198 Intestinal and Multivisceral Transplantation
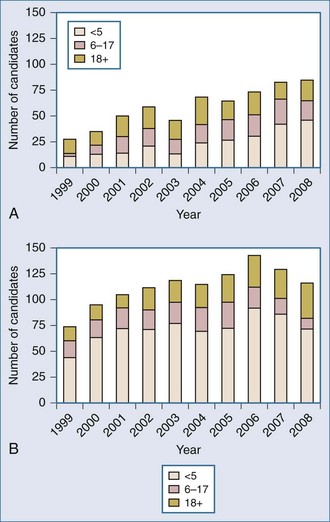
The ongoing development of intestinal and multivisceral transplantation remains a dynamic process moved forward by advances in multidisciplinary care of intestinal failure, surgical technique, innovative immunosuppressive strategies, and an improved understanding of intestinal transplantation immunology. Recognition of intestinal transplantation as an established modality for select intestinal failure patients and better outcomes over the past decade have led to an increasing number of candidates referred for intestinal transplantation each year (Figure 198-1) and allowed more patients to benefit. In the United States alone, nearly 700 patients are alive with a functioning intestinal allograft as of December 2007.1 Although the time interval between listing and intestinal transplant has decreased over the past decade (Figure 198-2), waitlist mortality remains high, particularly for infants and adults with concomitant liver failure.2 Immunosuppression for intestinal and multivisceral transplantation now commonly involves perioperative antibody induction. The inability to prevent and treat chronic rejection in isolated intestinal allografts continues to be a fundamental barrier to achieving successful long-term outcomes and is the subject of rigorous investigation. Long-term data on nutritional outcomes and transplantation morbidity will help further define the optimal timing and role of intestinal and multivisceral transplantation in patients with intestinal failure.
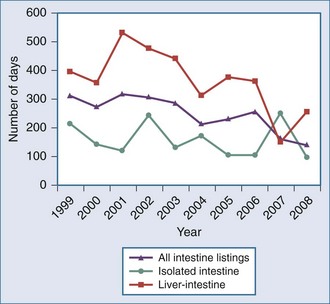
Figure 198-2 Median time to transplant for intestine waiting list registrants, 1999-2008.
(Adapted from Mazariegos GV, Steffick DE, Horslen S, Farmer D, Fryer J, Grant D et al. Intestine transplantation in the United States, 1999-2008. Am J Transplant 2010;10:1020-34.)
 Management of Intestinal Failure
Management of Intestinal Failure
Intestinal failure is clinically defined as the loss of nutritional autonomy secondary to bowel dysfunction. Patients with intestinal failure are initially managed by administration of total parenteral nutrition (TPN) through central venous access. The duration of intestinal failure is variable and in certain patients unpredictable, from short-term to lifelong, and depends largely on the adaptation capacity of the remaining viable intestine. Improved long-term outcomes in TPN-dependent pediatric patients have been reported recently by single centers.3,4,5 Nonetheless, there remains a significant subset of patients who develop irreversible intestinal failure and require indefinite TPN therapy with its attendant complications. Intestinal transplantation may be lifesaving in this group of patients.6
Optimal management of the patient with intestinal failure is achieved after a detailed multidisciplinary evaluation.7,8 Obtaining a comprehensive history is critical and must include birth and disease history, past surgical procedures, infections, number and location of previous central venous lines, presence of central venous thrombosis, a detailed nutrition history including duration of TPN, details of TPN prescriptions and maximal enteral feeding tolerance, as well as medication history and frequency/volume of stools. A careful history and physical examination by the intestine rehabilitation team is critical to the process of achieving a complete pretransplant workup. Further investigations may include upper gastrointestinal (GI) contrast study with small-bowel follow-through, contrast enema if indicated, abdominal sonogram, ultrasound exam of central venous anatomy, endoscopy with small-intestinal aspiration for quantitative microbial culture and mucosal biopsy, and liver biopsy if there is evidence of liver dysfunction or portal hypertension.
Management of the patient with intestinal failure focuses on optimization of gut adaptation and recovery of intestinal function to achieve enteral autonomy. Surgical therapies that have a role in adaptation after intestinal failure include serial transverse enteroplasty (STEP).9 Alternatively, if gut dysfunction is considered irreversible, management of these patients concentrates on maintaining optimal growth in children and nutritional repletion in adults to prepare them for eventual intestinal transplantation.
