Chapter 17 Intradiscal Therapies
Intradiscal decompression for chronic intractable discogenic pain can be achieved by means of various methods, such as intradiscal electrothermal therapy (IDET), nucleoplasty, and percutaneous discectomy. Tables 17.1 and 17.2 summarize these three techniques.
Table 17.2 The Evidence for Efficacy Intradiscal Electrothermal Therapy (IDET) and Nucleoplasty in Management of Chronic Discogenic Low Back Pain
| Therapy | Evidence of Short-Term Relief | Evidence of Long-Term Relief |
|---|---|---|
| IDET | Strong | Moderate |
| Nucleoplasty | Limited | Limited |
Data from Boswell MV, Shah RV, Everett CR, et al. Interventional techniques in the management of chronic spinal pain: Evidence-based practice guidelines. Pain Physician 2005;8:1-47.
Intradiscal electrothermal therapy
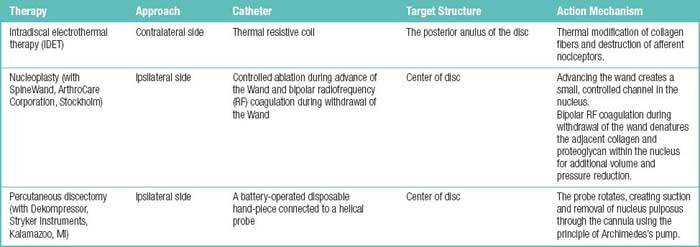
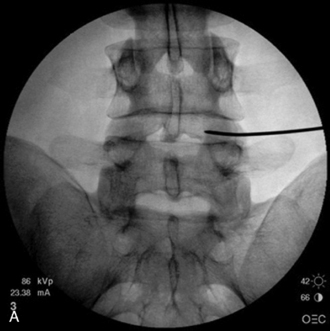
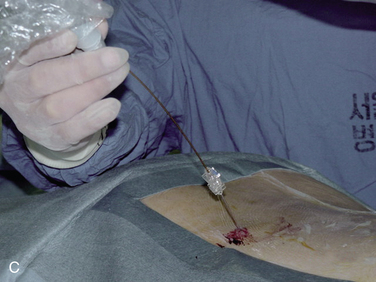
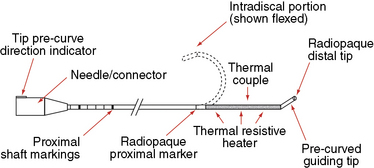
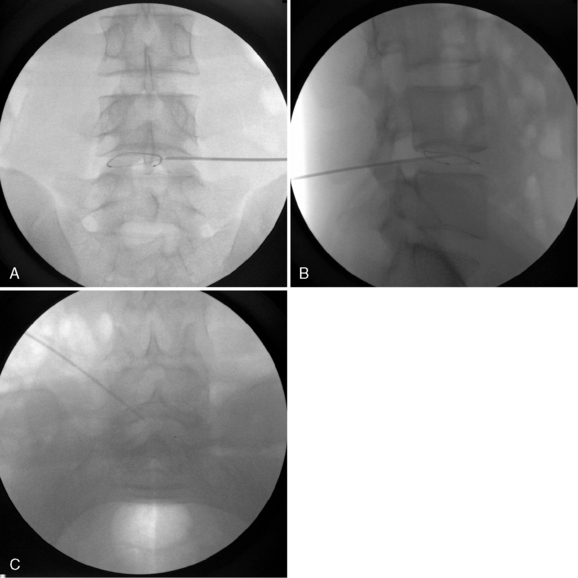
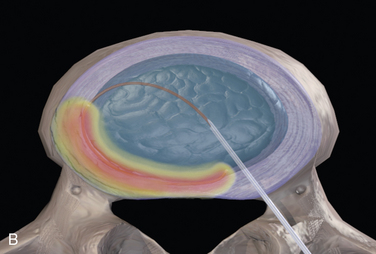
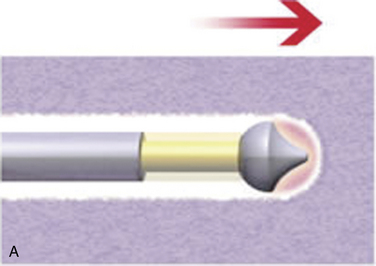
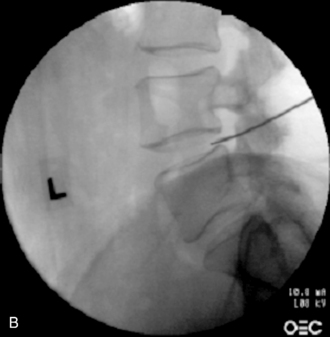
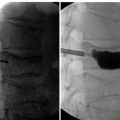
In IDET, the collagen fibers of the disc are modified and the nociceptors causing axial back pain are destroyed by thermal energy delivered through the percutaneous threading of a flexible catheter, composed of a thermal resistive coil, into the disc from the side contralateral to the lesion under fluoroscopic guidance (Fig. 17-1).
