CHAPTER 96 Intradiscal Electrothermal Annuloplasty
INTRODUCTION
Spine specialists have been frustrated by the lack of effective treatments for chronic low back pain. The intervertebral disc is believed to be the source of low back pain in as many as 40% of patients with chronic low back pain.1–4 The results from conservative therapies are frequently poor in this patient population. Over the years, a number of intradiscal techniques to either shrink or remove disc material believed to be causing lumbar pain and/or radiculopathy have been described.5 These techniques include interbody fusion, posterolateral fusion, microdiscectomy, arthroscopic discectomy, automated percutaneous lumbar discectomy, chymopapain, as well as other procedures.6–10 Recently, a new technique has been developed that involves the percutaneous insertion of a thermal resistance probe with controlled heating of the disc material.11–14 This technique is known as intradiscal electrothermal annuloplasty (or IDET).
Intradiscal electrothermal annuloplasty was developed as a minimally invasive procedure for the treatment of pain due to degenerative disc disease.15 The procedure has been used in the lumbar spine of patients who have failed conservative treatment regimens and who might otherwise be candidates for a spinal fusion procedure. The IDET procedure was developed by two brothers, Drs. Jeffrey and Joel Saal, specialists in physical medicine and rehabilitation. The procedure was developed to offer patients with chronic discogenic low back pain an option other than chronic pain management for spinal fusion.16 The IDET procedure is specifically devised to treat degenerative disc disease resulting in chronic low back pain. Thermal energy had been shown to induce tissue shrinkage in cadaveric and animal models.17–20 Saal and Saal hypothesized that thermal energy might have a role in the treatment of so-called ‘internal disc disruption,’ and, thus, in chronic low back pain.13 This proposition led to the development of a catheter to deliver intradiscal electrothermal annuloplasty therapy.17
PATHOPHYSIOLOGY OF DISC DEGENERATION AS IT RELATES TO IDET
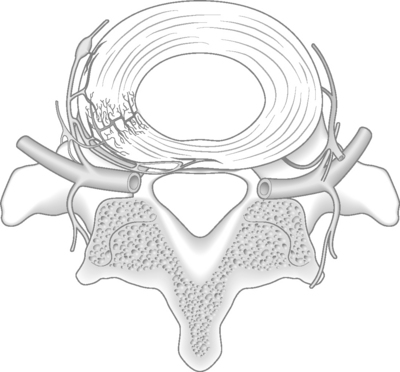
The pathophysiology and origins of low back pain of discogenic origin are incompletely understood.21 One theory hypothesizes that small, post-traumatic peripheral tears of the anulus fibrosus lead to an acceleration in the dehydration of the intervertebral disc, with resultant fraying of the nucleus pulposus.17,22 Studies using annular trauma in a sheep model support this theory.23 The intervertebral disc is surrounded by an external continuous plexus of interlacing nerve fibers. Contributions to this network occur ventrally from a plexus surrounding the anterior longitudinal ligament and dorsally from a plexus surrounding the posterior longitudinal ligament.21 Vascular ingrowth also has been observed in peripheral tears of the anulus. Nociceptors may accompany this vascular growth and account for the presence of sensory nerve supply in the inner anulus (Fig. 96.1).
In the normal intervertebral disc, sensory nerves do not penetrate beyond the outer one-third of the anulus fibrosus.1 In degenerative disc disease, however, an association has been demonstrated between the ingrowth of nerves expressing substance P and disc degeneration. Disc degeneration and disc injury are associated with centripetal growth of nerve fibers in the disc, which would provide a morphologic basis for true discogenic pain. It is this ingrowth of nerve fibers that is believed to be the source of back pain of discogenic origin.
