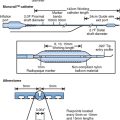
Chapter 17 Intracoronary brachytherapy
 Coronary vascular brachytherapy (VBT), using beta and gamma emitters, has revolutionized the treatment of in-stent restenosis (ISR).
Coronary vascular brachytherapy (VBT), using beta and gamma emitters, has revolutionized the treatment of in-stent restenosis (ISR).INTRODUCTION TO RADIATION BIOLOGY AND SYSTEMS
Radiation inhibits smooth muscle cell (SMC) proliferation and intimal hyperplasia by intervening in the cell cycle to cause cell death to radiosensitive cells, especially those undergoing mitosis following vascular injury. Radiation acts by absorbing into the target molecules such as DNA, RNA, or enzymes, by interacting with these molecules via formation of highly reactive free radicals, or by inducing programmed cell death, called apoptosis. Radiation may reduce restenosis by inhibiting the first wave of cell proliferation in the adventitia and the media, by inducing favorable remodeling,1 and by suppression of macrophages and adventitial myofibroblasts.2,3
EXPERIMENTAL FOUNDATION OF BRACHYTHERAPY
Brachytherapy evolved into clinical practice based on firm and elaborate experimental animal data. These involved external beam radiation by Schwartz et al.,4 catheter based systems by several investigators utilizing gamma radiation with 192Ir (Waksman et al. at Emory University,5,6 Wiedermann et al. at Columbia University,7,8 and Raizner et al. at Baylor University9), using beta radiation with 90Sr/Y (Verin et al.11, Waksman12), and beta radiation with 32P by Raizner et al. All these investigators have shown reduction in neointimal hyperplasia in the irradiated arteries utilizing doses ranging from 6-56 Gy.
Further experiments have been conducted using radioactive stents by Fischell et al.13 Hehrlein et al.,14,15 Laird et al.16,17 Radioactive stents, which were implanted in an atherosclerotic pig model, failed to show superiority over control non-radioactive stents with any of the treated doses at six months.17
Waksman et al.,18 Weinberger et al.,19 Robinson et al.,20 Makkar et al.,21 and Kim et al.22 used liquid isotope-filled balloons to irradiate porcine coronaries. The emitters used for this technology were 133Xenon, 188Re (14 Gy), and 166Ho (9, 18 Gy). These pre-clinical studies showed reduction in neointimal tissue as assessed by IVUS and histomorphometry. The concept of the radioisotope filled balloons is attractive because it has the advantages of centering and ease of use; however, a potential leakage hazard is of great concern.
CLINICAL TRIALS
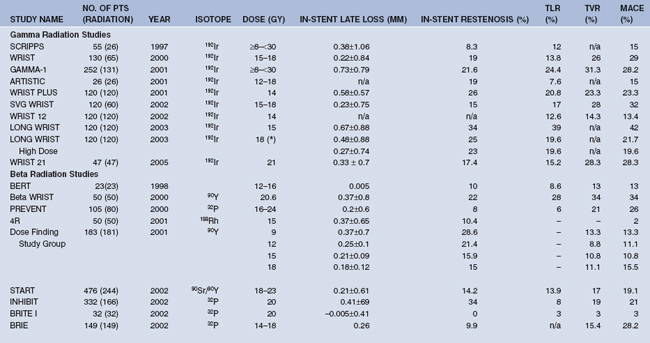
Several series of clinical trials were conducted to understand the safety, efficacy, and durability of VBT mainly in the US and Europe. While both gamma and beta radiations were studied for the treatment of ISR, only beta sources were studied for de novo lesions. Detailed discussion of the clinical trials is out of scope for this chapter, but a brief summary of the landmark clinical trials conducted thus far with gamma and beta emitters is presented. Salient features of the studies published in major journals are shown in Table 17.1.
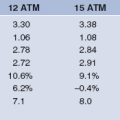
TABLE 17.1 RESULTS OF MAJOR CLINICAL TRIALS WITH GAMMA AND BETA RADIATION PUBLISHED IN PEER REVIEWED JOURNALS, ARRANGED IN CHRONOLOGICAL ORDER
Clinical trials of gamma radiation
The first study of intracoronary radiation in human coronary arteries was conducted in 1994 by Condado et al. in which 21 patients (22 lesions – two-thirds being de novo lesions) were treated with 192Ir after routine balloon angioplasty. On angiographic follow up at six months, a binary restenosis rate of 28.6% was reported,23 which remained the same at five years. Angiographic complications included four aneurysms (two procedure related and two occurring within three months). At three and five years, all aneurysms except one remained unchanged and no other angiographic complications were observed.24
Gamma radiation for in-stent restenosis
SCRIPPS trials
Scripps Coronary Radiation to Inhibit Proliferation Post-Stenting (SCRIPPS) was the first randomized trial to evaluate the safety and efficacy of intracoronary γ radiation as adjunctive therapy to stents. Follow up at six months and three years showed significantly lower restenosis rates in the 192Ir group − 17% and 33%, respectively, compared to placebo (54% and 63%). A subgroup analysis of the 35 patients enrolled due to ISR showed a 70% reduction in the recurrence rate in the irradiated group compared to placebo.25,26 There were no evident clinical complications resulting from the radiation treatment, and clinical benefits were maintained at five years, with a significant reduction in the need for target lesion revascularization (TLR).27
Washington Radiation for In-Stent restenosis Trial (WRIST) series
Original WRIST was the first study to evaluate the effectiveness of radiation therapy in patients with ISR. In this study, 130 patients (100 with native coronaries and 30 with saphenous vein grafts) with ISR lesions (up to 47 mm in length) were randomized to receive either 192Ir or placebo. At six months, the radiation group showed a reduction in restenosis (19% vs. 58% in placebo) and 79% and 63% reduction in the need for revascularization and MACE, respectively, compared to placebo.28 Extended follow up of these patients showed durable beneficial effect of radiation at one year, three years29 and five years30 in MACE rates compared to placebo. MACE rates were significantly lower at five years follow up, albeit at the expense of repeat revascularization procedures suggesting that radiation may delay, in part, the biological processes and that a late catch-up phenomena or late thrombosis will reduce the long-term benefit of radiation.
