trials will likely require cooperation among multiple institutions and countries. Future treatment approaches should include “standard” courses of EBRT ± concurrent chemotherapy and surgical resection with the integration of novel radiation sensitizers, protectors, and targeted biologic agents with IORT.102
2 Abe M, Fukada M, Yamano K, et al. Intraoperative radiation in abdominal and cerebral tumors. Acta Radiol. 1971;10:408-416.
4 Okunieff P, Sundararaman S, Chen Y. Biology of large dose per fraction radiation therapy. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative Irradiation—Techniques and Results. Totowa, NJ: Humana Press; 1999:25-46.
5 Gunderson LL, Nagorney DM, Martenson JA, et al. External beam plus intraoperative radiation for gastrointestinal cancers. World J Surg. 1995;19:191-197.
7 Garton GR, Gunderson LL, Nagorney DM, et al. High dose preoperative external beam and intraoperative irradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 1993;27:1153-1157.
9 Mohiuddin M, Regine WF, Stevens J, et al. Combined intraoperative radiation in perioperative chemotherapy for unresectable cancers of the pancreas. J Clin Oncol. 1995;13:2764-2768.
11 Sindelar WF, Kinsella TJ, Chen PW, et al. Intraoperative radiotherapy and retroperitoneal sarcomas. Final results of a prospective, randomized, clinical trial. Arch Surg. 1993;128:402-410.
17 Fletcher GH. Clinical dose response curves of human malignant epithelial tumors. Br J Radiol. 1973;46:1-12.
18 Fletcher GH, Shukovsky LJ. The interplay of radiocurability and tolerance in the irradiation of human cancers. J Radiol Electrol. 1975;56:383-400.
27 Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949-955.
30 Sauer R, Becker H, Hoheberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740.
32 Nag S, Gunderson LL, Willett CG, et al. Intraoperative irradiation with electron beam or high dose rate brachytherapy. Methodological comparisons. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative Irradiation—Techniques and Results. Totowa, NJ: Humana Press; 1999:111-130.
35 Kinsella TJ, DeLuca AM, Barnes M, et al. Threshold dose for peripheral neuropathy following intraoperative radiotherapy (IORT) in a large animal model. Int J Radiat Oncol Biol Phys. 1991;20:697-701.
39 Sindelar WF, Johnstone PA, Hoekstra H, et al. Normal tissue tolerance to IORT: The NCI experimental studies. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative Irradiation—Techniques and Results. Totowa, NJ: Humana Press; 1999:131-146.
49 Mathis K, Nelson H, Pemberton J, et al. Unresectable colorectal cancer can be cured with multi-modality therapy. Ann Surg. 2008;248:592-598.
50 Haddock M, Miller R, Nelson H, et al. Intraoperative electron radiation for locally recurrent colorectal cancer. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:50.
53 Kusters M, Valentini V, Calvo F, et al. Results of a European pooled analysis of IORT- containing multi-modality treatment for locally advanced rectal cancer. Adjuvant chemotherapy prevents local recurrence rather than distant metastases. Ann Oncol. 2010;21:1279-1284.
59 Garton GR, Gunderson LL, Nagorney DM, et al. High-dose preoperative external beam and intraoperative irradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 1993;27:1153-1157.
61 Willett CG, Del Castillo CF, Shih HA, et al. Long-term results of intraoperative electron beam irradiation (IOERT) for patients with unresectable pancreatic cancer. Ann Surg. 2005;241:295-299.
62 Gunderson LL, Moss A, Callister MG, et al. Preoperative chemoradiation and IOERT for unresectable or borderline resectable pancreas cancer. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:32-33.
65 Early Breast Cancer Trialist Collaborative Group. Favourable and unfavourable effect on long-term survival of radiotherapy for early breast cancer. An overview of the randomized trials. Lancet. 2000;355:1757-1770.
68 Veronesi U, Orecchia R, Luini A, et al. Full-dose intraoperative radiotherapy with electrons during breast-conserving surgery. Experience with 590 cases. Ann Surg. 2005;242:101-106.
69 Bartelink A, Horiot J, Poortmans P, et al. Impact of a higher radiation dose on local control and survival on breast-conserving therapy of early breast cancer. 10-Year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25:3259-3265.
