Chapter 6 Intracranial Atherosclerotic Disease
Narrowing of the large intracranial arteries is a surprisingly common cause of stroke in non-Caucasian populations, and one often overlooked in the evaluation of cerebrovascular patients. Various imaging modalities can effectively interrogate the intracranial vascular tree, at least the large intracranial vessels, to determine the extent of this condition. Although various pathologies can affect intracranial large arteries, including moyamoya disease, sickle cell disease, spasm, and arteritis, the most common cause is atherosclerosis. This chapter reviews the frequency of intracranial atherosclerotic disease, available diagnostic modalities, and treatment options.
EPIDEMIOLOGY AND NATURAL HISTORY
In the United States, between 8% and 10% of all strokes are deemed to be due to intracranial atherosclerotic disease.1,2 However, the burden of this condition has clear ethnic differences, affecting Asians, African Americans, and Latinos more than Caucasians. Some reports have cited that as many as 25% of all strokes in Asians are due to intracranial stenosis.3 In addition, intracranial disease may coexist with extracranial disease; 6% of patients with symptomatic extracranial carotid disease have tandem intracranial disease.4
The progression of severity of intracranial stenosis has not been carefully evaluated. Small studies have reported that between one third and one half of vessels develop worsening stenosis during a mean follow-up of 27 months.5,6 More important, progression of arterial narrowing strongly correlates with development of ischemic symptomatology.6 Therefore,
MECHANISMS OF CEREBRAL ISCHEMIA
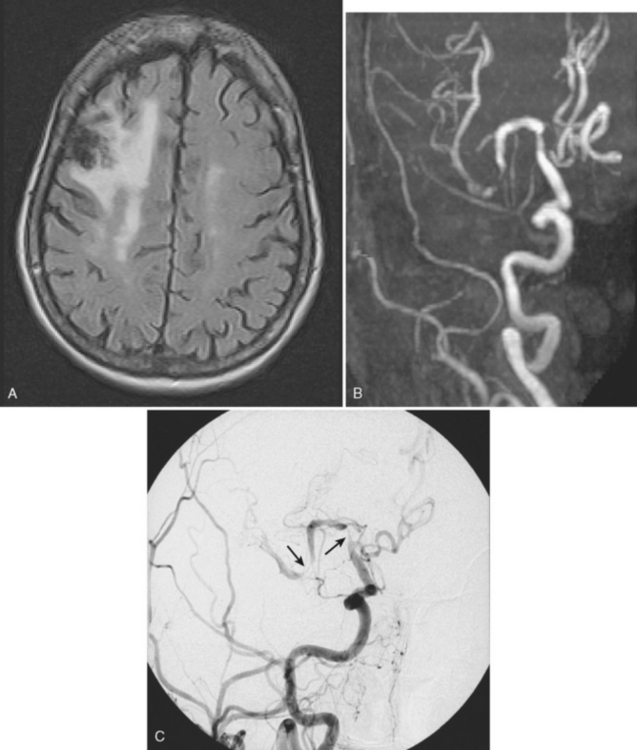
The mechanisms by which intracranial arterial disease causes cerebral ischemia are varied and include occlusion of the ostium of a penetrating artery producing a small subcortical (lacunar) stroke, occlusion of multiple penetrators resulting in a large subcortical infarct (such as a striatocapsular stroke), hemodynamic reduction in blood flow with a subsequent