CHAPTER 119 Intestinal Obstruction
SMALL BOWEL OBSTRUCTION
ETIOLOGY
The most common cause of small bowel obstruction (SBO) is intra-abdominal adhesions following laparotomy; this accounts for about three fourths of all cases.1,2 Peritoneal adhesions are common after laparotomy and are exacerbated by intra-abdominal infection, the tissue ischemia attending wound closure (e.g., an anastomosis), external beam radiation, and the presence of foreign material, such as sutures. Although SBO can occur any time after laparotomy, the risk is greatest in the first few postoperative years. Lower abdominal or pelvic operations have a higher risk of adhesive SBO than do upper abdominal procedures, such as cholecystectomy.
Several population-based and large case studies have defined the risk of adhesive obstruction after common abdominal operations. For example, the risk of adhesive SBO after appendectomy is about 1% with 30 years of follow-up; the risk is greater for patients with perforated appendicitis (2.76% at 30 years) than nonperforated appendicitis (0.75% at 30 years).3 The incidence of adhesive SBO after gynecologic operations is similar to that of appendectomy except for cesarean delivery, which has a lower risk of subsequent SBO. Using Medicare data, Beck and colleagues found that the incidence of adhesive obstruction after intestinal resection and anastomosis was 14.3% within two years.4
About one fourth of patients who present with SBO have an etiology other than intraperitoneal adhesions. Of these, the most common causes are Crohn’s disease (7%) (see Chapter 111), intra-abdominal neoplasms (5%), and abdominal wall hernias (2%).2 A comprehensive list of causes of intestinal obstruction is shown in Table 119-1.
| Intrinsic Bowel Lesions |
Abdominal wall hernias (e.g., umbilical) and incisional hernias, as well as inguinal and femoral hernias, are much more common causes of SBO than internal or intra-abdominal hernias (e.g., paraduodenal hernias). Less than 10% of patients presenting to the hospital with SBO have a hernia as the cause of their obstruction,2,5 and up to 30% of patients requiring an operation for SBO have an incarcerated hernia as the etiology of their obstruction.1
Intestinal obstruction caused by a hernia has a particularly high risk of strangulation, failure to resolve spontaneously, and recurrence when it is not surgically corrected. In one study of 877 patients, three fourths of patients presenting with incarcerated hernias and SBO had ischemic bowel at the time of operation; the bowel was necrotic in 27%.1 In contrast, only 29% of patients presenting with SBO due to adhesions had a strangulated obstruction, and only 11% had nonviable bowel.1 The increased risk of obstruction and strangulation is due, at least in part, to the rigid fascial defect through which the herniated intestine passes. Femoral hernias, in particular, pose a high risk of intestinal strangulation. Although SBO from a groin hernia can occur at any age, it is particularly prevalent in the elderly; advanced age, concomitant chronic illnesses, and treatment delay are associated with unfavorable outcomes in this subset of patients presenting with SBO.6
Internal hernias may be congenital (e.g., paraduodenal) or acquired (e.g., hernias through mesenteric defects created in the performance of intestinal anastomoses). The 3% incidence of internal herniation of the Roux limb after gastric bypass for weight loss is a particularly important example of an internal hernia, given the frequency with which this procedure is performed today.7 Congenital and acquired internal hernias are discussed in greater detail in Chapter 24.
Various authors have reported herniation of a portion of the bowel wall (Richter’s hernia) or a whole segment of the intestine through a laparoscopic trocar site with resultant intestinal obstruction.8,9 The incidence of trocar site hernias is 1% to 2%, and intestinal obstruction is significantly less common.8 In these instances, the hernias usually occur at 10-mm port sites positioned at or close to the midline.9 Intestinal obstruction after laparoscopic transabdominal preperitoneal herniorrhaphy usually is due to herniation of the bowel through a defect in the peritoneal closure. In these instances, the bowel is tethered by adhesions between the partially peritoneal-covered prosthesis and the intestine, with formation of a kink or a point of torsion.
Neoplasms are a relatively unusual cause of SBO, accounting for less than 10% of cases2,10; adenocarcinoma accounts for more than 50% of instances of colonic obstruction by neolasms. In more than 90% of such neoplastic SBO, the small intestine becomes obstructed by extrinsic compression or local invasion from advanced gastrointestinal or gynecologic malignancies. Advanced colorectal cancer and ovarian adenocarcinoma are the two most common malignancies associated with SBO. Hematogenous metastases from breast cancer, melanoma, or Kaposi’s sarcoma also can involve the small intestine, with subsequent obstruction. Primary neoplasms of the small intestine, of which adenocarcinoma and carcinoid tumors are most common, are the cause of SBO in less than 3% of cases (Chapter 121).
PATHOPHYSIOLOGY
The duration and degree of obstruction and the presence and severity of ischemia determine the local and systemic consequences of intestinal obstruction. The intestinal mucosa is an important and early site of injury in both simple and strangulated intestinal obstruction. Microscopic evidence of epithelial injury occurs within the first four to six hours of simple intestinal obstruction and progresses to focal epithelial necrosis within 8 to 12 hours.11 Strangulated obstruction exacerbates the injury, causing extensive mucosal necrosis and sloughing.
Failure of normal intestinal motility causes bacterial overgrowth within the small intestine and loss of the normally increasing concentration gradient of bacteria from the jejunum to the ileum. Disruption of the ecologic balance of the normal enteric microflora is associated with the translocation of bacteria to mesenteric lymph nodes and systemic organs. In a study by Deitch, enteric bacteria, particularly Escherichia coli, were cultured from mesenteric lymph nodes in nearly 60% of patients with simple intestinal obstruction compared with only 4% of controls.12 These observations are consistent with experimental studies that described the translocation of bacteria into the submucosa within 36 minutes of simple SBO.13 Together, these data are consistent with the hypothesis that translocating enteric bacteria contribute to the septic consequences of SBO.
CLINICAL FEATURES
Physical Examination
In general, patients with simple SBO appear to be acutely ill, with abdominal distention and systemic evidence of intravascular volume depletion. Auscultation of the abdomen reveals periods of increased bowel sounds separated by intervals of relative quiet. The quality of the bowel sounds usually is described as high-pitched or musical. Borborygmi are pronounced rumbling bowel sounds that correspond with paroxysms of cramping abdominal pain. In the setting of prolonged obstruction, bowel sounds disappear as intestinal motility decreases. As alluded to earlier, the abdomen generally is distended and only minimally tender. Abdominal tenderness with guarding or other evidence of peritonitis suggests strangulation of the obstruction and necessitates urgent laparotomy. Patients with proximal SBO can have minimal abdominal distention if they have been vomiting. Patients with closed-loop obstructions can present with pain out of proportion to the physical findings, much like that of other causes of acute mesenteric ischemia, such as embolus in the superior mesenteric artery (SMA) (see Chapter 114). The presence of a tender mass at the site of an inguinal, femoral, or umbilical hernia strongly suggests that this is the etiology of the obstruction.