INTRADISCAL ELECTROTHERMAL THERAPY
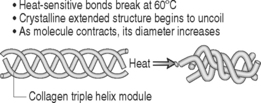
The theoretic basis for IDET is that targeted thermal energy within the pathologic disc is designed to shrink collagen fibrils, cauterize granulation tissue, and coagulate nerve tissue in the posterior anulus fibrosus.13,16 When temperatures above 65°C were applied to cadaveric shoulder capsules, studies have shown shrinkage of the specimen and, histologically, hyalinization of the collagen (Fig. 96.2).18 The procedure is supported by basic science research performed by investigators who assess in vitro temperature mapping and demonstrated that thermal resistance catheter heating conducted temperatures sufficient to coagulate nerve endings and to contract collagen.24 Bono et al.25 documented in a cadaver model the temperature sufficient for collagen denaturation and nociceptive ablation were detected at distances greater than previously documented. These data suggest that the proposed heat-dependent mechanism of action in intradiscal electrothermal therapies are achieved in most discs. Among other factors, intraspecimen variability of maximum temperatures may help explain the somewhat inconsistent clinical results following intradiscal electrothermal therapy. One in vitro study, however, suggested that the temperatures developed during intradiscal electrothermal therapy were insufficient to alter collagen architecture or stiffen the treated motion segment acutely.26 Kleinstueck et al.26 also investigated the biomechanical effects of IDET on vertebral motion. It is plausible that the heated catheter denatures and shrinks the collagen fibrils, thus stabilizing the motion segment. In this cadaveric study, there was an increase in motion at the IDET-treated levels. This investigation, however, did not take into account the effects of subsequent scarring that would occur over time in vivo. Subsequent scarring may result in stiffening and stabilization of the motion segment over a period of time consistent with the clinical relief of pain at 1–3 months. Other studies27 have shown a similar triad of biologic repair in the therapeutic effect (maintenance of shrinkage, secondary scarring and thickening, and destruction of sensory fibers). Gross pathology before and after treatment demonstrates shrinkage of the nuclear matrix.17 Although the in vivo response has not yet been proven, IDET is likely successful in denervation of the anulus fibrosus through temperature modulation.28
INDICATIONS FOR INTRADISCAL ELECTROTHERMAL
The indications for IDET are generally felt to be similar to those for interbody fusion, and apply the same diagnostic and treatment criteria. As with most treatments, patient selection may be the single most important criterion for a successful outcome. Discogenic low back pain is the most common condition treated with IDET, and its presence is the primary selection criterion. Patients should have chronic low back pain in the absence of other readily identifiable structural abnormalities. Discogenic pain is most often defined as unremitting, persistent low back pain that is worse with axial loading and improved with recumbency. The low back pain is greater than leg pain. This is a nociceptive pain and not a neuropathic pain syndrome.29
Patient selection criteria for the IDET procedure are similar to those used for spinal fusion for degenerative disc disease of the lumbar spine (Table 96.1).15 Several authors have published strict inclusion criteria as well as exclusion criteria (Table 96.2).13, 30, 31 The primary indication is unremitting, persistent low back pain. An additional criterion is a failure of satisfactory improvement with a nonoperative care program that includes back education, activity modification, a progressive and intensive exercise program, a trial of physical therapy, and use of oral nonsteroidal antiinflammatory drugs. These authors have described the failure of the conservative (nonoperative) treatment as a minimum 6-month period of comprehensively applied nonoperative treatment with the patient reporting persistent pain and disability, dissatisfaction with quality of life, and a desire to pursue alternative treatment options. Physical examination should include a normal neurological examination with a negative straight leg raising sign.
| Intrusive low back pain for more than 3 months |
| Failure to achieve adequate improvement with comprehensive nonoperative treatment |
| No medical or other systemic causes for the low back pain |
Table 96.2 Contraindications for APLD
| INCLUSION CRITERIA | |
| Duration of symptoms of at least 3–6 months | |
| Failed 6 weeks conservative care | |
| Abnormal disc morphology | |
| Predominance of low back pain symptoms | |
| Concordant pain reproduced on discography | |
| EXCLUSION CRITERIA | |
| Abnormal neurological findings/compressive lesion | |
| Severe disc degeneration | |
| Segmental instability | |
| Other medical conditions | |
| Consideration of previous surgery | |
Imaging studies are necessary in the diagnostic evaluation process. Magnetic resonance imaging (MRI) confirms the presence of disc degeneration, desiccation, high-intensity zones (Modic endplate changes), and loss of disc height (Fig. 96.3). Contained disc fragment herniations may also be present. Extruded and free fragments of disc cannot be effectively treated using this procedure, but patients with these lesions may be treated for discogenic low back pain with the proviso that the fragments will be unchanged after treatment. We do not feel that a focal protrusion of a disc is a contraindication to performing the IDET procedure. The authors have not seen a single clinical case in which a disc herniation occurred after the IDET procedure. There does not appear to be any increased risk if undertaken in such discs.