80 Petersen I, Haddock M, Stafford S, et al. Use of intraoperative radiation therapy for retroperitoneal sarcomas. Update of the Mayo Clinic Rochester Experience. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:57.
83 Krempien R, Roeder F, Buchler M, et al. Intraoperative radiation therapy (IORT) for primary and recurrent retroperitoneal soft tissue sarcoma. First results of a pooled analysis. ISIORT 2008 Proceedings. Rev. Cancer. 2008;22:56.
95 Suzuki K, Gunderson L, Devine R, et al. Intraoperative radiation after palliative surgery for locally recurrent rectal cancer. Cancer. 1995;75:939-952.
97 Wallace HJ, Willett CG, Shellito PC, et al. Intraoperative radiation therapy for locally advanced recurrent rectal and rectosigmoid cancer. J Surg Oncol. 1995;60:122-127.
1 Medina R, Casas F, Calvo F. Radiation oncology in Spain. Historical notes for the radiology centennial. Int J Radiat Oncol Biol Phys. 1996;35:1075-1097.
2 Abe M, Fukada M, Yamano K, et al. Intraoperative radiation in abdominal and cerebral tumors. Acta Radiol. 1971;10:408-416.
3 Rubin P, Cassarett GW. Clinical Radiation and Pathology. Philadelphia: WB Saunders; 1968.
4 Okunieff P, Sundararaman S, Chen Y. Biology of large dose per fraction radiation therapy. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative Irradiation—Techniques and Results. Totowa, NJ: Humana Press; 1999:25-46.
5 Gunderson LL, Nagorney DM, Martenson JA, et al. External beam plus intraoperative radiation for gastrointestinal cancers. World J Surg. 1995;19:191-197.
6 Roldan GE, Gunderson LL, Nagorney DM, et al. External beam versus intraoperative and external beam irradiation for locally advanced pancreatic cancer. Cancer. 1988;61:1110-1116.
7 Garton GR, Gunderson LL, Nagorney DM, et al. High dose preoperative external beam and intraoperative irradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 1993;27:1153-1157.
8 Whittington R, Solin L, Mohiuddin M, et al. Multimodality therapy of unresectable pancreatic adenocarcinoma. Cancer. 1984;54:1991-1998.
9 Mohiuddin M, Regine WF, Stevens J, et al. Combined intraoperative radiation in perioperative chemotherapy for unresectable cancers of the pancreas. J Clin Oncol. 1995;13:2764-2768.
10 Mohiuddin M, Cantor RJ, Bierman W, et al. Combined modality treatment of localized unresectable adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1988;14:79-84.
11 Sindelar WF, Kinsella TJ, Chen PW, et al. Intraoperative radiotherapy and retroperitoneal sarcomas. Final results of a prospective, randomized, clinical trial. Arch Surg. 1993;128:402-410.
12 Brierly JD, Cummings BJ, Wong CS, et al. Adenocarcinoma of the rectum treated by radical external radiation therapy. Int J Radiat Oncol Biol Phys. 1995;31:255-259.
13 O’Connell MJ, Childs DS, Moertel CG, et al. A prospective controlled evaluation of combined pelvic radiotherapy and methanol extraction residue of BCG (MER) for locally unresectable or recurrent rectal carcinoma. Int J Radiat Oncol Biol Phys. 1982;8:1115-1119.
14 Gunderson LL, Cohen AC, Dosoretz DD, et al. Residual, unresectable or recurrent colorectal cancer. External beam irradiation and intraoperative electron beam boost + resection. Int J Radiat Oncol Biol Phys. 1983;9:1597-1606.
15 Tewfik HH, Bushsbuam HJ, Latourette HB, et al. Para-aortic lymph node irradiation in carcinoma of the cervix after exploratory laparotomy and biopsy-proven aortic nodes. Int J Radiat Oncol Biol Phys. 1982;8:13-18.
16 Piver MS, Barlow JJ. High dose irradiation to biopsy confirmed aortic node metastases from carcinoma of the uterine cervix. Cancer. 1977;39:1243-1246.
17 Fletcher GH. Clinical dose response curves of human malignant epithelial tumors. Br J Radiol. 1973;46:1-12.
18 Fletcher GH, Shukovsky LJ. The interplay of radiocurability and tolerance in the irradiation of human cancers. J Radiol Electrol. 1975;56:383-400.