RADIOLOGIC FINDINGS
Abdominal Plain Films
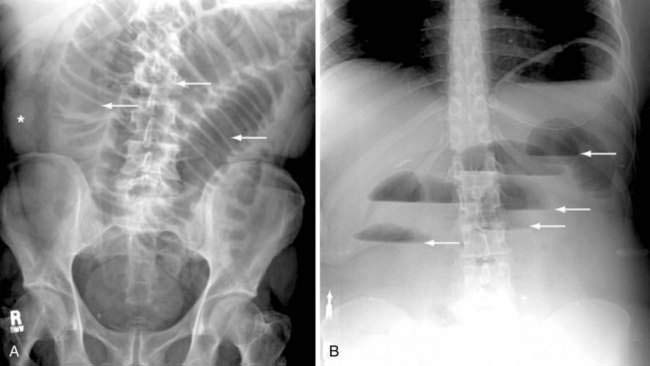
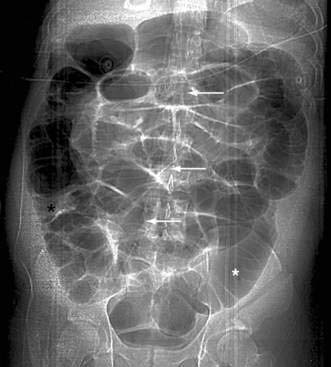
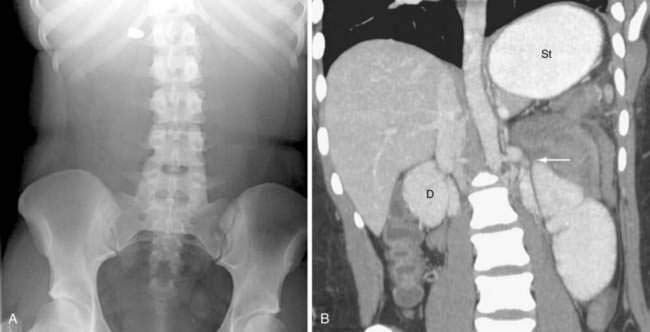
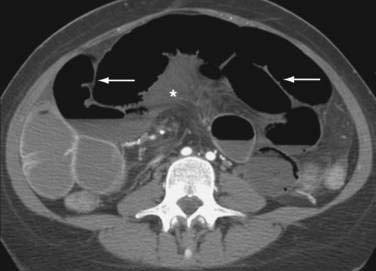
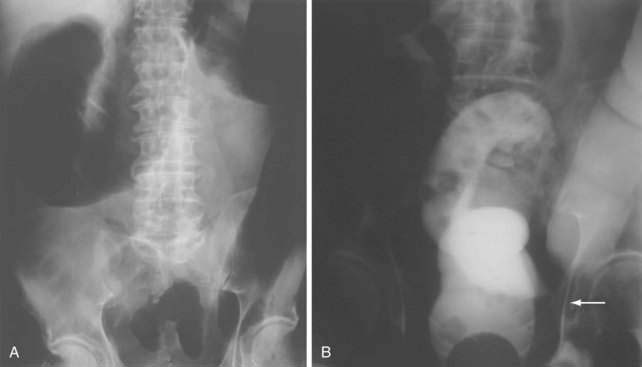
Classically, the abdominal films of patients with SBO demonstrate multiple dilated gas- or fluid-filled loops of small intestine with a decompressed colon (Fig. 119-1). The finding of dilated small bowel loops containing air-fluid levels is insufficient to distinguish SBO from ileus; however, when combined with an absence of colonic gas, the diagnosis of SBO becomes very likely. When dilated small bowel loops are accompanied by colonic distention, ileus or large bowel obstruction become more likely (Fig. 119-2). A gasless abdomen may be seen in patients with a very proximal SBO or those in whom the intestine is filled with fluid (Fig. 119-3).
Lappas and colleagues reviewed 12 radiologic findings associated with SBO and found that the combination of air-fluid levels of different heights in the same bowel loop and a mean air-fluid level diameter of 2.5 cm were most predictive of a high-grade partial or complete SBO.14 Thompson and coworkers corroborated the predictive value of these types of air-fluid levels and reported their sensitivity for SBO to be 59% to 93%.15 The limitations of abdominal plain films in determining the presence of intestinal obstruction are well recognized: 20% to 30% of patients with proven SBO have equivocal or normal studies.16,17 False-negative plain films are most likely to occur with low-grade, proximal, or closed-loop obstructions, and in such cases further imaging may be diagnostic (see Fig. 119-3).
Computed Tomography
Many studies support the use of abdominal computed tomography (CT) to evaluate patients with suspected intestinal obstruction.18,19 Advances in CT hardware and software provide high-resolution reconstructed images in any plane, thus enhancing image resolution and diagnostic confidence.20,21 Overall, CT is 90% to 95% sensitive, 96% specific, and 95% accurate in determining the presence of complete or high-grade SBO, and it provides important information regarding the site of obstruction and etiology in up to 95% of instances.
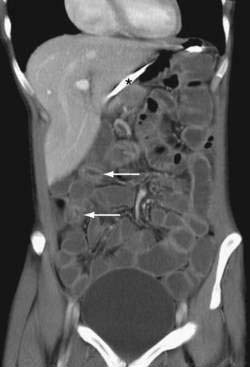
CT findings of mechanical SBO are listed in Table 119-2 and illustrated in Figures 119-4 to 119-7. The demonstration of dilated, fluid- or gas-filled loops of proximal bowel and collapsed loops of distal bowel supports the diagnosis of intestinal obstruction. A transition point between bowel loops with disparate calibers may be identified (see Fig. 119-4A) and the degree of obstruction estimated by the amount of enteral contrast passing through the obstruction. Tapered bowel at the transition point can form a beak (see Fig. 119-3B), and a thorough search of this area can suggest the cause of obstruction. Although peritoneal adhesions are not usually seen on imaging studies, the presence of a transition point without another identifiable cause strongly favors adhesive obstruction. CT, however, can demonstrate tumors and other nonadhesive causes of bowel obstruction (see Fig. 119-6).
Table 119-2 Computed Tomography Findings in Patients with Small Intestinal Obstruction
| Simple Complete Intestinal Obstruction |
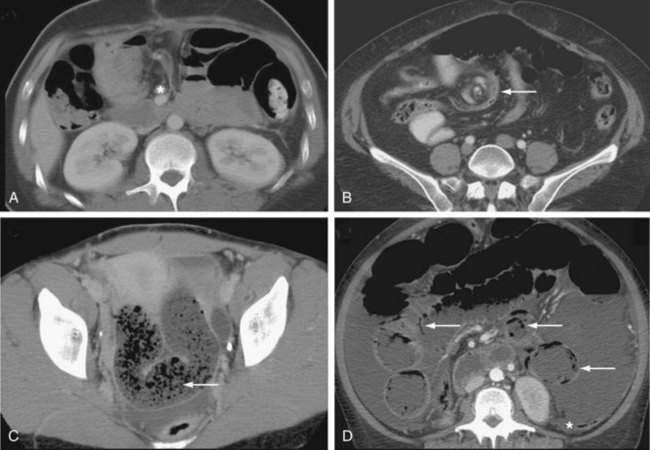
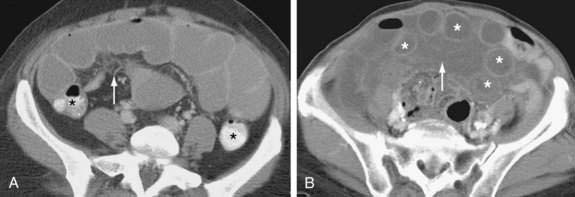
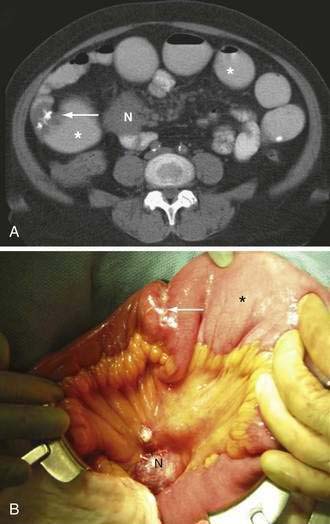
Figure 119-5. Computed tomography signs in small bowel obstruction. A, Abnormal position of mesenteric vessels: The superior mesenteric artery (*) is anterior to the superior mesenteric vein. B, Whirl sign (arrow). C, Small bowel feces sign (arrow). D, Pneumatosis (arrow) and large-volume ascites. The colon (*) is collapsed (see Table 119-2).
Many classic CT signs have been proposed to distinguish simple from strangulated SBO (see Table 119-2)22–28; these include circumferential bowel wall thickening and edema (see Fig. 119-4B), ascites (see Fig. 119-5D), mesenteric engorgement, abnormal vessel course (see Fig. 119-5A), altered enhancement of the bowel wall (see Fig. 119-7), and a bowel configuration suggesting a closed-loop obstruction or volvulus (see Fig. 119-4B). The small bowel feces sign refers to the presence of a mottled admixture of particulate matter and gas within the dilated bowel proximal to a low-grade obstruction or in the setting of intestinal ischemia (see Fig. 119-5C).24,27 A closed-loop obstruction or small bowel volvulus is suggested by U– or C-shaped dilated bowel loops and a radial distribution of stretched mesenteric vessels converging toward a point of torsion (see Fig. 119-4B).22 The whirl sign25 also suggests intestinal torsion or volvulus and refers to a swirling mass of soft tissue and fat density that occurs as the bowel rotates on its mesentery (see Fig. 119-5B).