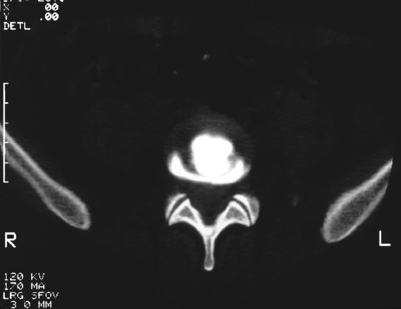
Karasek and Bogduk11,30 state that the major indication for IDET is internal disc disruption, not merely discogenic pain. This internal disc disruption may be diagnosed by a combination of disc stimulation (‘provocative discography’) that produces concordant pain with no pain replication at adjacent levels, and a computed tomography (CT) scan after discography that reveals an annular tear. Both criteria must be met in order for the discogram to be considered ‘positive.’ The author often employs provocative discography, conducted by an unbiased interventional spine physician.13 This diagnostic study is used to confirm the clinical suspicions, and its results may be used as exclusionary criteria for patients with multilevel disease. Postdiscography CT also provides technical information regarding placement of the intradiscal catheter by evaluating the location and degree of annular disruption (Fig. 96.4). In addition, if a skilled interventional spine physician has difficulty placing a 20-gauge needle into the L5–S1 disc space, one can reasonably expect to have similar difficulties placing an even large needle into the same intervertebral space for the IDET procedure.
Another group of patients considered for IDET are those with multilevel degenerative disc disease in whom multilevel interbody fusion is being considered. These patients may benefit from combined-modality treatment including a fusion at the most affected level and IDET at the less symptomatic level. Kapural et al. reported that the pain relief and Pain Disability Index were significantly better in patients with a single- or two-level degenerative disc disease than in the multiple degenerative disc disease group.32
The exclusion criteria for IDET initially presented by Saal and Saal12 included patients with inflammatory arthritides, nonspinal conditions that could mimic lumbar pain, and prior surgery at the symptomatic levels. The authors’ exclusion criteria include lumbar spinal instability that would require fusion and the presence of infection or malignancy at the level requiring treatment. Multilevel disease (three or more levels of disc disease documented on MRI or discography) is a relative but not necessarily absolute contraindication to IDET. Finally, a greater than 75% loss of normal disc height is also a relative contraindication to the procedure.
IDET TECHNIQUE
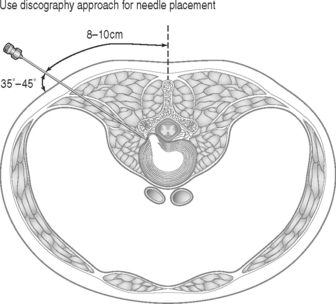
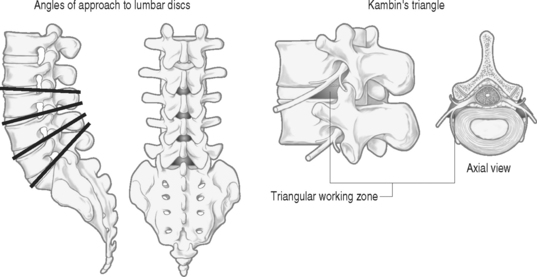
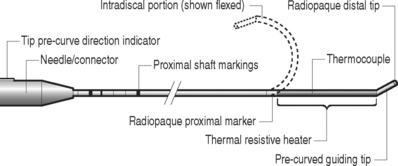
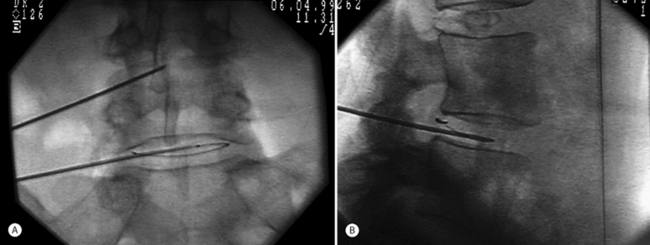
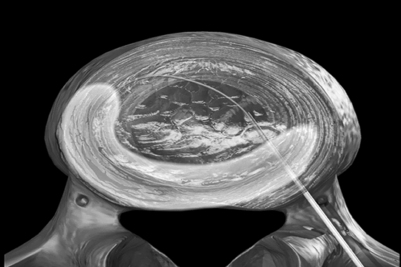
The authors perform the IDET procedure in a standard operating room in an outpatient setting. The technique consisted of placing the patient on a Jackson Table (Orthopaedic Systems, Inc., Union City, CA) in a prone position. Intravenous sedation is given and C-arm fluoroscopy is utilized to obtain anteroposterior, oblique (‘needle eye view’), and lateral images. The treatment level is localized and local anesthesia is applied to the skin 6–9 cm lateral to the midline (Fig. 96.5). In this approach, the anatomical triangular working zone described by O’Neill et al., Kambin, and Parke is used (Fig. 96.6).5, 9, 33 This ‘working triangle’ avoids the exiting nerve root. The IDET set includes a 17-gauge needle and stylet. The needle is directed toward the center of the disc under fluoroscopic guidance, and the anulus is punctured. The thermal-resistance catheter is inserted through the needle into the disc (Fig. 96.7). The catheter is coiled