19 Griscom NT, Wang CC. Radiation therapy in inoperable breast carcinoma. Radiology. 1962;79:18-23.
20 Tepper J. Clonogenic potential of human tumors. A hypothesis. Acta Radiol Oncol. 1981;20:283-288.
21 Ramsay J, Suit HD, Sedlacek R. Experimental studies on the incidence of metastases after failure of radiation treatment and the effect of salvage surgery. Int J Radiat Oncol Biol Phys. 1988;14:1165-1168.
22 Suit HD. Local control in patient survival. Int J Radiat Oncol Biol Phys. 1992;23:653-660.
23 Suit HD, Sedlacek RS, Gillette EL. Examination for a correlation between probabilities of development of distant metastasis and of local recurrence. Radiology. 1970;95:189-194.
24 Suit HD. Potential for improving survival rates for the cancer patient by increasing efficacy of treatment of the primary lesion. Cancer. 1982;50:1227-1234.
25 Fuks Z, Leibel SA, Wallner KE, et al. The effect of local control in metastatic dissemination in carcinoma of the prostate. Long-term results in patients treated with I-125 implantation. Int J Radiat Oncol Biol Phys. 1991;21:537-547.
26 Leibel SA, Scott CB, Mohiuddin M, et al. The effect of local-regional control on distant metastatic dissemination in carcinoma of the head and neck. The results of an analysis of the RTOG head and neck database. Int J Radiat Oncol Biol Phys. 1991;21:549-556.
27 Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949-955.
28 Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas—616 patients. Results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567-579.
29 Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997;15:928-937.
30 Sauer R, Becker H, Hoheberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740.
31 Gunderson LL, Shipley WU, Suit HD, et al. Intraoperative irradiation. A pilot study combining external beam photons with “boost” dose intraoperative electrons. Cancer. 1982;49:2259-2266.
32 Nag S, Gunderson LL, Willett CG, et al. Intraoperative irradiation with electron beam or high dose rate brachytherapy. Methodological comparisons. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative Irradiation—Techniques and Results. Totowa, NJ: Humana Press; 1999:111-130.
33 Tepper JE, Gunderson LL, Orlow E, et al. Complications of intraoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1984;10:1831-1839.
34 Johnstone PA, Sindelar WF, Kinsella TJ. Experimental and clinical studies of intraoperative radiation. Int J Radiat Oncol Biol Phys. 1986;12:1687-1695.
35 Kinsella TJ, DeLuca AM, Barnes M, et al. Threshold dose for peripheral neuropathy following intraoperative radiotherapy (IORT) in a large animal model. Int J Radiat Oncol Biol Phys. 1991;20:697-701.
36 Kinsella TJ, Sindelar WF, DeLuca AM, et al. Tolerance of the canine bladder to intraoperative radiation therapy. An experimental study. Int J Radiat Oncol Biol Phys. 1988;14:939-946.
37 Sindelar WF, Tepper JE, Travis EL. Tolerance of bile duct to intraoperative irradiation. Surgery. 1982;92:533-546.
38 Sindelar WF, Tepper JE, Kinsella TJ. Late effects of intraoperative radiation therapy on retroperitoneal structures, intestine, and bile duct in a large animal model. Int J Radiat Oncol Biol Phys. 1994;29:781-788.
39 Sindelar WF, Johnstone PA, Hoekstra H, et al. Normal tissue tolerance to IORT. The NCI experimental studies. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative Irradiation—Techniques and Results. Totowa, NJ: Humana Press; 1999:131-146.
40 LeCouteur RA, Gillette EL, Powers EL, et al. Peripheral neuropathies following experimental intraoperative radiation therapy (IORT). Int J Radiat Oncol Biol Phys. 1989;17:583-590.
41 Gillette EL, Gillette SM, Vujaskovic Z, et al. Influence of volume on canine ureters and peripheral nerves irradiated intraoperatively. In: Schildberg FW, Willich N, Krämling H, editors. Intraoperative Radiation Therapy—Proceedings of the 4th International IORT Symposium. Munich, 1992. Essen, Germany: Verlag Die Blaue Eule; 1993:61-63.