Other landmark trials in this series were SVG-WRIST which evaluated the effect of radiation therapy in patients with diffuse ISR lesions in saphenous vein grafts,31 Long WRIST in patients with diffuse ISR in native coronary arteries (lesion length 36 to 80 mm),32 Long WRIST High Dose which tested the efficacy of an 18 Gy dose of radiation, WRIST Plus and WRIST 12 which tested the efficacy of prolonged Clopidogrel therapy (up to 6 months and 12 months, respectively) to reduce the incidence of late thrombosis, and WRIST 21 which tested whether escalation of radiation dose to 21 Gy would improve the clinical outcomes beyond Long WRIST High Dose. These studies have demonstrated superiority of radiation therapy in the treatment of ISR in vein graft disease (SVG-WRIST) and diffuse lesions (Long WRIST). The Long WRIST High Dose registry showed that a 3 Gy increase in the dose, from 15 to 18 Gy, provided additional reduction in MACE rates.33 The strategy of prolonged antiplatelet therapy for six months in WRIST Plus reduced thrombosis rates from 9.6% to 2.5% – levels comparable to non-irradiated controls.34 WRIST 12 has demonstrated further reduction in MACE and TLR with 12 months of Clopidogrel therapy.35 Based on these observations, it has become standard practice to provide at least 12 months of Clopidogrel therapy for patients undergoing radiation therapy for ISR. Further escalation of dose from 18 Gy to 21 Gy was studied in WRIST 21 but has not been shown to improve the results. Hence, a dose of 18 Gy may be sufficient to treat ISR with γ radiation.
GAMMA trials
GAMMA-1, a multicenter, randomized trial conducted with IVUS-guided dosimetry, showed significant reductions in the in-stent and in-lesion restenosis rates compared to 50.5% and 55.3% in the control group. The greatest benefit was obtained in patients with long lesions and/or diabetes. In this study, it was observed that the late thrombosis phenomenon was more frequent in patients treated with radiation therapy than with placebo (5.3% vs. 0.8%). All patients in the 192Ir group who presented with late thrombosis had new stents placed within the in-stent target lesion at the time of the procedure.36 This trial demonstrated the efficacy of intracoronary gamma radiation for the prevention of ISR recurrence, and increased awareness regarding the correlation between late thrombosis and an increased risk of myocardial infarction (MI).
Gamma-2, a registry of 125 patients including complex lesions such as calcific lesions requiring rotablation, used a fixed dose of 14 Gy at 2 mm from the center of the source and showed a reduction of 52% and 40% in in-stent and in-lesion restenosis, respectively, and a reduction of 48% and 36% in TLR rates and MACE rates, respectively.37
ARTISTIC I and II
Angiorad Radiation Technology for In-Stent restenosis Trial In native Coronaries (ARTISTIC) examined the usage of the AngioRad system in patients with ISR in native coronary arteries. At three years, the cumulative MACE rate was 24.1% in placebo patients and 22.8% in the AngioRad group.38 ARTISTIC II used the same system in 236 patients and tested the efficacy as measured by a composite clinical end-point at nine months after radiation. These results were compared to the historical control group of 104 patients from the ARTISTIC I and WRIST studies. The Kaplan-Meier estimate of freedom from target vessel failure (TVF) for the placebo group was 52% while in the irradiated group it was 85.5%.39
Beta Radiation for ISR
BETA WRIST
In this study, beta radiation was shown to be effective in the treatment of ISR in 50 patients. These patients demonstrated a 58% reduction in the rate of TLR and a 53% reduction in TVR at six months compared to the historical control group of WRIST.40 The clinical benefit was maintained at two-year follow up with a reduction in TLR (42 % vs. 66%), TVR (46% vs.72%), and MACE (46 % vs. 72%) compared to placebo. This study showed that the efficacy of beta and gamma emitters for the treatment of ISR appeared similar at longer-term follow up.
START and START 40/20
In the START (Stents And Radiation Therapy) Trial, 476 patients were randomized to either placebo or an active radiation train 30 mm in length using the BetaCath system.41 Late thrombosis as a complication of brachytherapy was first recognized during this trial and antiplatelet therapy was prolonged to at least 90 days.
During multiple studies of radiation, including START, the medical community became aware of the mismatch between the interventional injury length and radiation length – the so-called ‘geographic miss’ phenomenon – with the potential to compromise clinical outcome.42
INHIBIT and GALILEO INHIBIT
INHIBIT (Intimal Hyperplasia Inhibition with Beta In-stent Trial) examined the efficacy of the Galileo system for the treatment of ISR in 332 patients.43
At nine months, 32P significantly reduced rates of TLR and MACE. Tandem positioning to cover diffuse lesions >22 mm with 32P was safe and effective. Galileo INHIBIT was an international, multicentered registry of 120 patients with ISR in which 32P was delivered at 20 Gy at 1 mm into the vessel wall. There was a reduction in the primary clinical end point defined as MACE-TLR by 49% and reduction in angiographic endpoint of binary restenosis by 74% in the stented segment and by 27% in the analysis segment.44 The thrombosis rate was low, 1.5%, and similar to the control group, 1.2%.
Balloon catheter-based beta radiation Trials for ISR
BRITE, BRITE-II, 4R, and CURE
Beta radiation using the Radiance system was administered in 32 patients in the feasibility study called BRITE (Beta Radiation to prevent In-sTent rEstenosis). Seventy percent of the dose was administered when the balloon was inflated. At six months, TVR (3%), MACE (3%), and in-stent binary restenosis rates (0%) were the lowest reported to-date in any vascular brachytherapy series.45 The BRITE II study evaluated the efficacy of beta radiation using the RDX system in 429 patients randomized to either radiation (n=321) or placebo (n=108). The RDX system demonstrated safety characterized by high technical success rates (>95%), low periprocedural complications (<1%), and low 30-day MACE (<1%). The most prevalent location of restenosis was within the radiated vessel outside the injured zone despite lower rates of geographic miss (8.5%).46 The RDX system demonstrated a very low ISR rate (10. 9% vs. 46.1%) and proved to optimize the results when compared to historical studies.