watershed stroke, and finally generation of a thrombus on the surface of the plaque with artery-to-artery embolism causing a cortical infarct. Figure 6-1 shows a cortical infarct in a patient with a middle cerebral artery stenosis. Indeed, in the extracranial-intracranial artery bypass surgery trial, 61% of those with middle cerebral artery stenosis had a cortical infarct.7 The role of artery-to-artery embolism in the production of strokes related to intracranial arterial disease has been confirmed by the finding of microembolic signals during transcranial Doppler (TCD) monitoring of the affected artery. Vessels with microembolic signals tend to be more stenotic and have an increased risk of becoming symptomatic.8
The mechanisms of infarction intracranial atherosclerosis are:
DIAGNOSIS OF INTRACRANIAL ATHEROSCLEROTIC DISEASE
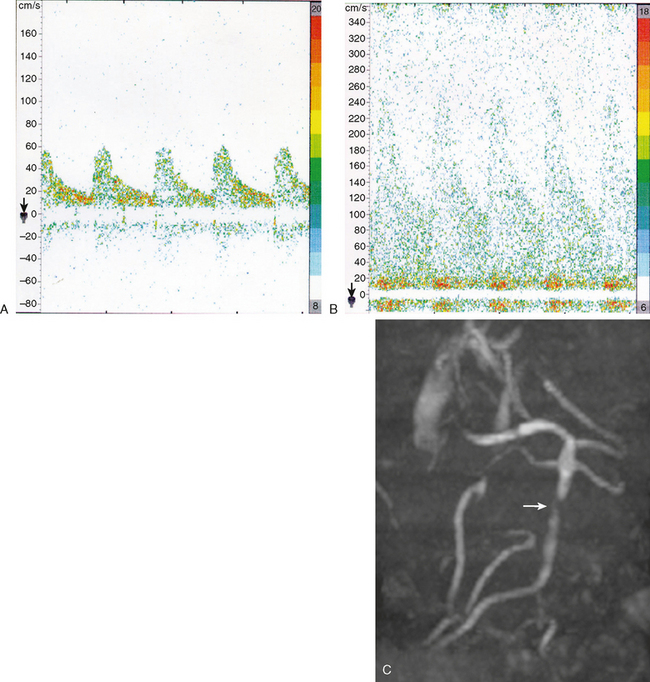
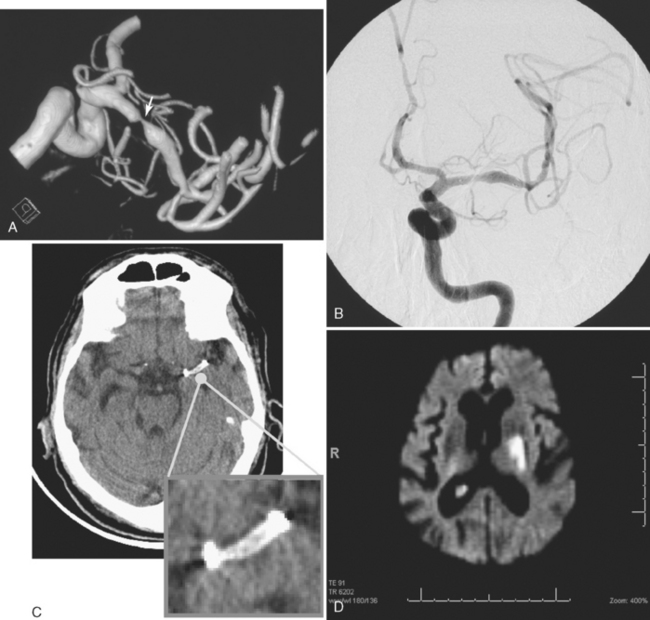
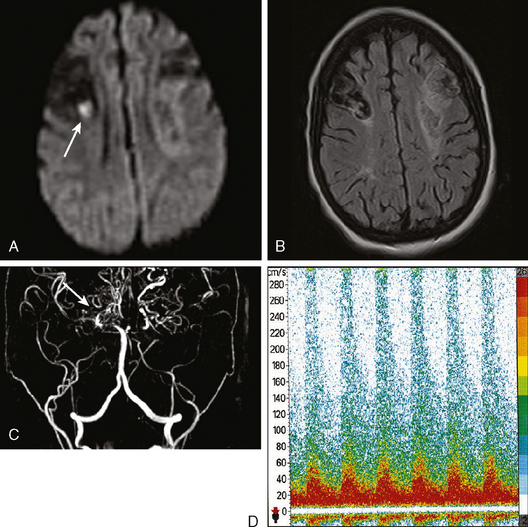
The gold standard method to detect intracranial arterial disease remains conventional catheter angiography. Nonetheless, this procedure is invasive, expensive, and has associated risks.9 Therefore, there is interest in determining the accuracy of available noninvasive tests. The recent Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) study evaluated the role of TCD and magnetic resonance angiography (MRA) in detecting 50% to 99% stenosis of the large intracranial arteries.10 Both TCD and MRA had strong negative predictive values of 86% and 91%, respectively, but the positive predictive values were lower at 36% and 59%. The TCD and MRA criteria for diagnosing 50% to 99% stenosis are outlined in Table 6-1. Data for sensitivity and specificity of computed tomography angiography (CTA) were unavailable from SONIA because of small numbers, but there is information indicating that it has good correlation with angiography, with one report showing a positive predictive value of 93%.11 Therefore, a normal TCD or MRA is a relatively trustworthy indication that there is no significant stenosis, but an