Mechanisms of Action
Thermal modification of collagen fibers acts as follows:
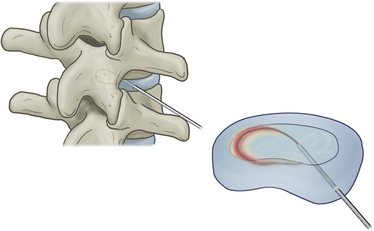
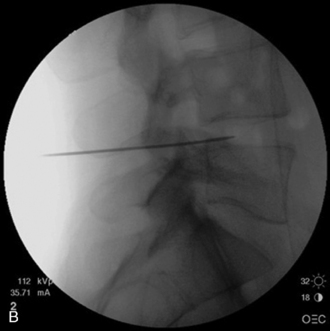
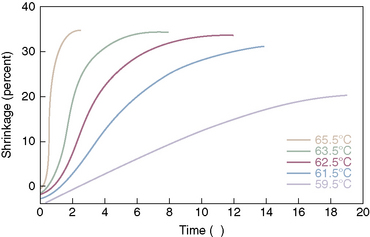
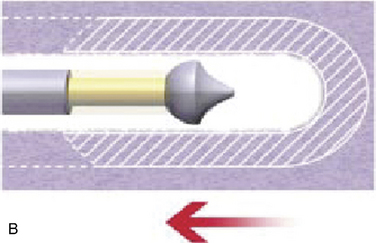
 With disc temperatures reaching 65° C, collagen may contract as much as 35% from its original size (Fig. 17-2).
With disc temperatures reaching 65° C, collagen may contract as much as 35% from its original size (Fig. 17-2).Indications and Contraindications
Ideal candidates for IDET have the following features:
Indications
The indications for IDET are as follows:
 Failure of a minimum of 6 weeks of conservative treatment (including pain medication and physical therapy) to improve the symptoms
Failure of a minimum of 6 weeks of conservative treatment (including pain medication and physical therapy) to improve the symptoms The presence of degenerative disc disease on magnetic resonance imaging with global disc degeneration or posterior or posterolateral anular tear evident
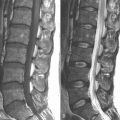
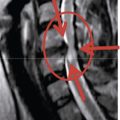
The presence of degenerative disc disease on magnetic resonance imaging with global disc degeneration or posterior or posterolateral anular tear evident The presence of one- or two-level symptomatic disc degeneration as determined by provocative lumbar discography at L3-L4, L4-L5, L5-S1 and with an adjacent asymptomatic “control” disc
The presence of one- or two-level symptomatic disc degeneration as determined by provocative lumbar discography at L3-L4, L4-L5, L5-S1 and with an adjacent asymptomatic “control” discRelated Anatomy and Pathophysiology
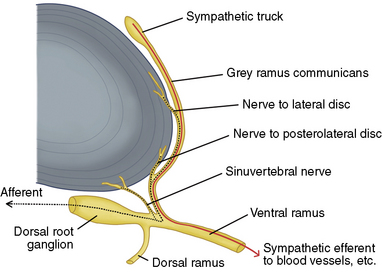
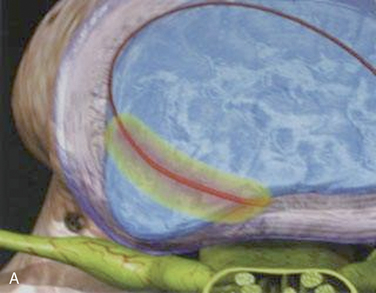
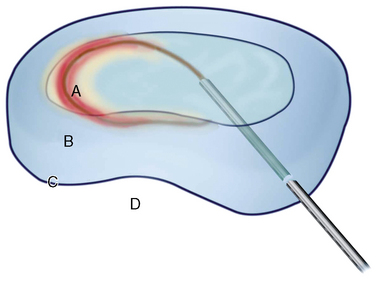
Figure 17-3 illustrates innervations of an intervertebral disc related to IDET.
Procedure
Limitations
IDET has some limitations, which are as follows:
 It is difficult to navigate and place the catheter at the exact portion of the anulus that contains the tear.
It is difficult to navigate and place the catheter at the exact portion of the anulus that contains the tear. If the temperature of the catheter tip is 90° C, that of the outer anulus is only 42° C. It is difficult to ablate afferent type C sensory innervation at 42° C.
If the temperature of the catheter tip is 90° C, that of the outer anulus is only 42° C. It is difficult to ablate afferent type C sensory innervation at 42° C.Nucleoplasty
Nucleoplasty is a procedure in which the disc nucleus is decompressed using both energy and heat. We use the Perc-D (DLR in larger discs and DLG longer access) SpineWand (AthroCare Corporation, Stockholm), which is a 1-mm-diameter bipolar instrument designed for this procedure. The tip of the wand has a slight C curve to allow for channeling. The wand is connected to the standard ArthroCare power generator. Nucleoplasty utilizes the company’s patented Coblation (controlled ablation) technology, which has found applications in other areas of medical care [2]. This process generates a unique low-temperature plasma field (see later) for precise, controlled ablation with minimal risk of thermal injury (Fig. 17-11).
