CHAPTER 16 Intestinal Gas
COMPOSITION AND VOLUME OF GASTROINTESTINAL GAS
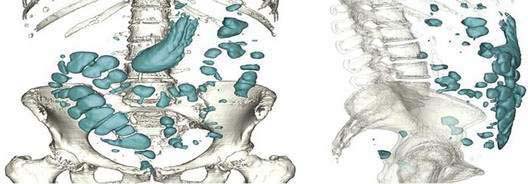
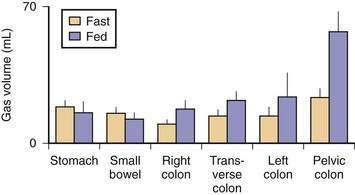
Five gases—N2, O2, CO2, H2, and methane (CH4)—account for more than 99% of intestinal gas; many additional gases are present in trace concentrations. In a study of 10 healthy fasting subjects, the composition of gas within the gastrointestinal tract was assessed using a washout technique in which a rapid infusion of argon into the jejunum was used to flush out the intestinal gases.1 N2 was usually predominant, O2 was present in low concentrations, and the concentrations of CO2, H2, and CH4 were highly variable. The volume of each gas within the intestinal lumen reflects the balance between the input and output of that gas. Input may result from swallowing, chemical reactions, bacterial fermentation, and diffusion from the blood, whereas output involves belching, bacterial consumption, absorption into the blood, and anal evacuation. Measurements of intestinal gas volume, originally obtained using a body plethysmograph and later using a washout technique, have indicated that the volume of intestinal gas is approximately 200 mL in healthy subjects. Similar data have been reported using a specifically designed and validated computed tomography (CT) technique.2 In the fasting state, the healthy gastrointestinal tract contains only about 100 mL of gas distributed almost equally among six compartments—stomach, small intestine, ascending colon, transverse colon, descending colon, and distal (pelvic) colon. Postprandially, the volume of gas increases by 65%, primarily in the pelvic colon (Figs. 16-1 and 16-2).
GAS METABOLISM AND EXCRETION
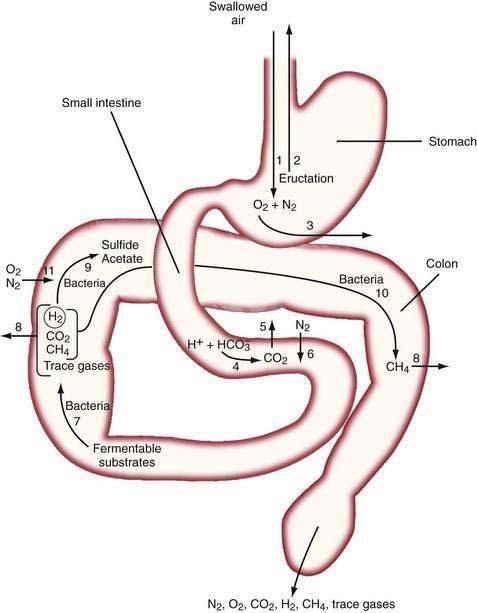
The diffusivity of a gas across the mucosa of the gastrointestinal tract depends on its solubility in water; for a given partial pressure difference, CO2 diffuses much more rapidly than H2, CH4, N2, and O2. The rate and direction of diffusion of each gas is a function of the diffusivity, partial pressure difference between lumen and blood, and exposure of the gas to the mucosal surface.3 H2 and CH4 absorbed from the bowel are excreted in expired air, and breath analysis provides a simple means of assessing the volume of these gases in the gastrointestinal tract. Figure 16-3 schematically depicts the processes regulating the volume and composition of gas in various segments of the gastrointestinal tract.
MOUTH TO STOMACH
The absence of the gastric bubble in subjects with advanced achalasia indicates that air swallowing (rather than intraluminal production) is the major source of stomach gas. Ultrafast CT studies have shown that an average of 17.7 mL of air (14 mL of N2) is swallowed with a 10-mL bolus of liquid.4 Most of this swallowed air presumably is regurgitated, because neither absorption of intestinal gas nor excretion in flatus can account for the relatively enormous quantities of N2 that would be swallowed with the daily ingestion of 1500 mL of liquid.5 Swallowed air contains minimal CO2, and CO2 diffuses from blood into the stomach bubble. The Po2 of swallowed air is higher than that of blood, and O2 is absorbed from the stomach.3
SMALL INTESTINE
In the upper small intestine, CO2 is liberated from the interaction of bicarbonate and acid. Bicarbonate is delivered to the intestinal lumen via biliary, pancreatic, and small intestinal secretions, and the acid is delivered via gastric secretion (about 30 mEq/hr after meals6) or fatty acids released during digestion of triglycerides (about 100 mEq of acid/30 g of fat). The Pco2 of duodenal contents after a meal rises to 300 mm Hg in control subjects and 500 mm Hg in patients with duodenal ulcers,7 which indicates that CO2 comprises about 40% and 66% of duodenal gas in these two states, respectively. This high Pco2 causes the luminal Pn2 to fall below that of blood, and N2 diffuses from blood into the lumen.
Although CO2 is absorbed rapidly during its passage through the small intestine, a study using CT-based volumetric analysis has shown that in the postprandial period, small bowel gas volume remains constant, whereas gas increases in the pelvic colon (see Fig. 16-2). This colonic increment occurs 99 ± 22 minutes after the meal, earlier than would be expected for gas derived from colonic fermentation of food substrates. Thus, gas (N2 or CO2) of proximal origin presumably is propelled caudally to increase the volume of gas in the pelvic colon.8
COLON
In the colon, luminal bacteria produce and consume intestinal gases, and this activity frequently is the primary determinant of anal gas output. The composition of the colonic microflora, which varies considerably among individuals, is determined by early environmental conditions as well as factors encountered later in life, such as antibiotic and dietary exposures.9 For example, chronic ingestion of high doses of an intestinally malabsorbed disaccharide (lactulose by patients with constipation or lactose by persons with intestinal lactase deficiency) results in diminished breath H2 excretion following a challenge dose of the same disaccharide.10 This phenomenon appears to result from the colonic proliferation of organisms such as Bifidobacterium spp. that ferment lactose or lactulose via non-H2 releasing pathways.11 The production of the bacterial gases CO2, H2, and CH4 may indirectly increase luminal gas volumes by reducing luminal Pn2 to a value less than that of blood, thereby causing N2 to diffuse from the blood to the lumen. Similarly, the low Po2 of colonic gas results in diffusion of O2 from the blood to the lumen; however, O2 is rapidly consumed by intestinal organisms (see Fig. 16-3).
