CHAPTER 43 INTRACRANIAL HEMORRHAGE: ANEURYSMAL, IDIOPATHIC, AND HYPERTENSIVE
Bleeding in the cranial space, or intracranial hemorrhage (ICH), often results in disastrous consequences for patients. ICH can be classified in terms of location, being either intraparenchymal or within spaces such as the subarachnoid, ventricular, subdural, or extradural space. Bleeding may be spontaneous or traumatic; sometimes no cause is found. It should be remembered that intracranial vascular abnormalities can also manifest without bleeding; early signs may be caused by compression of surrounding anatomical structures.
The effect of ICH on the general population can be fully grasped when viewed within the broader category of stroke, currently the leading cause of neurological deficit in the world and the third leading cause of death in the United States, accounting for 15% of all U.S. deaths annually.1 ICH accounts for 10% to 17% of all strokes, and the rate of mortality from ICH is considerably higher than that from nonhemorrhagic or ischemic stroke; some studies quote a mortality rate as high as 90%.2 This chapter focuses on ICH with particular regard to cerebral aneurysm and hypertensive and idiopathic hemorrhage.
ANEURYSMAL INTRACRANIAL HEMORRHAGE
Epidemiology
Cerebral aneurysm has been recognized as a cause of human illness since the end of the 19th century, but the actual incidence is difficult to estimate. In autopsy studies, the prevalence ranges from 0.2% to 7.9%. Other studies indicate a prevalence of 5%.3 The ratio of ruptured aneurysm to unruptured (incidental) aneurysm is 5:3 to 5:6 (rough estimate is 1:1; i.e., 50% of these aneurysms rupture).4 Only 2% of aneurysms manifest during childhood.5
Etiology
The exact pathophysiology of the development of aneurysms is controversial, but abnormal vascular anatomy and flow dynamics are important factors. In contrast to extracranial blood vessels, there is less elastic in the tunica media and adventitia of cerebral blood vessels, the media has less muscle, the adventitia is thinner, and the internal elastic lamina is more prominent.6,7 Outpouching is more likely to occur because of this, but it is also noteworthy that aneurysms are usually found at vessel bifurcations at sites of greatest flow. This, together with the fact that large cerebral blood vessels lie within the subarachnoid space with little supporting connective tissue,8,8a may predispose these vessels to the development of saccular aneurysms. Aneurysm formation is more common in women and in smokers. Beyond the third decade in particular, SAH is usually caused by rupture of a cerebral aneurysm. Other than size of aneurysm alone, the “aspect ratio” (aneurysm depth to aneurysm neck) has also been shown to be a potentially useful predictor of rupture (Figs. 43-1 and 43-2).9
The etiology of aneurysms may be any of the following:
 “Atherosclerotic” or hypertensive, the presumed etiology of most saccular aneurysms; probably interacts with congenital predisposition
“Atherosclerotic” or hypertensive, the presumed etiology of most saccular aneurysms; probably interacts with congenital predispositionOf all saccular aneurysms, 85% to 95% are located in the carotid system, with the following three most common locations: (1) the anterior communicating artery (ACom) for 30% (aneurysms in the ACom and anterior cerebral artery are more common in male patients); (2) the posterior communicating artery for 25%; and (3) the middle cerebral artery for 20%. Of the remaining saccular aneurysms, 5% to 15% are located in posterior circulation (vertebrobasilar); 10%, at the basilar tip (most common), followed by the basilar artery-superior cerebellar artery junction, basilar artery-vertebral artery junction, and anterior inferior cerebellar artery; and 5%, on the vertebral artery (vertebral artery-posterior inferior cerebellar artery junction most common). Twenty percent to 30% of patients with aneurysm have multiple aneurysms.10
Manifestation of Aneurysms
Major rupture is the most frequent manifestation leading to SAH. Clinical features may vary from sudden headache to a moribund comatose state. Neurological deficits, if present, depend on location and size of the hematoma. Signs of meningism with photophobia, neck stiffness, and vomiting are often seen. Progressive neurological deficit or decreasing con scious level may occur as a result of mass effect from a growing hematoma or the development of hydrocephalus. Ruptured cerebral aneurysm may be accompanied by the following:
 Intracerebral hemorrhage, which occurs in 20% to 40% of affected patients (more common with aneurysms distal to the circle of Willis; e.g., middle cerebral artery aneurysms).