4R, a South Korean registry evaluated β-radiation therapy with 188Re-MAG3-filled balloon following rotational atherectomy for diffuse ISR in 50 patients. The mean dose was 15 Gy and the mean irradiation time was 201.8 ± 61.7 seconds. No adverse events occurred during the follow up period. The six-month binary angiographic restenosis rate was 10.4%. Two potential limitations of this technology included reduced dosing at the balloon margins (edge effect) and the risks of balloon rupture with radiation spill. In the event of balloon rupture using 188Re, concomitant administration of potassium perchlorate may mitigate thyroid uptake.47
The Columbia University Restenosis Elimination (CURE) study evaluated liquid 188Re injected into a perfusion balloon. Thirty patients were treated with balloon alone and 30 patients were stented (with subsequent 188Re therapy). The delivered dose was 20 Gy to the balloon surface with a dwell time of 6.9 ± 2.2 minutes. At 12 months follow up in the first 37 patients, the rate of TLR-free survival was 75%.48
RENO and BRIE
Registry Novoste (RENO) is a registry of 1098 consecutive patients using the Novoste BetaCath system. Six-month follow up data showed non-occlusive restenosis in 18.8% of patients, total occlusion in 5.7%, and a MACE rate of 18.7% (1.9% deaths from any cause, 2.6% from acute MI, 13.3% from TVR by PCI and 3.3% from TVR by CABG).49 The Beta Radiation in Europe (BRIE) study evaluated the safety and efficacy of the BetaCath system in patients with up to two discrete lesions, de novo and restenotic, in different vessels. The binary in-stent restenosis was 9.9%, excluding total occlusions, was 4.9%.50 This study highlights the full potential of brachytherapy, provided late total occlusions are minimized by prolonged antiplatelet therapy.
Tungsten WRIST
In a feasibility study using Tungsten (199W) wire dose range (18–25 Gy), in 30 patients with ISR in native coronaries, Waksman et al. have shown the safety and comparable results to other beta and gamma sources.51
Beta radiation for de novo lesions
Several clinical studies were undertaken to test the effectiveness of beta radiation for de novo lesions concurrent to the trials of ISR. Important early studies included the Geneva trial,52 the dose-finding study BERT (Beta Energy Restenosis Trial) which used 90Y,53 and the Proliferation REeduction with Vascular ENergy Trial (PREVENT)54 that utilized 32P.
BERT
BERT was a feasibility study of 23 patients with de novo lesions using the Novoste system.53 No complications or adverse events were noticed at 30 days and at nine months follow up. Three patients underwent repeat revascularization to the target lesion. The Canadian arm of this study included 30 patients, and at six months follow up, the angiographic restenosis rate was 10% with negative late loss and late loss index.55 The European arm of BERT (BERT 1.5) was conducted at the Thoraxcenter in Rotterdam, The Netherlands in an additional 30 patients who were treated successfully with balloon angioplasty. Angiographic restenosis in this cohort was higher than reported in the US and Canadian trials. Overall, the restenosis rate based on 64 of the 80 patients in the BERT series is 17%, and the late loss and late loss indexes were below 5%. Over 50% of the patients had larger minimal lumen diameter (MLD) at follow up compared to control, and a sub study of IVUS demonstrated vessel remodeling at the irradiated site.56
PREVENT
PREVENT was a randomized study of 105 patients with de novo (70%) or restenotic (30%) lesions. Angiographic restenosis at six months was 8.2% (target site) and 22.4% (target site plus adjacent segments) compared to 39.1% and 50.0% in the control group, respectively.54 The one-year MACE (death, MI, and TLR) was less in radiation group though not statistically significant. The occurrence of MI due to thrombotic events after discharge occurred in seven patients who received radiation therapy and in none in the control group.
DOSE-FINDING STUDY GROUP
This was the only study which tested optimization of dose in a randomized fashion to 9, 12, 15, and 18 Gy using Yttrium-90 in 183 patients. Stenting after radiation was required in 47% of patients because of residual stenosis or major dissection. At six-month angiographic follow up, a significant dose-dependent benefit was evident. Thrombosis or late occlusion of the target vessel occurred in 3.3% of patients who were treated with only balloon angioplasty and in 14.3% patients who received new stents. This study demonstrated a marked reduction in restenosis in non-stented arteries after administration of 18 Gy of beta radiation, especially in patients who underwent plain balloon angioplasty, suggesting that beta radiation therapy should be evaluated as an adjunctive to PTCA.57
BETA-CATH
In this large randomized study, 1455 patients with sub optimal results after balloon angioplasty were treated with a stent and then assigned to 90Sr/Y or placebo treatment.58 In patients treated with balloon angioplasty alone, radiated patients (n=264) tended to have lower TVF (14.2%) compared to placebo patients (20.4%, n=240). In the 452 patients treated with extended antiplatelet therapy who received a new stent, there were no differences in the 240-day TVF rate. Comparison of placebo and radiation treatment from pooled PTCA and stent groups (antiplatelet therapy ≥60 days) showed similar eight-month TLR and TVF-MACE.