Parenteral nutrition–associated liver disease (PNALD), also referred to as intestinal failure–associated liver disease (IFALD), remains a critical problem in this patient population, affecting infants disproportionately. The 1-year mortality of patients with PNALD exceeds 80% in the absence of TPN weaning or transplantation. Although not always feasible, the best strategy to prevent and treat PNALD involves a commitment to the advancement of enteral nutrition. Despite a conscientious approach to TPN therapy, many children and adults still develop cholestasis relatively early in their clinical course. Prevention and timely treatment of infection, minimizing SBBO, preventing overfeeding with dextrose, providing adequate amino acids, cycling TPN, providing TPN-free days when possible, and providing taurine to neonates are probably all important measures to slow the progression of PNALD.10 Stasis of bile in the non-stimulated biliary system and gallbladder can lead to sludge buildup and cholelithiasis. In the authors’ experience, cholecystectomy rarely improves liver function and is not indicated for PNALD alone. None of the components of standard parenteral nutrition solutions have been conclusively shown to cause or contribute to PNALD, but excessive glucose and improper ratios of glucose to amino acid have been associated with hepatic steatosis. Recently, interest in the manipulation of the lipid component of TPN has led some to advocate for the removal of soy-based lipid solutions or their substitution with Omegaven (a fish-oil-based, intravenous [IV] lipid solution rich in omega-3 fatty acids); however, substantive evidence that these measures retard or reverse the progression of liver disease has not yet been demonstrated.11,12,13 Many clinicians will add but not entirely substitute fish-oil-based lipids for soy-based solutions only after liver function tests demonstrate abnormalities.
Indications For Transplant
In October 2000, the Center for Medicare and Medicaid Services approved intestinal, combined liver-intestine, and multivisceral transplantation as a standard of care for patients with irreversible intestinal failure who could no longer be maintained with TPN. Intestinal and multivisceral transplantation are now considered for patients with irreversible intestinal failure who fail TPN therapy due to complications, who cannot tolerate quality-of-life limitations associated with TPN therapy, or who must undergo native bowel resection for potentially life-limiting indications. The myriad causes of bowel dysfunction can be subcategorized into acute and chronic pathophysiologies. Common causes of acute dysfunction include necrotizing enterocolitis, volvulus, and mesenteric thrombosis. Common causes of chronic dysfunction include Crohn’s disease and radiation enteritis. These disease processes can alternatively be classified as either surgical due to resection leading to short bowel syndrome (SBS) or nonsurgical due to congenital enterocyte disorders leading to dysmotility or malabsorption. Unlike patients with SBS, patients with nonsurgical causes of intestinal failure may have native intestine which demonstrates normal gross morphology and anatomic length. Table 198-1 lists the already well-described indications for intestinal and multivisceral transplantation.
TABLE 198-1 Indications for Intestinal and Multivisceral Transplantation
| Pediatric Patients | Adult Patients |
|---|---|
| Volvulus | Superior mesenteric artery thrombosis |
| Gastroschisis | Crohn’s disease/irritable bowel disease (IBD) |
| Necrotizing enterocolitis | Desmoid tumor |
| Pseudo-obstruction | Volvulus |
| Microvillus inclusion disease | Trauma |
| Intestinal polyposis | Familial polyposis |
| Hirschsprung’s disease | Gastrinoma |
| Trauma | Budd-Chiari disease |
| Intestinal adhesions | |
| Pseudo-obstruction | |
| Radiation enteritis |
Owing to the particularly high morbidity and mortality of children with PNALD, increasing efforts have been made by the pediatric medical community to optimize timing of referral of these patients to specialized intestine-failure rehabilitation centers and transplant centers to improve overall outcomes. A recent expert consensus panel14 recommended the following pediatric criteria for consultation or referral for small-bowel transplant assessment: (1) children with massive small-bowel resection, (2) children with severely diseased bowel and unacceptable morbidity, (3) continuing prognostic or diagnostic uncertainty, (4) microvillus inclusion disease or intestinal epithelial dysplasia, (5) persistent hyperbilirubinemia (>6 g/dL), (6) thrombosis of 2 of 4 upper body central veins, (7) the request of the patient or family.