within the disc as it is deflected by the annular fibers (Fig. 96.8). The tip of the catheter is directed to the posterior aspect of the disc in such a manner that the heating elements of the catheter remained on the symptomatic side. Therefore, the needle is inserted on the side contralateral to the patient’s pain (or annular tear seen on postdiscography axial CT images) (Fig. 96.9). The catheter temperature is increased along an electronically programmed protocol over 13 minutes to 90°C and is allowed to remain at that temperature for 4 minutes. The temperature of the catheter is initially 65°C and is increased by 1°C every 30 seconds until the target temperature is reached. A single heating treatment is administered. The authors do not inject antibiotics into the disc space. The catheter and needle are then removed as a single unit.
Other authors have also described their technique for the IDET procedure. Derby et al.34 stated that when there has been significant posterior annular disruption, it is sometimes difficult to navigate the catheter anywhere but the outermost annular fibers. In these cases, one can easily reach temperatures that denervate the outer anulus at temperatures and times at or below 60°C tissue temperature which would correspond to 85°C measured temperature in the original IDET catheter. Karasek and Bogduk30 emphasize that the catheter position at all times should be buried between the lamellas of the anulus fibrosis some 5 mm deep to its outer surface. Of note is that Freeman et al.35 stated that the IDET did not produce denervation of the experimentally induced posterior annular lesion in a sheep model. After assessing the temperatures in thermal dose distribution during IDET, Kleinstueck et al.36 reported temperatures were not reliably produced in clinically relevant regions, such as the posterior anulus. A more recent study by Bono et al.25 have refuted this finding.
Patients with multilevel disease undergo treatment at other involved level(s) using the same protocol. New catheters and needles are used at each level. Patients with only back pain and no leg pain undergo treatment on the opposite disc side (i.e. bilateral) as well. Patients are discharged after 2 hours of observation. Following the procedure, patients are instructed to resume their usual activities as tolerated after 24 hours. The authors do not routinely prescribe a lumbar orthoses after the procedure. This is the authors’ own protocol. Many practitioners place different restrictions upon their patients as well as prescribe a lumbar orthoses after the procedure.
REVIEW OF PUBLISHED CLINICAL DATA
Since 1998, there have been over 40 peer-reviewed articles and abstracts that have presented clinical outcomes associated with the IDET procedure.2, 13, 30, 37 The initial publication regarding IDET was from the Saal brothers in 2000.12 These investigators reported a series of 91 patients who were chosen from a population pool of 1116 consecutive patients presenting with chronic low back pain of >3 months duration. Of the 91 patients who did not respond to nonoperative care, 62 underwent IDET as an alternative to spinal fusion and 29 remained in a control group to assess the impact of natural history on symptom resolution.38 At 12 and 24 months, the mean decrease in Visual Analog Scale (VAS) score was 3.52±2.30 and 3.41±1.96, respectively. Based upon these initial data, the authors concluded that a statistically significant improvement in pain was obtained in patients with chronic discogenic low back pain treated with IDET.