Decreased bowel wall enhancement is the most specific finding of intestinal ischemia (see Fig. 119-7), but its sensitivity is relatively low in most series (34% to 56%).23,24,28 Porto-mesenteric venous gas, pneumoperitoneum, and pneumatosis intestinalis linearis (see Fig. 119-5D) may be seen very late in the natural history of strangulated obstruction and suggest the presence of extensive necrosis. Despite these many signs, the early diagnosis of strangulated SBO remains a challenge with reported sensitivity, specificity, and accuracy of these various criteria to detect strangulated obstruction ranging from 14% to 95%.24
Detection of low-grade or intermittent bowel obstruction may be extremely difficult using standard radiologic and CT approaches; diagnostic accuracy varies from 48% to 66%.29 In these settings, CT enteroclysis (Fig. 119-8) combines the advantages of active luminal distention with the mural and extraenteric evaluation of cross-sectional imaging; this technique has been shown to raise the accuracy for detecting small bowel diseases to nearly 100%.30,31 Magnetic resonance imaging also has been used to detect SBO and to characterize benign and malignant causes,32 although its greater cost, lower spatial resolution, and lack of incremental diagnostic gain over CT has limited its widespread implementation.33
Barium and Water-Soluble Small Bowel Contrast Studies
Fluoroscopic studies of the gastrointestinal tract with enteral contrast agents (barium sulfate or Gastrografin) have long been used to evaluate patients with suspected SBO, particularly when the clinical presentation is atypical, abdominal plain films are nondiagnostic, and lower grades of bowel obstruction are suspected. Contrast radiography with barium sulfate has been shown to provide useful information—definite diagnosis, no obstruction, high-grade or complete obstruction—in 50% to 80% of patients examined,34 and several studies suggest that passage of orally administered Gastrografin into the colon within six to 24 hours can identify patients most likely to respond to nonoperative management.35–37 The disadvantages of luminal study approaches, including prolonged examination times, the need to ingest large volumes of contrast agent, and limited visibility of mucosa at transition point, have caused fluoroscopic small bowel studies to be largely replaced with CT or CT enteroclysis.
In patients with chronic partial SBO, fluoroscopic imaging of the site of obstruction may be facilitated by barium enteroclysis, which can characterize the site of obstruction accurately in 86% to 100% of instances (Fig. 119-9).17 After intubating the duodenum or proximal jejunum, contrast material is delivered directly into the small intestine, and the small bowel then is evaluated for distensibility, stenoses, masses, and inflammatory changes. The degree of intestinal obstruction may be determined by challenging luminal distention and by identifying the arrival and passage of contrast through an area of narrowing. This study is, of course, contraindicated in patients presenting acutely with obstructive symptoms or evidence of a complete or high-grade SBO. In patients with combined small bowel and colonic distention, a Gastrografin contrast enema or CT with rectal contrast may be used to distinguish ileus from a distal large bowel obstruction.
TREATMENT AND OUTCOME
Medical Management
In the absence of clinical evidence of strangulated obstruction or an incarcerated hernia, 64% to 73% of patients with SBO can be managed successfully with fluid and electrolyte resuscitation and NG aspiration.5,38 Foster and colleagues reported that 76% of 32,583 patients with SBO admitted to acute care hospitals in California in 1997 were managed without operation.5 In contrast, less than half of patients presenting with a complete SBO are managed successfully nonoperatively.38
Most patients who can be successfully managed nonoperatively have substantial improvement within the first 48 hours of treatment. Clinical deterioration, or even the failure to improve, mandates urgent operation. Of those patients in whom nonoperative management fails, almost 20% have strangulated obstruction.38 As alluded to earlier, patients whose SBO is most likely to resolve without operation may be identified by the appearance of oral contrast within the cecum on abdominal films within four to 24 hours of administration (sensitivity, 0.96; specificity, 0.96).39 CT, with enteral and parenteral contrast, can provide the same prognostic information with regard to the passage of oral contrast into the cecum; in addition, this modality also can provide radiologic evidence of ischemic bowel.
Although some studies have suggested that orally administered Gastrografin may enhance the likelihood of successful nonoperative management of SBO,37 the preponderance of data suggests that it has no therapeutic effect.39 Similarly, few data suggest that placement of a long tube into the small intestine improves the likelihood of successful nonoperative management compared with a standard NG tube for decompression. It is our opinion that if long intestinal tubes have a place in the management of patients with SBO, it is in patients with prior episodes of intestinal obstruction from adhesions, radiation enteritis, or Crohn’s disease.
Surgical Management
Although laparoscopy has revolutionized the management of many gastrointestinal diseases, its use for patients with SBO, first reported in 1991, has not been implemented as widely as for other conditions. The advantages of this operative approach include a quicker resolution of postoperative ileus, a reduction in the length of hospital stay, and fewer complications.40–42 Laparoscopic treatment of SBO, however, is limited by the technical difficulties associated with exploring the abdomen in the presence of markedly distended loops of small intestine and the perceived heightened risk of injury to the distended and thin-walled bowel.
In experienced hands, laparoscopic treatment of SBO is successful in about one half to two thirds of patients,40,41,43 especially when there has been only one or two previous abdominal operations, and if the antecedent procedure was an appendectomy. Patients who present to the hospital relatively early after the onset of symptoms and are operated on within 24 hours of admission do better than those who have a longer trial of nonoperative management. The most common reasons for conversion to laparotomy are the presence of dense adhesions,40 the need for intestinal resection, an iatrogenic injury to the bowel, or an inability to identify the site of the obstruction.43
The morbidity and mortality rates associated with the laparoscopic management of patients with SBO compare favorably with those of open approaches. In a systematic review of 19 studies that included 1061 patients, Ghosheh and Salameh reported that the overall complication rate for laparoscopic treatment of SBO was 15.5%; the mortality rate was 1.5%.43 The incidence of iatrogenic perforation of the bowel was 8.4% in the largest series of patients undergoing laparoscopic treatment of SBO (308 patients from 35 surgical centers);40 this figure is similar to the average incidence of 6.5% reported in Ghosheh and Salameh’s collective review.43 The risk of intraoperative bowel injury can be mitigated by using endoscopic scissors rather than monopolar electrocautery to lyse adhesions; beginning the bowel exploration from the cecum and collapsed distal small bowel loops, and working proximally toward the transition zone and dilated intestine; dividing only those adhesions relevant to the obstruction; gently manipulating the dilated bowel using atraumatic intestinal clamps placed on the mesentery instead of the intestinal wall; and using an open Hasson technique by which entry into the peritoneal cavity is performed under direct visualization, entering the abdomen at a site remote from any previous incisions.
