Intraoperative 3-D Echocardiography
Gian Paparcuri
Transesophageal echocardiography is probably the most frequently used imaging technique in cardiac surgery. This powerful technology provides timely information, about cardiac structures (anatomy) and function (hemodynamics) without disrupting the surgical workflow.
 Enhances the illusion of depth perception.
Enhances the illusion of depth perception.
 Offers a better appreciation of individual patient anatomy.
Offers a better appreciation of individual patient anatomy.
 Facilitates the understanding of complex cardiac pathology.
Facilitates the understanding of complex cardiac pathology.
 Improves appreciation of the relationship between cardiac structures by allowing visualization from different angles.
Improves appreciation of the relationship between cardiac structures by allowing visualization from different angles.
 Expedites the study of ventricular volumes by displaying geometrically complicated chambers, such as the right ventricle.
Expedites the study of ventricular volumes by displaying geometrically complicated chambers, such as the right ventricle.
 Eliminates the assumption of specific geometric shape of the ventricles, when quantifying volume and ejection fraction. 3-D does not assume the heart has a particular shape, because you see it as it is (quantification is more accurate).
Eliminates the assumption of specific geometric shape of the ventricles, when quantifying volume and ejection fraction. 3-D does not assume the heart has a particular shape, because you see it as it is (quantification is more accurate).
 Reduces measurement variability.
Reduces measurement variability.
 Offers the potential to slice the dynamic cardiac structures in infinite planes through the three dimensions.
Offers the potential to slice the dynamic cardiac structures in infinite planes through the three dimensions.
With 3-D echocardiography, people who aren’t echocardiographers can appreciate valve anatomy and physiology in three dimensions. Surgeons tend to very much appreciate 3-D images because the echo image looks exactly like what they see when the heart is open. Finally, for those of you taking the well-known echocardiography course in San Diego, expect to see a lot of 3-D. It seems like the speakers are not allowed to talk about echocardiography if they don’t complement their presentation by adding some 3-D images.
TEE Probes
Non-3-D echocardiography probes use a limited number (64–128) of piezoelectric elements (crystals) to scan tissue. Sequential (phased) activation of individual crystals generates an ultrasound beam that is steered back and forth over a 90° angle to sweep a flat, “pie-shaped” scanning plane or sector. 3-D echocardiographic probes have on their tips more than 2500 active elements, incorporated onto the probe, conforming a rectangular grid of 50 rows and 50 columns for a total of 2500 independent piezoelectric crystals, generating a matrix array.
 Conversion: raw data are placed into a Cartesian volume with each point assigned x-y-z coordinates and an echo intensity value. The product of this step is a group of points with distinctive echogenic characteristics and a known position in space, called voxels or volume of pixels, encrypting the physical characteristics and location of the smallest cube in the dataset, similar to pixel size in 2-D image resolution. Large voxels are generated when raw data are available for fewer points in the space and interpolation has to fill wider gaps. Still not a clear 3-D image.
Conversion: raw data are placed into a Cartesian volume with each point assigned x-y-z coordinates and an echo intensity value. The product of this step is a group of points with distinctive echogenic characteristics and a known position in space, called voxels or volume of pixels, encrypting the physical characteristics and location of the smallest cube in the dataset, similar to pixel size in 2-D image resolution. Large voxels are generated when raw data are available for fewer points in the space and interpolation has to fill wider gaps. Still not a clear 3-D image.
 Interpolation: filling the gaps or space between all the known points in space with data points of similar characteristics. The result is a smooth surface 3-D object.
Interpolation: filling the gaps or space between all the known points in space with data points of similar characteristics. The result is a smooth surface 3-D object.
Finally, step 4 or 3-D image display: makes 3-D dataset visible.
 1st step is segmentation: separates the object to be rendered from surrounding structures (differentiates between cardiac tissue, blood, pericardial fluid, blood, air; given their diverse physical properties and different ability to reflect US; this requires setting a threshold of echo intensity). The program excludes from further processing any point with echo intensity equal to or lower than blood, and delineates the 3-D surfaces of cardiac tissue.
1st step is segmentation: separates the object to be rendered from surrounding structures (differentiates between cardiac tissue, blood, pericardial fluid, blood, air; given their diverse physical properties and different ability to reflect US; this requires setting a threshold of echo intensity). The program excludes from further processing any point with echo intensity equal to or lower than blood, and delineates the 3-D surfaces of cardiac tissue.
 2nd step: The 3-D dataset undergoes 1 of 3 increasingly complex rendering techniques to create a visible 3-D object: wireframe rendering, surface rendering or volume rendering.
2nd step: The 3-D dataset undergoes 1 of 3 increasingly complex rendering techniques to create a visible 3-D object: wireframe rendering, surface rendering or volume rendering.