The wide variations in definitions of success that have been used to evaluate the outcomes of IDET therapy make comparisons among studies difficult.39 A meta-analysis published in 2001 reviewed 11 studies involving 704 patients.2, 30, 37, 40, 41 This meta-analyses showed a clinically significant improvement in pain and physical functioning post-IDET. The reported outcomes from the published and presented clinical studies seem to attest to the efficacy and safety of IDET. In eight studies that utilized the VAS to assess pain outcome, all reported decreases in the VAS. The results of the meta-analysis of changes in VAS scores derived from a total of 448 patients demonstrated a p-value of p<0.0001 for a significant decrease in pain of at least two points at a minimum of 6 months post-treatment. Combining a total of 448 patients in the literature, McGraw et al.38 found a mean decrease in pain following the IDET of 2.7 points on the VAS scale.
A meta-analysis of five studies was performed that evaluated the Physical Function Scale of the SF-36 for a total of 404 patients.38 The results of a meta-analysis addressing SF-36 Bodily Pain scores found an overall mean significantly greater than 7 points (p<0.0001), supporting a clinically significant improvement in bodily pain post-treatment.
Freedman et al.39 reported an overall success rate of 65% in the management of chronic discogenic low back pain in active-duty soldiers. Wetzel et al.28 published their review of several studies and concluded that the pain resulting from lumbar disc disease may be diminished by IDET. All the studies projected a positive therapeutic effect. They stated that all the studies, however, suffered from the same methodological flaws. All studies utilized a prospective cohort design or a nonrandomized, prospective design with a biased control. A review by Biyani et al.21 concluded that IDET is a potentially beneficial treatment for internal disc disruption in carefully selected patients as an alternative to spinal fusion. In the authors’ own study, IDET was found to be effective in 75% of patients in improving their chronic low back pain at 1 year.31
Karasek et al.11,30 summarize the literature by stating that strict adherence to their proposed selection criteria results in a 60% chance of obtaining at least 50% relief of pain and a 20% chance of obtaining complete pain relief. Bogduk and Karasek42 similarly found 54% of patients had a 50% reduction in pain, and 20% achieved complete relief of their pain. With a 2-year follow-up, these investigators found the long-term results of IDET to be stable and enduring. Derby et al.2 and Lee et al.43 also found this long-term duration of pain relief.
Finally, a randomized, double-blind, placebo-controlled trial evaluating the efficacy of IDET for the treatment of chronic discogenic low back pain with 6-month outcomes data has recently been published.44 In this study of 64 patients, a statistically significant improvement in pain levels was documented when compared with the control group as determined by the outcome instruments of SF-36, Visual Analog Scale, Oswestry Disability Index, Beck Depression Inventory (BDI), and Work Status Questionnaire. Primary eligibility criteria for this study were as follows: age 18–65 years; low back greater than leg pain present for greater than 6 months duration; failure to improve after at least 6 weeks of nonoperative care, including antiinflammatory and analgesic medications and a physical therapy and/or home directed lumbar exercise program; low back pain exacerbated by sitting or standing and relieved by lying down; a score less than 20 on the Beck Depression Scale; no surgical interventions within the previous 3 months and less than 20% disc height narrowing on lateral plane film radiographs. Of 1360 individuals who were prepared to submit to randomization, 260 were found potentially eligible after clinical examination and 64 became eligible after discography. The primary objective of the study was to compare the improvement in pain and physical function between groups. Post hoc analyses were conducted that summarized the proportions of patients who at 6 months obtained 25%, 50%, and 75% relief of pain relative to pretreatment levels. Percentage relief of pain at follow-up was calculated for each patient as the difference between the postoperative pain score from baseline, divided by the baseline score, and converted to a percentage. Both groups exhibited significant improvements in pain scores, but the improvement in the IDET group was significantly greater than in the sham group (p=0.045). A similar pattern of improvement occurred in the Bodily Pain Scores of the SF-36 but was not significantly different between the two groups (p=0.086). Both groups improved with respect to Physical Functioning on the SF-36 (p=0.050), although with no significant differences between groups; but on the Oswestry Disability Scale, the IDET group achieved significantly better outcomes (p=0.05).