Fermentation of Unabsorbed Substrates
The colon harbors large number of hydrogen-producing bacteria that ferment undigested substrates (carbohydrates and protein), with the release of hydrogen and carbon dioxide.12–14 Newborn infants, as well as germ-free rats, excrete no H2, whereas H2 is detected within hours of bacterial contamination of the gastrointestinal tract. Therefore, bacterial metabolism is the sole source of intestinal H2.15
Various carbohydrates are incompletely absorbed by the intestines of healthy subjects (Table 16-1). Most of the world’s adult population malabsorbs lactose as a result of a genetically programmed reduction in lactase synthesis. Fruits and vegetables (particularly legumes) contain indigestible oligosaccharides such as stachyose and raffinose that are readily fermented by colonic bacteria.16 A fraction of the complex carbohydrate in wheat (pasta, white bread), oats, potatoes, and corn is not absorbed in the small bowel.17 In part, this malabsorption reflects the presence of starch in a physical form that resists amylase digestion. Resistance to amylase is further enhanced when starches are refrigerated and then reheated, a process that results in crystallization (retrogradation) of the starch.18 White rice flour is the only complex carbohydrate that is almost totally absorbed. A sizable fraction of the healthy population cannot completely absorb the large quantity of fructose present in soft drinks. The poor absorption of sorbitol has led to its use as a low-calorie sugar substitute; however, this compound is readily fermented by colonic bacteria. Although fermentable fiber is commonly assumed to provide substrate for gas production, the standard dose of a psyllium results in a minimal increase in H2 excretion.19 Some components of normal meals interfere with absorption of nutrients and thereby increase colonic gas production; for example, fiber increases starch malabsorption,20 and a pancreatic amylase inhibitor in beans slows starch digestion and absorption.21 The high fasting H2 excretion observed in small intestinal bacterial overgrowth or untreated celiac disease has been attributed to fermentation of large quantities of mucus secreted by the intestine in these conditions.22
Table 16-1 Carbohydrate-Containing Foods That May Be Malabsorbed in the Healthy Human Small Intestine
| FOOD | MALABSORBED CARBOHYDRATE |
|---|---|
| Complex carbohydrates (wheat, corn, potatoes) | Resistant and retrograded starch (see text) |
| Dairy products (milk, ice cream, cottage cheese, yogurt) | Lactose |
| Dietetic candies and chewing gum | Mannitol, sorbitol, xylitol |
| Grains, fruits, vegetables | Fiber (hemicellulose, pectin, gums, mucilage) |
| Legumes (baked beans, soy beans) | Stachyose, raffinose |
| Soft drinks, honey | Fructose |
Gas-Consuming Flora
Some colonic bacteria consume intraluminal gases (H2, CO2, and O2), and this catabolism may account for a considerable proportion of intraluminal gas disposal.14,23,24 The vast majority of H2 released in the intestine normally is consumed by other bacteria, and the marked interindividual differences in breath H2 excretion observed after ingestion of a given dose of lactulose probably reflects differences in bacterial H2 consumption rather than differences in production. Sulfate-reducing bacteria use H2 to reduce sulfate to sulfide. Methanogenic bacteria use H2 to reduce CO2 to CH4.25–27 This reaction uses 5 moles of gas to produce 1 mole of CH4, and thus results in a reduction in bowel gas. Although methanogens are present in the feces of almost all adults, only about 40% of adults have sufficient concentrations of methanogens (106/g) to yield detectable breath CH4 concentrations.28 Sulfate-reducing bacteria and methanogens compete for H2, and feces of subjects usually contain one or the other type of organism. Analysis of flatus, however, has shown that subjects who excrete methane also can excrete appreciable H2S, suggesting that sulfate-reducing bacteria remain active in the proximal colons of these subjects.
The fraction of H2 that escapes consumption is determined by the efficiency of fecal stirring and the quantity and type of H2-consuming bacteria. Because a higher hydrogen tension is maintained in poorly stirred feces, a greater fraction of total H2 production is consumed in the semisolid feces of the left colon than in the liquid feces of the right colon. Consumption is markedly enhanced by the presence of methanogens, which oxidize H2 more rapidly than other H2-consuming bacteria. Studies of strongly methanogenic feces have suggested that if similar high concentrations of methanogens were present throughout the colon, almost no H2 would escape consumption. CH4-producing bacteria, however, are normally present in a high concentration only in the left colon.29 As a result, H2 liberated in the right colon is not acted on by methanogens until it reaches the left colon, thus explaining the reduced but still appreciable breath H2 excretion observed in most CH4-producing subjects.30 The inability of some subjects to increase breath H2 excretion after intestinal carbohydrate malabsorption probably reflects extremely efficient consumption of H2 by methanogens rather than a failure to produce H2.
Odoriferous Gases
None of the quantitatively important gases has an odor, and the unpleasant odor of feces results from gases present in trace quantities. The intensity of the noxious odor of flatus samples positively correlates with the concentrations of hydrogen sulfide and methanethiol.31 Hydrogen sulfide is released during bacterial metabolism of sulfate, cysteine, and mucin; therefore, both exogenous and endogenous compounds supply the substrate for this reaction. Methionine appears to be the favored substrate for methanethiol production. In addition to their noxious odor, hydrogen sulfide and methanethiol have toxicities similar to that of cyanide. The colonic mucosa protects itself from the damaging effect of these compounds via a highly developed system that metabolizes these gases to thiosulfate.32 This detoxification mechanism is so efficient that negligible quantities of these gases enter the blood perfusing the colon, and hydrogen sulfide and methanethiol of intestinal origin are not excreted in the breath.33 By contrast, an odoriferous sulfur-containing gas (allyl methyl sulfide) derived from garlic is not metabolized by the intestinal mucosa and is absorbed from the gut and excreted in expired air.33
ELIMINATION
The intestinal gas that is not absorbed or metabolized is eliminated via anal evacuation. Despite the many beliefs concerning which foods cause gas, scientific data concerning diet-related increases in rectal gas evacuation are sparse. Collection of gas using a rectal tube has shown a postprandial excretion rate of 15 mL/hr with a low-fiber diet, 93 mL/hr with a normal diet, 140 mL/hr after ingestion of Brussels sprouts, and 176 mL/hr with a pork and beans diet.16 Another study in which 24-hour gas evacuation was measured has shown that with a normal diet containing 200 g of beans, 705 mL (range, 476 to 1491 mL) was excreted per anus, and 50% of this gas was hydrogen, whereas with a fiber-free diet, the evacuation rate fell to 214 mL, with a low hydrogen content.34
The average frequency of passages of gas per rectum by healthy subjects is roughly 10 times daily, with an upper limit of normal of about 20 times daily.35 Neither age nor gender significantly correlates with flatus frequency.16 The composition of flatus is highly variable, depending on fermentative activity in the colon. In the presence of fermentable residues in the colon, the volume of flatus increases as a result of more rapid production of H2, CO2, and methane (in subjects with methanogenic flora), whereas N2 and O2 concentrations decline. Because absorbed H2 and CH4 are not metabolized, breath excretion of these gases equals their rates of absorption. Breath H2 excretion is the product of the alveolar ventilation rate and alveolar H2 concentration. Because alveolar ventilation is relatively constant in sedentary persons, the end-alveolar breath H2 concentration can be used as a simple indicator of total breath H2 excretion. Long-term simultaneous measurements of rectal and breath H2 excretion have been made in adult subjects maintained in an air-tight environment.36 Normally, breath H2 excretion averages about 50% of total (breath plus rectal) excretion; however, this percentage increases to 65% when production is low and decreases to 20% when production is high. Because the intestinal absorption process for H2 is not saturatable, the decreasing proportion of H2 excreted in the breath with rapid production presumably is a result of more rapid propulsion of this gas to the anus.