Intracerebral hemorrhage, which occurs in 20% to 40% of affected patients (more common with aneurysms distal to the circle of Willis; e.g., middle cerebral artery aneurysms).Intraventricular hemorrhage occurs with 13% to 28% of ruptured aneurysms in clinical series (higher in autopsy series)11 and may lead to obstructive hydrocephalus. The presence of intraventricular hemorrhage is an indicator of poor prognosis11 (64% rate of mortality); ventricular size on admission is the most important prognostic factor (large ventricles being worse). Patterns that may occur include the following:
 ACom aneurysm, which tends to produce intraventricular hemorrhage by rupture through the lamina terminalis into the lateral or anterior third ventricles.
ACom aneurysm, which tends to produce intraventricular hemorrhage by rupture through the lamina terminalis into the lateral or anterior third ventricles.Other Possible Presentations
Mass effect can give rise to possible “warning signs”: for example, giant aneurysms can include brainstem compression, which produces hemiparesis and cranial neuropathies; cranial neuropathy (average latency from symptom to SAH was 110 days) can include non-pupil-sparing third nerve palsy produced by expanding posterior communicating artery aneurysm; rarer cranial neuropathies produce visual loss as a result of compressive optic neuropathy12 (ophthalmic artery aneurysms) and chiasmal syndromes (ophthalmic, ACom, or basilar apex aneurysms). Facial pain syndromes in the ophthalmic or maxillary nerve distribution that may mimic trigeminal neuralgia can occur with intracavernous or supraclinoid aneurysms.13,14
Also, intrasellar aneurysm may produce disturbances in the endocrine system.15 Warning or sentinel hemorrhage (minor bleeding) may occur; this condition has the shortest latency (10 days) between symptom onset and SAH.16 Headache without hemorrhage may occur; this may be acute, severe, and “thunderclap” in nature, sometimes described as the “worst headache of my life.” It may be caused by aneurysmal expansion, thrombosis, or intramural bleeding17 (all without rupture). Alternatively, it can be chronic, having usually been present for more than 2 weeks, is unilateral (often retro-orbital or periorbital) in about one half of cases, and is possibly caused by dural irritation. In the other one half of cases, it is diffuse or bilateral, possibly caused by mass effect that produces raised intracranial pressure. Asymptomatic aneurysm may be discovered incidentally on angiography, computed tomographic (CT) scan, or magnetic resonance imaging obtained for other reasons.
Treatment
Exclusion of the Aneurysm
Traditionally, aneurysmal occlusion was achievable only with craniotomy and dissection of the aneurysm in the basal cisterns and surgical clipping of the aneurysm neck. However, endovascular occlusion with platinum coils is being increasingly used as an alternative to craniotomy. This trend has been influenced by the results of the International Subarachnoid Aneurysm Trial (ISAT), which concluded that coiling is a safer treatment option than surgery (Molyneux et al, 2002).18 This remains a controversial area, however, and long-term studies investigating the risk of rebleeding (i.e., efficacy) after coiling are particularly required.
Cerebral Vasospasm
Cerebral vasospasm is constriction of the cerebral arterial vasculature that leads to delayed cerebral ischemia. The pathophysiology is poorly understood. Traditionally, rebleeding was the major concern after rupture of a cerebral aneurysm. However, the general shift to early occlusive therapy has minimized the effect of rebleeding. Indeed, except for the initial hemorrhage, cerebral vasospasm is now recognized as the most significant cause of death and disability, killing 7% of patients and leading to severe disability in another 7%.19 It is present in 20% to 30% of cases of aneurysmal SAH and is seen in 30% to 70% of angiograms at day 7 after SAH with and without clinical deficit. Vasospasm typically occurs at days 3 to 5 but has been reported up until day 21. Common manifestations include reduced Glasgow Coma Scale (GCS) score, confusion, and focal deficit.
Management of vasospasm combines triple-H therapy (hypervolemia, hypertension, and hemodilution) with the calcium channel antagonist nimodipine20 and transcranial Doppler ultrasonography. Hypertension is achieved with inotropes and is facilitated by early occlusion of the aneurysm. Several studies have shown the benefit of volume expansion in reversing deficits,21 but triple-H therapy has yet to be tested in a randomized controlled trial. In total, 11 trials of calcium channel antagonists have been reported, and the evidence suggests that they reduce the risk of vasospasm, although the results depended mainly on one trial with nimodipine.22 The exact mechanism of action of nimodipine remains unknown, but it is now standard therapy for vasospasm prophylaxis. Detection and monitoring of vasospasm are also possible with measurement of transcranial Doppler velocities, but this technique is unreliable: It is not “on-line,” and interoperator variability is large.