SVG BRITE
This study examined the RDX system using a 32P emitter at a dose of 20 Gy at 1 mm from the balloon surface in saphenous vein grafts of 49 patients; 24 of whom were treated for de novo lesions. New stents were implanted in most patients with de novo lesions. The outcome of the patients with de novo lesions was encouraging with only 8% TVR and 12% angiographic restenosis rate.59
ECRIS
EndoCoronary-Rhenium-Irradiation-Study (ECRIS) was a randomized trial in which 225 patients (71% de novo lesions) were randomly assigned to receive β-irradiation using 188Re-filled balloon catheter or no additional intervention. Clinical and procedural data did not differ between the groups except that there was a higher rate of stenting in the control group (63%) compared with the rhenium-188 group (45%). After six months of follow up, late loss was significantly lower in the irradiated group compared with the control group, both of the target lesion (0.11±0.54 versus 0.69±0.81 mm) and of the total segment (0.22±0.67 versus 0.70±0.82 mm). This was also evident in the subgroup of patients with de novo lesions and independent from stenting. Binary restenosis rates and TVR were significantly lower after rhenium-188 brachytherapy compared with the control group.60
BRIDGE
The BRIDGE study is a multicenter, randomized, controlled trial of 112 patients which evaluated the acute and long-term efficacy of intravascular brachytherapy with 32P (20 Gy at 1 mm in the coronary wall) immediately following direct stenting in de novo lesions. TVR and MACE rates at one year in the irradiated group (20.4% and 25.9%, respectively) were higher than in the control group (12.1% and 17.2%, respectively).61
Clinical trials with radioactive emitting stents
In contrast to brachytherapy using removable sources, radioactive stents (permanent implants) have beta emitting atoms (32P) on the stent surface and emit beta particles that are absorbed by neointimal tissue to a depth of 1–2 mm into the vessel wall over longer periods of time. The first clinical trials included IRIS, the Milan dose-finding study, and the Vienna experience. In IRIS (Isostent for Restenosis Intervention Study) pilot trials, 32P radioactive Palmaz-Schatz stents were developed with varying radioactivities and were implanted with a high rate of technical success but limited by an angiographic restenosis rate (stent and edges) of 40% at six months follow up.62 The Milan dose-finding study showed that activities >3 μCi (32P stents 0.75–12 μCi) profoundly inhibited intimal hyperplasia, however restenosis still occurred in >40% of lesions at the stent edges, that is, the ‘candy wrapper’ effect.63,64 These findings were replicated in the Vienna study using high activity (6–21 μCi) stents.65 An effort to circumvent edge restenosis by ‘hot’ and ‘cold’ end stents failed to prevent edge effect with an angiographic restenosis rate of 33% in 56 implanted stents.66 In summary, while radioactive stents effectively inhibit intrastent neointimal hyperplasia, the problem of edge restenosis has not been solved with either less aggressive stent implantation or modification of the stent ends. Currently there is no indication for their clinical use.
Beta versus gamma brachytherapy
While contemporaneous clinical trials to-date have demonstrated comparability of clinical efficacy of beta and gamma brachytherapy,67 beta systems have several inherent advantages, such as reduced radiation exposure to operators and catheterization laboratory personnel, less shielding requirements, and easier handling of sources that have lead to more widespread acceptance of this technology. Potential limitations of beta sources in larger vessels (for example, vein grafts, peripheral arteries and renal access grafts) may be offset by the use of sources with higher activity, centering mechanisms, and appropriately longer dwell times.
Restenosis in the peripheral arterial system
Restenosis following PTA is mainly seen in small and medium peripheral arteries, such as the saphenous femoral-popliteal arteries (SFA) and renal arteries, with diffuse atherosclerotic disease. The mechanisms for a high rate of recurrence after intervention in the peripheral arterial system (PAS) are mainly attributed to exuberant healing response with smooth muscle proliferation,68,69 early and late recoil after balloon angioplasty,70,71 mechanical problems with stents, such as stent fractures and crushing, in-stent restenosis, and aggressive progression of the atherosclerotic disease.
Clinical trials
Superficial femoral artery
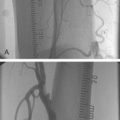
Liermann and Schopohl were the first to perform VBT for the treatment of in-stent restenosis in the peripheral arteries. Known as the Frankfurt Experience, this pilot study was conducted in 30 patients with ISR in their SFAs.72–75 Patients underwent atherectomy and PTA followed by endovascular radiation using the MicroSelectron HDR afterloader and a noncentering catheter with Ir-192. No adverse effects from the radiation treatment were reported at up to seven-year follow up. The five-year patency rate of the target vessel was 82%, with only 11% stenosis within the treated segment reported. Late total occlusion developed in 7% of the treated vessels after 37 months.
The Vienna experience
Vienna I was a pilot study with an indication of radiation safety after PTA that showed only 60% patency at one year.76 The Vienna II trial had 113 patients with de novo or recurrent femoropopliteal lesions who were randomized to PTA + brachytherapy (n=57) or PTA alone (n=56). The primary end point of cumulative patency rates at 12-month follow up was higher in the PTA + brachytherapy group (63.6%) compared to the PTA group (35.3%). The patients from this study were followed-up to 36 months and demonstrated durability of the results.77 In Vienna III, a centering catheter that was used for the same patient population with a dose of 18 Gy showed a restenosis rate of 23.4% in the irradiated group compared to 53.3% in the placebo arm.78 Vienna IV was a pilot study examining radiation with stenting of the SFA; and Vienna V was a randomized study for similar indications. Both Vienna IV and V demonstrated an increase rate of subacute and late thrombosis when stents were combined with radiation, with up to 16.7% in the radiation group versus 4.3% in the control stenting without radiation. Once thrombosis was controlled, the radiation group had less restenosis.79
The PARIS trials
The Paris Radiation Investigational Study (PARIS) is the first FDA-approved, multicenter, randomized, double-blind, control study involving 300 patients following PTA to SFA stenosis using a gamma radiation 192-Ir source. Utilizing the MicroSelectron HDR afterloader, a treatment dose of 14 Gy is delivered via a centered segmented end-lumen balloon catheter. The primary objectives of this study are to determine angiographic evidence of patency and a reduction of >30% of the restenosis rate of the treated lesion at six months. A secondary endpoint is to determine the clinical patency at 6 and 12 months by treadmill exercise and by the ankle-brachial index (ABI). In the feasibility phase of PARIS, 40 patients with claudication were enrolled. The mean lesion length was 9.9±3.0 cm with a mean reference vessel diameter of 5.4±0.5 mm. The six-month angiographic follow up was completed on 30 patients; 13.3% of them had evidence of clinical restenosis.80
Due to poor enrolment, only 203 patients with claudication and femoropopliteal disease were enrolled in the study. After successful PTA, a segmented centering balloon catheter was positioned to cover the PTA site. The patients were transported to the radiation oncology suite and randomized to receive either radiation therapy using the MicroSelectron HDR afterloader with Ir-192 at a dose of 14 Gy at 2 mm into the vessel wall (105 patients), or treatment with a sham control in 98 patients. Patients were followed for 12 months, with clinic visits at 1, 6, and 12 months and follow up angiography at 12 months. The restenosis rate at follow up was similar in both groups (28.6% brachytherapy vs. 27.5% placebo). There was no significant difference in MLD, late loss, or the number of total occlusions. Exercise ABI, resting ABI, and maximum walking time were not different between treatment groups. For patients older than 65 years, maximum walking times at 6 and 12 months were better in the brachytherapy group. In the subgroups of patients with diabetes, male patients, or patients receiving Clopidogrel or who have a proximal/medial lesion, maximum walking time in the brachytherapy group was better than in the placebo at 6 months but not different at 12 months.