abnormal result has a suboptimal predictive value. The relatively low positive predictive value for TCD may be understandable because it is a blind procedure in which the skill of the operator comes into play. The Doppler shift is greatest if the insonation is parallel to the plane of flow; perpendicular insonation will not detect this Doppler shift and therefore will be unable to detect flow velocity reliably. Figure 6-2 shows an intracranial carotid (cavernous segment) detected by MRA and confirmed by catheter angiography; TCD performed through the orbital window could not detect the increase in flow velocity in this case. However, straight arterial segments may be easier to interrogate by TCD, such as the vertebral, basilar, and M1 segments of the middle cerebral arteries. Figure 6-3 shows a significant basilar stenosis detected by a focal increase in flow velocity on TCD also noted on MRA.
TABLE 6-1 TCD and MRA cutoff parameters to detect ≥50% stenosis of large intracranial arteries.
| TCD (mean flow velocity) | |
|---|---|
| MCA | 100 cm/sec |
| ICA | 90 cm/sec |
| Vertebral | 80 cm/sec |
| Basilar | 80 cm/sec |
| MRA | |
|---|---|
| 50% stenosis or flow gap | |
MRA, magnetic resonance angiography; TCD, transcranial Doppler.
(Adapted from Feldmann E, Wilterdink JL, Kosinski A, Lynn M, Chimowitz MI, Sarafin J, et al., Neurology 2007; 68:2099–2106.)
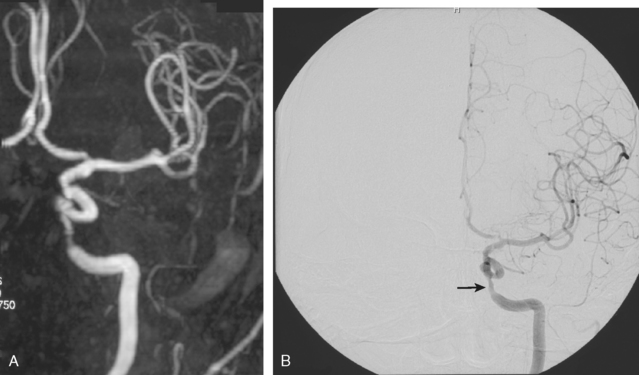
Figure 6-2 (A) A cavernous segment intracranial carotid stenosis confirmed by catheter angiography (B).
If a risky or invasive procedure is planned, then confirmatory conventional angiography is required. However, if medical treatment will not be significantly altered by the test results, it is reasonable to rely on noninvasive diagnostic modalities. As reviewed subsequently, current medical interventions are not optimal, and further research on the valve of revascularization will determine whether it is an effective and safe option; therefore, the relevance of the choice of diagnostic test will be amplified as more therapeutic choices become available.