INTESTINAL PROPULSION, ACCOMMODATION, AND TOLERANCE OF GAS
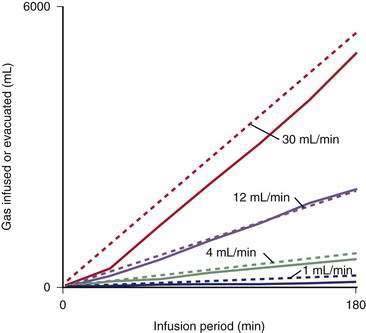
The rate of propulsion of gas in the intestine toward the anus is a crucial determinant of the volume of gas present in each segment of the gastrointestinal tract and the total volume in all segments at any moment. Gas transit determines the residence time of gas in the intestinal lumen; hence, the absorption and bacterial consumption of gas are influenced by transit time, as is the composition of gas evacuated from the rectum.37 Intestinal gas transit and tolerance have been measured using a gas challenge test, in which a mixture of gases is constantly infused into the jejunum and anal gas output is quantified (Fig. 16-4). An initial dose-response study using infusion rates of up to 30 mL/min (1.8 L/hr) has shown that most healthy subjects evacuate gas as rapidly as it is infused, with little or no discomfort.38 The intestine actively propels gas, with transit of gas more effectively in the erect than in the supine posture.39 Transit of gas, like that of solids and liquids, is modulated by a series of reflex mechanisms; intraluminal nutrients, particularly lipids, delay gas transit,40 whereas mechanical stimulation of the intestine (e.g., mild rectal distention) has a strong prokinetic effect.41 Gas is moved along the gastrointestinal tract far more rapidly than solids and liquids, but the type of motor activity that determines gas transit is not known. Infusion of gas does not induce detectable changes in small intestinal motility as recorded by manometry.42 By contrast, studies in which tonic (sustained motor) activity in the intestine was measured by means of a barostat have suggested that gas infusion induces tonic changes—contraction orad to the infusion site and relaxation distal to the collection site. Conceivably, movement and displacement of large masses of low-resistance gas is produced by subtle changes in tonic activity and capacitance of the intestine that do not affect the movement of solids and liquids.43 Gas boluses infused into the left colon have been shown to elicit forceful peristaltic contractions that precede small gas expulsions,44 but this type of phasic event has not been recorded during continuous gas infusion with a barostat located inside the rectum. Therefore, these phasic events could be a response to focal distention produced by abrupt delivery of intraluminal gas.
Gas transit is normally effective, but when an appreciable amount of gas is retained within the gastrointestinal tract (meteorism), subjects may develop abdominal distention and symptoms. Different experimental models of gas retention have been used to show that although abdominal distention is related to the volume of gas within the gut, perception of abdominal symptoms depends both on intestinal motor activity and on the intraluminal distribution of gas.45,46 When the propulsion of jejunal gas is inhibited with glucagon, gas accumulation is not associated with symptoms, suggesting that the reduction in bowel tone reduces awareness of the increase in bowel gas. Gas retention is better tolerated in the colon than in the small intestine. Retention of gas in the intestine stimulates an abdominal accommodation reflex that adapts the muscular activity of the anterior abdominal wall and the diaphragm to the volume load. Considerable colonic gas retention produces relatively small increments in girth in healthy persons, because the anterior abdominal wall contracts and the diaphragm relaxes.47 Therefore, this abdominophrenic coordination determines the increase in abdominal girth that results from an increase in intestinal contents.
CLINICAL GAS PROBLEMS
REPETITIVE ERUCTATION
Pathophysiology
The occasional belch expels gas swallowed with ingested solids or liquids. Repetitive eructation results from the inadvertent aspiration of air into the hypopharynx, most of which is immediately expelled (with inexplicable satisfaction to the subject), and only a small fraction enters the stomach. Frequently, the process is triggered by emotional stress or dyspeptic symptoms that patients misinterpret as excessive gas in the gastrointestinal tract. If appreciable swallowed air enters the stomach, discomfort may increase, resulting in more air swallowing—that is, a vicious cycle develops. Thus, chronic eructation is almost always a behavioral disorder, and radiographic and endoscopic evaluation should be reserved for patients who have associated symptoms or signs suggestive of thoracic or abdominal pathology.48,49 Difficulty with eructation after a fundoplication for gastroesophageal reflux disease results in the gas-bloat syndrome (see Chapter 43).
VOLUMINOUS FLATUS
Pathophysiology
Excessive flatulence usually results from intestinal flora proficient at producing gas (most likely because of reduced gas consumption), particularly when associated with a diet rich in fermentable residues. Most subjects who complain of excessive passage of rectal gas have no apparent bowel disease and produce excess gas on a seemingly normal diet. In a minority of persons, however, excess flatulence may be attributable to a condition that results in carbohydrate malabsorption (e.g., lactose malabsorption or celiac disease). A well-documented case of severe flatulence secondary to air swallowing has been reported.50 Frequent eructation and large volumes of gas in the stomach and small bowel suggest that the problem is caused by air swallowing. Gas chromatographic analysis of flatus collected via a rectal tube can differentiate air swallowing (N2 predominant) from intraluminal production (H2, CO2, and CH4 predominant) as the source of the gas, but this test is too complex to use in clinical practice.