42 Gillette EL, Gillette SM, Powers BE. Studies at Colorado State University of normal tissue tolerance of beagles to IOERT, EBRT or a combination. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative Irradiation—Techniques and Results. Totowa, NJ: Humana Press; 1999:147-164.
43 Shaw EG, Gunderson LL, Martin JK, et al. Peripheral nerve and ureteral tolerance of intraoperative radiation therapy. Clinical and dose response analysis. Radiother Oncol. 1990;18:247-255.
44 Miller R, Haddock M, Petersen I, et al. Intraoperative electron-beam radiotherapy and ureteral obstruction. Int J Radiat Oncol Biol Phys. 2006;64:792-798.
45 Johnstone PA, Sindelar WF, Kinsella TJ. Experimental and clinical studies of intraoperative radiation therapy. Curr Probl Cancer. 1994;18:249-292.
46 Kinsella TJ, DeLuca AM, Barnes M, et al. Threshold dose for peripheral neuropathy following intraoperative radiotherapy (IORT) in a large animal model. Int J Radiat Oncol Biol Phys. 1991;20:697-701.
47 Gunderson LL, Nelson H, Martenson JA, et al. Intraoperative electron and external beam irradiation with or without 5-fluorouracil and maximum surgical resection for previously unirradiated, locally recurrent colorectal cancer. Dis Colon Rectum. 1996;39:1379-1395.
48 Gunderson LL, Nelson H, Martenson JA, et al. Locally advanced primary colorectal cancer. Intraoperative electron and external beam irradiation ± 5-FU. Int J Radiat Oncol Biol Phys. 1997;37:601-614.
49 Mathis K, Nelson H, Pemberton J, et al. Unresectable colorectal cancer can be cured with multi-modality therapy. Ann Surg. 2008;248:592-598.
50 Haddock M, Miller R, Nelson H, et al. Combined modality therapy including intraoperative electron irradiation for locally recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 2011;79:143-150.
51 Gomez M, Calvo F, Gonzalez C, et al. Timing and intensity of neoadjuvant treatment in rectal cancer. Results of pre (+ IOERT) versus post (no IOERT) chemoradiation. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:45.
52 Rutten H, Valentini V, Krempien R, et al. Treatment of locally advanced rectal cancer by intraoperative electrobeam radiotherapy containing multimodality treatment. Results of a European pooled analysis. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:45-46.
53 Kusters M, Valentini V, Calvo F, et al. Results of a European pooled analysis of IORT- containing multi-modality treatment for locally advanced rectal cancer. Adjuvant chemotherapy prevents local recurrence rather than distant metastases. Ann Oncol. 2010;21:1279-1284.
54 Vujaskovic Z. Structural and physiological properties of peripheral nerves after intraoperative radiation. J Peripher Nerv Syst. 1997;2:343-349.
55 Azinovic I, Martinez-Monge R, Javier-Aristu J, et al. Intraoperative radiotherapy electron boost followed by moderate doses of external beam radiotherapy in resected soft-tissue sarcoma of the extremities. Radiother Oncol. 2003;67:331-337.
56 Gunderson LL, Willett CG, Harrison LB, Calvo FA. Intraoperative Irradiation—Techniques and Results. Totowa, NJ: Humana Press; 1999.
57 Gunderson LL, Willett CG, Calvo FA, Harrison LB. Intraoperative Irradiation—Techniques and Results, 2nd ed. Totowa, NJ: Humana Press/Springer; 2011.
58 Roldan GE, Gunderson LL, Nagorney DM, et al. External beam vs external beam and intraoperative irradiation for locally advanced pancreatic cancer. Cancer. 1988;61:1110-1116.
59 Garton GR, Gunderson LL, Nagorney DM, et al. High-dose preoperative external beam and intraoperative irradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 1993;27:1153-1157.
60 Tepper JE, Shipley WU, Warshaw AL, et al. The role of misonidazole combined with intraoperative radiation therapy in the treatment of pancreatic carcinoma. J Clin Oncol. 1987;5:579-584.
61 Willett CG, Del Castillo CF, Shih HA, et al. Long-term results of intraoperative electron beam irradiation (IOERT) for patients with unresectable pancreatic cancer. Ann Surg. 2005;241:295-299.
62 Gunderson LL, Moss A, Callister MG, et al. Preoperative chemoradiation and IOERT for unresectable or borderline resectable pancreas cancer. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:32-33.