MEDICAL THERAPY
Symptomatic intracranial arterial disease was, up to recently, commonly treated with anticoagulants. This approach was based on a small case series showing a decrease in mortality in patients with vertebrobasilar symptoms treated with anticoagulants,12 and a subsequent retrospective study of 151 patients treated with either aspirin or warfarin, in which the anticoagulated patients fared better in stroke and death risk.13 Nonetheless, a randomized controlled study was necessary to answer the question adequately. The recently completed Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) study has provided valuable information on the medical treatment of this condition.14 This trial studied patients with a recent stroke or transient ischemic attack (TIA) due to intracranial arterial stenosis (50%–99% reduction of the luminal diameter of a major intracranial vessel) and randomized participants to receive aspirin (1300 mg/d) or warfarin (adjusted to maintain an international normalized ratio of 2–3). There was no difference in the combined primary outcome measure of ischemic stroke, intracerebral hemorrhage, or vascular death between both treatment arms, which occurred at a staggering rate of 22% during the 1.8-year average follow-up period. Almost two thirds of the events in the combined primary outcome measure were due to recurrent stroke in the territory of the stenotic vessel. Most of the events occurred in the first couple of weeks after randomization. There was a greater than twofold incidence of major hemorrhage or death in the anticoagulated group that led to early termination of the trial. No subgroup of patients in whom warfarin was superior to aspirin was identified.15 Therefore, warfarin appears to be no more effective and riskier than aspirin for secondary stroke prevention in patients with intracranial stenosis.
The control of vascular risk factors remains an important aspect of the approach to the patient with intracranial arterial disease. The WASID study demonstrated that greater control of low-density lipoprotein (LDL) to a goal of less than 100 mg/dl reduced the risk of recurrent events. In addition, good control of blood pressure also reduced the risk of stroke recurrence.16 This is important because some clinicians had recommended maintenance of elevated blood pressure to prevent hemodynamic (watershed) events in patients with intracranial disease. The data from WASID show that good blood pressure control, regardless of the degree of stenosis, is indicated for most patients. This recommendation should be individualized, and it does not apply to the very early period after a cerebral ischemic event, but, in general, the Joint National Committee (JNC 7) recommendations for blood pressure management17 should be followed, with no indication to maintain an elevated blood pressure for extended periods of time, except perhaps in the acute period after a stroke.18
WASID also allowed the identification of a group at high risk of recurrent stroke in the territory of the stenotic vessel. High-risk factors included severe stenosis (defined as ≥70%), female sex, and enrollment soon after the onset of symptoms.15 The impact of higher degree of stenosis mirrors the experience with extracranial carotid stenosis and is not unexpected. It should be recalled that the large intracranial arteries have a diameter of approximately 3 mm, and thus a 70% stenosis would result in a small residual diameter. Women had an increased risk of stroke recurrence, perhaps because of smaller diameter of their vessels, although hormonal factors might also play a role. The effect of early recruitment into the study as a risk factor for recurrence may be due to recruitment bias, but it is also clear that the early weeks after an event constitute the most vulnerable period. This has led some to suggest that more aggressive antithrombotic therapy for two to four weeks after the stroke might be considered,19 although this approach has not yet been studied in a clinical trial. Interestingly, neither the vessel involved nor the length of the stenosis were shown to predict an increased risk of recurrence. Therefore, anterior circulation and posterior circulation intracranial stenosis should not be viewed differently in regard to risk or treatment considerations. The identification of the high-risk group may permit risk stratification, which would be particularly useful if a risky but effective intervention such as endovascular revascularization became increasingly available. Table 6-2 outlines the risk of recurrence according to degree of stenosis.
TABLE 6-2 Probability of stroke recurrence at 2 years in the territory of the symptomatic vessel in intracranial arterial disease.
| Risk of recurrence | ||
|---|---|---|
| First Event | Stenosis 50%–69% | Stenosis 70%–99% |
| Stroke | 11% | 25% |
| TIA | 8% | 14% |
TIA, transient ischemic attack.