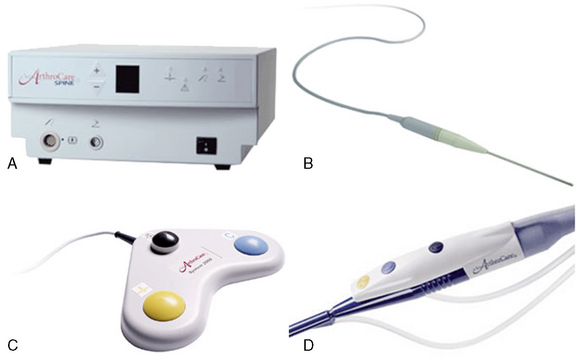
Figure 17–11 A, System 200, (ArthroCare Corporation, Stockholm). B, Patient cable. C, Foot control. D, Hand switch control.
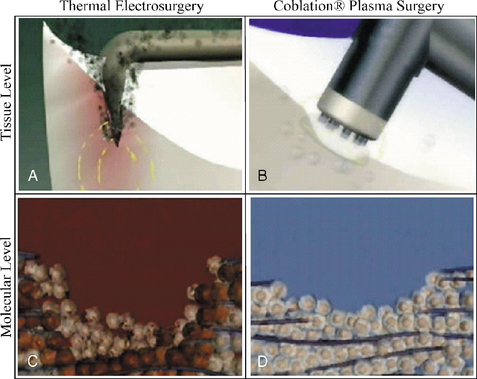
Coblation removes tissue by using radiofrequency energy to excite the electrolytes in a conductive medium, such as saline solution, to create precisely focused plasma. The energized particles in the plasma have sufficient energy to break molecular bonds, excising or dissolving soft tissue at relatively low temperatures (typically 40° to 70° C), thereby preserving the integrity of surrounding healthy tissue (Fig. 17-12).
Ablation generates approximately 120 volts of energy at the tip of the wand with resultant tip temperatures of 50° to 70° C. A plasma field is generated at the tip, which is a millimicron-thick field of highly energized particles that result in molecular dissociation of the disc material directly in front of the tip. This creates a channel from the posterolateral anulus to the anteromedial anulus (Fig. 17-13A) [3]. On withdrawal of the SpineWand, the coagulation mode is used. This mode uses 60V of energy and a tip temperature of 70° C. On complete withdrawal of the instrument, the thermal effect causes denaturization of the type II collagen with resultant shrinkage of the surrounding collagen and widening of the channel (Fig. 17-13B) [3].
Indications and Contraindications
Procedure
Nucleoplasty using the Perc-DLR or Perc-DLG SpineWand is performed as follows (Fig. 17-14A, B) [4]:
Nucleoplasty using the Perc-DC SpineWand is performed as follows (see Fig. 17-14C) [4]:
Percutaneous discectomy
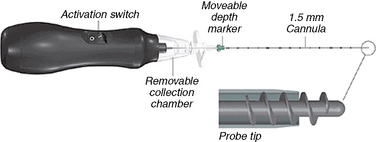
The Dekompressor 1.5-mm percutaneous discectomy probe (Stryker Instruments, Kalamazoo, MI) utilizes a highly efficient method for removal of intervertebral disc nucleus pulposus entirely under fluoroscopic control [5]. The probe consists of a battery-operated disposable handpiece connected to a helical probe (Fig. 17-17). It is intended for use in aspiration of disc material during percutaneous discectomies in the lumbar, thoracic, and cervical regions of the spine.
Indications and Contraindications
Benefits
Percutaneous discectomy with a probe has the following advantages over open discectomy:
Procedure
Percutaneous discectomy using the Dekompressor probe is performed as follows:
1 Freeman B.J., Fraser R.D., Cain C.M., et al. A randomized, double-blind, controlled trial: Intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine. 2005;30:2369-2377.
2 Singh V., Piryani C., Liao K., Nieschulz S. Percutaneous disc decompression using coblation (nucleoplasty) in the treatment of chronic discogenic pain. Pain Physician. 2002;5:250-259.
3 Sharps L.S., Isaac Z. Percutaneous disc decompression using nucleoplasty. Pain Physician. 2002;5:121-126.
4 ArthroCare Spine Coblation technology web page. Available at http://www.nucleoplasty.com/ Accessed 2007
5 Dekompressor Percutaneous Discectomy Probe. Kalamazoo, MI: Stryker Instruments, 2005. Available at http://www.stryker.com/stellent/groups/public/documents/web_prod/008450.pdf