With respect to categorical outcomes, statistically significant differences in favor of the IDET group occurred both for absolute change and for relative changes in pain scores as measured by the VAS. Furthermore, more substantial differences were found when the main outcomes were stratified according to baseline scores. It emerged that IDET was significantly more effective for patients with pain scores less than 70 at inception and for patients with poor function or greater disability at inception. Reciprocally, IDET had no significantly greater effect than sham treatment for patients with low disability or who had already had good physical function. Few interventions that the authors perform for spinal disorders have undergone such a rigorous, randomized, placebo-controlled trial as this one. The authors concluded that the efficacy of IDET could not be attributed wholly to a placebo effect. In summary, many studies by independent researchers have been published that suggest a positive therapeutic benefit of the IDET procedure.
COMPLICATIONS
Another treatment-related problem that may occur is difficulty in threading the catheter into the disc space once the needle is adequately positioned. Threading the catheter may be difficult if the needle tip is positioned against the endplate or if the disc is extremely degenerated (i.e. collapsed). A 75% or greater loss of disc height makes it extremely difficult to properly position the catheter within the disc space. Should this difficulty arise, it is extremely important not to force the catheter into the disc space because the catheter can be sheared off by the needle tip. Additionally, the catheter and needle must be removed as a single unit once the catheter is extended past the needle tip.29
McGraw et al. reported a single minor complication of temporary radicular pain in a series of 30 patient.38 Saal and Saal13 reported no complications in their study of 62 patients followed to 24 months. Wetzel et al.28 reported a minimal complication rate in a series of 75 patients. A study specifically addressing the risk factors for failure and complications of IDET found a 10% complication rate in a series of 79 patients.45 However, all of these complications were minor and self-limited. A single case of cauda equina syndrome caused by device malpositioning has been reported.46 Finally, Djurasovic et al.47 reported a single case of osteonecrosis of the adjacent vertebral bodies after an IDET procedure. Other major series report no additional complications for the IDET procedure.15,16,30,31,38,42,43,48
With over 50 000 IDET procedures performed in the United States to date, there have been no reports of discitis.1 However, this remains a potential complication. One explanation for the absence of discitis cases is the coaxial nature of the catheter system, such that the catheter that is applying the heat to the posterior anulus does not contact the skin, which would be the source of infection.34 In summary, IDET is a safe procedure, and there is no evidence to suggest that IDET is harmful to patients, either clinically or biomechanically.
SUMMARY
The IDET procedure was developed as a minimally invasive procedure for the treatment of pain due to degenerative disc disease. This chapter presents a summary of the currently available clinical data regarding the indications and clinical outcomes of the IDET procedure. In light of good and excellent reported outcomes, excellent patient satisfaction, and few reported complications, IDET has been advocated as a viable treatment option for chronic discogenic low back pain refractory to nonoperative therapy.35 The IDET technique is performed by a variety of physician specialties involved in the care and management of patients with chronic low back pain of discogenic origin. IDET is currently widely being used for the treatment of this condition. The IDET procedure has gained widespread popularity and is being performed in numerous centers throughout the United States. IDET is a safe procedure that appears to be moderately effective in relieving pain of discogenic origin. It may be an alternative to lumbar interbody fusion in patients who cannot or wish not to undergo a major fusion procedure. The complication rate is very low and recovery time is minimal, especially compared with an interbody fusion operation. Finally, there is no evidence to suggest that IDET is harmful to patients, either clinically or biomechanically.
1 Coppes M, Marani E, Thomeer R, et al. Innervation of ‘painful’ lumbar discs. Spine. 1997;22:2342-2350.
2 Derby R, Eek B, Chen Y, et al. Intradiscal electrothermal annuloplasty (IDET): a novel approach for treating chronic discogenic back pain. Neuromodulation. 2000;3:82-88.
3 Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22:181-187.
4 Schwarzer A, April C, Derby R, et al. The relative contributions of the disc and zygapophyseal joint in chronic low back pain. Spine. 1994;19:801-806.