Outcome
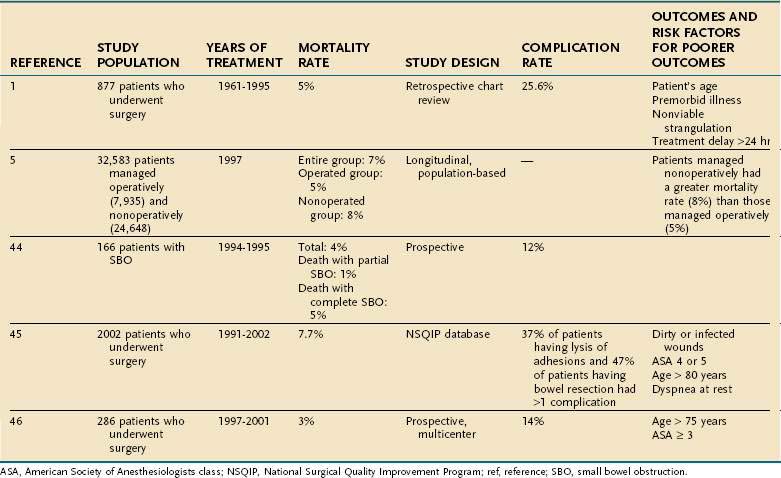
The outcome of patients with SBO may be considered in terms of immediate risk of morbidity and mortality and the long-term risk of recurrent obstruction. The risk of death associated with intestinal obstruction ranges from 2% to 8% in studies published since 2000 (Table 119-3).5,38,44–46 The most commonly cited risk factors for increased risk of death are advanced age and American Society of Anesthesiologists (ASA) class 3 or higher. Chronic illnesses, strangulated obstruction, and treatment delay also have been associated with an increased mortality risk.1,45,46 The incidence of major complications after operative treatment of SBO ranges from 12% to 47% depending upon the procedure performed; bowel resection poses a greater risk of complications than does simple lysis of adhesions. The most common medical complications include pneumonia and respiratory failure, pulmonary embolism, cardiac complications, and prolonged ileus. The most common surgical complications are wound infections, intra-abdominal infections, intra-abdominal bleeding, and intestinal necrosis and perforation.1,44–46
Once a patient has developed adhesive SBO, the rate of recurrent obstruction is high. In one retrospective study of 500 patients who underwent operative treatment of SBO, the rate of recurrent obstruction was 18% with 10 years of follow-up and 29% at 30 years.47 These figures are similar to those of Miller and coworkers, who found that one third of 410 patients presenting to the hospital with adhesive SBO subsequently were readmitted with recurrent obstruction.48 Similarly, Foster and colleagues reported that 18% of 32,583 patients admitted to California hospitals in 1997 with a diagnosis of SBO had a subsequent admission for the same diagnosis within five years.5
Although obstruction can recur at any time in the patient’s life, most (50% in Fevang’s study) occur within the first five years after the index operation for obstruction.47 Some evidence suggests that operative treatment of adhesive SBO is associated with a lower rate of recurrent obstruction47 and a longer interval between admissions than nonoperative management.5,48 Miller and coworkers related the likelihood of recurrent obstruction to the pattern of peritoneal adhesions: Obstruction from a single adhesive band had a 25% risk of recurrence, compared with a 49% incidence of recurrence when adhesions were dense and matted.48
SPECIAL CONSIDERATIONS
Early Postoperative Obstruction
As with other patients with SBO, initial management of postoperative SBO begins with intravascular volume resuscitation and NG aspiration. In one series, 20 of 23 such cases resolved with nonoperative management alone, all within six days of treatment.49 Although strangulated postoperative SBO is unusual, evidence of clinical deterioration with the development of severe abdominal pain, hemodynamic instability, or evidence of peritonitis suggests strangulation and mandates immediate laparotomy. In patients who develop SBO early after laparoscopy, the surgeon must suspect that a portion of the intestine herniated through a trocar site, and urgent operative relief of the obstruction is required.
Small Bowel Obstruction in Patients with Malignancies
Peritoneal spread of gastrointestinal and gynecologic malignancies is an important cause of SBO. In both colorectal and ovarian cancer, the risk of developing malignant obstruction is strongly linked to the initial stage of the disease. For example, Ellis and colleagues found that only 18% of patients who developed SBO after undergoing colectomy for early-stage colon cancer had recurrent cancer as the cause of their obstruction, whereas carcinomatosis was the cause of obstruction in 82% of patients with more-advanced disease.50 Similarly, Tunca and colleagues found that only 15% of patients with stage 1 ovarian cancer eventually developed malignant SBO compared with 35% of patients with more-advanced disease.51 In the absence of recurrent malignancy, intestinal obstruction may be due to postoperative intraperitoneal adhesions or fibrosis from radiation therapy. Gadolinium-enhanced MRI and positron emission tomography can assist in differentiating malignant from benign causes of intestinal obstruction in patients with a history of malignancy.
Because most instances of SBO from recurrent malignancy represent incurable disease, the goal of treatment is to improve the quality of life over the remainder of a limited life expectancy. In such instances, the purpose of surgery is to relieve the symptoms of obstruction. Factors to consider in planning operative therapy include the chance of successful palliation, the risk of repeat obstruction, the quality of life for the patient after surgery, the ability to administer future chemotherapy, and the risk of operative morbidity and mortality. Surgical options include resection with reanastomosis, surgical bypass with an enteroenterostomy, or diverting ileostomy. In many instances, symptoms can be palliated successfully by operation, at least for a time.52 In one retrospective study of 64 patients with recurrent ovarian cancer, the obstruction was relieved surgically in 84% of cases, and 71% of patients were able to tolerate a diet for at least 60 days postoperatively53; the median survival of those patients in whom the obstruction was relieved was about 12 months. Unfortunately, the incidence of operative and postoperative complications is high for patients undergoing an operative approach to malignant intestinal obstruction.52
Intussusception
Intussusception is the invagination of a proximal segment of bowel (intussusceptum) into an adjacent distal segment (intussuscipiens) (Figs. 119-10 and 119-11). Although more often recognized as a cause of SBO in children, about 5% of cases occur in adults. In contrast to children, in whom there is rarely an anatomic abnormality of the intestine, small bowel intussusception in adults is associated with an underlying pathologic process in 80% to 90% of cases. Benign tumors are the most common lead point in the small intestine, whereas adenocarcinoma is the most common lead point for ileocolic and colocolic intussusceptions.
The incidence of intussusception peaks in children between the ages of four and seven months, and most cases occur before the child is one year of age. Most children present with the acute onset of vomiting, bloody stools, and abdominal pain; only about 20% have all three of these classic symptoms. In one study of 244 children with intussusception, only 6% had all three of these symptoms and an abdominal mass on examination.54 Adults with intussusception present with cramping abdominal pain and symptoms of intestinal obstruction that often are several weeks in duration. An abdominal mass and evidence of gastrointestinal bleeding or anemia are present in a minority of cases.55–57
In most medical centers, ultrasonography (US) is the initial procedure of choice for diagnosing intussusception in the pediatric population. The accuracy of this technique is greater than 90% in experienced hands,58 and its noninvasive nature and lack of ionizing radiation represent significant advantages over contrast enemas.59 Most practices reserve the air or liquid enema for reduction of intussusceptions found by US in suitable candidates.
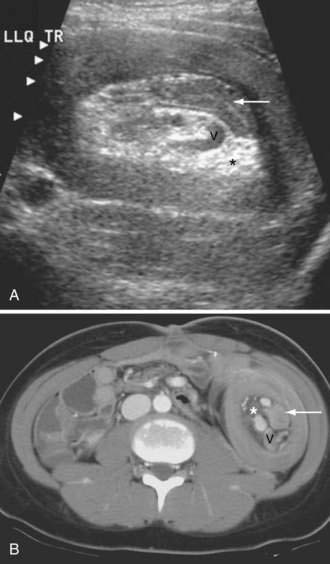
In nonpregnant adults, the procedure of choice for diagnosing intussusception is CT. CT and US detect the double bowel of the intussusceptum and intussuscipiens along with variable amounts of mesenteric fat (see Fig. 119-10). Concentric rings of inner and outer bowel appear hypoechoic on US (intermediate density on CT), with the characteristic hyperechoic fat (low density on CT) between the intussusceptum and intussuscipiens. Images obtained in long axis or obliquity might appear reniform or sausage shaped. Most pediatric ileocolic intussusceptions occur in the subhepatic region.
Seventy percent to 80% of intussusceptions in the pediatric population may be successfully managed nonoperatively by reduction with air- or liquid-contrast enemas under fluoroscopic or US guidance (see Fig. 119-11). The successful reduction of pediatric intussusception is higher with air-contrast enemas than with liquid enemas, and the success of this technique diminishes substantially as the duration of symptoms exceeds 24 hours.60 The overall rate of bowel perforation is low, and recurrent intussusception occurs in about 10% of cases.59 Obvious contraindications to enema reduction include peritonitis, shock, sepsis, or pneumoperitoneum.