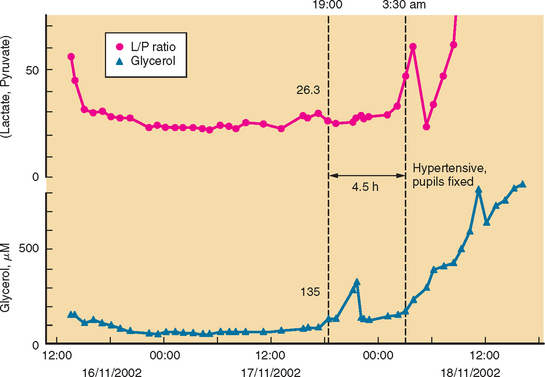
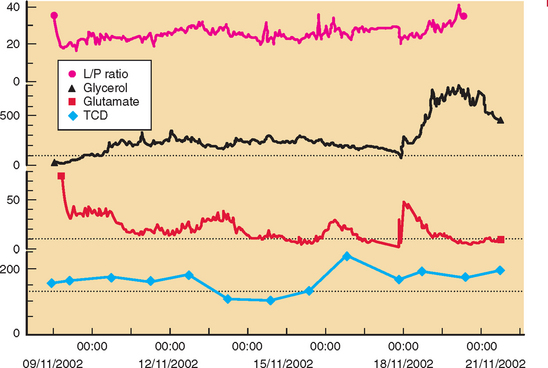
Developments such as the application of microdialysis to the injured human brain may improve monitoring and detection of cerebral vasospasm.23 There is good evidence that an increase in ratio of lactate to pyruvate precedes onset of vasospasm by several hours.24 Other studies point to the possibility that outcome after SAH is genetically predetermined.25
HYPERTENSIVE INTRACRANIAL HEMORRHAGE
Epidemiology
Stroke is the third most common cause of mortality and the leading cause of chronic neurological morbidity worldwide.26–30 Spontaneous intracerebral hemorrhage accounts for approximately 10% to 15% of all cases of stroke, with an estimated annual incidence of 37,000 cases in the United States; hypertension causes 40% to 60% of spontaneous intracerebral hemorrhages.28,36 ICH is associated with a much higher rate of mortality than are ischemic forms of stroke.27,28,56,57
Etiology
 Acute rise in blood pressure32 after (sympathomimetic) rise in blood pressure induced by drugs (amphetamines, cocaine, pseudoephedrine, and heroin); cold exposure; trigeminal stimulation; and acute increase in cerebral blood flow with resulting intracranial arterial hypertension after carotid endarterectomy or cardiac surgery
Acute rise in blood pressure32 after (sympathomimetic) rise in blood pressure induced by drugs (amphetamines, cocaine, pseudoephedrine, and heroin); cold exposure; trigeminal stimulation; and acute increase in cerebral blood flow with resulting intracranial arterial hypertension after carotid endarterectomy or cardiac surgeryPathology
Chronic hypertension is believed to alter vascular wall structure, resulting in degenerative weakening and consequent rupture, but the exact pathological process involved remains controversial.31a,33
Jean-Martin Charcot and Charles-Joseph Bouchard first described miliary aneurysms, which were thought to be responsible for hypertensive hemorrhages, inasmuch as they are commonly found on the perforating arteries of the basal ganglia and pons (common sites of hypertensive hemorrhages) and were believed to be more common in hypertensive patients. Fischer demonstrated these to be false aneurysms representing small (0.2- to 1.0-mm) healed foci of arteriolar bleeds. He attributed hypertensive hemorrhages to lipohyalinosis (found closer to sites of rupture than Charcot-Bouchard miliary aneurysms), as well as to plasmatic destruction and atherosclerosis of the vessel wall.38,39 It has also been hypothesized that the walls of arteries supplying regions that commonly bleed are thinner than vessels of similar size and are, in addition, subject to higher pressures as they originate from larger vessels.42
Distribution of Hypertensive Hemorrhage
In most hypertensive hemorrhages, bleeding is primarily into the brain parenchyma. This may be associated with extensions into the ventricular system, the subarachnoid space, and, in rare cases, the subdural space. Subarachnoid and intraventricular hemorrhages, occurring in the absence of parenchymal bleeding, usually result from other pathology, such as aneurysms.52 Table 43-1 shows the distribution (frequency) of hypertensive parenchymal hemorrhage.59