63 Ogawa K, Karasawa K, Ito Y, et al. Intraoperative radiotherapy for resected pancreatic cancer. A multi-institutional retrospective analysis of 210 patients. Int J Radiat Oncol Biol Phys. 2010;77:734-742.
64 Valentini V, Calvo F, Rani M, et al. Intraoperative radiotherapy (IORT) pancreatic cancer. Joint analysis of the ISIORT-Europe experience. Radiother Oncol. 2009;91:54-59.
65 Early Breast Cancer Trialist Collaborative Group. Favourable and unfavourable effect on long-term survival of radiotherapy for early breast cancer. An overview of the randomized trials. Lancet. 2000;355:1757-1770.
66 Lemanski C, Azria D, Gourgon-Bourjade S, et al. Intraoperative radiotherapy in early-stage breast cancer. Results of the Montpellier phase II trial. Int J Radiat Oncol Biol Phys. 2010;76:698-703.
67 Majewski W, Wydmanski J, Kanieska-Dorsz Z, et al. Early results of a targeted intra-operative radiation therapy (TARGIT) as a boost in breast conserving treatment. ISIORT 2008 Proceedings. Cancer. 2008;22:17.
68 Veronesi U, Orecchia R, Luini A, et al. Full-dose intraoperative radiotherapy with electrons during breast-conserving surgery. Experience with 590 cases. Ann Surg. 2005;242:101-106.
69 Bartelink A, Horiot J, Poortmans P, et al. Impact of a higher radiation dose on local control and survival on breast-conserving therapy of early breast cancer. 10-Year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25:3259-3265.
70 Battle JA, DuBois JB, Merrick HW, et al. IORT for breast cancer. In: Gunderson LL, Willett CG, Harrison LB, Calvo FA, editors. Intraoperative Irradiation—Techniques and Results. Totowa, NJ: Humana Press; 1999:521-526.
71 Reitsamer R, Peintinger F, Kopp M, et al. Local recurrence rated in breast cancer patients treated with intraoperative electron-boost radiotherapy versus postoperative external beam electron boost irradiation. Strahlenther Onkol. 2004;1:38-44.
72 Wenz F, Welzel G, Blank E, et al. Intraoperative radiotherapy as a boost during breast-conserving surgery using low kilovoltage x-rays. The first 5 years of experience with a novel approach. Int J Radiat Oncol Biol Phys. 2010;77:1309-1314.
73 Veronesi U, Gatti G, Luini A, et al. Full-dose intraoperative radiotherapy with electrons during breast-conserving surgery. Arch Surg. 2003;138:1253-1256.
74 Arcangeli G, Arcangeli S, Giordano C, et al. Intraoperative (IORT) versus standard radiotherapy (EBRT) in breast cancer. An update of an ongoing Italian multicenter, randomized study. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:13.
75 Cuncins-Harn A, Saunders C, Walsh D. A systematic review of intraoperative radiotherapy in early breast cancer. Breast Cancer Res Treat. 2004;85:271-280.
76 Vaidya J, Baum M, Tobias J, et al. Targeted intraoperative radiotherapy (TARGIT) yields very low recurrence rates when given as a boost. Int J Radiat Oncol Biol Phys. 2006;66:1335-1338.
77 Sedlmayr F, Fastner G, Merz F, et al. IORT with electrons as boost strategy during breast-conserving therapy in limited stage breast cancer. Results of a IORT pooled analysis. Strahlenther Onkol. 2007;183:32-34.
78 Sedlmayer F, Fastner G, Merz F, et al. ISIORT pooled analysis on linac-based IORT as boost strategy during breast conserving therapy. ISIORT 2008 Proceedings. on behalf of the ISIORT-Europe]. Rev Cancer. 2008;22:21-22.
79 Petersen IA, Haddock MG, Donohue JH, et al. Use of intraoperative electron beam radiotherapy in the management of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Bio Phys. 2002;52:469-475.
80 Petersen I, Haddock M, Stafford S, et al. Use of intraoperative radiation therapy for retroperitoneal sarcomas. Update of the Mayo Clinic Rochester Experience. ISIORT 2008 Proceedings. Cancer. 2008;22:57.