Gallstone Ileus
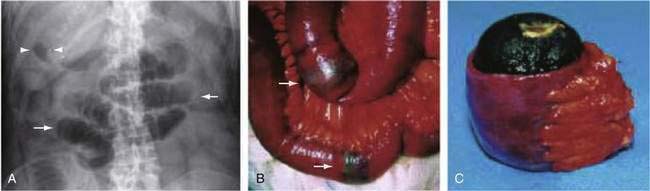
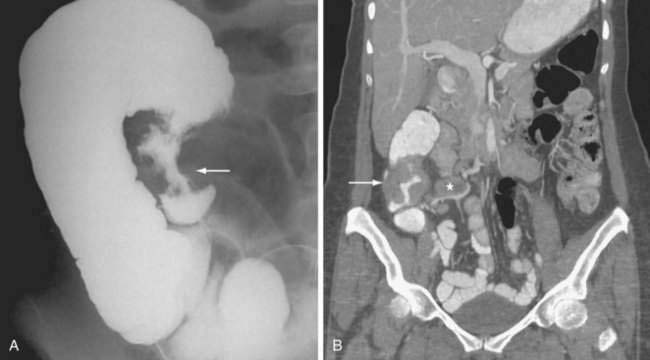
Gallstone ileus is an unusual cause of intestinal obstruction, accounting for about 1% to 4% of all cases (see Chapters 65 and 66).61 This complication of cholelithiasis is more common in the elderly, most patients presenting in the seventh or eighth decades of life. The term “gallstone ileus” is a misnomer, because this condition represents a true mechanical obstruction of the intestine by a gallstone or gallstones within the lumen of the bowel. Most commonly, gallstones large enough to cause obstruction enter the bowel via a cholecystoduodenal fistula. As the stone migrates through the intestinal tract, it produces intermittent obstruction, with resultant waxing and waning of symptoms, thereby confounding early diagnosis. The most common site of obstruction is the ileum, accounting for about 60% of cases; this is followed by the jejunum (15%), stomach (15%), and colon (5%). In the absence of an intestinal stricture, a gallstone of at least 2 cm is required to cause intestinal obstruction (Fig. 119-12).
The diagnosis of gallstone ileus is delayed in up to half of the patients because of nonspecific and inconsistent symptoms. Only 50% to 70% of patients have clinical features of SBO. The classic radiologic features of gallstone ileus include pneumobilia, intestinal obstruction, aberrant gallstone location, and a change in the location of a previously observed stone. Only about 10% of gallstones are sufficiently calcified to be identified on abdominal plain films, although the increasing use of radiologic imaging in the emergency department has led to descriptions of gallstone ileus on CT, MR, and US. In 2005, Lassandro and colleagues reported on the use of CT in 40 patients with surgically proven gallstone ileus; pneumobilia and the biliary-enteric fistula were identified in nearly 90% and 12% of cases, respectively.62
Treatment of gallstone ileus is focused on removing the obstructing stone, usually by operative enterolithotomy; endoscopic removal of the stone with or without lithotripsy also has been reported. In general, enterolithotomy alone is the appropriate initial treatment given the emergent nature of this procedure, the advanced age of many of these patients, and the common presence of a complex right upper quadrant mass containing the cholecystoenteric fistula. Together, these factors argue against identification and repair of the fistula at the time of emergent laparotomy for intestinal obstruction. Elective cholecystectomy with repair of the fistula generally is performed after the patient has recovered from the initial operation because up to 17% of patients develop recurrent gallstone ileus or other biliary complications after enterolithotomy alone.63 Lobo and coworkers, however, reported 11 elderly patients who presented with gallstone ileus and were treated with enterolithotomy alone; none of these patients developed subsequent biliary symptoms with nearly four years of follow-up.61
Midgut Volvulus
Midgut volvulus due to intestinal rotational anomalies is an important cause of intestinal obstruction, particularly in neonates. The anatomy and embryology that underlies the development of duodenal obstruction from Ladd’s bands and midgut volvulus is discussed in Chapter 96. It is worth reiterating, however, that the most common anomaly associated with midgut volvulus is nonrotation, in which there is inadequate counterclockwise rotation of the midgut loop around the SMA. This limited rotation results in the duodenojejunal junction and the entire small intestine being located to the right of the midline, while the colon resides in the left abdomen and the cecum is positioned near the midline. The narrow mesenteric pedicle predisposes the patient to midgut volvulus, and the peritoneal attachments (Ladd’s bands), which extend anterior and lateral to the duodenum to fix the cecum to the posterior body wall, can obstruct the duodenum.
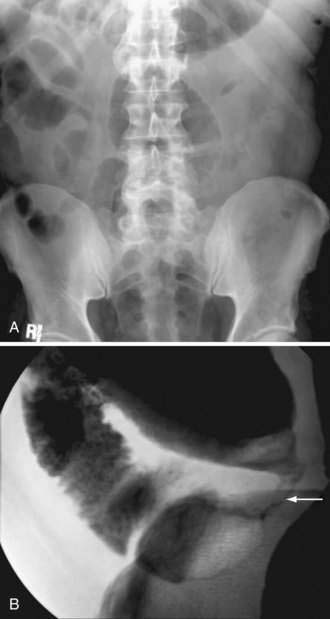
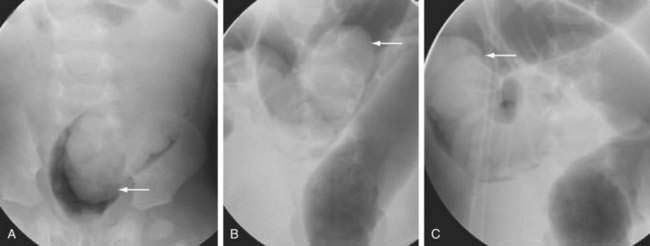
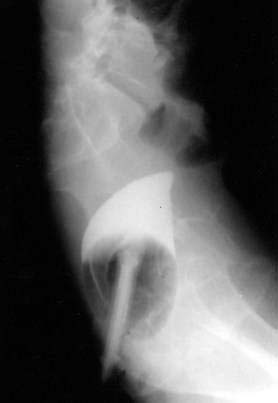
The high risk of intestinal ischemia and necrosis from midgut volvulus and the associated very high mortality rate mandates early diagnosis and aggressive management of neonatal intestinal obstruction. An abdominal plain film that demonstrates a distended stomach and proximal duodenum with a paucity of small bowel gas suggests obstruction, which can obviate further radiologic evaluation. A normal plain film does not exclude malrotation, however, and direct imaging of the stomach and small intestine with an upper gastrointestinal series (UGIS) is needed to confirm or exclude mechanical causes of bilious vomiting. A diagnostic UGIS demonstrates duodenojejunal junction malposition and can illustrate the characteristic corkscrew or coiled appearance of the duodenum and proximal jejunum when malrotation is complicated by midgut volvulus (Fig. 119-13). The use of a contrast enema to demonstrate malrotation is less direct than UGIS and can have false positive and false negative rates of 15% and 20%, respectively.
COLONIC OBSTRUCTION
ETIOLOGY
Colonic volvulus is the axial twisting of the colon on its vascular pedicle. The anatomic factors necessary for the development of volvulus include a redundant segment of bowel that is freely movable within the peritoneal cavity, a long movable mesocolon, and close approximation of two points of fixation of the colon. Typically, a closed-loop obstruction is produced that causes ischemia of the bowel wall due to twisting of the vascular pedicle and increased wall tension from colonic distention. The sigmoid colon and cecum are the most common sites of colonic volvulus, accounting for about 75% and 22% of all cases, respectively. Rarely, volvulus occurs at the transverse colon (2%) or the splenic flexure (1%). Although colonic volvulus causes only 15% of colonic obstructions in the United States and other western countries, it is a significantly more common cause of colonic obstruction in parts of Africa and the Middle East. Less than 10% of cases of colonic obstruction are due to a fibrotic inflammatory stricture caused by acute diverticulitis. The complications of diverticulitis are discussed further in Chapter 117.
CLINICAL FEATURES
Patients with left-sided colonic tumors or benign fibrotic strictures often have noted a change in stool caliber over the recent months. Blood in the stool, or an iron-deficiency anemia, is highly suggestive of carcinoma, as is the occurrence of weakness, weight loss, and anorexia. Vomiting, when present, is usually a late finding. Symptoms of malignant and diverticular-associated obstruction often are insidious in onset, with a median duration of three months. In one study, 25% of patients with obstruction from colorectal cancer had symptoms for six to 24 months before diagnosis.64
Patients with sigmoid volvulus are usually in their sixth to eighth decades of life and often have concomitant chronic illnesses. Acute abdominal distention is the most common presentation for patients with colonic volvulus, occurring in about two thirds of patients. About 20% of patients have abdominal pain, nausea, vomiting, and constipation. The duration of symptoms of colonic volvulus is significantly less than that seen with a malignant or diverticular stricture. In one series of 228 patients with colonic volvulus, the average duration of symptoms before presentation was 73 hours.65 Abdominal tenderness is present in less than one third of patients with volvulus, and peritonitis suggests impending or actual colonic necrosis and perforation.