TABLE 43-1 Distribution (Frequency) of Hypertensive Parenchymal Hemorrhage
| Location | Frequency |
|---|---|
| Supratentorial | 80% |
| Infratentorial | 20% |
| Putamen | 55% |
| Thalamus | 10% |
| Subcortical white matter | 15% |
| Cerebellum | 10% |
| Pons | 10% |
Pathophysiology
After a parenchymal hemorrhage, a circular hematoma forms and directly destroys and may compress and displace adjacent brain tissue, which results in neurological deficit. Surrounding the hematoma is a salvageable ischemic area of brain, the penumbra, loss of which results in secondary damage.31 Bleeding is usually brief but may continue for some hours, resulting in large or irregular-shaped hematomas and may occur in patients with a bleeding diathesis.41,51,53,58 A hematoma may track along white matter tracts or dissect brain parenchyma with extension into the ventricular system or subarachnoid space. Parenchyma immediately surrounding a hematoma undergoes necrosis and is invaded by inflammatory cells with resolution of liquefied clot, leaving a smaller hemosiderin-stained cavity surrounded by an area of gliosis.43
Clinical Features
Clinical features depend on the location and size of hemorrhage, and manifestation may be asymptomatic. The more common features are summarized as follows:44
 Putaminal hemorrhage: hemiplegia, dysphasia, and conjugate gaze paresis with deviation toward the side with hematoma.
Putaminal hemorrhage: hemiplegia, dysphasia, and conjugate gaze paresis with deviation toward the side with hematoma. Thalamic hemorrhage: hemiplegia, hemisensory loss, dysphasia, abnormalities of vertical gaze, and pupillary changes.
Thalamic hemorrhage: hemiplegia, hemisensory loss, dysphasia, abnormalities of vertical gaze, and pupillary changes. Cerebellar hemorrhage: severe headache (occipital), nausea and vomiting, and ataxia; motor deficit is not usual.
Cerebellar hemorrhage: severe headache (occipital), nausea and vomiting, and ataxia; motor deficit is not usual.Diagnosis
Definitive diagnosis of ICH requires neuroimaging. CT scan shows the location, size, and mass effect (parenchymal and ventricular shift or compression) of hemorrhages, hydrocephalus, and subarachnoid or intraventricular extension. Magnetic resonance imaging and angiography are less useful in the acute phase but may be useful in the differential diagnosis.36,37
Treatment
Medical treatment is the mainstay in the initial management of hypertensive ICH.26 Treatment is aimed at several objectives:
 Intensive monitoring and care in specialist units, including serial neuroimaging in the early stages.
Intensive monitoring and care in specialist units, including serial neuroimaging in the early stages. Control of raised intracranial pressure: hyperventilation, head elevation, diuretics (mannitol, frusemide), ventricular drainage.
Control of raised intracranial pressure: hyperventilation, head elevation, diuretics (mannitol, frusemide), ventricular drainage. Treatment of systemic disorders and complications: coagulation; fever; hypoxia; seizures; gastrointestinal, endocrine, pulmonary, and cardiovascular disease; infections; and electrolyte abnormalities.