Excessive passage of gas per anus may be a source of social embarrassment, but most affected persons do not complain of abdominal discomfort, because the normal intestine is able to propel and evacuate large gas loads without symptoms. Abdominal discomfort generally indicates associated irritable bowel syndrome (IBS).51
Treatment
Commercial preparations of β-galactosidase (Beano) are touted to enhance the digestion of the indigestible oligosaccharides present in legumes and other vegetables52; however, efficacy has only been demonstrated for the liquid preparation; tablets containing this enzyme may not be effective. Activated charcoal has been reported to reduce breath H2 excretion,53 but a second study has shown that charcoal does not bind H2 (nor any other quantitatively important intestinal gas) and does not reduce breath H2 excretion.54
Antibiotics, particularly rifaximin, have been claimed to reduce intestinal gas production,55 although decreased rectal gas excretion has not been demonstrated. Given the problems associated with chronic antibiotic therapy as well as the lack of clear-cut benefit, it seems inadvisable to use antibiotics to manipulate the flora of flatulent patients.
EXCESSIVELY ODORIFEROUS FLATUS
Pathophysiology
The odor of rectal gas results from trace gases, rather than the gases present in large volumes in flatus. As noted, sulfur-containing gases are associated with an offensive odor. Excessively malodorous flatus may be related to an overly proficient sulfate-reducing flora and the availability of sulfate-containing substrates (e.g., cruciferous vegetables, some amino acids) in the colon. These gases are rapidly absorbed across the mucosa (half-time in the lumen of less than one minute56; therefore, the passage of these gases in flatus is sensitive to the time between their release from the fecal mass and arrival at the rectum.
Treatment
Theoretically, a diet low in sulfur-containing compounds (e.g., cruciferous vegetables, beer, protein) should limit the production of sulfur-containing gases; however, the ability of dietary manipulations to reduce gas odor is totally anecdotal. Dietary measures that reduce total gas production (see earlier) also may be helpful. Several commercial devices use activated charcoal to adsorb odoriferous gases. These devices consist of charcoal-impregnated pads, underwear, and cushions. The efficacy of these devices has been tested by infusing hydrogen sulfide at the anus and measuring the fraction of this gas absorbed by the device. Cushions have been shown to be less effective than the pads or underwear.31,57 Orally administered products that have been tested for their ability to reduce the release of sulfur gases include activated charcoal (eight 260-mg tablets daily), which is ineffective,58 and the maximal dosage of bismuth subsalicylate, which is effective. The potential toxicity of long-term administration of bismuth subsalicylate, however, probably precludes this mode of therapy.
IMPAIRED GAS EVACUATION
In contrast to the patients with excessive flatus, some patients complain of gas retention associated with impaired fecal evacuation. Normally, evacuation of gas results from a mild increase in intra-abdominal pressure coupled with anal relaxation.59 Incoordination of this process produces functional outlet obstruction that may be associated with the sensation of difficult gas evacuation and constipation (see Chapter 18). Fecal retention prolongs colonic fermentation and could increase gas production. Patients with gas retention resulting from impaired anal evacuation may benefit from biofeedback treatment, which can help improve gas elimination and fecal evacuation.
ABDOMINAL BLOATING AND DISTENTION
Pathophysiology
Symptoms commonly attributed to too much gas, such as abdominal bloating and distention, are among the most frequently encountered gastrointestinal complaints.60 Bloating is an ambiguous term that refers to subjective sensations of a swollen abdomen, full belly, abdominal pressure, or excess gas. Abdominal distention refers to an objective increase in girth. Distention usually develops following meals or at the end of the day and resolves after an overnight rest. Tape measure and x-ray measurements have demonstrated objectively a clear-cut increase in abdominal girth with episodes of bloating, a finding confirmed by measurements using inductance plethysmography and CT imaging.61–65 Some patients with IBS, particularly those with rectal hypersensitivity, however, complain of bloating in the absence of objective distention.52,63 Understanding the complex relationship between gas and bloating complaints is important, because rational treatment is based on the altered physiology. A major question is to what extent subjective bloating and objective distention are associated with or caused by an increased rate of production or volume of intestinal gas.
Patients with IBS (who frequently complain of bloating) have been reported to have increased gas production caused by small intestinal bacterial overgrowth55 or malabsorption (see Chapter 118).66 This finding, however, has not been supported by other well-designed studies.67 A one-week study68 of hourly breath H2 concentrations during waking hours has shown no differences between patients with IBS and healthy subjects, despite a greater perception of bloating by the patients with IBS. Another study,69 in which total (breath plus anal) gas excretion was measured in a small number of patients with IBS and healthy subjects housed in a gas-tight environment, has demonstrated that the patients with IBS excrete more H2 but the total of H2 plus CH4 does not differ between the two groups.
Multiple studies have attempted to determine whether the gastrointestinal tracts of patients with bloating contain excessive gas. Intestinal gas washout studies have shown similar volumes of bowel gas in patients with IBS and healthy controls.2,70,71 Although some studies using plain abdominal radiography72,73 found that intra-abdominal gas content was greater in patients with IBS than in healthy subjects (by 54% to 118%), no significant correlations were observed between intestinal gas content and bloating. The most compelling evidence for the lack of an association between bloating and the volume of bowel gas was provided by a study in which a validated CT technique was used to show that most patients with bloating have normal volumes of bowel gas. Excessive gas was observed, however, in patients with severe motility disorders.65,74
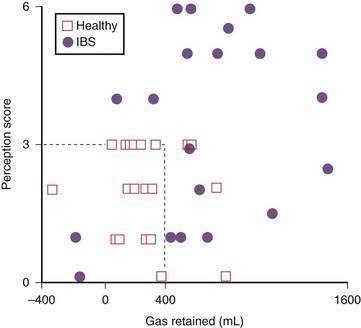
Although the volume of bowel gas seemingly is normal in persons with bloating, multiple studies using intestinal gas infusion have shown consistently that these patients have impaired handling of the infused gas. In response to an exogenous gas load, these patients exhibit gas retention, abdominal symptoms, or both (Fig. 16-5).2,70,71 These abnormalities apparently reflect impaired reflex control of gas transit40,51,75,76 as well as the frequently demonstrated hypersensitivity to bowel distention that characterizes patients with IBS.77–79 Therefore, gas transit studies seem to provide a sensitive method of identifying subtle intestinal motor disturbances not detectable by conventional diagnostic tests. Although total gas volume is not increased in patients with IBS, disturbances in gas propulsion may lead to localized gas accumulations (e.g., the splenic flexure syndrome) that cause symptoms in the hyperreactive intestines of patients with bloating.