More studies to support the effectiveness of gamma radiation for in-stent restenosis were recently published by Krueger et al.81 In this study, 30 patients who underwent PTA for de novo femoropopliteal stenoses were randomly assigned to undergo 14 Gy centered endovascular irradiation (irradiation group, n=15) or no irradiation (control group, n=15). Intra-arterial angiography was performed 6, 12, and 24 months after treatment; and duplex ultrasonography was performed the day before and after PTA and at 1, 3, 6, 9, 12, 18, and 24 months later. Baseline characteristics did not differ significantly between the two groups. Mean absolute individual changes in degree of stenosis, compared with the degrees of stenosis shortly after PTA in the irradiation group versus in the control group were 10.6% ± 22.3 versus 39.6% ± 24.6 (P < .001) at 6 months, 2.0% ± 34.2 versus 40.6% ± 32.6 (P =.002) at 12 months, and 7.4% ± 43.2 versus 37.7% ± 34.5 (p= .043) at 24 months. The rates of target lesion restenosis at six months (p=.006) and 12 months (p=.042) were significantly lower in the irradiation group. The authors concluded that endovascular radiation was effective for patients who were treated with angioplasty for de novo femoropopliteal lesions.
Restenostic lesions and VBT
The effectiveness of VBT for restenotic SFA lesions was examined in another randomized study reported by Zehnder et al. In this study, gamma radiation was used at a dose of 12 Gy. The primary endpoint was >50% restenosis at 12 months assessed by duplex Doppler. The recurrence rate in the radiation arm was 23% versus 42% in the PTA alone group.82 This study demonstrated that VBT can be effective in restenotic lesions.
Brachytherapy and Probucol
In another randomized four-arm study for patients with PTA lesions, patients were randomized to VBT, VBT and probucol, probucol alone, or placebo. The recurrence rate in the radiation arm alone was 17%, VBT and probucol was 20%, probucol alone was 27%, and the placebo group was 42%. This study confirms prior observations regarding the effectiveness of VBT for the treatment of SFA lesions without additional benefit of probucol when compared to PTA alone.83
Drug-eluting stents and brachytherapy
Evidence is favorable for the efficacy of DES to reduce restenosis in de novo lesions and experience is growing with other lesions including in-stent restenosis of BMS. In the randomized ISAR-DESIRE study, Kastrati et al. reported restenosis rates of 14.3% with sirolimus-eluting stents and 21.7% with paclitaxel-eluting stents.84 Recent randomized and non randomized studies comparing vascular brachytherapy and DES confer that DES are showing similar safety and efficacy profile when compared to vascular brachytherapy. In the SISR trial there was reduction in target lesion revascularization in the DES group compared with VBT although this study borrowed patients from the historical Gamma 1 and Gamma 2 it appears that DES are effective as vascular brachytherapy even for diffuse ISR lesions.85,86 Thus the role of vascular brachytherapy in the coronary arteries remained for the treatment of DES restenosis. The RESCUE (Radiation for drug Eluting Stent in Coronary FailUrE) study was an international registry of patients who present with ISR of a previously placed coronary DES and assigned to treatment with either beta or gamma radiation following PCI. Eight months follow up data in 61 patients showed 6 (10%) MACE and similar TLR and TVR.87
1 Waksman R, Rodriquez JC, Robinson KA, et al. Effect of intravascular irradiation on cell proliferation, apoptosis and vascular remodeling after balloon overstretch injury of porcine coronary arteries. Circulation. 1997;96:1944-1952.
2 Rubin P, Williams JP, Riggs PN, et al. Cellular and molecular mechanisms of radiation inhibition of restenosis. Part I: role of the macrophage and platelet-derived growth factor. Int J Radiat Oncol Biol Phys. 1998;40:929-941.
3 Wang H, Griendling KK, Scott NA, et al. Intravascular radiation inhibits cell proliferation and vascular remodeling after angioplasty by increasing the expression of p21 in adventitial myofibroblasts. Circulation. 1999;100:I-700.
4 Schwartz RS, Koval TM, Edwards WD, et al. Effect of external beam irradiation on neointimal hyperplasia after experimental coronary artery injury. J Am Coll of Cardiol. 1992;19:1106-1113.
5 Waksman R, Robinson KA, Crocker IR, et al. Endovascular low-dose irradiation inhibits neointima formation after coronary artery balloon injury in swine. A possible role for radiation therapy in restenosis prevention. Circulation. 1995;91:1533-1539.
6 Waksman R, Robinson KA, Crocker IR, et al. Intracoronary radiation before stent implantation inhibits neointima formation in stented porcine coronary arteries. Circulation. 1995;92:1383-1386.
7 Wiedermann JG, Marboe C, Amols H, et al. Intracoronary irradiation markedly reduces restenosis after balloon angioplasty in a porcine model. J Am Coll Cardiol. 1994;23:1491-1498.
8 Wiedermann JG, Marboe C, Amols H, et al. Intracoronary irradiation markedly reduces neointimal proliferation after balloon angioplasty in swine: persistent benefit at 6-months follow up. J Am Coll Cardiol. 1995;25:1451-1456.