Treatment of systemic disorders and complications: coagulation; fever; hypoxia; seizures; gastrointestinal, endocrine, pulmonary, and cardiovascular disease; infections; and electrolyte abnormalities.Surgical treatment is generally reserved for cases in which medical treatment fails to control intracranial pressure or to relieve compression and herniation, and it is more often performed for infratentorial hemorrhages, because of the high risk of herniation. Ventriculostomy involves placement of catheters in the cerebral ventricles and enables monitoring of intracranial pressure and drainage of cerebrospinal fluid when decompression is needed. Hematomas may necessitate evacuation through stereotactic aspiration or open surgery.34
IDIOPATHIC INTRACRANIAL HEMORRHAGE
The cause of ICH is not always apparent on initial investigation and is often multifactorial in nature (e.g., hypertension may coexist with other disorders and may be a reflex response to raised intracranial pressure). It is important, however, to establish the cause of all hemorrhages in order to target management strategies appropriately. The various causes of ICH are listed as follows:46,47,49
 Vascular: aneurysms, arteriovenous malformations and fistulas, amyloid angiopathy, cavernous and venous angiomas, telangiectasias, arterial dissection, caroticocavernous fistula
Vascular: aneurysms, arteriovenous malformations and fistulas, amyloid angiopathy, cavernous and venous angiomas, telangiectasias, arterial dissection, caroticocavernous fistula Drugs: sympathomimetic drugs (cocaine, amphetamine, pseudoephedrine), anticoagulants, antiplatelet drugs, thrombolysis
Drugs: sympathomimetic drugs (cocaine, amphetamine, pseudoephedrine), anticoagulants, antiplatelet drugs, thrombolysisDifficulty in establishing a definite cause of hemorrhage should prompt extensive and thorough reassessment of the history, which may contribute to clarification of the diagnosis. Initial CT scan does not always reveal the underlying pathology, and it may be useful to consider reviewing or repeating the scan and other investigations or to investigate the patient with magnetic resonance imaging or angiography. Despite intensive investigation, some vascular lesions remain occult.26
Spontaneous Intracranial Hemorrhage: Early Surgery or Initial Conservative Management?
The role of surgery in spontaneous supratentorial ICH has been a point of controversy for many years. However, a prospective randomized trial was performed to compare early surgery with initial conservative treatment for such patients59 and appears to have shed some light on the appropriate course of management. The authors used a parallel-group trial design. Early surgery combined hematoma evacuation (within 24 hours of random assignment) with medical treatment. Initial conservative treatment entailed medical treatment, although later evacuation was allowed if necessary. In this study, 1033 patients from 83 centers in 27 countries were randomly assigned to undergo early surgery (503) or receive initial conservative treatment (530). At 6 months, 51 patients could not be monitored for follow-up, and 17 were alive with unknown status. Of 468 patients who underwent early surgery, 122 (26%) had a favorable outcome, in comparison with 118 (24%) of 496 who received initial conservative treatment (odds ratio = 0.89 [95% confidence interval = 0.66 to 1.19], P = 0.414); the absolute benefit was 2.3% (-3.2 to 7.7), and the relative benefit was 10% (-13 to 33). The authors concluded that patients with spontaneous supratentorial intracerebral hemorrhage in neurosurgical units show no overall benefit from early surgery in comparison with initial conservative treatment.
Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000;342:29-36.
Kase CS, Mohr JP, Caplan LR. Intracerebral hemorrhage. In Barnett HJM, Mohr JP, Stein BM, et al, editors: Stroke: Pathophysiology, Diagnosis, and Management, 2nd ed., New York: Churchill Livingstone, 1992.
Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
van Gijn J, Rinkel GJ. Subarachnoid hemorrhage: diagnosis, causes, and management. Brain. 2001;124:249-278.
Woo D, Haverbusch M, Sekar P, et al. Effect of untreated hypertension on hemorrhagic stroke. Stroke. 2004;35:1703-1708.
1 Kuller LH. Incidence, rates of stroke in the 80s. Stroke. 1989;20:841-843.
2 Wolf PA, Kannel WB, McGee DL, et al. Epidemiology of strokes in North America. In: Barnett HJM, Mohr JP, Stein BM, et al, editors. Stroke: Pathophysiology, Diagnosis, and Management. New York: Churchill Livingstone; 1986:19-29.
3 Wiebers DO, Whisnant JP, Sundt TM, et al. The significance of unruptured intracranial saccular aneurysms. J Neurosurg. 1987;66:23-29.
4 Fox JL. Intracranial Aneurysms. New York: Springer-Verlag, 1983.
5 Almeida GM, Pindaro J, Plese P, et al. Intracranial arterial aneurysms in infancy and childhood. Childs Brain. 1977;3:193-199.
6 Fang H. A comparison of blood vessels of the brain and peripheral blood vessels. In: Wright IS, Millikan CH, editors. Cerebral Vascular Diseases. New York: Grune & Stratton; 1958:17-22.
7 Wilkinson IMS. The vertebral artery: extracranial and intracranial structure. Arch Neurol. 1972;27:392-396.
8 Youmans JR, editor. Neurological Surgery, 3rd ed., Philadelphia: WB Saunders, 1990.
8a Nader-Sepahi A, Casimiro M, Sen J, et al. Is aspect ratio a reliable predictor of aneurysmal rupture? Neurosurgery. 2004;54:1343-1348.