DIAGNOSTIC EVALUATION
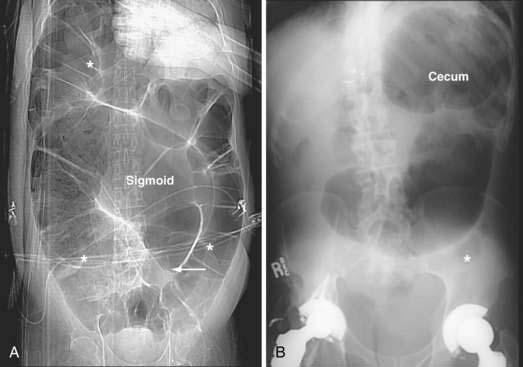
The initial diagnostic approach to patients with suspected colonic obstruction is similar to that of patients with SBO. Plain abdominal films in the supine and upright positions should be obtained to determine the site of the obstruction and whether the obstruction is partial or complete. As alluded to earlier, small bowel distention may or may not be present in patients with colonic obstruction depending upon the competency of the ileocecal valve (Fig. 119-14). Abdominal plain films also may be diagnostic, or at least highly suggestive, of sigmoid and cecal volvulus. As described by Agrez and Cameron,66 the standard radiologic feature of sigmoid volvulus is a distended ahaustral sigmoid loop—a bent inner-tube appearance—whose apex is directed toward the right shoulder (Fig. 119-15A). Radiologic features of cecal volvulus include a massively dilated cecum located in the epigastrium or left upper quadrant, a distended kidney bean-shaped cecum, distended loops of small bowel suggesting SBO, and a single long air-fluid level present on upright or decubitus films (see Fig.119-15B). Although these classic radiographic findings of colonic volvulus are seen in only 40% to 60% of cases, the diagnosis of colonic volvulus can be made with abdominal films alone in up to 85% of cases.
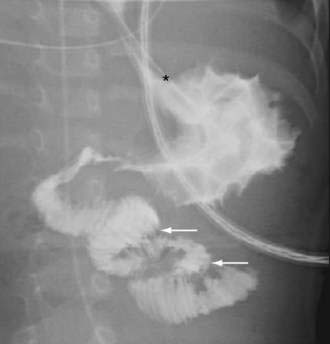
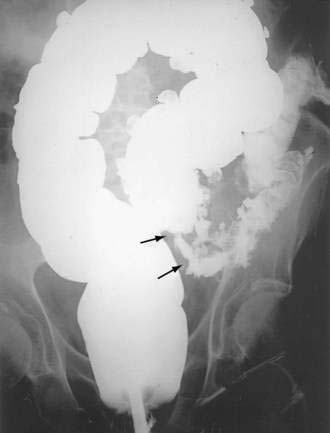
Performing further diagnostic studies in patients with suspected colonic obstruction is predicated on the presence or absence of peritonitis and the degree of obstruction (partial or complete). Patients with peritonitis should undergo resuscitation and urgent laparotomy without additional diagnostic procedures. In contrast, patients whose abdominal plain film suggests a distal obstruction and who do not have evidence of peritonitis or strangulated obstruction should undergo a water-soluble contrast enema or CT with rectal contrast to confirm and localize the site of obstruction (Figs. 119-16 and 119-17). In patients with suspected sigmoid or cecal volvulus, a water-soluble contrast enema may be helpful by demonstrating a point of torsion (a mucosal spiral pattern or beak sign) without the risk of barium peritonitis in the case of unrecognized perforation (Fig. 119-18).
TREATMENT AND OUTCOME
Malignant and Benign Colonic Strictures
Numerous studies have documented the successful use of colonoscopically placed self-expanding metal stents to relieve malignant colonic obstruction before definitive resection or to palliate obstructive symptoms in patients with advanced disease. Successful management has been shown in up to 80% to 90% of patients in whom colonoscopic stent placement has been attempted.67–69 This approach allows mechanical bowel preparation with subsequent partial colectomy and primary anastomosis using either laparoscopic or open operative techniques or treatment of significant concomitant medical illnesses.67 In Khot’s systematic review of 262 cases in which colonic stents were used as a bridge to definitive resection, 223 were successful (85%).69 Other authors have placed self-expanding colonic metallic stents in patients with obstructing colon cancers and nonresectable metastases, allowing earlier administration of chemotherapy70 and earlier discharge from the hospital.71 Complications associated with colonic stent placement include perforation, stent migration, and recurrent obstruction.
If colonic stenting is unsuccessful, patients who are medically fit and have relatively small tumors or inflammatory masses of the left colon or sigmoid should undergo the appropriate segmental colectomy with either an end colostomy or primary anastomosis. The classic operative approach to such patients is a left colectomy with an end colostomy and Hartmann’s pouch, although numerous investigators have reported on the safety of a primary anastomosis after emergent left colectomy for obstructing colorectal cancer and diverticular strictures72–74 or sigmoid volvulus.75 These studies have shown rates of anastomotic leakage (1% to 8%), wound infection (5% to 16%), and mortality (1% to 6%) comparable to earlier data with resection and end colostomy.64 Lim and colleagues compared manual decompression and primary anastomosis with intraoperative colonic irrigation and found that intraoperative irrigation offered no improvement in outcome compared with manual decompression alone.73
Colonic obstructions located proximal to the splenic flexure are most often adenocarcinomas and are treated with partial colectomy and primary ileocolic anastomosis in all but the most unfit patients. The presence of nonviable colon necessitates resection in even the most severely compromised patient. In such an instance, resection with end ileostomy and distal mucous fistula obviates the risk of performing an anastomosis in a grossly contaminated field or in a critically ill patient in whom the risk of anastomotic dehiscence may be particularly high. Colonoscopically placed self-expanding metal stents also have been used with good results in the management of patients with obstructing proximal colon cancers, although most are treated with primary resection and anastomosis.76
Volvulus
Decompression of a sigmoid volvulus may be accomplished with a flexible or rigid sigmoidoscope and the placement of a rectal tube. In a classic study, Ballantyne compiled 19 American series totaling 595 patients and found that sigmoidoscopy, either alone or combined with a rectal tube, successfully reduced the volvulus in 70% to 80% of attempts.77 Placement of a rectal tube for 48 hours can minimize the possibility of early recurrence. Endoscopic reduction of sigmoid volvulus alone is associated with a recurrence rate of 25% to 50%,65,77 and therefore, sigmoid resection and coloproctostomy or, in medically compromised patients, end colostomy, should follow endoscopic decompression and mechanical preparation of the bowel. Recurrence rates with this approach are 3% to 6%.65,77 Patients requiring emergent laparotomy for strangulated sigmoid volvulus should have sigmoid resection with end colostomy and a Hartmann’s pouch or primary anastomosis as discussed earlier.75 Right colectomy with primary ileotransverse colostomy is the procedure of choice for cecal volvulus.
Regardless of the approach, emergent operations to relieve colonic obstruction are associated with significantly greater morbidity and mortality risks than those performed electively. Grossmann and colleagues found that the mortality rate was 24% for patients undergoing emergency operation for sigmoid volvulus compared with 6% for those undergoing elective resection.65 Similarly, Sjo and coworkers reported that the early postoperative mortality rate was 19% after emergency operation for colorectal cancer compared with 3.5% for patients undergoing elective resection.78 Emergency operation, American Society of Anesthesiologists class 3 or 4, necrotic bowel, and advanced tumor stage have been consistently identified as independent predictors of early postoperative mortality.65,78–80
The long-term outlook for patients who present with obstructing colon cancers is significantly worse than that for patients without obstruction. Wang and coworkers found that despite similar tumor stages, patients who presented with obstructing right-sided colon cancers had significantly higher rates of tumor recurrence than did patients who presented without obstruction (49% vs. 22%, respectively). Similarly, the five-year survival was worse for patients presenting with obstruction than for those patients without obstruction (36% vs. 77%, respectively).81 Even in the setting of a potentially curative resection, the cancer-specific survival at five years was 75% for patients without obstruction compared with 52% for those patients presenting with obstruction.82
Abbas S, Bissett IP, Parry BR. Oral water soluble contrast for the management of adhesive small bowel obstruction. Cochrane Database Syst Rev 2007; (3):CD004651. (Ref 39.)