5 O’Neill C, Derby R, Kenderes L. Precision injection techniques for diagnosis and treatment of lumbar disc disease. Sem Spine Surg. 1999;11:104-118.
6 Turner JA, Ersek M, Herron L, et al. Patient outcomes after lumbar spinal fusions. JAMA. 1992;268:907-911.
7 Fritzell P, Hagg O, Wessberg P, et al. Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26:2521-2532.
8 Welch WC. Intradiscal electrothermy (IDET) – patient selection criteria. Cleveland Clinic/University of Cincinnatti Winter Neuroscience Symposium (Snowmass, Colorado); 2001.
9 Kambin P. Alternative to open lumbar discectomy: Arthroscopic microdiscectomy. In: Lange A, editor. Operative spinal surgery. Stanford, CT: Appleton & Lange, 1999.
10 Onik G, Maroon J, Davis GW. Automated percutaneous discectomy at the L5–S1 level. Use of a curved cannula. Clin Orthop. 1989;238:71-76.
11 Karasek M, Bogduk N. Intradiscal electrothermal annuloplasty: percutaneous treatment of chronic discogenic low back pain. Techn Reg Anesthes Pain Manage. 2001;5:130-135.
12 Saal J. Intradiscal electrothermal therapy for the treatment of chronic discogenic low back pain. Operat Tech Orthopaed. 2000;10:271-281.
13 Saal JA, Saal JS. Intradiscal electrothermal treatment for chronic discogenic low back pain: a prospective outcome study with minimum 1-year follow-up. Spine. 2000;25:2622-2627.
14 Saal JS, Saal JA. Management of chronic discogenic low back pain with a thermal intradiscal catheter: a preliminary study. Spine. 2000;25:382-388.
15 Heary RF. Intradiscal electrothermal annuloplasty: The IDET Procedure. In: Resnick DK, Haid RWJr, editors. Surgical management of low back pain. Rolling Meadows: Kristine Rynne Mednansky; 2001:143-150.
16 Saal JA, Saal JS. Intradiscal electrothermal treatment for chronic discogenic low back pain. Spine. 2002;27:966-974.
17 Wetzel TF, McNally TA. Treatment of chronic discogenic low back pain with intradiskal electrothermal therapy. J Am Acad Orthop Surg. 2003;11:6-11.
18 Hayashi K, Thabit GI, et al. The effect of thermal heating on the length and histologic properties of the glenohumeral joint capsule. Am J Sports Med. 1997;25:107-112.
19 Obrzut S, Hecht P, Hayashi K, et al. The effect of radiofrequency energy on the length and temperature properties of the glenohumeral joint capsule. Arthroscopy. 1998;14:395-400.
20 Naseef GI, Foster T, Trauner K, et al. The thermal properties of bovine joint capsule: The basic science of laser-and radiofrequency-induced capsular shrinkage. Am J Sports Med. 1997;25:670-674.
21 Biyani A, Andersson GBJ, Chaudhary H, et al. Intradiscal electrothermal therapy a treatment option in patients with internal disc disruption. Spine. 2003;28:S8-S14.
22 Otsi O, Vernon-Roberts B, Moore R, et al. Annular tears and disc degeneration in the lumbar spine: A post-mortem study of 135 discs. J Bone Joint Surg [Br]. 1992;74:678-682.
23 Otsi O, Vernon-Roberts B, Fraser R. Annulus tears and intervertebral disc degeneration: An experimental study using an animal model. Spine. 1990;15:762-767.
24 Ashley J, CGharpuray V, Saal J, et al. Temperature distribution in the intervertebral disc: a comparison of intranuclear radiofrequency needle to a novel heating catheter. Proceedings of the 1999 Bioengineering Conference, 1999;77.
25 Bono CM, Iki J, Jolata A, et al. Temperatures within the lumbar disc and endplates during intradiscal electrothermal therapy: formulation of a predictive temperature map in relation to distance from the catheter. Spine. 2004;29(10):1124-1131.
26 Kleinstueck F, Diederich C, Nau W, et al. Acute biomechanical and histological effects of intradiscal electrothermal therapy on human lumbar discs. Spine. 2001;26:2198-2207.