(Adapted from Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al., Circulation 2006; 113:555–563.)
INTERVENTIONAL APPROACHES
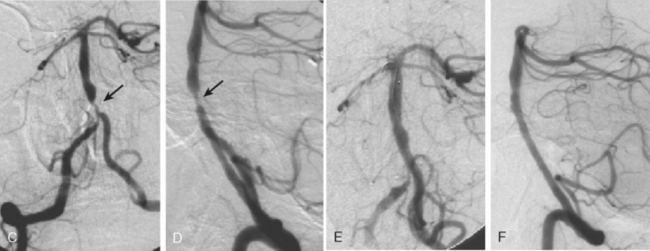
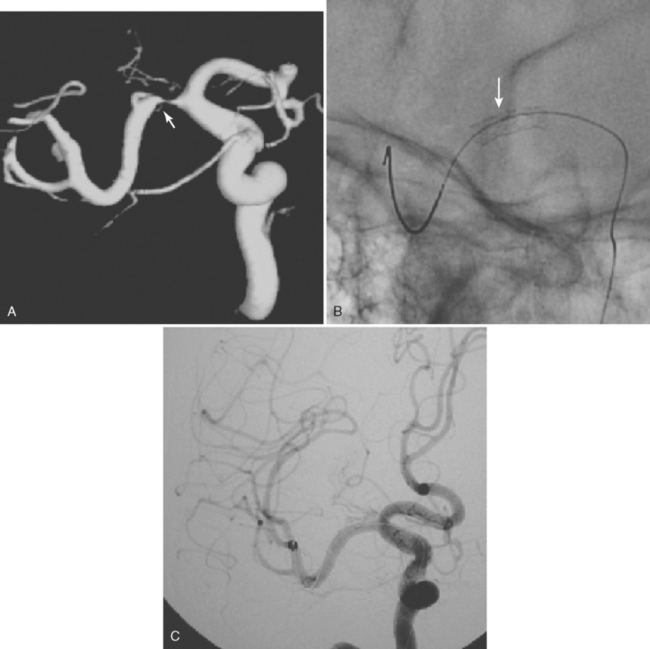
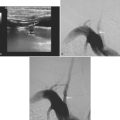
According to the WASID study, there is a significant risk of stroke recurrence of approximately 15% and of stroke recurrence in the territory of the stenotic vessel of approximately 12% in the first year after the initial symptom, despite antithrombotic therapy in the form of aspirin or warfarin.12 This elevated risk has prompted the search for more efficient methods of stroke prevention. Prior surgical interventions such as extracranial to intracranial bypass were unsuccessful in improving outcomes,20 which has led to the consideration of endovascular approaches to treat the stenotic vessel. These interventions include angioplasty and stenting. A number of nonrandomized and controlled series have reported that angioplasty alone is feasible but is often complicated by intimal dissection and plaque disruption, intraluminal thrombus formation, and even vascular rupture.21 Therefore, there is considerable excitement about the use of angioplasty with stenting to avoid these potential complications. The initial experience with stents expanded by balloons at high pressure still raised concerns for plaque disruption and occlusion of penetrating arteries at the level of the stent.21 Technical advances in the stents available and increasing expertise among neurointerventionalists promise to improve outcomes in the near future. Examples of basilar artery and middle cerebral artery angioplasty are illustrated in Figures 6-5 and 6-6.