9 Nehls DG, Flom RA, Carter LP, et al. Multiple intracranial aneurysms: determining the site of rupture. J Neurosurg. 1985;63:342-348.
10 Mohr G, Ferguson G, Khan M, et al. Intraventricular hemorrhage from ruptured aneurysm: retrospective analysis of 91 cases. J Neurosurg. 1983;58:482-487.
11 Yeh HS, Tomsick TA, Tew JM. Intraventricular hemorrhage due to aneurysms of the distal posterior inferior cerebellar artery. J Neurosurg. 1985;62:772-775.
12 Raps EC, Galetta SL, Solomon RA, et al. The clinical spectrum of unruptured intracranial aneurysms. Arch Neurol. 1993;50:265-268.
13 Sano H, Jain VK, Kato Y, et al. Bilateral giant intracavernous aneurysms: technique of unilateral operation. Surg Neurol. 1988;29:35-38.
14 White JC, Ballantine HT. Intrasellar aneurysms simulating hypophyseal tumors. J Neurosurg. 1961;18:34-50.
15 Okawara SH. Warning signs prior to rupture of an intracranial aneurysm. J Neurosurg. 1973;38:575-580.
16 Verweij RD, Wijdicks EFM, van Gijn J. Warning headache in aneurysmal subarachnoid hemorrhage: a case-control study. Arch Neurol. 1988;45:1019-1020.
17 Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
18 Kassel NF, Torner JC, Haley ECJr. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1. Overall management results. J Neurosurg. 1990;73:18.
19 Sen J, Belli A, Albon H, et al. Triple-H therapy in the management of aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2003;2:614-621.
20 Kassel NF, Peerless SJ, Durward QJ. Treatment of ischemic deficits from vasospasm with intravascular volume expansion and induced arterial hypertension. Neurosurgery. 1982;11:337-343.
21 Rinkel GJE, Feigin VL, Algra A, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage (Cochrane Review). In: The Cochrane Library, Issue 2. Oxford: Update Software; 2003.
22 Sen J, Belli A, Petzold A, et al: Extracellular fluid S100B in the CNS: a future surrogate marker of acute brain injury. Acta Neurochir, in press.
23 Unterberg AW, Sakowitz OW, Sarrafzadeh AS, et al. Role of bedside microdialysis in the diagnosis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2001;94:740-749.
24 Morgan L, Montgomery H, Grieve J, et al. Association of interleukin 6–174G/C and–572G/C promoter polymorphisms with cerebral aneurysms. Interv Neuroradiol. 2003;9:168.
25 Batjer HH, Kopitnik TAJr, Friberg L. Spontaneous intracerebral and intracerebellar haemorrhage: differential diagnosis. Youmans JR, editor. Neurological Surgery, 4th ed., vol 2. Philadelphia: WB Saunders, 1996;1449-1464.
26 Bozzola FG, Gorelick PB, Jensen JM. Epidemiology of intracranial hemorrhage. Neuroimaging Clin North Am. 1992;2:1.
27 Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage: a powerful and easy to use predictor of 30-day mortality. Stroke. 1993;24:987-993.
28 Broderick JP, Brott T, Tomsick T, et al. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg. 1993;78:188-191.
29 Brown RD, Whisnant JP, Sicks JD, et al. Stroke incidence, prevalence and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27:373-380.
30 Camarata PJ, Heros RC, Latchaw RD. “Brain attack:” the rationale for treating stroke as a medical emergency. Neurosurgery. 1994;34:144-158.
31 Caplan L. Intracerebral hemorrhage revisited. Neurology. 1988;38:624-627.
31a Challa VR, Moody DM, Bell MA. The Charcot-Bouchard aneurysm controversy: impact of a new histologic technique. J Neuropathol Exp Neurol. 1992;51:264-271.
32 Crowell RM, Ojemann RG, Ogilvy CS. Brain hemorrhage. In: Ojemann RG, Ogilvy CS, Crowell RM, et al, editors. Surgical Management of Neurovascular Disease. 3rd ed. Baltimore: Williams & Wilkins; 1995:561-580.
33 Dandapani BK, Suzuki S, Kelley RE, et al. Relation between blood pressure and outcome in intracerebral hemorrhage. Stroke. 1995;26:21-24.