9 Mazur W, Ali MN, Khan MM, et al. High dose rate intracoronary radiation for inhibition of neointimal formation in the stented and balloon injured porcine models of restenosis: angiographic, morphometric and histopathological analyses. Int J Radiot Oncol Biol Phy. 1996;36:777-788.
10 Wiedermann JG, Leavy JA, Amols H, et al. Effects of high dose intracoronary irradiation on vasomotor function and smooth muscle histopathology. Am J Physiol. 1994;267:H125-H132. (Heart Circ Physiol.36)
11 Verin V, Popowski Y, Urban P, et al. Intra-arterial beta irradiation prevents neointimal hyperplasia in a hypercholesterolemic rabbit restenosis model. Circulation. 1995;92:2284-2290.
12 Waksman R, Robinson KA, Crocker IR, et al. Intracoronary low-dose beta-irradiation inhibits neointima formation after coronary artery balloon injury in the swine restenosis model. Circulation. 1995;92:3025-3031.
13 Fischell TA, Kharma BK, Fischell DR, et al. Low dose beta particle emission from stent wire results in complete localized inhibition of smooth muscle cell proliferation. Circulation. 1994;90:2956-2963.
14 Hehrlein C, Kniser S, Kollum M, et al. Effects of very low dose endovascular irradiation via an activated guidewire on neointima formation after stent implantation. Circulation. 1995;92:I-69.
15 Hehrlein C, Gollan C, Donges K, et al. Low dose radioactive endovascular stent prevent smooth muscle cell proliferation and neointimal hyperplasia in rabbits. Circulation. 1995;92:1570-1575.
16 Laird JR, Carter AJ, Kufs WM, et al. Inhibition of neointimal proliferation with low-dose irradiation from a beta-particle-emitting stent. Circulation. 1996;93:529-536.
17 Carter AJ, Laird JR, Bailey LR, et al. Effects of endovascular radiation from a beta-particle-emitting stent in a porcine coronary restenosis model. A dose-response study. Circulation. 1996;94:2364-2368.
18 Waksman R, Chan RC, Vodovotz Y, et al. Radioactive 133-xenon gas-filled angioplasty balloon: A novel intracoronary radiation system to prevent restenosis. J Am Coll Cardiol. 1998;31:356A.
19 Weinberger J. Waksman R, Serruys P, editors. Solution-applied beta emitting radioisotope (SABER) system: Handbook of Vascular Brachytherapy, 1st Ed, London: Martin Dunitz Ltd, 1998.
20 Robinson KA, Pipes DW, Bibber RV, et al. Dose response evaluation in balloon injured pig coronary arteries of a beta emitting 186Re liquid filled balloon catheter system for endovascular brachytherapy. Advances in Cardiovascular Radiation Therapy II, Washington DC, 1998, March 8–10.
21 Makkar R, Whiting J, Li A, Cordero H, et al. A beta-emitting liquid isotope filled balloon markedly inhibits restenosis in stented porcine coronary arteries. J Am Coll Cardiol. 1998;31:350A.
22 Kim HS, Cho YS, Kim JS, et al. Effect of transcatheter endovascular holmium-166 irradiation on neointimal formation after balloon injury in porcine coronary artery. J Am Coll Cardiol. 1998;31:277A.
23 Condado JA, Waksman R, Gurdiel O, et al. Long-term angiographic and clinical outcome after percutaneous transluminal coronary angioplasty and intracoronary radiation therapy in humans. Circulation. 1997;96:727-732.
24 Condado JA, Waksman R, Saucedo JF, et al. Five-year clinical and angiographic follow up after intracoronary iridium-192 radiation therapy. Cardiovasc Radiat Med. 2002;3:74-81.
25 Teirstein PS, Massullo V, Jani S, et al. Catheter-based radiotherapy to inhibit restenosis after coronary stenting. N Engl J Med. 1997;336:1697-7703.
26 Teirstein PS, Massullo V, Jani S, et al. Two-year follow up after catheter-based radiotherapy to inhibit coronary restenosis. Circulation. 1999;99:243-247.
27 Grise MA, Massullo V, Jani S, et al. Five-Year Clinical Follow Up After Intracoronary Radiation, Results of a Randomized Clinical Trial. Circulation. 2002;105:2737-2740.
28 Waksman R, White RL, Chan RC, et al. Intracoronary radiation therapy for patients with in-stent restenosis: 6-month follow up of a randomized clinical study. Circulation. 1998;98:17. I-651:3421.
29 Ajani AE, Waksman R, Sharma AK, et al. Three-year follow up after intracoronary gamma radiation therapy for in-stent restenosis. Original WRIST. Washington Radiation for In-Stent Restenosis Trial. Cardiovasc Radiat Med. 2001;2:200-204.
30 Waksman R, Ajani AE, White RL, et al. Five-Year Follow Up After Intracoronary Gamma Radiation Therapy for In-Stent Restenosis. Circulation. 2004;109:340-344.
31 Waksman R, Ajani AE, White RL, et al. Intravascular Gamma Radiation For In-Stent Restenosis In Saphenous-Vein Bypass Grafts. N Engl J Med. 2002;346:1194-1199.
32 Waksman R, Cheneau E, Ajani AE, et al. Intracoronary Radiation Therapy Improves the Clinical and Angiographic Outcomes of Diffuse In-Stent Restenotic Lesions Results of the Washington Radiation for In-Stent Restenosis Trial for Long Lesions (Long WRIST) Studies. Circulation. 2003;107:1744-1749.
33 Javed MH, Mintz GS, Waksman R, et al. Serial intravascular ultrasound assessment of the efficacy of intracoronary y radiation therapy for preventing recurrence of very long, diffuse, in-stent restenosis lesions. Circulation. 2000;104:856-859.
34 Waksman R, Ajani AE, White RL, et al. Prolonged antiplatelet therapy to prevent late thrombosis after intracoronary gamma-radiation in patients with in-stent restenosis: Washington Radiation for In-Stent Restenosis Trial plus 6 months of clopidogrel (WRIST PLUS). Circulation. 2001;103:2332-2335.