 Transplantation Procedures
Transplantation Procedures
Brief descriptions of recipient operations are provided. The multivisceral donor procurement operation has already been well described.15
Isolated Intestinal Transplant
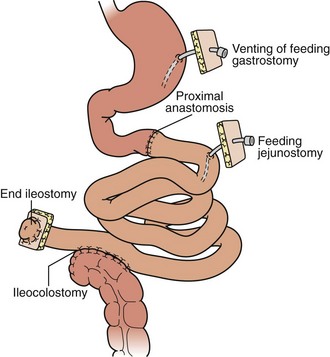
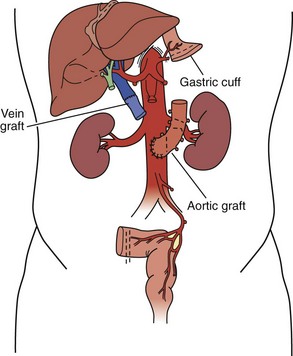
For isolated intestinal transplant (Figure 198-3), the donor intestinal graft (jejunum and ileum) is procured along with donor vascular conduits, including an artery (iliac and/or carotid) and a vein (iliac). The donor superior mesenteric vessels are occasionally anastomosed directly to the recipient superior mesenteric artery and vein if adequate length is achieved. More commonly, interposition vascular conduits are anastomosed to the recipient infrarenal aorta and recipient superior mesenteric vein (portal drainage) or inferior vena cava (systemic drainage) to provide sufficient length and proper orientation for the allograft.
Combined Small-Bowel And Liver Transplant
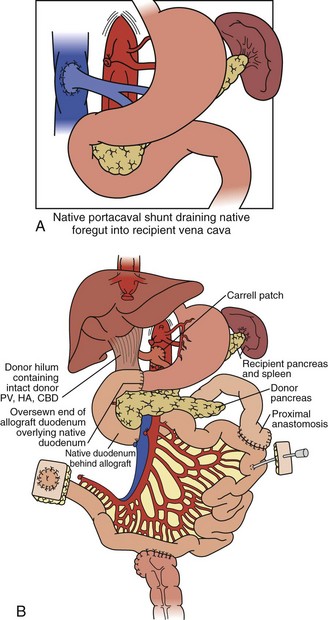
For combined small-bowel and liver transplant (Figure 198-4), the recipient hepatectomy is performed with preservation of the native retrohepatic inferior vena cava. The recipient foregut including stomach, native pancreas, and proximal duodenum is also preserved, and its outflow maintained with a permanent end-to-side portocaval shunt. The composite donor allograft includes the primary organs (liver and small bowel) as well as the donor duodenum and pancreas, allowing for maintenance of donor hepatobiliary continuity. Arterial inflow to the composite donor allograft is achieved using an arterial interposition conduit from the recipient infrarenal aorta. Liver venous outflow commonly involves the well-described “piggyback” technique, anastomosing donor suprahepatic inferior vena cava to the confluence of the recipient hepatic veins and cava. Intestinal reconstruction is performed in a similar fashion to an isolated intestinal transplant. Feeding tubes are placed as indicated.
Full Multivisceral Transplant
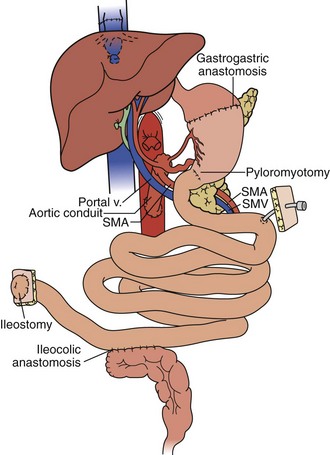
In the full multivisceral transplant procedure (Figure 198-5), prior to implantation, the recipient distal stomach, duodenum, pancreas, liver, and remaining small bowel are resected. The recipient inferior vena is meticulously preserved. The absence of remaining foregut or midgut precludes the need for portocaval shunt. Vascular inflow is similar to composite liver-bowel transplant but now includes celiac inflow to the stomach as well. Vascular outflow is identical to composite liver-bowel transplant. The donor spleen is removed from the composite allograft on the backtable prior to reperfusion.