A study of patients with IBS in which CT images were compared under basal conditions and during an episode of severe distention has demonstrated that the sensation of distention is associated with an increase in the anteroposterior diameter of the abdomen and a significant diaphragmatic descent, but only a modest increment in intestinal gas content.65 Electromyographic studies have shown that the abdomen normally adapts to an increase in contents via a coordinated abdominophrenic response.47 Patients with IBS have an incoordination (dyssynergia) between diaphragmatic contraction and anterior wall relaxation in response to increased intra-abdominal volume loads.80,81 The result is that minor increases in bowel contents that are well tolerated by healthy subjects cause discomfort, a sense of abdominal distention, and an increase in abdominal girth in patients with bloating.
Treatment
Because patients with bloating and abdominal distention seem to have a common variant of IBS, the basic approach to treatment should be similar to that prescribed for IBS (see Chapter 118). These patients, however, may have a disorder resulting from a number of altered pathophysiologic mechanisms. A hypersensitive gut, for example, may be associated with impaired anal evacuation, particularly in patients with constipation-predominant IBS, and symptoms will worsen if gas production is increased. In these patients, the treatment strategy may need to be modified.82 Many therapies have been claimed to relieve bloating, but the few well-controlled studies were directed toward symptoms of IBS in general, not bloating specifically.
Nonpharmacologic Therapies
Intestinal clearance of perfused gas is increased by mild exercise and the erect posture, which may explain anecdotal observations that activity (as opposed to resting in the supine posture) improves bloating symptoms.39,83,84 Although intestinal gas volumes appear to be normal in patients with bloating, the sensitivity of their intestines to normal volumes of bowel contents suggests that limiting gas production to a minimum may be beneficial. Therefore, dietary manipulations to reduce gas production (described earlier) may be beneficial. Low-fiber85 and low-residue diets also have been reported to improve symptoms.86 More than 20 reports on the use of probiotics to reduce symptoms of IBS have been published. The results are promising but inconsistent, possibly depending on the bacterial species used, doses, duration of treatment, and endpoints used for evaluation. Hypnosis has been reported to reduce symptoms of IBS, including bloating.87
Pharmacologic Therapies
Although studies have suggested that antibiotics, particularly rifaximin, can reduce symptoms of IBS,55 IBS may first appear after antibiotic therapy.88,89 Until more data are available, using antibiotics to treat bloating seems inadvisable. Simethicone has defoaming properties that eliminates bubbles that might trap gas,90 but it does not reduce the volume of gas. The effectiveness of this compound in the treatment of gas symptoms remains controversial.91
Neostigmine, a potent prokinetic agent, has been reported to reduce abdominal symptoms resulting from an intestinal infusion of gas. Chronic administration of pyridostigmine improves symptoms in patients complaining of bloating but has only marginal effects on intestinal gas content.71,74 In placebo-controlled trials, the prokinetic agents metoclopramide and the restricted drug cisapride have produced statistically significant reductions in complaints of abdominal distention.92,93 Tegaserod (no longer available in the United States) also has been shown to reduce bloating and distention in some, but not all, controlled trials carried out with this agent.94
As noted, patients with bloating have reduced tolerance to gas infused in the intestine, and the perception of intestinal distention by healthy subjects is reduced when intestinal motor activity is inhibited by glucagon. Therefore, drugs that reduce intestinal motility theoretically could enhance the tolerance of the hypersensitive gut to normal volumes of gas. Anticholinergic drugs such as hyoscyamine, dicyclomine, or scopolamine inhibit intestinal motility and may reduce the response to bowel gas. A meta-analysis of the efficacy of smooth muscle relaxants in the treatment of IBS46,95 has concluded that these drugs are superior to placebo in the management of symptoms, specifically abdominal pain and distention. These drugs, however, may enhance gas retention, and anecdotal evidence suggests that their administration may actually worsen abdominal distention in some patients. Peppermint oil has an antispasmodic effect on the gastrointestinal tract because of the calcium channel blocker activity of its active constituent, menthol.96 Although some studies have reported improvement in abdominal distention and reduction in flatus emission with peppermint oil, a meta-analysis97 has indicated that its benefit in IBS is questionable. Drugs that act on the efferent nerves of the intestine also might be effective. For example, low-dose tricyclic antidepressants and possibly selective serotonin reuptake inhibitors have proven useful in the treatment of abdominal pain and symptoms of IBS, an effect probably related to their antinociceptive action (see Chapter 118).98
PNEUMATOSIS CYSTOIDES INTESTINALIS
Pneumatosis cystoides intestinalis and coli is a condition characterized by the presence of gas-filled cysts in the wall of the small bowel, colon, or both (see Chapter 124). The cysts may be asymptomatic or associated with diarrhea, bloating, or abdominal pain. Many patients with pneumatosis have extremely high breath H2 concentrations, a finding indicative of high luminal concentrations of H2.99,100 The feces of three patients with pneumatosis of the colon were found to have unusually low concentrations of H2-consuming organisms, and a patient with pneumatosis limited to the small intestine had small bowel contents that produced but could not consume H2. Therefore, the high luminal H2 of these subjects appears to reflect H2 production that is relatively unopposed by H2 consumption. An association between pneumatosis and chronic administration of chloral hydrate seemingly is explained by the ability of chloral hydrate to inhibit H2 consumption by intestinal flora.101
How a high luminal H2 tension results in pneumatosis is controversial. One proposal is that the high luminal H2 results in supersaturation of tissue with H2. As a result, H2 bubbles form via a process similar to that which results in tissue collections of gas in deep sea divers.102 A second theory proposes that small intramural gas collections normally occur with some frequency, but are quickly absorbed into the circulation. In the presence of high H2 production, rapid diffusion of luminal H2 into the cyst dilutes other cyst gases (e.g., N2).100 Thus, the cyst N2 tension remains lower than or equal to that in the blood. As a result, N2 in the cyst cannot be absorbed and the cyst persists. The most effective treatment to eliminate the cysts is the administration of high concentrations of O2 via inhalation.103 This maneuver reduces the blood N2 tension to a value below that of the cyst, allowing N2 to diffuse from the cyst into the blood, with resolution of the cyst. Other forms of therapy that may be effective are heliox (a low-density gas mixture), antibiotics that inhibit H2 production (ciprofloxacin has been used successfully in a patient with small intestinal bacterial overgrowth and pneumatosis cystoides intestinalis), and dietary manipulations, such as lactose restriction, that reduce the delivery of fermentable substrate to the colonic bacteria.
Accarino A, Perez F, Azpiroz F, et al. Intestinal gas and bloating: Effect of prokinetic stimulation. Am J Gastroenterol. 2008;103:2036-42. (Ref 74.)
Agrawal A, Houghton LA, Lea R, et al. Bloating and distention in irritable bowel syndrome: The role of visceral sensation. Gastroenterology. 2008;134:1882-9. (Ref 63.)