35 Waksman R, Ajani AE, Pinnow E, et al. Twelve Versus Six Months of Clopidogrel to Reduce Major Cardiac Events in Patients Undergoing γ-Radiation Therapy for In-Stent Restenosis. Washington Radiation for In-Stent restenosis Trial (WRIST) 12 Versus WRIST PLUS. Circulation. 2002;106:776-778.
36 Leon MB, Teirstein PS, Moses JW, et al. Localized intracoronary gamma-radiation therapy to inhibit the recurrence of restenosis after stenting. N Engl J Med. 2001;344:250-256.
36 Kim HS, Ajani AE, Waksman R. Vascular brachytherapy for in-stent restenosis. J Interv Cardiol. 2000;13:417-423.
37 Waksman R, Bhargava B, Chan RC, et al. Intracoronary radiation with gamma wire inhibits recurrent in-stent restenosis. Cardiovasc Radiat Med. 2001;2:63-68.
38 Kereikas DJ, Waksman R, Mehra A, et al. Multi-center experience with a novel Ir-192 vascular brachytherapy device for in-stent restenosis: final results of the AngioRad™ T radiation therapy for in-stent restenosis intracoronaries II (ARTISTIC II) trial. J Am Coll Cardiol. 2003;41:49A.
39 Waksman R, White RL, Chan RC, et al. Intracoronary beta radiation therapy inhibits recurrence of in-stent restenosis. Circulation. 2000;101:1895-1898.
40 Popma JJ, Suntharalingam M, Lansky AJ. Randomized trial of 90Sr/90Y beta-radiation versus placebo control for treatment of in-stent Restenosis. Circulation. 2002;106:1090-1096.
41 Kim HS, Waksman R, Cottin Y, et al. Edge stenosis and geographical miss following intracoronary gamma radiation therapy for in-stent restenosis. J Am Coll Cardiol. 2001;15:1026-1030.
42 Waksman R, Raizner AE, Yeung AC, et al. Use of localized intracoronary beta radiation in treatment of in-stent restenosis: the INHIBIT randomized controlled trial. Lancet. 2002;359:551-557.
43 Waksman R on behalf of the Galileo INHIBIT and INHIBIT investigators. Manual stepping of 32P fl-emitter for diffuse in-stent restenosis lesions, tandem versus single position. Clinical and Angiographic Outcome from a Multicenter Randomized study. Presented at AHA 2001.
44 Waksman R, Buchbinder M, Reisman M, et al. Balloon-Based Radiation Therapy for Treatment of In-Stent Restenosis in Human Coronary Arteries: Results From the BRITE I Study. Cathet Cardiovascular Intervent. 2002;57:286-294.
45 Waksman R on behalf of the BRITE II investigators. Balloon based radiation for coronary in-stent restenosis: 9 months results from the BRITE II study. Presented at ACC 2003.
46 Park S-W, Kong M-K, Moon DH, et al. Treatment of diffuse in-stent restenosis with rotational atherectomy followed by radiation therapy with a rhenium-188-mercaptoacetyltriglycine-filled balloon. J Am Coll Cardiol. 2001;38:631-637.
47 Weinberger J, Giedd KN, Simon AD, et al. Radioactive beta-emitting solution filled balloon treatment prevents porcine coronary restenosis. Cardiovasc Radiat Med. 1999;1:252-256.
48 Urban P, Serruys P, Baumgart D, et al. A multicentre European registry of intraluminal coronary beta brachytherapy. Eur Heart J. 2003;24:604-612.
49 Serruys PW, Sianos G, van der Giessen W, et al. Intracoronary β-radiation to reduce restenosis after balloon angioplasty and stenting. The Beta Radiation in Europe (BRIE) Study. Eu Heart Journal. 2002;23:1351-1359.
50 Waksman R, Ajani AE, Dilcher CE, et al. Intracoronary Radiation Therapy Using a Novel Beta Emitter for In-Stent Restenosis: Tungsten WRIST. J Am Coll Cardiol. 2004;43:A90.
51 Verin V, Urban P, Popowski Y, et al. Feasibility of intracoronary beta-irradiation to reduce restenosis after balloon angioplasty. A clinical pilot study. Circulation. 1997;95:1138-1144.
52 King SBIII, Williams DO, Chougule P, et al. Endovascular beta-radiation to reduce restenosis after coronary balloon angioplasty. Results of the beta energy restenosis trial (BERT). Circulation. 1998;97:2025-2030.
53 Raizner AE, Oesterle SN, Waksman R, et al. Inhibition of restenosis with β-emitting radiotherapy report of the proliferation reduction with vascular energy trial (PREVENT). Circulation. 2000;102:951-958.
54 Bonan R, Arsenault A, Tardif JC, et al. Beta Energy Restenosis trials, Canadian Arm. Circulation. 1997;96:I-219.
55 Gijzel AL, Wardeh AJ, van der Giessen WJ, et al. B-energy to prevent restnosis: the Rotterdam contribution to the BERT 1.5 trial — 1 yr. Follow up. Eur Heart J. 370, 1999. (abstract 1945).
56 Verin V, Popowski Y, deBruyne B, et al. Endoluminal beta-radiation therapy for the prevention of coronary restenosis after balloon angioplasty. N Engl J Med. 2001;344:243-249.
57 Results from Late-breaking clinical trails sessions at ACC 2001. J Am Coll Cardiol. 2001;38:595-612.
58 Stone GW, Mehran R, Midei M, et al. Usefulness of beta radiation for de novo and In-Stent restenotic lesions in saphenous vein grafts. J Am Coll Cardiol. 2003;92:312-314.
59 Höher M, Wöhrle J, Wohlfrom M, et al. Intracoronary _-Irradiation with a Rhenium-188-Filled Balloon Catheter A Randomized Trial in Patients with De Novo and Restenotic Lesions. Circulation. 2003;7:3022-3027.
60 Serruys PW, Wijns W, Sianos G, et al. Direct stenting versus direct stenting followed by centered beta-radiation with IVUS-guided dosimetry and long-term antiplatelet treatment; results of a randomized trial (the BRIDGE trial). J Am Coll Cardiol. 2004;44:528-537.