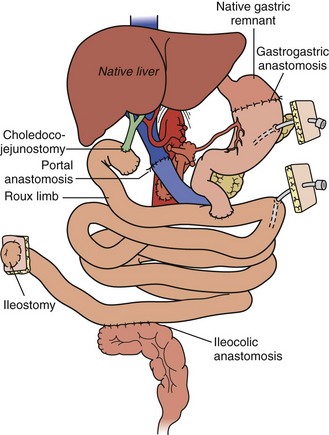
A “modified” multivisceral transplant (Figure 198-6) involves transplantation of a full composite allograft without a liver. The recipient liver is preserved along with its vasculature and extrahepatic biliary system. Vascular conduits are used routinely (Figure 198-7). This procedure involves disruption of hepatobiliary continuity, commonly requiring in children a recipient-to-donor Roux-en-Y hepatojejunostomy, and in adults a choledochocholedochostomy (duct-to-duct) anastomosis, as well as vascular anastomoses to the recipient common hepatic artery and portal vein.
 Immunosuppression
Immunosuppression
Two classes of immunomodulatory drugs have recently been introduced for intestinal transplantation and have been associated with improvements in 1-year patient and graft survival. Depleting antilymphocyte antibody therapies include rabbit antithymocyte globulin (rATG, Thymoglobulin [Genzyme Corp., Cambridge, Massachusetts]) and alemtuzumab (Campath-1H [Genzyme Corp.]). The individual use of these agents by high-volume single centers has demonstrated improved short-term survival and decreased rejection rates as well as severity.16,17,18 Associated with similar improvements in survival and decreased incidence of acute rejection and severity, induction with nondepleting interleukin (IL)-2 receptor antagonists, daclizumab (Zenapax) and basiliximab (Simulect), has also gained increasing acceptance by many intestinal transplant programs. Immunosuppression for intestinal and multivisceral transplantation now involves perioperative antibody induction in 60% of cases.
Immunologic Monitoring
The gold standard for monitoring and diagnosing rejection in intestinal and multivisceral transplant recipients remains routine ileoscopy and proximal enteroscopy with histopathologic examination of multiple random mucosal biopsies. Significant investigation is underway toward the development of tools to guide and monitor the immunologic state of the intestinal transplant recipient. Ideally, noninvasive markers such as serologic, proteomic, or genomic markers may identify those patients who are at increased risk of rejection and, conversely, those who might benefit from decreased levels of immunosuppression.19,20 Preformed antibody and de novo antidonor-specific antibody measurement may be of assistance in determining risk of rejection.21,22 When technically feasible, the presence of circulating donor cells in the recipient peripheral blood should be serially evaluated after transplantation by either flow cytometry or polymerase chain reaction (PCR). Monoclonal antibodies specific for donor HLA class I molecules are used for single-color immunofluorescence analysis. The presence of donor-specific antibodies in intestinal transplant recipients at the University of Pittsburgh prompts aggressive therapy with serial plasmapheresis and intravenous immunoglobulin (IVIG) until clearance of antibodies has been confirmed. For PCR analysis, primers specific for donor HLA class II alleles or else the sex-determining region of the Y chromosome (in male donor to female recipients) can be used. The use of fecal calprotectin or serum citrulline as noninvasive biochemical markers of allograft rejection does not appear to be warranted based upon currently available data.23,24
 Postoperative Management
Postoperative Management
Assessment Of Intestinal Allograft
Surveillance for intestinal allograft rejection in the early postoperative period focuses on clinical evaluation and gross morphologic examination of the stoma and distal ileum. Frequent routine enteroscopy surveillance is the most reliable method for achieving an early diagnosis of intestinal rejection (Figure 198-8). Endoscopic evaluations are performed initially twice a week through the allograft ileostomy; upper endoscopy is reserved for occasions where clinical changes are not well explained by distal allograft evaluation and biopsy. Common changes to the normal appearance of an intestinal allograft include edema, cyanosis, congestion, and increased stomal output. These changes should prompt an immediate workup, with a differential diagnosis that includes preservation injury (Figure 198-9), sepsis, rejection, and enteritis.