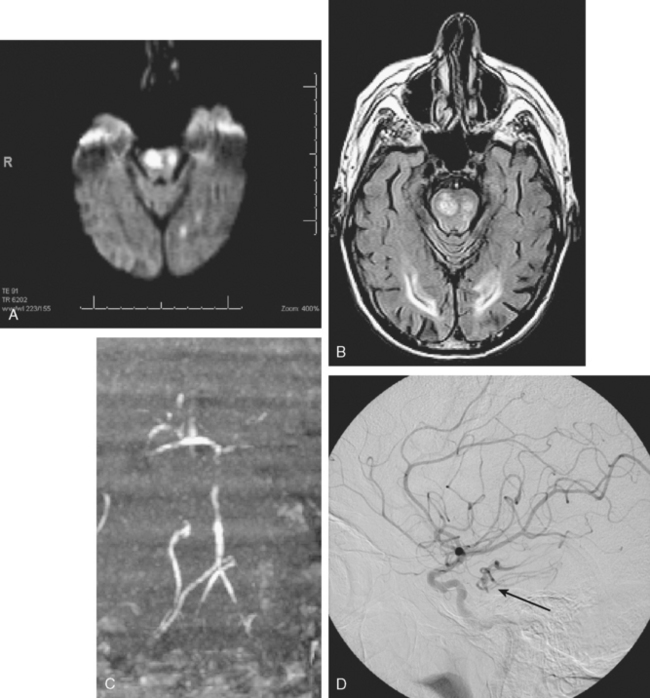
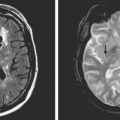
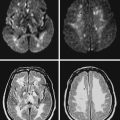
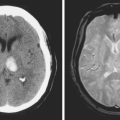
A 74 year old man with dislipidemia and an old right hemispheric stroke due to right middle cerebral artery occlusion presented with spells of transient aphasia and right hemiparesis which recurred in spite of different antithrombotic regimens. Angiography confirmed a high-grade left middle cerebral artery stenosis (Figure 6-6); incidentally, the right middle cerebral artery was occluded. Given the clinical picture and the contralateral middle cerebral artery occlusion, it was decided to proceed with endovascular treatment of the symptomatic lesion. The left middle cerebral artery was treated with angioplasty and stenting with a Wingspan stent with good angiographic results. After the procedure he developed persistent mild aphasia and right hemiparesis. A left capsular infarct in the distribution of the penetrating vessels at the level of the stent was seen on DWI MRI.
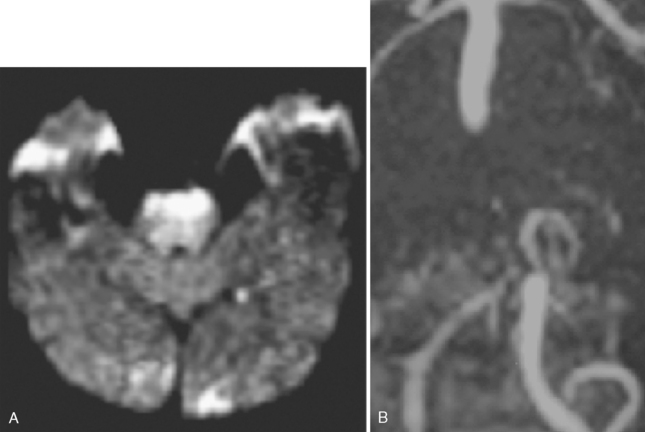
The SSYLVIA study employed a coronary stent in 43 intracranial stenoses with a 30-day risk of stroke in the stented vessel of 6.6% plus a 7.3% subsequent stroke risk for up to 1 year.23 This is actually slightly worse than the risk of stroke with medical therapy.14 These disappointing results led to the development of a new self-expandable stent that presumably causes less plaque disruption. The Wingspan stent was recently approved by the U.S. Food and Drug Administration for intracranial atherosclerotic disease; initial experience with this device has been associated with a 1-year risk of ipsilateral stroke and death of 9.3%.24 It is possible that this new generation of stents will be helpful for high-risk patients with intracranial arterial disease, but this remains to be proved in a randomized study. In selected cases, many centers are deploying intracranial stents successfully, as illustrated in Figure 6-7. This author believes that, at the present time, intracranial stenting should be reserved for those patients at high risk of recurrent stroke who have failed medical therapy; similar recommendations have recently been put forward.25 Future stent and catheter development, and further evidence about their safety and efficacy, might allow for a more liberal use of this technology.