Azpiroz F, Malagelada J-R. Abdominal bloating. Gastroenterology. 2005;129:1060-78. (Ref 60.)
Bredenoord AJ, Smout AJ. Physiologic and pathologic belching. Clin Gastroenterol Hepatol. 2007;5:772-5. (Ref 5.)
Houghton LA, Lea R, Agrawal A, et al. Relationship of abdominal bloating to distention in irritable bowel syndrome and effect of bowel habit. Gastroenterology. 2006;131:1003-10. (Ref 62.)
Levitt MD, Furne J, Aeolus MR, et al. Evaluation of an extremely flatulent patient: Case report and proposed diagnostic and therapeutic approach. Am J Gastroenterol. 1998;11:2276-81. (Ref 50.)
Levitt MD, Furne J, Olsson S. The relation of passage of gas an abdominal bloating to colonic gas production. Ann Intern Med. 1996;124:422-4. (Ref 19.)
Levitt M, Olsson S. Pneumatosis cystoides intestinalis and high breath H2 excretion: Insights into the role of H2 in this condition. Gastroenterology. 1995;108:1560-5. (Ref 100.)
Passos MC, Tremolaterra F, Serra J, et al. Impaired reflex control of intestinal gas transit in patients with abdominal bloating. Gut. 2005;54:344-8. (Ref 75.)
Perez F, Accarino A, Azpiroz F, et al. Gas distribution within the human gut: Effect of meals. Am J Gastroenterol. 2007;102:842-9. (Ref 8.)
Posserud I, Stotzer PO, Bjornsson ES, et al. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802-8. (Ref 67.)
Salvioli B, Serra J, Azpiroz F, et al. Origin of gas retention and symptoms in patients with bloating. Gastroenterology. 2005;128:574-9. (Ref 51.)
Suarez F, Furne J, Springfield J, et al. Insights into human colonic physiology obtained from the study of flatus composition. Am J Physiol. 1997;272:G1028-33. (Ref 14.)
Suarez FL, Springfield J, Levitt MD. Identification of gases responsible for the odour of human flatus and evaluation of a device purported to reduce this odour. Gut. 1998;43:100-4. (Ref 31.)
Tremolaterra F, Villoria A, Azpiroz F, et al. Impaired viscerosomatic reflexes and abdominal wall dystony associated with bloating. Gastroenterology. 2006;130:1062-8. (Ref 81.)
1. Levitt MD. Volume and composition of human intestinal gas determined by means of an intestinal washout technique. N Engl J Med. 1971;284:1394-8.
2. Serra J, Azpiroz F, Malagelada J-R. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut. 2001;48:14-9.
3. Levitt MD, Bond JH, Levitt DG. Gastrointestinal gas. In: Johnson LR, editor. Physiology of the gastrointestinal tract. New York: Raven Press; 1981:497.
4. Pouderoux P, Ergun GA, Lin S, et al. Esophageal bolus transit imaged by ultrafast computerized tomography. Gastroenterology. 1996;110:1422-8.
5. Bredenoord AJ, Smout AJ. Physiologic and pathologic belching. Clin Gastroenterol Hepatol. 2007;5:772-5.
6. Fordtran JS, Walsh JH. Gastric acid secretion rate and buffer content of the stomach after eating: Results in normal subjects and in patients with duodenal ulcer. J Clin Invest. 1973;52:645-57.
7. Rune SJ. Acid-base parameters of duodenal contents in man. Gastroenterology. 1972;62:533-9.
8. Perez F, Accarino A, Azpiroz F, et al. Gas distribution within the human gut: Effect of meals. Am J Gastroenterol. 2007;102:842-9.
9. Stephen AM, Cummings JH. Mechanism of action of dietary fibre in the human colon. Nature. 1980;284:283-4.
10. Hertzler SR, Savaiano DA. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am J Clin Nutr. 1996;64:232-6.
11. Hertzler SR, Savaiano DA, Levitt MD. Fecal hydrogen production and consumption measurements. Response to daily lactose ingestion by lactose maldigesters. Dig Dis Sci. 1997;42:348-53.
12. Levitt MD, Bond JH. Volume, composition, and source of intestinal gas. Gastroenterology. 1970;59:921-9.
13. Levitt MD. Intestinal gas production—recent advances in flatology. N Engl J Med. 1980;302:1474-5.
14. Suarez F, Furne J, Springfield J, et al. Insights into human colonic physiology obtained from the study of flatus composition. Am J Physiol. 1997;272:G1028-33.
15. Levitt MD. Production and excretion of hydrogen gas in man. N Engl J Med. 1969;281:122-7.
16. Steggerda FR. Gastrointestinal gas following food consumption. Ann. N Y Acad Sci. 1968;150:57-66.
17. Levitt MD, Hirsh P, Fetzer CA, et al. H2 excretion after ingestion of complex carbohydrates. Gastroenterology. 1987;92:383-9.
18. Scheppach W, Bach M, Bartram P, et al. Colonic fermentation of potato starch after a freeze-thaw cycle. Dig Dis Sci. 1991;36:1601-5.
19. Levitt MD, Furne J, Olsson S. The relation of passage of gas an abdominal bloating to colonic gas production. Ann Intern Med. 1996;124:422-4.
20. Hamberg O, Rumessen JJ, Gudmand-Hoyer E. Inhibition of starch absorption by dietary fibre. A comparative study of wheat bran, sugar-beet fibre, and pea fibre. Scand J Gastroenterol. 1989;24:103-9.
21. Boibin M, Flourié B, Rizza RA, et al. Gastrointestinal and metabolic effects of amylase inhibition in diabetics. Gastroenterology. 1998;1988:387-94.
22. Perman JA, Modler S. Glycoproteins as substrates for production of hydrogen and methane by colonic bacterial flora. Gastroenterology. 1982;82:911-16.
23. Strocchi A, Levitt MD. Factors affecting hydrogen production and consumption by human fecal flora. The critical roles of hydrogen tension and methanogenesis. J Clin Invest. 1992;89:1304-11.
24. Gibson GR, Cummings JH, Macfarlane GT, et al. Alternative pathways for hydrogen disposal during fermentation in the human colon. Gut. 1990;31:679-83.
25. Flourie B, Pellier P, Florent C, et al. Site and substrates for methane production in human colon. Am J Physiol. 1991;260:G752-7.
26. Kajs TM, Fitzgerald JA, Buckner RY, et al. Influence of a methanogenic flora on the breath H2 and symptom response to ingestion of sorbitol or oat fiber. Am J Gastroenterol. 1997;92:89-94.