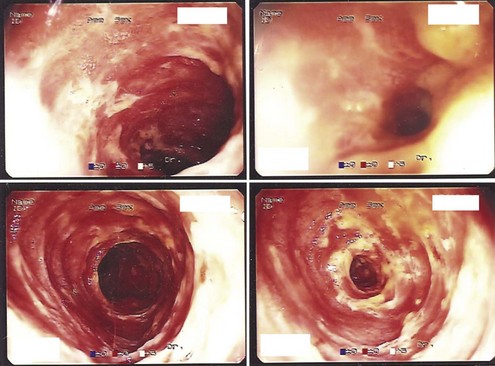
Figure 198-8 Enteroscopic findings consistent with acute cellular rejection of intestinal allograft.
(Courtesy Kareem Abu-Elmagd, MD.)
 Management of Allograft Rejection
Management of Allograft Rejection
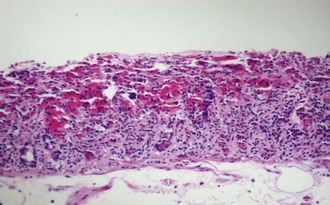
Allograft rejection (Figure 198-10) is strongly associated with graft loss and patient death and remains a significant obstacle to achieving successful long-term outcomes for intestinal and multivisceral transplant recipients. Historically, acute cellular rejection was reported in 70% to 90% of intestinal allografts within 90 days post transplant. In contrast, rejection rates of 30% to 40% are currently reported by large centers thanks to advances in allograft histopathologic surveillance, immunosuppression, and immunologic monitoring. Unlike liver allograft rejection, the natural history of rejection of intestinal allograft is unforgiving, making early diagnosis and treatment critical for successful reversal of the rejection process.
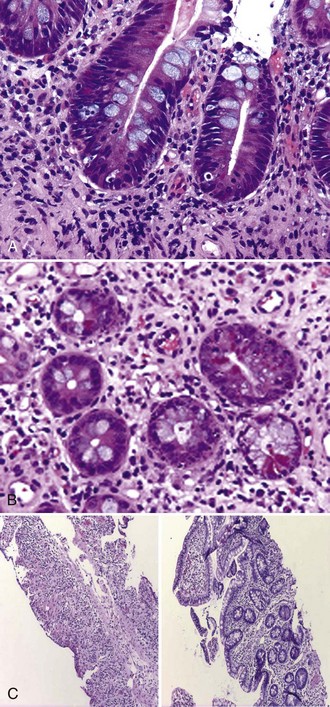
Figure 198-10 Acute cellular rejection of intestinal allograft: mild (A), moderate (B), and severe (C).
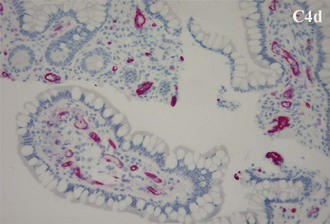
Antibody-mediated rejection (AMR) of the intestinal allograft (Figure 198-11) is characterized by intestinal dysfunction, diffuse C4d staining on allograft biopsy, and usually identification of donor-specific antibodies. Treatment of AMR consists of plasmapheresis in combination with IVIG and steroids. Rituximab or bortezomib can be used in select recipients.
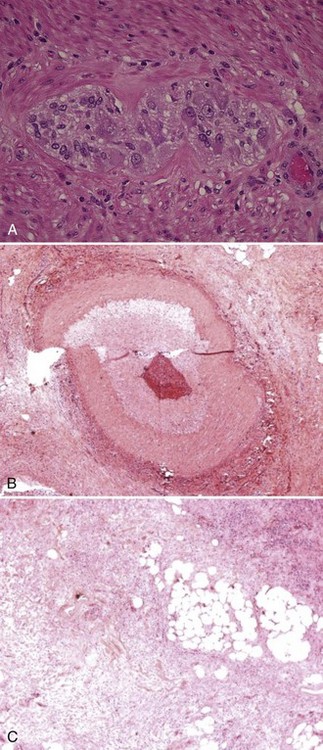
Chronic rejection (Figure 198-12) is observed in 10% to 15% of pediatric and adult intestinal allografts, occurring more commonly in isolated intestinal allografts. In adult recipients at the University of Pittsburgh, multivisceral transplants including a liver allograft demonstrated a significantly better chronic rejection-free survival compared with the liver-free intestinal and other multivisceral transplant recipients.25 Risk factors for chronic rejection include type of allograft and retransplantation. The clinical presentation of chronic rejection may include weight loss, chronic diarrhea, intermittent fevers, distal intestinal allograft obstruction, or GI bleeding. Histologically, chronic rejection is characterized by villous blunting, focal ulcerations, epithelial metaplasia, and scant cellular infiltrates on endoscopic mucosal biopsies. Full-thickness biopsies of intestinal allograft with chronic rejection demonstrate obliterative thickening of intestinal arterioles.