1 Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction: the Northern Manhattan Stroke Study. Stroke. 1995;26:14-20.
2 Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27:1974-1980.
3 Wong KS, Huang YN, Gao S, Lam WW, Chan YL, Kay R. Intracranial stenosis in Chinese patients with acute stroke. Neurology. 1998;50:812-813.
4 Kappelle LJ, Eliasziw M, Fox AJ, Sharpe BL, Barnett HJM. Importance of intracranial atherosclerotic disease in patients with symptomatic stenosis of the internal carotid artery. Stroke. 1999;30:282-286.
5 Akins PT, Pilgram TK, Cross DTIII, Moran CJ. Natural history of stenosis from intracranial atherosclerosis by serial angiography. Stroke. 1998;29:433-438.
6 Arenillas JF, Molina CA, Montaner J, Abilleira S, González-Sánchez MA, Álvarez-Sabín J. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke. 2001;32:2898-2904.
7 Bogousslavsky J, Barnett HJ, Fox AJ, Hachinski VC, Taylor W. Atherosclerotic disease of the middle cerebral artery. Stroke. 1986;17:1112-1120.
8 Gao S, Wong KS, Hansberg T, Lam WWM, Droste DW, Ringelstein EB. Microembolic signal predicts recurrent cerebral ischemic events in acute stroke patients with middle cerebral artery stenosis. Stroke. 2004;35:2832-2836.
9 Bendszus M, Stoll G. Silent cerebral ischaemia: hidden fingerprints of invasive medical procedures. Lancet Neurol. 2006;5:364-372.
10 Feldmann E, Wilterdink JL, Kosinski A, Lynn M, Chimowitz MI, Sarafin J, et althe Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial Investigators. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial. Neurology. 2007;68:2099-2106.
11 Bash S, Villablanca JP, Jahan R, Duckwiler G, Tillis M, Kidwell C, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. Am J Neuroradiol. 2005;26:1012-1021.
12 Millikan CH, Siekert RG, Shick RM. Studies in cerebrovascular disease. III. The use of anticoagulant drugs in the treatment of insufficiency or thrombosis within the basilar arterial system. Mayo Clin Proc. 1955;30:116-126.
13 Chimowitz MI, Kokkinos J, Strong J, Brown MB, Levine SR, Silliman S, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45:1488-1493.
14 Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et alfor the Warfarin–Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305-1316.
15 Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et alfor the Warfarin–Aspirin Symptomatic Intracranial Disease Trial Investigators. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555-563.
16 Chaturvedi S, Turan TN, Lynn MJ, Kasner SE, Romano J, Cotsonis G, et alWASID Study Group. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology. 2007;69:2063-2068.
17 Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JLJr, et alNational Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
18 Adams HP, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke. Stroke. 2007;38:1655-1711.
19 Koroshetz WJ. Warfarin, aspirin, and intracranial vascular disease. N Engl J Med. 2005;352:1368-1370.
20 The EC/IC Bypass Study Group. Failure of extracranial–intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med. 1985;313:1191-1200.
21 Cruz-Flores S, Diamond AL Angioplasty for intracranial artery stenosis. Cochrane Database Syst Rev. 2006;3:CD004133. CD004133.pub2.
22 Jiang WJ, Srivastava T, Gao F, Du B, Dong KH, Xu XT. Perforator stroke after elective stenting of symptomatic intracranial stenosis. Neurology. 2006;66:1868-1872.
23 SSYLVIA Study Investigators. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke. 2004;35:1388-1392.
24 Bose A, Hartmann M, Henkes H, Liu HM, Teng MM, Szikora I, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke. 2007;38:1531-1537.
25 Hankey GH, Cruz-Flores S, Diamond AL. Angioplasty with or without stenting for intracranial artery stenosis. Stroke. 2006;37:2858-2859.