Wireframe rendering: The simplest technique. Defines and connects equidistant points on the surface of a 3-D object with lines (wires) to create a mesh of small polygonal tiles. Smoothing algorithms refine the narrow angles making rudimental object appear more real. This technique is used for relatively flat surfaces such as the LV and the atrial cavities. It cannot display objects/structures with complex shapes, such as valves (requires greater anatomic detail for meaningful analysis). Processes small amount of data (fast and efficiently performed on basic computers).
Surface rendering: similar to wireframe but defines more points on the surface of a 3-D object making the lines joining them visible. Displays details of a 3-D surface and makes morphologic assessment of the corresponding anatomic structure feasible. Generates 3-D objects with rendered surfaces and a hollow core.
Volume rendering: displays 3-D objects with a rendered surface and details of its inner structure. Enables the potential display of every voxel of the 3-D object permitting a “virtual dissection”.
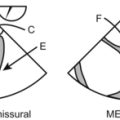
Mitral Valve
 Spatial manipulation, assessment in anatomical and surgical orientation for discussion with the surgeon.
Spatial manipulation, assessment in anatomical and surgical orientation for discussion with the surgeon.
The MV can be easily imaged using all the 3-D imaging modes described:
 Live (from 2-D ME 4C) yields only a portion of the MV.
Live (from 2-D ME 4C) yields only a portion of the MV.
 Zoom is the modality of choice to view detailed anatomy of the entire MV with adequate temporal and spatial resolution. The zoom acquisition starts with imaging the entire MV in 2-D, preferably with the AV in view. The displayed pyramid-shaped 3-D image can be manipulated on screen in real time to view the MV from any perspective.
Zoom is the modality of choice to view detailed anatomy of the entire MV with adequate temporal and spatial resolution. The zoom acquisition starts with imaging the entire MV in 2-D, preferably with the AV in view. The displayed pyramid-shaped 3-D image can be manipulated on screen in real time to view the MV from any perspective.
Stored MV 3-D images can be cropped on any plane to further delineate leaflet morphology.
The role of 3-D transesophageal echocardiography is expanding to become a powerful tool guiding surgical repair (treatment of choice for MR). In mitral stenosis, 3-D echo can consistently identify MV commissural fusion and predict the success of MV balloon valvuloplasty. Some authors have proposed planimetry by real-time 3-D echocardiography as a “gold standard” in the assessment of MS.
LV Assessment
 2-D limitations: “eyeballing assessment”, time consuming, geometrical assumptions, foreshortened views.
2-D limitations: “eyeballing assessment”, time consuming, geometrical assumptions, foreshortened views.
 3-D benefits: largest cardiac structure (in normal hearts) and 3-D (full volume) is the only imaging modality that can capture the entire LV volume at sufficient frame rate (25 Hz) allowing dynamic assessment.
3-D benefits: largest cardiac structure (in normal hearts) and 3-D (full volume) is the only imaging modality that can capture the entire LV volume at sufficient frame rate (25 Hz) allowing dynamic assessment.
LV Volume
 3-D-guided biplanes: by simultaneously displaying 2 perpendicular 2-D planes (ME 4C and ME 2C combination minimizes LV foreshortening) cutting the LV along its long axis at the true apex. Allows calculation (by applying modified Simpson biplane methods of disks to ES and ED frames) of LV volume, EF, mass. Limitations: still relies on geometric assumptions.
3-D-guided biplanes: by simultaneously displaying 2 perpendicular 2-D planes (ME 4C and ME 2C combination minimizes LV foreshortening) cutting the LV along its long axis at the true apex. Allows calculation (by applying modified Simpson biplane methods of disks to ES and ED frames) of LV volume, EF, mass. Limitations: still relies on geometric assumptions.
 Direct volumetric analysis: rendering a cast of the LV cavity and measure its volume throughout the cardiac cycle. Required identification of 4 LV walls and apex derived from full-volume 3-D dataset. Semiautomatic endocardial border detection creates a dynamic cast of the LV endocardial cavity. EDV and ESV are measured and SV and EF calculated. It is a more accurate method for LV volume in patients with abnormal ventricular shape or regional wall motion abnormalities due to a better alignment through the cardiac apex—inclusion of more endocardial surface during analysis and the lack of geometric shape assumptions (more reliable especially for less experienced users).
Direct volumetric analysis: rendering a cast of the LV cavity and measure its volume throughout the cardiac cycle. Required identification of 4 LV walls and apex derived from full-volume 3-D dataset. Semiautomatic endocardial border detection creates a dynamic cast of the LV endocardial cavity. EDV and ESV are measured and SV and EF calculated. It is a more accurate method for LV volume in patients with abnormal ventricular shape or regional wall motion abnormalities due to a better alignment through the cardiac apex—inclusion of more endocardial surface during analysis and the lack of geometric shape assumptions (more reliable especially for less experienced users).
RV Assessment
RV 2-D assessment is difficult because of its crescent shape and complex geometry (a lot of assumptions). 3-D overcomes 2-D limitations allowing full-volume 3-D dataset acquisition for RV size, volume and function with good correlation with MRI and better reproducibility than 2-D.