81 Gieschen HL, Spiro IJ, Suit HD, et al. Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Bio Phys. 2001;50:127-131.
82 Pierie J, Betensky R, Choudry U, et al. Outcomes in a series of 103 retroperitoneal sarcomas. Eur J Surg Oncol. 2006;32:1235-1241.
83 Krempien R, Roeder F, Buchler M, et al. Intraoperative radiation therapy (IORT) for primary and recurrent retroperitoneal soft tissue sarcoma. First results of a pooled analysis. ISIORT 2008 Proceedings. Cancer. 2008;22:56.
84 Gunderson LL, Nagorney DM, McIlrath DC, et al. External beam and intraoperative electron irradiation for locally advanced soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 1993;25:647-656.
85 Calvo FA, Azinovic I, Martinez R, et al. Intraoperative radiotherapy for the treatment of soft tissue sarcomas of central anatomic sites. IORT 94-5th International Symposium Abstracts. Hepatogastroenterology. 1994;41:4.
86 Dubois JB, Hay MH, Gely S, et al. Intraoperative radiation therapy (IORT) in soft tissue sarcomas. IORT 94-5th International Symposium Abstracts. Hepatogastroenterology. 1994;41:3.
87 Pisters PWT, Ballo MT, Fenstermacher MJ, et al. Phase I trial of preoperative concurrent doxorubicin and radiation therapy, surgical resection, and intraoperative electron-beam radiation therapy for patients with localized retroperitoneal sarcoma. J Clin Oncol. 2003;21:3092-3097.
88 Haddock MG, Petersen IA, Webb MJ, et al. Intraoperative radiotherapy for locally advanced gynecologic malignancies. Front Radiat Ther Oncol. 1997;31:256-259.
89 Haddock MG, Petersen IA, Webb MJ, et al: Intraoperative radiation therapy for locally advanced gynecological (GYN) malignancies. Presented before the 3rd International ISIORT Meeting, Aachen, Germany. ISIORT Abstract 5.555, 2002.
90 Haddock MG: Intraoperative radiation therapy for locally advanced gynecologic malignancies. ISIORT 2005 Proceedings; personal communication, 2005
91 Martinez-Monge R, Jurado M, Arist J, et al. Intraoperative electron beam radiotherapy during radical surgery for locally advanced and recurrent cervical cancer. Gynecol Oncol. 2001;80:538-543.
92 Zi L, Ying G. Ten year experience using IOERT to treat stage IIB cervical cancer. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:30.
93 Tran P, Su Z, Hara W, et al. Long-term survivors using intraoperative radiotherapy for recurrent gynecologic malignancies. Int J Radiat Oncol Bio Phys. 2007;69:504-511.
94 Willett CG, Shellito PC, Tepper JE, et al. Intraoperative electron beam radiation therapy for primary locally advanced rectal and rectosigmoid carcinoma. J Clin Oncol. 1991;9:843-849.
95 Suzuki K, Gunderson L, Devine R, et al. Intraoperative radiation after palliative surgery for locally recurrent rectal cancer. Cancer. 1995;75:939-952.
96 Willett CG, Shellito PC, Tepper JE, et al. Intraoperative electron beam radiation therapy for recurrent locally advanced rectal and rectosigmoid carcinoma. Cancer. 1991;67:1504-1508.
97 Wallace HJ, Willett CG, Shellito PC, et al. Intraoperative radiation therapy for locally advanced recurrent rectal and rectosigmoid cancer. J Surg Oncol. 1995;60:122-127.
98 Abuchaibe O, Calvo FA, Tangeo E, et al. Intraoperative irradiation in locally advanced recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 1993;26:859-867.
99 Dresen R, Goesns M, Martijm H, et al. Radical resection after IOERT containing multimodality treatment is an important determinant for outcomes in patients treated for locally recurrent rectal cancer. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:45-46.
100 Haddock MG, Gunderson LL, Nelson H, et al. Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int J Radiat Oncol Biol Phys. 2001;49:1267-1274.
101 Meurk ML, Goer DA, Spalek G, Cock T, et al. The Mobetron. A new concept for intraoperative radiotherapy. Front Radiat Ther Oncol. 1997;31:65-70.
102 Merrick HW, Gunderson LL, Calvo FA. Future directions in intraoperative radiation therapy. Surg Oncol Clin North Am. 2003;12:1099-1105.