Fevang B-T, Fevang J, Lie SA, et al. Long-term prognosis after operation for adhesive small bowel obstruction. Ann Surg. 2004;240:193-201. (Ref 47.)
Fevang BT, Fevang J, Stangeland L, et al. Complications and death after surgical treatment of small bowel obstruction. A 35-year institutional experience. Ann Surg. 2000;231:529-37. (Ref 1.)
Foster NM, McGory ML, Zingmond DS, Ko CY. Small bowel obstruction: a population-based appraisal. J Am Coll Surg. 2006;203:170-6. (Ref 5.)
Grossman EM, Longo WE, Stratton MD, et al. Sigmoid volvulus in Department of Veterans Affairs Medical Centers. Dis Colon Rectum. 2000;43:414-18. (Ref 65.)
Kaiser AD, Applegate KE, Ladd AP. Current success in the treatment of intussusception in children. Surgery. 2007;142:469-77. (Ref 54.)
Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg. 2002;89:1096-102. (Ref 69.)
Levard H, Boudet M-J, Msika S, et al. Laparoscopic treatment of acute small bowel obstruction: a multicentre retrospective study. ANZ J Surg. 2001;71:641-46. (Ref 40.)
Margenthaler JA, Longo WE, Virgo KS, et al. Risk factors for adverse outcomes following surgery for small bowel obstruction. Ann Surg. 2006;243:456-64. (Ref 45.)
McArdle CS, McMillan DC, Hole DJ. The impact of blood loss, obstruction, and perforation on survival in patients undergoing curative resection for colon cancer. Br J Surg. 2006;93:483-8. (Ref 82.)
1. Fevang BT, Fevang J, Stangeland L, et al. Complications and death after surgical treatment of small bowel obstruction. A 35-year institutional experience. Ann Surg. 2000;231:529-37.
2. Miller G, Boman J, Shrier I, et al. Etiology of small bowel obstruction. Am J Surg. 2000;180:33-6.
3. Andersson RE. Small bowel obstruction after appendectomy. Br J Surg. 2001;88:1387-91.
4. Beck DE, Opelka FG, Bailey HR, et al. Incidence of small-bowel obstruction and adhesiolysis after open colorectal and general surgery. Dis Colon Rectum. 1999;42:241-8.
5. Foster NM, McGory ML, Zingmond DS, Ko CY. Small bowel obstruction: a population-based appraisal. J Am Coll Surg. 2006;203:170-6.
6. Alvarez JA, Baldonedo RF, Bear IG, et al. Incarcerated groin hernias in adults: presentation and outcome. Hernia. 2004;8:121-6.
7. Podnos YD, Jimenez JC, Wilson SE, et al. Complications after laparoscopic gastric bypass. A review of 3464 cases. Arch Surg. 2003;138:957-61.
8. Tonouchi H, Ohmori Y, Kobayashi M, Kusunoki M. Trocar site hernia. Arch Surg. 2004;139:1248-56.
9. Bowrey D, Blom D, Crookes P, et al. Risk factors and prevalence of trocar site herniation after laparoscopic fundoplication. Surg Endosc. 2001;15:663-6.
10. Miller G, Boman J, Shrier I, et al. Small-bowel obstruction secondary to malignant disease: an 11-year audit. Can J Surg. 2000;43:353-8.
11. Kabaroudis A, Papaziogas B, Koutelidakis I, et al. Disruption of the small intestine mucosal barrier after intestinal occlusion: a study with light and electron microscopy. J Invest Surg. 2003;16:23-8.
12. Deitch EA. Simple intestinal obstruction causes bacterial translocation in man. Arch Surg. 1989;124:699-701.
13. Samel S, Keese M, Kleczka M, et al. Microscopy of bacterial translocation during small bowel obstruction and ischemia in vivo—a new animal model. BMC Surg. 2002;2:6.
14. Lappas JC, Reyes BL, Maglinte DT. Abdominal radiography findings in small bowel obstruction: Relevance to triage for additional diagnostic imaging. Am J Radiol. 2001;176:167-74.
15. Thompson WM, Kilani RK, Smith BB, et al. Accuracy of abdominal radiography in acute small bowel obstruction: Does reviewer experience matter? Am J Radiol. 2007;188:W233-38.
16. Maglinte DDT, Reyes BL, Harmon BH, et al. Reliability and role of plain film radiography and CT in the diagnosis of small-bowel obstruction. Am J Radiol. 1996;167:1451-5.
17. Shrake PD, Rex DK, Lappas JC, et al. Radiographic evaluation of suspected small bowel obstruction. Am J Gastroenterol. 1991;86:175-8.
18. Urban BA, Fishman EK. Tailored helical CT evaluation of acute abdomen. RadioGraphics. 2000;20:725-49.
19. Furukawa A, Yamasaki M, Furuichi K, et al. Helical CT in the diagnosis of small bowel obstruction. RadioGraphics. 2001;21:341-45.
20. Jaffe TA, Martin LC, Thomas J, et al. Small-bowel obstruction: coronal reformations from isotropic voxels at 16-section multi-detector row CT. Radiology. 2005;238:135-42.
21. Filippone A, Cianci R, Storto ML. Bowel obstruction: comparison between multi-detector row CT axial and coronal planes. Abdom Imaging. 2007;32:310-16.
22. Balthazar EJ, Birnbaum BA, Megibow AL, et al. Closed loop and strangulating intestinal obstruction: CT signs. Radiology. 1992;185:769-75.
23. Kim JH, Ha HK, Kim JK, et al. Usefulness of known computed tomography and clinical criteria for distinguishing strangulation in small bowel obstruction: analysis of true and false interpretation groups in computed tomography. World J Surg. 2004;28:63-8.
24. Sheedy SP, Earnest F, Fletcher JG, et al. CT of small bowel ischemia associated with obstruction in emergency department patients: diagnostic performance evaluation. Radiology. 2006;241:729-36.
25. Khurana B. The whirl sign. Radiology. 2003;226:69-70.
26. Duda JB, Bhatt S, Dogra VS. Utility of CT whirl sign in guiding management of small-bowel obstruction. Am J Radiol. 2008;191:743-7.
27. Jacobs SL, Rozenblit A, Ricci Z, et al. Small bowel faeces sign in patients without small bowel obstruction. Clin Radiol. 2007;62:353-7.
28. Jancelewicz T, Vu LT, Shawo AE, et al. Predicting strangulated small bowel obstruction: an old problem revisited. J Gastrointest Surg. 2009;13(1):93-9.
29. Maglinte DDT, Gage SN, Harmon BH. Obstruction of the small intestine: accuracy and role of CT in diagnosis. Radiology. 1993;188:61-4.
30. Boudiaf M, Jaff A, Soyer P, et al. Small bowel diseases: prospective evaluation of multi-detector row helical CT enteroclysis in 107 consecutive patients. Radiology. 2004;233:338-44.
31. Maglinte DDT, Sandrasegaran K, Lappas JC, Chiorean M. CT enteroclysis. Radiology. 2007;245:661-71.
32. Low RN, Chen SC, Baron R. Distinguishing benign from malignant bowel obstruction in patients with malignancy: findings at MR imaging. Radiology. 2003;228:157-65.
33. Ryan ER, Heaslip IS. Magnetic resonance enteroclysis compared with conventional enteroclysis and computed tomography enteroclysis: a critically appraised topic. Abdom Imaging. 2008;33:34-7.
34. Riveron FA, Obeid FN, Horst HM, et al. The role of contrast radiography in presumed bowel obstruction. Surgery. 1989;106:496-501.
35. Biondo S, Pares D, Mora L, et al. Randomized clinical study of Gastrografin administration in patients with adhesive small bowel obstruction. Br J Surg. 2003;90:542-46.