 Management of Complications
Management of Complications
Renal Complications
Deterioration of renal function in intestinal transplant recipients remains a significant clinical challenge. Pretransplant renal dysfunction is exacerbated by higher target levels of immunosuppression, repeated exposure to nephrotoxic antibiotics, and episodes of dehydration with intestinal allograft dysfunction. The incidence of chronic renal failure for intestinal transplant recipients at 5 years post transplant exceeds 20%.26 Overall, a review of Scientific Registry of Transplant Recipients (SRTR) data shows that patients without severe pretransplant renal dysfunction who do not receive a kidney as part of the composite allograft will generally demonstrate a 50% increase in serum creatinine at 5-year follow-up.
Posttransplant Lymphoproliferative Disorder
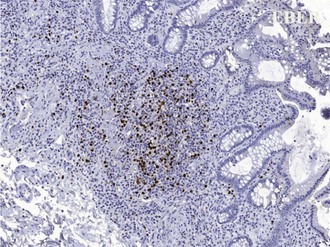
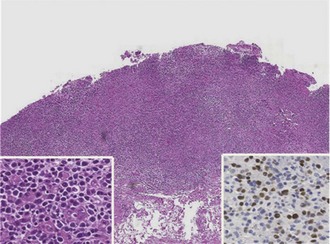
The development of PTLD is almost always associated with EBV infection. Posttransplant infection with EBV results in a spectrum of diseases, from mononucleosis syndromes and plasma cell hyperplasia to neoplastic PTLD (Figure 198-13). In a series of 500 intestinal and multivisceral transplants at the University of Pittsburgh, all but 2 of 57 recipients with PTLD developed the disorder as a consequence of confirmed EBV infection. Early studies found that primary tacrolimus use in pediatric patients was associated with a 15% long-term risk of PTLD, with almost 80% of these cases occurring within the first 2 years after transplant. Achieving an optimal immunosuppression steady state and avoiding excessive therapy intervals appear to be keys to minimizing EBV/PTLD complications. Cumulative PTLD-free survival for intestinal transplant recipients undergoing induction immunosuppression has improved to nearly 90%, possibly attributable to a lower incidence of acute rejection (and thus decreased need for escalation of immunosuppression) as well as improved EBV viral load monitoring.
Graft-Versus-Host Disease
Acute graft-versus-host disease (GVHD) results from immunocompetent donor T cells causing damage to recipient tissues after transplantation. The incidence of GVHD (Figure 198-14) after intestinal transplantation ranges between 5% and 10% and usually occurs within the first 6 months post transplant.27 The major targets of GVHD are epithelial cells of skin, intestine, and liver. Cardiac muscle involvement is not common but has been described. A recipient with GVHD commonly presents with fever and a maculopapular rash on the upper torso, neck, or palms of hands and feet, which may coalesce to form blisters or more diffuse erythema. Other clinical signs and symptoms include oral lesions, diarrhea, intestinal mucosal ulceration, native liver dysfunction, lymphadenopathy,28 and bone marrow suppression with pancytopenia. The variability of GVHD focality and severity leads to a wide spectrum of disease, from mild GVHD presenting with fevers and self-limiting rash to more severe forms leading to end-organ damage.