61 Moses J. IRIS trials low activity 32P stent. Advances in cardiovascular radiation therapy III, Washington DC 1999; 387–388 (abstract).
62 Albeiro R, Adamian M, Kobayashi N, et al. Short and intermediate-term results of (32) P radioactive beta emitting stent implantation in patients with coronary artery disease: The Milan Dose-Response Study. Circulation. 2000;101:18-26.
63 Albiero R, Nishida T, Adamian M, et al. Edge restenosis after implantation of high activity (32)P radioactive beta-emitting stents. Circulation. 2000;101:2454-2457.
64 Wexberg P, Siostrzonek P, Kirisits C, et al. High activity radioactive BX-stents for reduction of restenosis after coronary interventions: the Vienna P-32 dose response study. Circulation. 1999;100:I-156.
65 Wardeh A, Wijns W, Albiero R, et al. Angiographic follow up after 32-P beta emitting radioactive “cold ends” Isostent implantation. Results from Aalst, Milan and Rotterdam. Circulation. 2000;102:II-442. (abstract).
66 Shirai K, Lansky AJ, Mintz GS, et al. Comparison of the angiographic outcomes after beta versus gamma vascular brachytherapy for treatment of in-stent restenosis. Am J Cardiol. 2003;92:1409-1413.
67 Haude M, Erbel R, Issa H, et al. Quantitative analysis of elastic recoil after balloon angioplasty and after intracoronary implantation of balloon-expandable Palmaz-Schatz stents. J Am Coll Cardiol. 1993;21:2634.
68 Consigny PM, Bilder GE. Expression and release of smooth muscle cell mitogens in arterial wall after balloon angioplasty. J Vasc Med Biol. 1993;4:1-8.
69 Mintz GS, Popma JJ, Pichard AD, et al. Arterial remodeling after coronary angioplasty. A serial intravascular ultrasound study. Circulation. 1996;94:35-43.
70 Isner JM. Vascular remodeling. Honey, I think I shrunk the artery. Circulation. 1994;89:2937-2941.
71 Liermann DD, Bottcher HD, Kollath J, et al. Prophylactic endovascular radiotherapy to prevent intimal hyperplasia after stent implantation in femoropopliteal arteries. Cardiovasc Intervent Radiol. 1994;17:12-16.
72 Bottcher HD, Schopohl B, Liermann D, et al. Endovascular irradiation-a new method to avoid recurrent stenosis after stent implantation in peripheral arteries: technique and preliminary results. Int J Rad Oncol Biol Phys. 1994;29:183-186.
73 Liermann D, Kirchner J, Schopohl B, et al. Brachytherapy with iridium-192 HDR to prevent restenosis in peripheral arteries: an update. Herz. 1998;23:394-400.
74 Sidawy AN, Weiswasse JM, Waksman R. Peripheral vascular brachytherapy. J Vasc Surg. 2002;35:1041-1047.
75 Minar E, Pokrajac B, Ahmadi R, et al. Brachytherapy for prophylaxis of restenosis after long-segment femoropopliteal angioplasty: pilot study. Radiology. 1998;208:173-179.
76 Minar E, Pokrajac B, Maca T, et al. Endovascular Brachytherapy for Prophylaxis of Restenosis After Femoropopliteal Angioplasty: Results of a Prospective Randomized Study. Circulation. 2000;102:2694-2699.
77 Pokrajac B, Schmid R, Poetter R, et al. Endovascular brachytherapy prevents restenosis after femoropopliteal angioplasty: results of the Vienna-3 multicenter study. Int J Radiat Oncol Biol Phys. 2003;57(suppl):S250.
78 Wolfram RM, Pokrajac B, Ahmadi R, et al. Endovascular brachytherapy for prophylaxis against restenosis after long-segment femoropopliteal placement of stents: initial results. Radiology. 2001;220:724-729.
79 Waksman R, Laird JR, Jurkovitz CT, et al. Intravascular radiation therapy after balloon angioplasty of narrowed femoropopliteal arteries to prevent restenosis: results of the PARIS feasibility clinical trial. J Vasc Interv Radiol. 2001;12:915-921.
80 Krueger K, Zaehringer M, Bendel M, et al. De novo femoropopliteal stenoses: endovascular gamma irradiation following angioplasty — angiographic and clinical follow up in a prospective randomized controlled trial. Radiology. 2004;231:546-554.
81 Zehnder T, von Briel C, Baumgartner I, et al. Endovascular brachytherapy after percutaneous transluminal angioplasty of recurrent femoropopliteal obstructions. J Endovasc Ther. 2003;2:304-311.
82 Greiner, Gallino, Mahler, et al. The effects of probucol and brachytherapy on restenosis after angioplasty of the femoropopliteal arteries a randomized multicenter trial. Submitted for publication 2004.
83 Kastrati A, Mehilli J, Beckerath N, et al. Sirolimus-Eluting Stent or Paclitaxel-Eluting Stent vs Balloon Angioplasty for Prevention of Recurrences in Patients With Coronary In-Stent Restenosis: A Randomized Controlled Trial. JAMA. 2005;293:165-171.
84 Werner GS, Emig U, Krack A, et al. Sirolimus-eluting stents for the prevention of restenosis in a worst-case scenario of diffuse and recurrent in-stent restenosis. Catheter Cardiovasc Interv. 2004;63:259-264.
85 Iofina E, Haager PK, Radke PW, et al. Sirolimus- and paclitaxel-eluting stents in comparison with balloon angioplasty for treatment of in-stent restenosis. Catheter Cardiovasc Interv. 2005;64:28-34.
86 Kuchulakanti P, Torguson R, Canos D, et al. Optimizing Dosimetry with High Dose Intracoronary Gamma Radiation (21 Gy) for Patients with Diffuse. In-Stent Restenosis Cardiovasc Revasc Med. 2005;6:108-112.
87 Torguson R, Sabate M, Deible R, et al. Intravascular Brachytherapy versus drug eluting stents for the treatment of patients with DES Restenosis. Am J Cardiol. 2006;98:1340-1344.