27. Strocchi A, Furne J, Ellis C, et al. Methanogens outcompete sulphate reducing bacteria for H2 in the human colon. Gut. 1994;35:1098-101.
28. Weaver GA, Krause JA, Miller TL, et al. Incidence of methanogenic bacteria in a sigmoidoscopy population: An association of methanogenic bacteria and diverticulosis. Gut. 1986;27:698-704.
29. Flourie B, Etanchaud F, Florent C, et al. Comparative study of hydrogen and methane production in the human colon using caecal and faecal homogenates. Gut. 1990;31:684-5.
30. Cloarec D, Bornet F, Gouilloud S, et al. Breath hydrogen response to lactulose in healthy subjects: Relationship to methane producing status. Gut. 1990;31:300-4.
31. Suarez FL, Springfield J, Levitt MD. Identification of gases responsible for the odour of human flatus and evaluation of a device purported to reduce this odour. Gut. 1998;43:100-4.
32. Levitt MD, Furne J, Springfield J, et al. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J Clin Invest. 1999;104:1107-14.
33. Suarez F, Springfield J, Furne J, et al. Differentiation of mouth versus gut as site of origin of odoriferous breath gases after garlic ingestion. Am J Physiol. 1999;276:G425-30.
34. Tomlin J, Lowis C, Read NW. Investigation of normal flatus production in healthy volunteers. Gut. 1991;32:665-9.
35. Levitt MD, Furne J, Olson S. Relation of passage of gas and abdominal bloating to colonic gas production. Ann Intern Med. 1996;124:422-4.
36. Christl SU, Murgatroyd PR, Gibson GR, Cummings JH. Production, metabolism, and excretion of hydrogen in the large intestine. Gastroenterology. 1992;102:1269-77.
37. El Oufir L, Flourie B, des Varannes SB, et al. Relations between transit time, fermentation products, and hydrogen consuming flora in healthy humans. Gut. 1996;30:870-7.
38. Serra J, Azpiroz F, Malagelada J-R. Intestinal gas dynamics and tolerance in humans. Gastroenterology. 1998;115:542-50.
39. Dainese R, Serra J, Azpiroz F, et al. Influence of body posture on intestinal transit of gas. Gut. 2003;52:971-4.
40. Serra J, Salvioli B, Azpiroz F, et al. Lipid-induced intestinal gas retention in the irritable bowel syndrome. Gastroenterology. 2002;123:700-6.
41. Harder H, Serra J, Azpiroz F, et al. Reflex control of intestinal gas dynamics and tolerance. Am J Physiol. 2004;286:G89-94.
42. Galati JS, McKee DP, Quigley EM. Response to intraluminal gas in irritable bowel syndrome. Motility versus perception. Dig Dis Sci. 1995;40:1381-7.
43. Tremolaterra F, Serra J, Azpiroz F, et al. Intestinal tone and gas motion. Neurogastroenterol Mot. 2003;15:581.
44. Bassotti G, Germani U, Morelli A. Flatus-related colorectal and anal motor events. Dig Dis Sci. 1996;41:335-8.
45. Harder H, Serra J, Azpiroz F, et al. Intestinal gas distribution determines abdominal symptoms. Gut. 2003;52:1708-13.
46. Serra J, Azpiroz F, Malagelada J-R. Mechanisms of intestinal gas retention in humans:impaired propulsion versus obstructed evacuation. Am J Physiol. 2001;281:G138-43.
47. Villoria A, Azpiroz F, Soldevilla A, et al. Abdominal accommodation: A coordinated adaptation of the abdominal walls to its content. Am J Gastroenterol. 2008;103:2807-15.
48. Bredenoord AJ, Weusten BL, Sifrim D, et al. Aerophagia, gastric, and supragastric belching: A study using intraluminal electrical impedance monitoring. Gut. 2004;53:1561-5.
49. Bredenoord AJ, Weusten BL, Timmer R, et al. Psychological factors affect the frequency of belching in patients with aerophagia. Am J Gastroenterol. 2006;101:2777-81.
50. Levitt MD, Furne J, Aeolus MR, et al. Evaluation of an extremely flatulent patient: Case report and proposed diagnostic and therapeutic approach. Am J Gastroenterol. 1998;11:2276-81.
51. Salvioli B, Serra J, Azpiroz F, et al. Origin of gas retention and symptoms in patients with bloating. Gastroenterology. 2005;128:574-9.
52. Ganiats TG, Norcross WA, Halverson AL, et al. Does Beano prevent gas? A double-blind crossover study of oral alpha-galactosidase to treat dietary oligosaccharide intolerance. J Fam Pract. 1994;39:441-5.
53. Hall GHJr, Thompson H, Strother A. Effects of orally administered activated charcoal on intestinal gas. Am J Gastroenterol. 1981;75:192-6.
54. Potter T, Ellis C, Levitt MD. Activated charcoal: In vivo and in vitro studies of effect on gas formation. Gastroenterology. 1985;88:620-4.
55. Pimentel M, Park S, Mirocha J, et al. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: A randomized trial. Ann Intern Med. 2006;145:557-63.
56. Suarez FL, Furne JK, Springfield JR. Production and elimination of sulfur-containing gases in the rat colon. Am J Physiol. 1998;274:G727-33.
57. Ohge H, Furne JK, Springfield J, et al. Effectiveness of devices purported to reduce flatus odor. Am J Gastroenterol. 2005;100:397-400.
58. Suarez FL, Furne J, Springfield J, et al. Failure of activated charcoal to reduce the release of gases produced by the colonic flora. Am J Gastroenterol. 1999;94:208-12.
59. Azpiroz F, Enck P, Whitehead WE. Anorectal functional testing. Review of a collective experience. Am J Gastroenterol. 2002;97:232-40.
60. Azpiroz F, Malagelada J-R. Abdominal bloating. Gastroenterology. 2005;129:1060-78.
61. Maxton DG, Martin DF, Whorwell P, et al. Abdominal distention in female patients with irritable bowel syndrome: Exploration of possible mechanisms. Gut. 1991;32:662-4.
62. Houghton LA, Lea R, Agrawal A, et al. Relationship of abdominal bloating to distention in irritable bowel syndrome and effect of bowel habit. Gastroenterology. 2006;131:1003-10.
63. Agrawal A, Houghton LA, Lea R, et al. Bloating and distention in irritable bowel syndrome: The role of visceral sensation. Gastroenterology. 2008;134:1882-9.
64. Agrawal A, Whorwell PJ. Review article: Abdominal bloating and distention in functional gastrointestinal disorders—epidemiology and exploration of possible mechanisms. Aliment Pharmacol Ther. 2008;27:2-10.