 Outcomes
Outcomes
Patient And Graft Survival
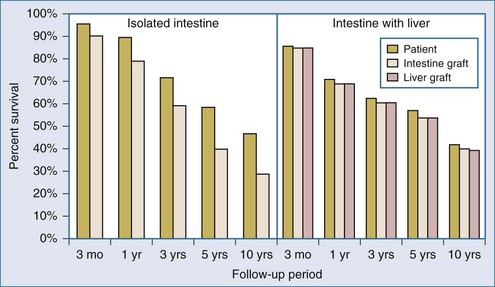
A significant improvement in early patient and graft survival after intestinal transplantation has been achieved over the past decade, with 1-year patient and graft survival (Figure 198-15) reaching 89.3% and 78.9% for intestine-only recipients and 71.5% and 69.0% for liver-intestine recipients, respectively. In 1998, the 1-year adjusted graft and patient survival after intestinal transplantation were only 52% and 69%, respectively. Updated outcomes for intestinal transplant recipients are now comparable to outcomes following pancreas, lung, and liver transplantation. Contributing factors to this marked improvement in outcomes after intestinal transplantation include increased experience among intestinal transplant teams, improvements in critical care, advances in immunosuppression, and advances in the detection and treatment of rejection. The hospitalization status of the recipient at the time of transplantation also remains a strongly predictive factor for patient survival, with an unadjusted 1-year survival rate of 83% for recipients not waiting in the hospital, 73% for recipients waiting in the hospital, and only 50% for recipients waiting in the ICU. In 1999, almost one-third of intestinal and multivisceral recipients were in intensive care at the time of transplantation, whereas in 2008, 70% were not in the hospital, and only 12% were in intensive care.
Long-Term Rehabilitation And Quality Of Life
Long-term functional outcomes after intestinal transplantation have not been fully characterized. A small preliminary study29 in pediatric recipients with functioning intestinal allografts more than 1-year post transplant found that quality of life was perceived by recipients to be comparable to that of their peers, while parental proxy assessments compared less favorably in terms of physical functioning, general health, and family activities. Younger recipients (5-10 years of age) demonstrated significantly worse outcomes than older recipients (11-18 years of age) in terms of global health assessments, general health perception, and family activities. There have been reports demonstrating significant improvement in certain aspects of psychiatric health after transition from parenteral nutrition to posttransplant TPN independence.30 In these reports, long-term physical and psychiatric rehabilitation were achieved in over 80% of intestinal transplant recipients who survived beyond the sixth postoperative month.
Abu-Elmagd KM, Costa G, Bond GJ, Soltys K, Sindhi R, Wu T, et al. Five hundred intestinal and multivisceral transplantations at a single center: major advances with new challenges. Ann Surg. 2009;250:567-581.
Beath S, Pironi L, Gabe S, et al. Collaborative strategies to reduce mortality and morbidity in patients with chronic intestinal failure including those who are referred for small bowel transplantation. Transplantation. 2008;85:1378-1384.
Fishbein TM. Current concepts: intestinal transplantation. N Engl J Med. 2009;361:999-1008.
Gupte GL, Beath SV. Update on intestinal rehabilitation after intestinal transplantation. Curr Opin Organ Transplant. 2009;14:1-7.
Mazariegos GV, Steffick DE, Horslen S, Farmer D, Fryer J, Grant D, et al. Intestine transplantation in the United States 1999-2008. Am J Transplant. 2010;10:1020-1034.
1 Mazariegos GV, Steffick DE, Horslen S, Farmer D, Fryer J, Grant D, et al. Intestine transplantation in the United States 1999-2008. Am J Transplant. 2010;10:1020-1034.
2 Berg CL, Steffick DE, Edwards EB, et al. Liver and intestine transplantation in the United States 1998-2007. Am J Transplant. 2009;9:907-931.
3 Spencer AU, Neaga A, West B, et al. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg. 2005;242:403-409.
4 Goulet O, Baglin-Gobet S, Talbotec C, et al. Outcome and long-term growth after extensive small bowel resection in the neonatal period: a survey of 87 children. Eur J Pediatr Surg. 2005;15:95-101.
5 Quiros-Tejeira RE, Ament ME, Reyen L, et al. Long-term parenteral nutrition support and intestinal adaptation in children with short bowel syndrome: a 25-year experience. J Pediatr. 2004;145:157-163.
6 Kocoshis SA, Beath SV, Booth IW, et al. Intestinal failure and small bowel transplantation, including clinical nutrition: Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterology Nutr. 2004;39:S655-S661.
7 Beath S, Pironi L, Gabe S, et al. Collaborative strategies to reduce mortality and morbidity in patients with chronic intestinal failure including those who are referred for small bowel transplantation. Transplantation. 2008;85:1378-1384.
8 Sudan D, DiBaise J, Torres C, et al. A multidisciplinary approach to the treatment of intestinal failure. J Gastrointest Surg. 2005;9:165-176.