36. Abbas SM, Bisset IP, Parry BR. Meta analysis of oral water soluble contrast agent in the management of adhesive small bowel obstruction. Br J Surg. 2007;4:404-11.
37. Choi HK, Chu KW, Law WL. Therapeutic value of Gastrografin in adhesive small bowel obstruction after unsuccessful conservative treatment: a prospective randomized trial. Ann Surg. 2002;236:1-6.
38. Fevang BT, Jensen D, Svanes K, Viste A. Early operation or conservative management of patients with small bowel obstruction? Eur J Surg. 2002;168:475-81.
39. Abbas S, Bissett IP, Parry BR. Oral water soluble contrast for the management of adhesive small bowel obstruction. Cochrane Database Syst Rev 2007; (3):CD004651.
40. Levard H, Boudet MJ, Msika S, et al. Laparoscopic treatment of acute small bowel obstruction: a multicentre retrospective study. ANZ J Surg. 2001;71:641-46.
41. Khaikin M, Schneidereit N, Cera S, et al. Laparoscopic vs. open surgery for acute adhesion small-bowel obstruction: patients’ outcome and cost-effectiveness. Surg Endosc. 2007;21:742-6.
42. Wullstein C, Gross E. Laparoscopic compared with conventional treatment of acute adhesive small bowel obstruction. Br J Surg. 2003;90:1147-51.
43. Ghosheh B, Salameh JR. Laparoscopic approach to acute small bowel obstruction: review of 1061 cases. Surgical Endoscopy. 2007;21:1945-9.
44. Williams SB, Greenspon J, Young HA, Orkin BA. Small bowel obstruction: conservative vs. surgical management. Dis Colon Rectum. 2005;48:1140-6.
45. Margenthaler JA, Longo WE, Virgo KS, et al. Risk factors for adverse outcomes following surgery for small bowel obstruction. Ann Surg. 2006;243:456-64.
46. Duron JJ, du Montcel ST, Berger A, et al. Prevalence and risk factors for mortality and morbidity after operation for adhesive postoperative small bowel obstruction. Am J Surg. 2008;195:726-34.
47. Fevang BT, Fevang J, Lie SA, et al. Long-term prognosis after operation for adhesive small bowel obstruction. Ann Surg. 2004;240:193-201.
48. Miller G, Boman J, Shrier I, et al. Natural history of patients with adhesive small bowel obstruction. Br J Surg. 2000;87:1240-7.
49. Ellozy SH, Harris MT, Bauer JJ, et al. Early postoperative small-bowel obstruction: a prospective evaluation in 242 consecutive abdominal operations. Dis Colon Rectum. 2002;45:1214-17.
50. Ellis CN, Boggs HWJr, Slagle GW, et al. Small bowel obstruction after colon resection for benign and malignant diseases. Dis Colon Rectum. 1991;34:367-71.
51. Tunca JC, Buchler DA, Mack EA. The management of ovarian cancer–caused bowel obstruction. Gynecol Oncol. 1981;12:186-92.
52. Mangili G, Aletti G, Frigerio L, et al. Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. Int J Gynecol Cancer. 2005;15:830-5.
53. Pothuri B, Vaidya A, Aghajanian C, et al. Palliative surgery for bowel obstruction in recurrent ovarian cancer: an updated series. Gyn Oncol. 2003;89:306-13.
54. Kaiser AD, Applegate KE, Ladd AP. Current success in the treatment of intussusception in children. Surgery. 2007;142:469-77.
55. Wang L-T, Wu C-C, Yu J-C, et al. Clinical entity and treatment strategies for adult intussusceptions: 20 years’ experience. Dis Colon Rectum. 2007;50:1941-49.
56. Chiang Y-M, Lin Y-S. Tumor spectrum of adult intussusception. J Surg Oncol. 2008;30:444-7.
57. Goh BKP, Quah H-M, Chow PKH, et al. Predictive factors of malignancy in adults with intussusception. World J Surg. 2006;30:1300-4.
58. Daneman A, Navarro O. Intussusception. Part 1: a review of diagnostic approaches. Pediatr Radiol. 2003;33:79-85.
59. Daneman A, Navarro O. Intussusception. Part 2: an update on the evolution of management. Pediatr Radiol. 2004;34:97-108.
60. Applegate KE. Clinically suspected intussusception in children: evidence-based and self-assessment module. Am J Radiology. 2005;185:S175-83.
61. Lobo DL, Jobling JC, Balfour TW. Gallstone ileus: diagnostic pitfalls and therapeutic successes. J Clin Gastroent. 2000;30:72-6.
62. Lassandro F, Romano S, Ragozzino A, et al. Role of helical CT in diagnosis of gallstone ileus and related conditions. Am J Radiol. 2005;185:1159-65.
63. Reisner RM, Cohen JR. Gallstone ileus: a review of 1001 reported cases. Am Surg. 1994;60:441-6.
64. Deans G, Krukowski Z, Irwin S. Malignant obstruction of the left colon. Br J Surg. 1994;81:1270-6.
65. Grossman EM, Longo WE, Stratton MD, et al. Sigmoid volvulus in Department of Veterans Affairs Medical Centers. Dis Colon Rectum. 2000;43:414-18.
66. Agrez M, Cameron D. Radiology of sigmoid volvulus. Dis Colon Rect. 1981;24:510-14.
67. Dauphine C, Tan P, Bert R, et al. Placement of self-expanding metal stents for acute malignant large-bowel obstruction. Ann Surg Oncol. 2002;9:575-9.
68. Meisner S, Hensler M, Knop F, et al. Self-expanding metal stents for colonic obstruction: experience from 104 procedures in a single center. Dis Colon Rectum. 2004;47:444-50.
69. Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg. 2002;89:1096-102.
70. Karoui M, Charachon A, Delbaldo C, et al. Stents for palliation of obstructive metastatic colon cancer: impact on management and chemotherapy administration. Arch Surg. 2007;142:619-23.
71. Carne PWG, Frye JNR, Robertson GM, Frizelle FA. Stents or open operation for palliation of colorectal cancer: a retrospective, cohort study of perioperative outcome and long-term survival. Dis Colon Rectum. 2004;47:1455-61.
72. Hsu T-C. Comparison of one-stage resection and anastomosis of acute complete obstruction of the left and right colon. Am J Surg. 2005;189:384-7.
73. Lim JF, Tang C-L, Seow-Choen F, Heah SM. Prospective, randomized trial comparing intraoperative colonic irrigation with manual decompression only for obstructed left-sided colorectal cancer. Dis Colon Rectum. 2005;48:205-9.
74. Zorcolo L, Covotta L, Carlomagno N, Bartolo DCC. Safety of primary anastomosis in emergency colo-rectal surgery. Colorectal Dis. 2003;5:262-9.
75. De U, Ghosh S. Single stage primary anastomosis without colonic lavage for left-sided colonic obstruction due to acute sigmoid volvulus: a prospective study of one hundred and ninety-seven cases. ANZ J Surg. 2003;73:390-2.
76. Repici A, Adler DG, Gibbs CM, et al. Stenting of the proximal colon in patients with malignant large bowel obstruction: techniques and outcomes. Gastrointest Endosc. 2007;66:940-4.
77. Ballantyne GH. Review of sigmoid volvulus: history and results of treatment. Dis Colon Rectum. 1982;25:494-501.
78. Sjo O, Larsen S, Lunde O, Nesbakken A. Short term outcome after emergency and elective surgery for colon cancer. Colorectal Dis. 2008 Jul 4. [Epub ahead of print]
79. Biondo S, Pares D, Frago R, et al. Large bowel obstruction: predictive factors for postoperative mortality. Dis Colon Rectum. 2004;47:1889-97.
80. Longo WE, Virgo KS, Johnson FE, et al. Risk factors for morbidity and mortality after colectomy for colon cancer. Dis Colon Rectum. 2000;43:83-91.
81. Wang H-S, Lin J-K, Mou C-Y, et al. Long-term prognosis of patients with obstructing carcinoma of the right colon. Am J Surg. 2004;187:497-500.
82. McArdle CS, McMillan DC, Hole DJ. The impact of blood loss, obstruction, and perforation on survival in patients undergoing curative resection for colon cancer. Br J Surg. 2006;93:483-8.