65. Perez F, Accarino A, Azpiroz F, et al. Abdominal bloating: Distinctive features of organic versus functional disorders. Gut. 2007;56:A39.
66. Rumessen JJ, Gudmand-Hoyer E. Functional bowel disease: Malabsorption and abdominal distress after ingestion of fructose, sorbitol, and fructose-sorbitol mixtures. Gastroenterology. 1988;95:694-700.
67. Posserud I, Stotzer PO, Bjornsson ES, et al. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802-8.
68. Haderstorfer B, Whitehead WE, Schuster MM. Intestinal gas production from bacterial fermentation of undigested carbohydrate in irritable bowel syndrome. Am J Gastroenterol. 1989;84:375-8.
69. King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352:1187-9.
70. Lasser RB, Bond JH, Levitt MD. The role of intestinal gas in functional abdominal pain. N Engl J Med. 1975;293:524-6.
71. Caldarella MP, Serra J, Azpiroz F, et al. Prokinetic effects in patients with intestinal gas retention. Gastroenterology. 2002;122:1748-55.
72. Chami TN, Schuster MM, Bohlman ME, et al. A simple radiologic method to estimate the quantity of bowel gas. Am J Gastroenterol. 1991;86:599-602.
73. Koide A, Yamaguchi T, Odaka T, et al. Quantitative analysis of bowel gas using plain abdominal radiograph in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1735-41.
74. Accarino A, Perez F, Azpiroz F, et al. Intestinal gas and bloating: Effect of prokinetic stimulation. Am J Gastroenterol. 2008;103:2036-42.
75. Passos MC, Tremolaterra F, Serra J, et al. Impaired reflex control of intestinal gas transit in patients with abdominal bloating. Gut. 2005;54:344-8.
76. Salvioli B, Serra J, Azpiroz F, et al. Impaired small bowel gas propulsion in patients with bloating during intestinal lipid infusion. Am J Gastroenterol. 2006;101:1853-7.
77. Accarino AM, Azpiroz F, Malagelada J-R. Selective dysfunction of mechanosensitive intestinal afferents in the irritable bowel syndrome. Gastroenterology. 1995;108:636-43.
78. Kellow JE, Azpiroz F, Delvaux M, et al. Applied principles of neurogastroenterology: Physiology/motility sensation. Gastroenterology. 2006;130:1412-20.
79. Azpiroz F. From sensation to perception: the gut brain connection. In: Pasricha J, Willis WD, Gebhart GF, editors. Chronic abdominal and visceral pain. Theory and practice. Boca Raton, Fla: CRC Press; 2007:193.
80. Villoria A, Azpiroz F, Malagelada J-R. Abdomino-phrenic dyssynergia, abdominal bloating and distension. Neurogastroenterol Mot. 2006;18:A229.
81. Tremolaterra F, Villoria A, Azpiroz F, et al. Impaired viscerosomatic reflexes and abdominal wall dystony associated with bloating. Gastroenterology. 2006;130:1062-8.
82. Azpiroz F, Serra J. Treatment of excessive intestinal gas. Curr Treat Options Gastroenterol. 2004;7:299-305.
83. Dainese R, Serra J, Azpiroz F, et al. Effect of physical activity on intestinal gas transit and evacuation in healthy subjects. Am J Med. 2004;116:536-9.
84. Villoria A, Serra J, Azpiroz F, et al. Physical activity and intestinal gas clearance in patients with bloating. Am J Gastroenterol. 2006;101:2552-7.
85. Francis CY, Whorwell PJ. Bran and irritable bowel syndrome: Time for reappraisal. Lancet. 1994;344:39-40.
86. Pimentel M, Constantino T, Kong Y, et al. A 14-day elemental diet is highly effective in normalizing the lactulose breath test. Dig Dis Sci. 2004;49:73-7.
87. Lea R, Houghton LA, Calvert EL, et al. Gut-focused hypnotherapy normalizes disordered rectal sensitivity in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:635-42.
88. Mendall MA, Kumar D. Antibiotic use, childhood affluence and irritable bowel syndrome (IBS). Eur J Gastroenterol Hepatol. 1998;10:59-62.
89. Maxwell PR, Rink E, Kumar D, et al. Antibiotics increase functional abdominal symptoms. Am J Gastroenterol. 2002;97:104-8.
90. Brecevic L, Bosan-Kilibarda I, Strajnar F. Mechanism of antifoaming action of simethicone. J Appl Toxicol. 1994;14:207-11.
91. Holtmann G, Gschossman JM, McAfee JG, et al. A randomized placebo-controlled trial of simethicone and cisapride for the treatment of patients with functional dyspepsia. Aliment Pharmacol Ther. 2002;16:1641-8.
92. Johnson AG. Controlled trial of metoclopramide in the treatment of flatulent dyspesia. BMJ. 1971;2:25-6.
93. Van Outryve M, Milo R, Toussaint J, et al. “Prokinetic” treatment of constipation—predominant irritable bowel syndrome: A placebo-controlled study of cisapride. J Clin Gastroenterol. 1991;13:49-57.
94. Evans BW, Clark WK, Moore DJ, et al. Tegaserod for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2004; (1):CD003960.
95. Poynard T, Regimbeau C, Benhamou Y. Meta-analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2001;15:355-61.
96. Hills JM, Aaronson PI. The mechanism of action of peppermint oil on gastrointestinal smooth muscle. Gastroenterology. 1991;101:55-65.
97. Pittler MH, Ernst E. Peppermint oil for irritable bowel syndrome: A critical review and methaanalysis. Am J Gastroenterol. 1998;93:1131-5.
98. Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: A meta-analysis. Am J Med. 2000;108:65-72.
99. Christl SU, Gibson GR, Murgatroyd PR, et al. Impaired hydrogen metabolism in pneumatosis cystoides intestinalis. Gastroenterology. 1993;104:392-7.
100. Levitt M, Olsson S. Pneumatosis cystoides intestinalis and high breath H2 excretion: Insights into the role of H2 in this condition. Gastroenterology. 1995;108:1560-5.
101. Florin TH. Alkyl halides, super hydrogen production and the pathogenesis of pneumatosis cystoides coli. Gut. 1997;41:778-84.
102. Florin TH, Hills BA. Does counterperfusion supersaturation cause gas cysts in pneumatosis cystoides coli, and can breathing heliox reduce them? Lancet. 1995;345:1220-2.
103. Forgacs P, Wright PH, Wyatt AP. Treatment of intestinal gas cysts by oxygen breathing. Lancet. 1973;1:579-82.