9 Ching YA, Fitzgibbons S, Valim C, et al. Long-term nutritional and clinical outcomes after serial transverse enteroplasty at a single institution. J Pediatr Surg. 2009;44:939-943.
10 ASPEN Board of Directors and Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enter Nutr. 2004;28(6):S39-S70.
11 Wiles A, Woodward JM. Recent advances in the management of intestinal failure-associated liver disease. Curr Opin Clin Nutr Metab Care. 2009;12:265-272.
12 Diamond IR, Sterescu A, Pencharz PB, et al. Changing the paradigm: Omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48:209-215.
13 Gura KM, Lee S, Valim C, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121:e678-e686.
14 Beath S, Pironi L, Gabe S, et al. Collaborative strategies to reduce mortality and morbidity in patients with chronic intestinal failure including those who are referred for small bowel transplantation. Transplantation. 2008;85:1378-1384.
15 Abu-Elmagd K, Fung J, Bueno J, et al. Logistics and technique for procurement of intestinal, pancreatic, and hepatic grafts from the same donor. Ann Surg. 2000;232:680-687.
16 Reyes J, Mazariegos GV, Abu-Elmagd K, Macedo C, Bond GJ, Murase N, et al. Intestinal transplantation under tacrolimus monotherapy after perioperative lymphoid depletion with rabbit anti-thymocyte globulin (thymoglobulin). Am J Transplant. 2005 Jun;5(6):1430-1436.
17 Abu-Elmagd KM, Costa G, Bond GJ, Wu T, Murase N, Zeevi A, et al. Evolution of the immunosuppressive strategies for the intestinal and multivisceral recipients with special reference to allograft immunity and achievement of partial tolerance. Transpl Int. 2009 Jan;22(1):96-109.
18 Nishida S, Levi DM, Moon JI, Madariaga JR, Kato T, Selvaggi G, et al. Intestinal transplantation with alemtuzumab (Campath-1H) induction for adult patients. Transplant Proc. 2004 Mar;38(6):1747-1749.
19 Zeevi A, Britz JA, Bentlejewski CA, et al. Monitoring immune function during tacrolimus tapering in small bowel transplant recipients. Transplant Immunol. 2005;15:17-24.
20 Fishbein T, Novitskiy G, Mishra L, et al. NOD2-expressing bone marrow-derived cells appear to regulate epithelial innate immunity of the transplanted human small intestine. Gut. 2008;57:323-330.
21 Wu T, Abu-Elmagd K, Bond G, Demetris AJ. A clinicopathologic study of isolated intestinal allografts with preformed IgG lymphocytotoxic antibodies. Hum Pathol. 2004;35:1332-1339.
22 Kato T, Mizutani K, Terasaki P, et al. Association of emergence of HLA antibody and acute rejection in intestinal transplant recipients: a possible evidence of acute humoral sensitization. Transplant Proc. 2006;38:1735-1737.
23 Sudan D, Vargas L, Sun Y, et al. Calprotectin: a novel noninvasive marker for intestinal allograft monitoring. Ann Surg. 2007;246:311-315.
24 Akpinar E, Vargas J, Kato T, et al. Fecal calprotectin level measurements in small bowel allograft monitoring: a pilot study. Transplantation. 2008;85:1281-1286.
25 Abu-Elmagd K, Costa G, Bond G, Soltys K, Sindhi R, Wu T, et al. Five hundred intestinal and multivisceral transplantations at a single center. Ann Surg. 2009;250:567-581.
26 Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-940.
27 Mazariegos GV, Abu-Elmagd K, Jaffe R, et al. Graft versus host disease in intestinal transplantation. Am J Transplant. 2004;4:1459-1465.
28 Nodit L, Murase N, Reyes JD, et al. Transient posttransplant graft-versus-host lymphadenopathy. Pediatr Dev Pathol. 2004;7:533-537.
29 Sudan D, Horslen S, Botha J, et al. Quality of life after pediatric intestinal transplantation: the perception of pediatric recipients and their parents. Am J Transplant. 2004;4:407-413.
30 Grant D, Abu-Elmagd K, Reyes J, Tzakis A, Langnas A, Fishbein T, et al. 2003 Report of the intestine transplant registry: a new era has dawned. Ann Surg. 2005;241:607-613.