34 Drury ID, Whisnant JP, Garraway WM. Primary intracerebral hemorrhage: impact of CT on incidence. Neurology. 1984;34:653.
35 Findlay JM. Current management of aneurysmal subarachnoid haemorrhage. Guidelines from the Canadian Neurological Society. Can J Neurol Sci. 1997;24:161-170.
36 Fischer CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30:536-550.
37 Fischer CM. Cerebral miliary aneurysms in hypertension. Am J Pathol. 1972;66:313-330.
38 Frankowski RF. Epidemiology of stroke and intracerebral hemorrhage. In: Kaufman HH, editor. Intracerebral Hematomas. New York: Raven Press, 1992.
39 Fujii Y, Tanaka R, Takeuchi S, et al. Hematoma enlargement in spontaneous intracerebral hemorrhage. J Neurosurg. 1994;80:51-57.
40 Garcia JH, Ho K-L. Pathology of hypotensive arteriopathy. Neurosurg Clin North Am. 1992;3:497.
41 Garcia JH, Ho K-L, Caccamo DV. Intracerebral hemorrhage: pathology of selected topics. In: Kase CS, Caplan LR, editors. Intracerebral Hemorrhage. Boston: Butterworth-Heineman, 1994.
42 Hamilton MG, Zabramski JM. Intracerebral hematomas. In: Carter LP, Spetzler RF, Hamilton MG, editors. Neurovascular Surgery. New York: McGraw-Hill; 1995:477-496.
43 Juvela S. Risk factors for impaired outcome after spontaneous intracerebral hemorrhage. Arch Neurol. 1995;52:1193-1200.
44 Juvela S, Hillbom M, Palomaki H. Risk factors for spontaneous intracerebral hemorrhage. Stroke. 1995;26:1558-1564.
45 Kase C. Intracerebral hemorrhage: non-hypertensive causes. Stroke. 1986;17:4590-4595.
46 Kase CS, Mohr JP, Caplan LR. Intracerebral hemorrhage. In Barnett HJM, Mohr JP, Stein BM, et al, editors: Stroke: Pathophysiology, Diagnosis, and Management, 2nd ed., New York: Churchill Livingstone, 1992.
47 Kaufman HH. Spontaneous intracerebral hematomas. In: Grossman RG, Hamilton WJ, editors. Principles of Neurosurgery. New York: Raven Press; 1991:65-78.
48 Lampl Y, Gilad R, Eshel Y, et al. Neurological and functional outcomes in patients with supratentorial hemorrhages: a prospective study. Stroke. 1995;26:2249-2253.
49 Lee K, Bae H, Yun I. Recurrent intracerebral hemorrhage due to hypertension. Neurosurgery. 1990;26:586-590.
50 Mayberg MR, Batjer HH, Dacey R, et al. Guidelines for the management of aneurysmal subarachnoid haemorrhage. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1994;25:2315-2328.
51 Mayer SA, Sacco RL, Shi T, et al. Neurologic deterioration in noncomatose patients with supratentorial intracerebral hemorrhage. Neurology. 1994;44:1379-1384.
52 Mori S, Sadoshima S, Ibayashi S, et al. Impact of thalamic hematoma on 6-month mortality and motor and cognitive functional outcome. Stroke. 1995;26:620-626.
53 Powers WJ. Acute hypertension after stroke: the scientific basis for treatment decisions. Neurology. 1993;43:461-467.
54 Sacco RL, Wolf PA, Bharucha NE, et al. Subarachnoid and intracerebral hemorrhage: Natural history, prognosis, and precursive factors in the Framingham study. Neurology. 1984;34:847.
55 Schuetz H, Dommer T, Boedeker R-H, et al. Changing pattern of brain hemorrhage during 12 years of computed axial tomography. Stroke. 1992;23:653.
56 Wijdicks EFM, Fulgham JR. Acute fatal deterioration in putaminal hemorrhage. Stroke. 1995;26:1953-1955.
57 Wiener H, Cooper P. The management of spontaneous intracerebral hemorrhage. Contemp Neurosurg. 1992;14(21):1-8.
58 Wityk RJ, Caplan LR. Hypertensive intracerebral hemorrhage: epidemiology and clinical pathology. Neurosurg Clin North Am. 1992;3:521.
59 Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387-397.