27 Arnoczky S, Aksan A. Thermal modification of connective tissues: Basic science considerations and clinical implications. J Am Acad Orthop Surg. 2000;8:305-313.
28 Wetzel FT, McNally TA, Phillips FM. Intradiscal electrothermal therapy used to manage chronic discogenic low back pain: new directions and interventions. Spine. 2002;27:2621-2626.
29 Welch WC, Gerszten PC. Alternative strategies for lumbar discectomy: intradiscal electrothermy and nucleoplasty. Neurosurg Focus. 2002;13:1-6.
30 Karasek M, Bogduk N. Twelve-month follow-up of a controlled trial on intradiscal thermal annuloplasty for back pain due to internal disc disruption. Spine. 2000;25:2601-2607.
31 Gerszten PC, Welch WC. Intradiscal electrothermy techniques. Congress of Neurological Surgeons Annual Meeting, Pain Section. San Antonio, Texas, Congress of Neurological Surgeons; 2000.
32 Kapural L, Mekhail N, Korunda Z, et al. Intradiscal thermal annuloplasty for the treatment of lumbar discogenic pain in patients with multilevel degenerative disc disease. Anesth Analg. 2004;99(2):472-476.
33 Parke W. Anatomy of the spinal nerve and its surrounding structures. Semin Orthop. 1991;6:65-71.
34 Derby R, Kleinstueck FS, Diederich CJ, et al. Temperature and thermal dose distributions during intradiscal electrothermal therapy in the cadaveric lumbar spine. WMJ. 2004;101(8):4-5.
35 Freeman BJ, Walters RM, Moore RJ, et al. Does intradiscal electrothermal therapy denervate and repair experimentally induced posterolateral annular tears in an animal model? Spine. 2003;28(23):2602-2608.
36 Kleinstueck FS, Diederich CJ, Nau WH, et al. Temperature and thermal dose distributions during intradiscal electrothermal therapy in the cadaveric lumbar spine. Spine. 2003;28(15):1700-1708. discussion 1709
37 Singh V. Intradiscal electrothermal therapy: a preliminary report. Pain Phys. 2000;3:367-373.
38 McGraw JK, Silber JS. Intradiscal electrothermal therapy for the treatment of discogenic back pain. Appl Radiol. 2001;30:1-6.
39 Freedman BA, Cohen SP, Kuklo TR, et al. Intradiscal electrothermal therapy (IDET) for chronic low back pain in active-duty soldiers: 2-year follow-up. Spine J. 2003;3:502-509.
40 Wetzel T, et al. IDET to treat discogenic low back pain: preliminary results of a multi-center prospective trial, presented at the annual meeting of the International Society for the Study of Lumbar Spine, Adelaide, Australia. Not yet published.
41 Liu B, Manos R, Criscitiello A, et al. Clinical factors associated with favorable outcomes using intradiscal electrothermal modulation (IDET). 15th Annual Meeting North American Spine Society. New Orleans; 2000.
42 Bogduk N, Karasek M. Two-year follow-up of a controlled trial of intradiscal electrothermal annuloplasty for chronic low back pain resulting from internal disc disruption. Spine J. 2002;2:343-350.
43 Lee MS, Cooper G, Lutz GE, et al. Intradiscal electrothermal therapy (IDET) for treatment of chronic lumbar discogenic pain: a minimum 2-year clinical outcome study. Pain Phys. 2003;6:443-448.
44 Pauza K, Howell S, Dreyfuss P, et al. A randomized, placebo controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4(1):27-35.
45 Cohen SP, Larkin T, Abdi S, et al. Risk factors for failure and complications of intradiscal electrothermal therapy: a pilot study. Spine. 2003;28:1142-1147.
46 Hsia A, Isaac K, Katz J. Cauda equina syndrome from intradiscal electrothermal theapy [Letter]. Neurology. 2000;55:320.
47 Djurasovic M, Glassman SD, Dimar JRII, et al. Vertebral osteonecrosis associated with the use of intradiscal electrothermal therapy. Spine. 2002;27:E325-E328.
48 Lutz C, Lutz GE, Cooke PM. Treatment of chronic lumbar diskogenic pain with intradiskal electrothermal therapy: a prospective outcome study. Arch Phys Med Rehabil. 2003;84:23-28.